Abstract
Objectives
Coxsackievirus B3 (CVB3) induced myocarditis is sex dependent with males developing more severe disease than females. Previous studies had shown that sex-associated hormones determine the sex bias with testosterone and progesterone promoting myocarditis while estrogen (E2) is protective. There are two major estrogen receptors: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). The goal of the current study was to determine the relative role of these receptors to myocarditis susceptibility and the mechanism of their action.
Methods
Female C57Bl/6 wild-type mice and C57Bl/6 mice deficient in ERα, or ERβ were infected intraperitoneally with 102 plaque forming units CVB3. After 7 days, hearts were evaluated for virus titers by plaque forming assay and myocardial inflammation. Lymphoid cells either from the spleen or infiltrating the heart were characterized by labeling with antibodies including CD4, CD25, FoxP3, IFNγ, IL-4, CD11b, CD1d, Vγ4, TCRβ, or with CD1d-tetramer and evaluated by flow cytometry. To confirm that signaling through distinct estrogen receptors controlled myocarditis susceptibility and T-regulatory cell response, male C57Bl/6 mice were treated with the ERα-specific agonist, propyl pyrazole triol (PPT), ERβ agonist, diarylpropionitrile (DPN), or 17-β-estradiol (E2) as a non-specific estrogen receptor agonist.
Results
Myocarditis, cardiac virus titers, and CD4+ Th1 (IFNγ) bias were increased in infected ERαKO and decreased in infected ERβKO mice compared to C57Bl/6 controls. CD4+Th1 bias and myocarditis severity correlated inversely with numbers of CD4+CD25+FoxP3+ T regulatory cells which were decreased in ERαKO and increased in ERβKO mice. Increased T-regulatory cells corresponded to a preferential activation of natural killer T (NKT) cells in ERβKO mice. Male C57Bl/6 mice treated with DPN showed increased myocarditis while those treated with PPT and E2 showed decreased myocarditis corresponding to either decreased (DPN) or increased (PPT/E2) T-regulatory cell responses in male C57Bl/6 mice. DPN and PPT treatment had no effect on T-regulatory cell responses in NKT KO or γδKO mice.
Conclusion
These results demonstrate that ERα and ERβ both modulated CVB3 myocarditis susceptibility but in opposite directions and that their predominant effect is mediated through their ability to alter NKT and Vγ4+ innate T cell responses in the infected host. It is these innate T cells which positively or negatively modulate T-regulatory cell responses.
Keywords: Coxsackievirus B3, Myocarditis, Estrogen receptor alpha, Estrogen receptor beta, T-regulatory cells, CD4+ cells, Immunosuppression
Introduction
Myocarditis is an inflammation of the myocardium which often follows microbial infections. While somewhat controversial, many clinical studies report an increased incidence of viral myocarditis in men compared to women, although women are more susceptible during pregnancy [1-3]. Mice infected with coxsackievirus B3 (CVB3) develop myocarditis with many similar characteristics to the human disease. The inflammatory infiltrate is predominantly comprised of mononuclear cells and inflammatory cells are intimately associated with necrotic myocytes while adjacent myocytes appear unharmed [4,5]. Male and pregnant female mice are highly susceptible to CVB3 induced myocarditis while virgin females are more resistant [6-10]. Sex associated hormones control myocarditis since castration of males is protective while restoration of testosterone increases susceptibility [11-13]. Furthermore, treating male mice with 17-β-estradiol (E2) significantly suppresses myocarditis while treatment of females with androgen enhances myocarditis [14]. The effects of E2 on both innate and adaptive immunity have been extensively studied and show a wide range of hormonal effects. The generally accepted estrogen effects include increasing immunoglobulin synthesis [15]; suppressing both T and B cell lymphopoiesis [16]; enhancing dendritic cell differentiation and antigen presentation [17]; suppressing TNFα and IL-6 expression [18,19]; while increasing IL-4 and IFNγ production [20,21]; inhibiting B cell apoptosis [22]; and promoting FoxP3+ T regulatory cell development [23,24]. Although the above effects are generally agreed upon by the majority of investigators, divergent reports exist. For example, while most published studies demonstrate a positive effect on IFNγ production by E2, some studies show either no effect on the level of this cytokine [21] or increased expression [25]. Thus, the effects of E2 might not be absolute but depend upon various factors including hormone concentration, cell type/tissue involved, or unknown variables.
There are two known estrogen receptors (ERα and ERβ) which bind with equivalent specificity and affinity to estrogen response elements (ERE) in gene promoter regions leading to modulation of gene expression [26]. ERα and ERβ have wide tissue distribution with ERα primarily found in the uterus, liver, kidney and heart while ERβ is primarily found in ovary prostate, lung, central nervous system, gastrointestinal tract, and bladder. Lymphocytes, macrophages and dendritic cells co-express both ERα and ERβ [27] but ERα primarily controls E2 modulation of dendritic cell maturation, T cell cytokine production and immunoglobulin response [21,28,29]. In contrast, signaling through ERβ up-regulates inducible nitric oxide synthase (iNOS) and nitric oxide generation while ERα suppresses this response [30]. Several important studies now show that where ERα and ERβ are co-expressed in the same cell, these receptors may exert opposing effects on gene expression and thus counter-balance each other [25,26,31,32].
Previous studies have also demonstrated that selective generation of CD4+CD25+FoxP3+ T regulatory cells prevent CVB3 induced myocarditis and that E2 treatment of infected mice promotes T regulatory cell activation [33]. This observation is consistent with previous published reports showing the same phenomenon [24,34]. However, the use of estradiol in the previous studies fails to distinguish between the hormonal signaling between the ERα and ERβ forms as the hormone binds to and causes signal transduction through both receptors. The goal of the current study was to determine whether there was a distinctive role of each receptor on CVB3 myocarditis and on immunoregulation. As shown here, the study found that in the CVB3 induced myocarditis model, signaling through the ERα is responsible for T regulatory cell response while signaling through ERβ inhibits T regulatory cell activation. The significance of this observation may be that selective activation of individual hormonal receptors may identify a potential therapeutic approach.
Materials and Methods
Mice
Female C57Bl/6 ERαKO (B6.129P2-Esr1<tm1Ksk>/J), female C57Bl/6 ERβKO (B6.129P2-Esr2<tm1Unc>/J), male C57Bl/6 GFP-FoxP3 (B6.Cg-Foxp3tm2Tch/J), male and female C57BL/6J mice, and C57Bl/6 δ KO (B6.129P2-Tcrdtm1Mom/J) 4-7 weeks of age, were purchased from Jackson Laboratories, Bar Harbor ME. Jα18 deficient were backcrossed for more than 10 generations to the C57Bl/6 background and were kindly supplied by Dr. Jon Boyson (University of Vermont, Burlington VT). These animals lack natural killer T cells (NKT KO) [35]. All experimental groups contained 4-6 mice each. All of the studies have been reviewed and approved by the University of Vermont Institutional Animal Care and Use Committee.
Virus
The H3 variant of CVB3 was made from an infectious cDNA clone as described previously [36].
Infection of mice
Mice were injected intraperitoneally (i.p.) with 102 plaque forming units (PFU) virus in 0.5 ml PBS. Animals were killed when mortibund or 7 days after infection. Controls were uninfected mice which were killed at the same time as infected animals.
Organ virus titers
Hearts were asceptically removed from the animals, weighed, homogenized in RPMI 1640 medium containing 5% fetal bovine serum (FBS), L-glutamine, streptomycin and penicillin. Cellular debris was removed by centrifugation at 300 × g for 10 min. Supernatants were diluted serially using 10-fold dilutions and titered on Hela cell monolayers by the plaque forming assay [37].
Hormone treatment
17-β-estradiol (Sigma Chemical Co., St. Louis, MO) was initially (120 μg/ml) diluted in 100% ethanol then diluted to 400 ng/ml in corn oil. Mice were injected subcutaneously (s.c.) with either 200 ng/mouse estradiol or ethanol/corn oil on days −4, 0 and +4 relative to infection. Mice treated with hormone included male C57Bl/6, NKTKO and Vγ4KO animals.
Estrogen receptor agonists
The ERα selective agonist, propyl pyrazole triol (PPT), and the ERβ selective agonist, diarylpropionitrile (DPN), were purchased from Tocris Co, Ellisville MO, initially dissolved in DMSO, then diluted 1:10 in corn oil to inject 0.05 mM/kg body weight (19.8 mg/kg). Mice were injected s.c. with the agonists or DMSO/corn oil vehicle on days −4, 0 and +4 relative to infection [38]. Mice treated with hormone included male C57Bl/6, NKTKO and Vγ4KO animals.
Histology
Hearts were fixed in 10% buffered formalin for 48 h, paraffin embedded, sectioned and stained by hematoxylin and eosin. Image analysis of cardiac inflammation was done as described previously [36].
Isolation of Inflammatory cells from the heart
The protocol for isolating inflammatory cells infiltrating the hearts of CVB3 infected mice has been published previously [39]. Hearts were perfused with 10 ml PBS, removed, minced finely then subjected to a 10 min digestion with 0.4% collagenase II (Sigma Chemical Co., St. Louis MO) and 0.25% pancreatin (Sigma) at 37°C and removal of the supernatant to a tube containing 10% FBS. The remaining tissue was pressed through a fine mesh screen to release additional lymphoid cells. The large cellular debris was allowed to settle and the cell suspension containing the inflammatory cells was added to the cells released by digestion and layered on Histopaque (Sigma-Aldrich, St. Louis MO) and centrifuged at 300 × g for 25 min. The cells at the interface were retrieved, and washed in PBS-2% FBS.
Flow cytometry
Details for flow cytometry and intracellular cytokine staining have been published previously [40]. Unless otherwise indicated, all antibodies were obtained from BD Biosciences, BD Sciences, Fair Lawn, NJ. As indicated in the text, lymphoid cells used for flow cytometry were either isolated from the heart as described above, or were isolated from spleens of mice. When isolated from the spleen, spleens were pressed through fine mesh screens to form single cell suspensions and the suspensions were layered on Histopaque and centrifuged at 300 × g for 25 min. The cells at the interface were retrieved, and washed in PBS-2% FBS. For intracellular cytokine staining, 106 lymphoid cells were cultured for 4 h in RPMI 1640 medium containing 5% FBS, 10 μg/ml Brefeldin A (BFA), 50 ng/ml phorbol myristate acetate (PMA), and 500 ng/ml ionomycin (Sigma). After culture, the cells were washed in PBS-1% bovine serum albumin (BSA; Sigma) containing BFA, and labeled with PerCP-Cy5.5-anti-CD4 (clone GK1.5) for 30 min, washed, then fixed in 2% paraformaldehyde for 10 min. The cells were resuspended in PBS-BSA containing 0.5% saponin, Fc Block and 1:100 dilutions of PE-anti-IFNγ (clone XMG 1.2), Alexa 647-anti-IL-4 (clone 11B11), or PE and Alexa 647- rat IgG1 (clone R3-34) and incubated for 30 min on ice. The cells were washed once in PBS-BSA-saponin and once in PBS-BSA, then resuspended in 2% paraformaldehyde. FoxP3 labeling was done using the eBioscience kit according to manufacturer's directions. Cells were labeled with Alexa647 anti-CD4, FITC-rat-anti-mouse CD1d (clone 1B1) and PerCP-Cy5.5 anti-CD25 (clone PC61) in PBS-1% BSA containing FcBlock, washed, fixed and permeabilized, then incubated with PE-anti-FoxP3 (clone FJK-16s, eBiosciences, San Diego CA) and FcBlock overnight at 4°C. Cells were washed and resuspended in PBS-2% paraformaldehyde. Additional cells were labeled with APC-Cy7 anti-CD11b (clone M1/70), FITC-anti-CD1d in PBS-BSA on ice for 30 min, washed and resuspended in 2% paraformaldehyde. To evaluate natural killer T (NKT) and Vγ4+T cells, inflammatory cells isolated from hearts as described above were stained with PE-mCD1d-tetramer (NIH Tetramer facility Yerkes) and FITC-anti-TCRβ (clone H57-597) for NKT cells or with PE-anti-Vγ4 (clone UC3) and FITC anti-CD69 (clone H1.2F3) for Vγ4+ cells. Cells were incubated for 30 min, washed and resuspended in 2% paraformaldehyde for analysis. Cells were analyzed using a Coulter Epics Elite flow cytometer with a single excitation wavelength (488 nm) and band filters for FITC (525 nm), PerCP-Cy5.5 (695/40 nm), and PE (575 nm). The excitation wavelength for Alexa 647 is 643 nm and a band filter of 660/20 nm. The excitation wavelength for APC-Cy7 was 595 nm and a band filter of 650 nm. The cell population was classified for cell size (forward scatter) and complexity (side scatter). At least 10,000 cells were evaluated. Positive staining was determined relative to isotype controls.
Isolation of mouse T-regulatory cells
Isolation of CD4+CD25+ T regulatory cells from the spleen was performed using the EasySep™ Mouse CD4+CD25+ Regulatory T Cell Isolation Kit (StemCell Technologies, Vancouver, Canada) according to manufacturer's directions.
Adoptive transfer of CD4+CD25+ T regulatory cells into ERαKO recipients
CD4+CD25+ T regulatory cells were isolated from C57Bl/6, ERαKO and ERβKO female mice infected 7 days earlier with 102 PFU CVB3 as described above. Cells were resuspended in PBS, and 0.2 mL containing either 1.0 × 104 or 1.0 × 105 cells was injected through the tail vein of anesthetized female ERαKO recipient mice on the same day that the recipient mice were infected with 102 PFU CVB3. Recipient mice were killed 7 days later.
Statistics
Data was analyzed for skewness and kurtosis using the SSPS for Windows program (Version 11.0. Chicago, IL: SPSS, Inc.; 2001) and showed that variance was not normally distributed for several groups. Statistical analysis was done by the nonparametric Mann-Whitney test using SPSS for Windows. Threshold for significance was 0.05 or better.
Results
ERα Protects against CVB3 induced myocarditis
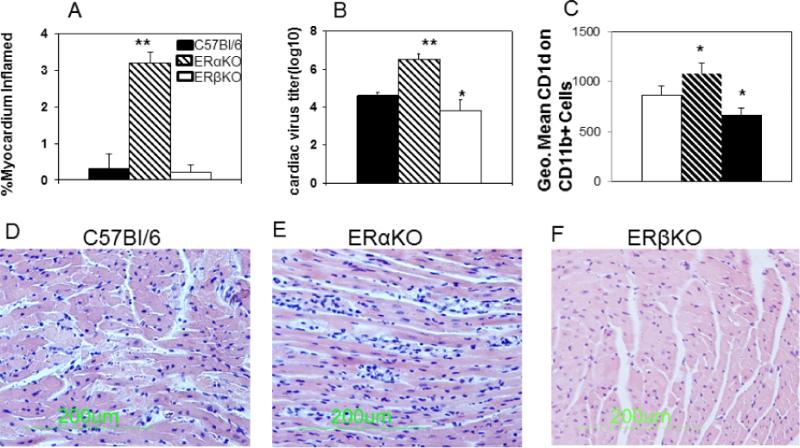
To determine whether estrogen-dependent protection requires either ERα or ERβ, wild-type C57Bl/6, ERαKO, and ERβKO female mice were infected with CVB3 and evaluated for myocarditis, and cardiac virus titers 7 days later (Figure 1). Neither C57Bl/6 nor ERβKO mice developed myocarditis (Figures 1A and 1F) while ERαKO mice were significantly more susceptible to myocarditis (Figures 1A and 1E; p<0.01). Cardiac virus titers were also significantly increased in infected ERαKO (p<0.01) mice and significantly decreased in infected ERβKO (p<0.05) females (Figure 1B). Spleen cells were labeled with anti-CD11b and anti-CD1d and evaluated by flow cytometry. Increased expression of CD1d on monocytes as determined by mean fluorescence intensity (MFI) staining was observed in ERαKO and decreased MFI staining was observed in ERβKO infected mice (Figure 1C; p<0.05).
Figure 1. ERα suppresses myocarditis susceptibility.
Female C57Bl/6, ERαKO and ERβKO mice, 4-7 weeks of age, were infected i.p. with 102 PFU CVB3 and killed 7 days later. Hearts were evaluated for (Sections A, D-F) myocarditis by staining formalin fixed sections with hematoxylin and eosin. Percentage myocardium inflamed for the total number of mice/group was determined by image analysis (A). Representative section from an individual mouse/group is shown in (D-F). Virus titers in the heart were determined by the plaque forming assay (B). Spleen lymphocytes were isolated from individual infected and uninfected mice, stained for CD1d and reported as mean fluorescence intensity gating on the CD11b+ population (C). Results are given as mean ± SEM from 5-6 mice/group. * and **Significantly different than C57Bl/6 mice at p<0.05 and p<0.01, respectively.
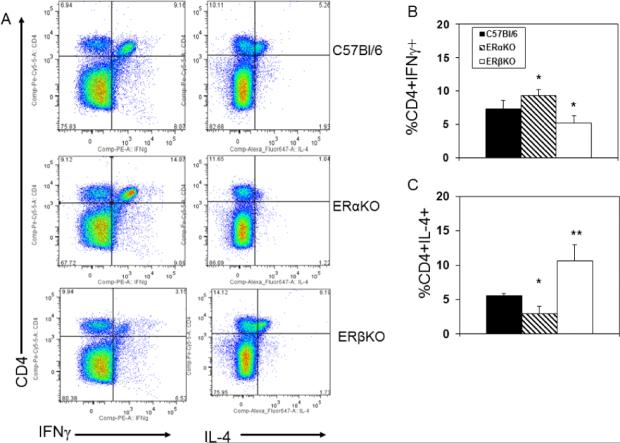
Effect of ERα and ERβ in IFNγ and IL-4 expression by CD4+ cells
C57Bl/6, ERαKO and ERβKO females were infected with CVB3 and killed 7 days later. Spleen cells were activated with PMA and ionomycin in the presence of brefeldin A, then labeled with antibody to CD4 and intracellularly with antibodies to IFNγ and IL-4 (Figure 2). Representative flow diagrams of cytokine expression from an individual mouse from each group are provided in Figure 2A and the summary of cytokine expression by all mice in each group are given in Figures 2B and 2C. IL-4 expression is significantly reduced in infected ERαKO mice (p<0.05) and increased in ERβKO (p<0.01) females. IFNγ expression is significantly increased in ERαKO mice but decreased in ERβKO animals (p<0.05 for both).
Figure 2.
ER modulates IL-4 and IFNγ expression by CD4+ T-cells. Spleen cells from C57Bl/6, ERαKO and ERβKO mice infected 7 days earlier with 102 PFU CVB3 were cultured for 4 h in medium containing PMA, ionomycin and brefeldin A, labeled with antibody to CD4, fixed, permeabilized, and labeled with antibodies to IFNγ and IL-4. Cells were evaluated by flow cytometry. (A) Representative flow diagrams from an animal in each group; (B) Mean (%) of cells staining positive for CD4 and IFNγ ± SEM. (C) Mean (%) cells staining positive for CD4 and IL-4 ± SEM. Groups consisted of 5-6 mice each. * and ** Significantly different than C57Bl/6 mice at p<0.05 and p<0.01, respectively.
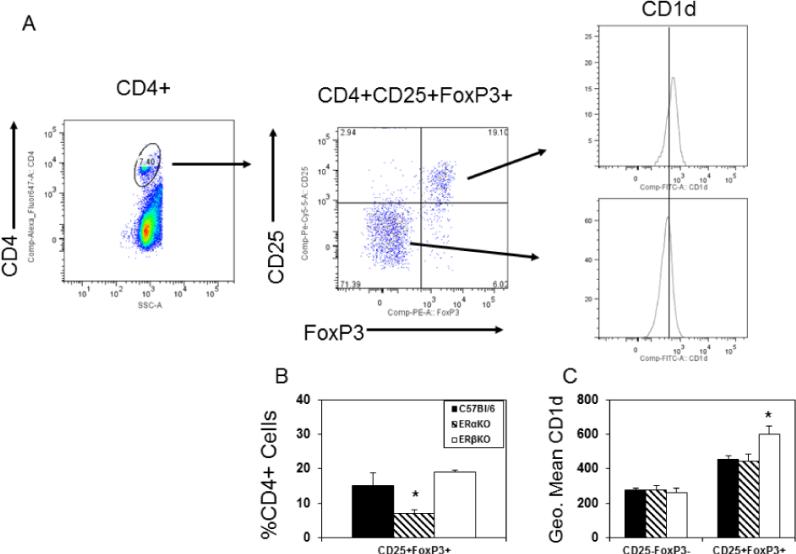
ERα promotes CD4+CD25+FoxP3+ T regulatory cell response during CVB3 infection
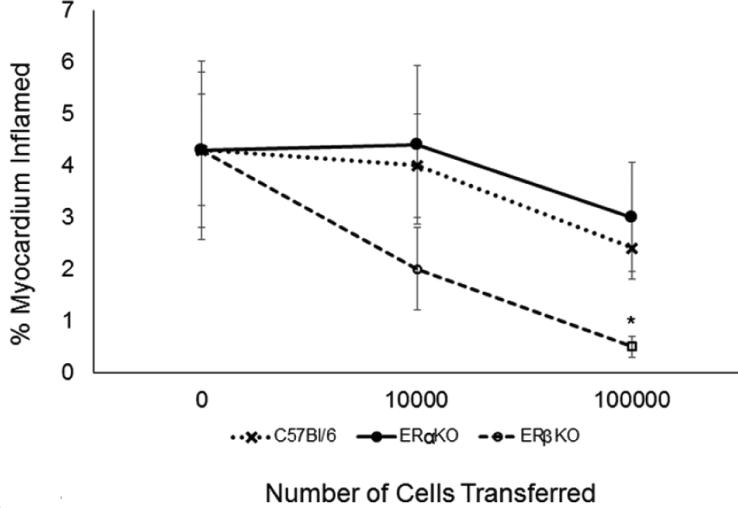
Prior studies found that females preferentially activate T regulatory cells when infected with CVB3 [33] and that this was mediated through the effects of estradiol. To determine whether the distinct estrogen receptors have different effects on T regulatory cell responses, spleen cells from C57Bl/6, ERαKO and ERβKO females infected 7 days previously with CVB3 were gated on the CD4+ cell population and evaluated for CD25 and FoxP3 expression (Figure 3). Approximately 24% of CD4+ cells in infected C57Bl/6 mice are CD25+FoxP3+ compared to only 15% of CD4+ cells from infected ERαKO mice indicating that significantly fewer T regulatory cells are generated in ERαKO mice (Figure 3B; p<0.05). No significant difference was observed in T regulatory cell numbers in infected ERβKO mice. CD4+CD25+FoxP3+ T cells express higher levels of CD1d than CD4+CD25−FoxP3− non-regulatory T cells (Figures 3A and 3C; p<0.05). However, when the CD4+CD25+FoxP3+ cells were evaluated for CD1d expression from the three different CVB3 infected mouse groups, ERβKO mice showed significantly increased MFI staining for CD1d than either C57Bl/6 or ERαKO females (Figure 3C; p<0.05). Since prior studies demonstrated CD1d expression levels by T regulatory cells correlated to their immunosuppressive activity in vivo [39], CD4+CD25+ cells were isolated from spleens of C57Bl/6, ERαKO and ERβKO female mice 7 days after CVB3 infection and either 104 or 105 cells were injected i.v. into female ERαKO mice on the same day as CVB3 infection. The recipients were killed 7 days later and evaluated for myocardial inflammation (Figure 4). Compared to infected ERαKO mice not receiving cells, both recipient groups given C57Bl/6 and ERαKO CD4+CD25+ T cells showed modest but not statistically significant suppression of myocarditis especially with 50,000 cells transferred. However, CD4+CD25+ cells from ERβKO donor mice were substantially more immunosuppressive (p<0.05).
Figure 3.
ERα promotes CD4+ T regulatory cell response. Spleen cells from C57Bl/6, ERαKO and ERβKO mice infected 7 days earlier with 102 PFU CVB3 were labeled with antibodies to CD4, CD1d and CD25, fixed, permeabilized and labeled with antibody to FoxP3 for flow cytometry analysis. (A) Cells were gated on the CD4+ cell population from an infected C57Bl/6 mouse, and then evaluated for CD25 and FoxP3 expression. The CD4+CD25−FoxP3− and CD4+CD25+FoxP3+ subpopulations were evaluated for CD1d expression. The line in the CD1d graph at far right indicates mean intensity fluorescence for the CD4+CD25−FoxP3− population and indicates that the CD4+CD25+FoxP3+ cells express higher levels of CD1d. (B) Summary of percentage of CD4+ cells which are CD25+FoxP3+ for 5-6 mice/group (mean ± SEM). (C) Summary of mean fluorescence intensity of staining for CD4+ cells which are either CD25−FoxP3− or CD25+FoxP3+ for 5-6 mice/group (mean ± SEM). *Significantly different than C57Bl/6 mice at p<0.05.
Figure 4.
Adoptive Transfer of CD4+CD25+ cells into CVB3 infected ERαKO female recipients. C57B/6, ERαKO and ERβKO female mice were infected with 102 PFU CVB3 and killed 7 days later. CD4+CD25+ T-cells were isolated from the spleen and 0, 104 or 105 cells were injected i.v. into infected female ERαKO recipients on the same day as infection. Recipients were killed 7 days later and evaluated for myocarditis. Results represent mean ± SEM of 4-6 mice/group. *Significantly different than C57Bl/6 mice at p<0.05.
Different effect of ERα and ERβ on NKT and Vγ4 T cell infiltration into the CVB3 infected heart
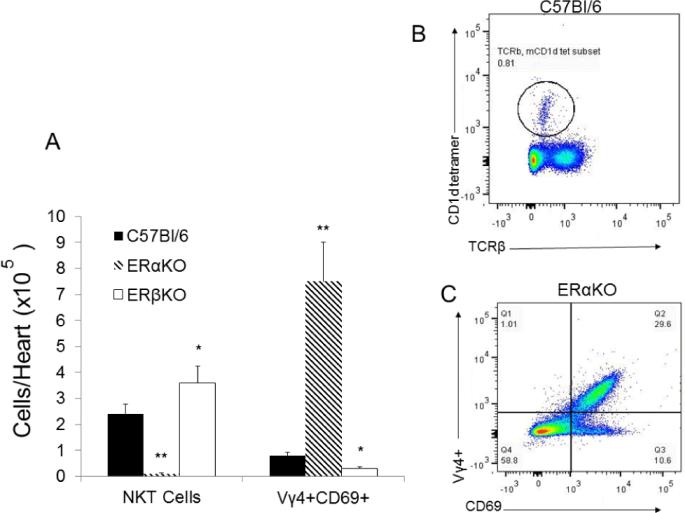
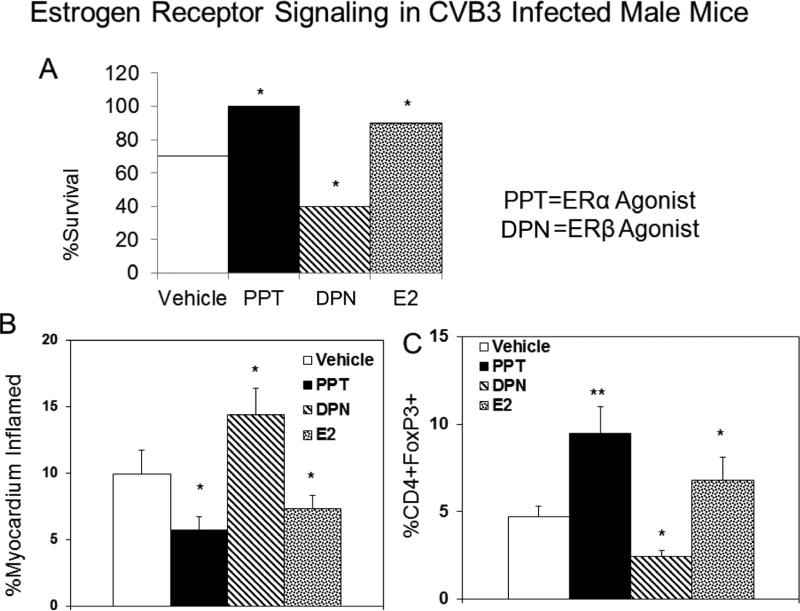
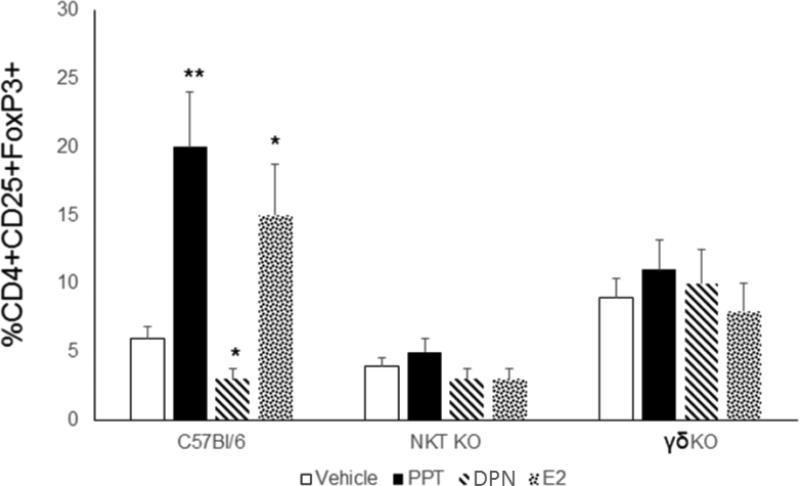
During CVB3 infections, innate T cells (NKT and γδ T cells) differentially control T regulatory cell activation [39,41,42]. Therefore, inflammatory lymphoid cells were isolated from hearts of female C57Bl/6, ERαKO and ERβKO mice 7 days after CVB3 infection evaluated for activated Vγ4+CD69+ T cells (Figure 5C) or NKT cells using the mCD1d-tetramer and antibody to TCRβ (Figure 5B). As shown in Figure 5A, ERαKO mice had few or no NKT cells infiltrating the hearts but had dramatically increased numbers of Vγ4+ cells compared to C57Bl/6 females (approximately 7-fold increase compared to C57Bl/6, p<0.01). ERβKO females showed slightly increased numbers of NKT (1.5 fold increase; p<0.05) and decreased Vγ4+ cells (p<0.05). To determine if the predominant immune modulatory role of estrogen receptors is mediated through these innate T cell mediators, initial studies confirmed that the receptor specific agonists PPT (ERα) and DPN (ERβ) replicated results using knockout mice. Male C57Bl/6 GFP-FoxP3 Tg mice were injected with PPT or DNP at 0.05 mM/kg on days −4, 0 and +4 relative to infection with CVB3. Male mice were also injected s.c. with estradiol (E2) as a non-specific ER agonist. Treatment of male mice with either E2 or PPT significantly reduced mortality (Figure 6A; p<0.05) and myocarditis (Figure 6B; p<0.05) while treatment with DPN actually promoted myocarditis (p<0.05). T regulatory cells were increased in males treated with PPT (p<0.01) and E2 (p<0.05) but were not increased by DPN treatment (Figure 6C). Finally, C57Bl/6, γδKO and NKT KO mice were treated with either vehicle, PPT, DNP or E2 and infected with CVB3 (Figure 7). While treating C57Bl/6 mice with PPT or E2 caused substantial increases in T regulatory cell responses compared to vehicle control (4-fold and 3-fold increase; p<0.01 and p<0.05), NKT KO or γδKO mice showed no difference between the treatment groups although NKTKO mice had fewer T regulatory cells while γδKO mice had greater numbers of T regulatory cells than C57Bl/6 mice.
Figure 5.
Estrogen receptor determines innate T-cell response in the heart. Female C57Bl/6, ERαKO and ERβKO mice were infected with CVB3 and killed 7 days later. Hearts were removed and infiltrating inflammatory cells were recovered and evaluated for natural killer T (NKT) cells using PE-mCD1d tetramer and FITC-anti-TCRβ. T-cells expressing the Vγ4 T-cell receptor (TCR) were identified by labeling cells with PE-anti-Vγ4 and FITC-anti-CD69. Summary of NKT and Vγ4+ cells in the different infected mouse strains is given in (A). Representative flow diagrams for NKT (B) and Vγ4+ (C) cells are provided. Results are given as mean ± SEM from 5-6 mice/group. * and **Significantly different than C57Bl/6 mice at p<0.05 and p<0.01, respectively.
Figure 6.
Treatment of male mice with either E2 or the ERα agonist protects against myocarditis and stimulates T regulatory cell response. Male C57Bl/6 GFP-FoxP3 Tg mice, 4-7 weeks of age, were injected s.c. with 0.05 mM/kg ERα agonist (PPT), ERβ agonist (DPN) or 200 ng/mouse E2 on days −4, 0, or +4 relative to infection with 102 PFU CVB3 on day 0. Control animals were injected s.c. with DMSO/corn oil only (vehicle). Groups contained 10 mice each. (A) Animal survival by day 7 after infection, (B) Percentage (%) of myocardium inflamed as determined by image analysis of hematoxylin and eosin stained sections, and (C) Percentage (%) of spleen cells which are positive for CD4 and the GFP-FoxP3. Results are given as mean ± SEM. * and **Significantly different than mice given vehicle at p<0.05 and p<0.01, respectively. Results are from 5-6 mice/group.
Figure 7.
ERα induction of T regulatory cells depends upon NK T-cells. Male C57Bl/6, NKT KO (Jα18KO) and γδKO mice were treated with 200 ng/mouse 17-β-estradiol or 0.05 mM/kg of the ERα and ERβ agonists (PPT and DPN) s.c., or with corn oil/DMSO (vehicle) as a control and infected with 102 PFU CVB3. Mice were killed 7 days after infection and spleens were evaluated for T-regulatory cells. Results are given as mean ± SEM. * and **Significantly different than mice given vehicle at p<0.05 and p<0.01, respectively. Results are from 5-6 mice/group.
Discussion
This paper shows that ERα and ERβ differentially regulate both myocarditis susceptibility and T regulatory cell responses primarily though their impact on the innate effector T cell populations NKT, and Vγ4+ cells. Previous studies in male mice showed that NKT cells preferentially activate T regulatory cells during CVB3 infections whereas T cells expressing the Vγ4 T cell receptor selectively abrogate immunosuppression by killing the CD1d+ CD4+CD25+FoxP3+ cell population [39,41-43]. Furthermore, studies showed that protection in females from CVB3-induced myocarditis was estrogen-dependent and was mediated through preferential activation of T regulatory cells in this sex [33,44]. However, the earlier study did not evaluate how the different estrogen receptors (ERα and ERβ) might selectively impact either CVB3 pathogenicity or T-regulatory cell responses. Sex hormones bind to specific nuclear and membrane-associated protein receptors resulting in diverse and often contradictory effects in innate and adaptive immunity. Evidence that membrane estrogen receptors (mER) are derived from the same genes as nuclear ERs comes from studies where deletion of the ERα or ERβ gene eliminates the function of both types of receptors [45]. A separate estrogen receptor is G protein-coupled ER (GPER), which is a 7-transmembrane protein that is unrelated to ERα or ERβ. Cells may express different combinations of the various ERs which may result in complex signaling patterns [46]. Among T cell populations, CD4+ cells in general express high levels of ERα but minimal or no ERβ [47]. However, this is not true for CD4+CD25+FoxP3+ T regulatory cells which express both ERs with high levels of ERβ (at least in patients with multiple sclerosis) [48]. Although this might have indicated that signaling through ERβ should preferentially promote immunosuppression through T regulatory cell activation, other studies have directly implicated ERα in T regulatory cell responses. One mechanism by which ERα might enhance T regulatory cell responses may be through direct upregulation of FoxP3 expression [34], although other mechanisms are also likely including impaired antigen presentation [23].
The significance of the current communication is that it provides another unique mechanism for hormonal control of T regulatory cell modulation different than those previously described. This study confirms that signaling through ERα promotes T regulatory responses leading to suppression of Th1 cell responses and protection from myocarditis during CVB3 infections of female mice. However, the effect does not appear to be mediated primarily through a direct effect on the T regulatory cell population since there was no increase in T regulatory cells in NKTKO or γδKO mice treated with PPT, the ERα agonist. If the effect of estrogen signaling is solely to upregulate FoxP3 expression in the CD4+CD25− cell population, lack of these innate T cells should not have hampered this response. The key may be in the level of CD1d expression which is substantially increased in mice lacking ERα. Prior studies have shown that CD1d expression levels are substantially higher in the myocarditis susceptible male than the myocarditis resistant female mice [49]. This suggests that ERα signaling either directly or indirectly influences CD1d expression. This could be either through hormone receptor binding to the CD1d promoter or through hormonal influence on the cytokine environment and antigen presenting cell activation altering interaction of macrophage/dendritic cells with the innate T cell subsets. Estrogen enhances dendritic cell maturation and antigen presentation resulting in increased MHC and accessory molecule expression, which may lead to stronger T cell-APC interactions [50]. Also sex hormones affect TLR expression and signaling. This may alter the cytokine environment in which the adaptive immune response develops [51,52].
Besides generating greater numbers of CD4+CD25+FoxP3+ T regulatory cells in CVB3 infected ERβKO mice, the suppressive activity of these cells is substantially increased compared to T regulatory populations from C57Bl/6 or ERαKO animals (Figure 4) on a per cell basis. Whether this indicates that these are a distinct subpopulation of T regulatory cells or whether activation of T regulatory cells through factors produced by NKT cells results in distinct gene expression profiles is currently unclear. Future studies will need to delineate why signaling through ERβ selectively allows activation of γδT cells whereas signaling through ERα selectively allows activation of NKT cells. Also, it is not clear where this signaling is important. One might assume that there might be differential estrogen receptor expression on the innate T cell effectors which control their activation profiles. Alternatively, and especially since CD1d expression levels on CD11b+ cells is significantly modulated through signaling by the different receptors (Figure 1), the effect may be indirect and mediated through interaction of either NKT or γδT cells with macrophage using the CD1d on the macrophage cell surface. Understanding the complexities of how estrogen signaling affects immune regulation during infection can have long-reaching impact not only for CVB3 and myocarditis, but might be relevant to other infectious, immunologic and autoimmune diseases as well.
Acknowledgement
This work was supported by National Institutes of Health grant HL108371. The PE-conjugated mCD1d tetramer was kindly supplied by the NIH Tetramer Core Facility (Yerkes) at Emory University. The author also wishes to thank Colette Charland and Roxana Del Rio Guerra for help with flow cytometry and Pamela Burton for help in preparing the manuscript.
References
- 1.Kühl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 2.Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101:425–484. [PMC free article] [PubMed] [Google Scholar]
- 4.Huber SA. Coxsackievirus-induced myocarditis is dependent on distinct immunopathogenic responses in different strains of mice. Lab Invest. 1997;76:691–701. [PubMed] [Google Scholar]
- 5.Woodruff JF, Woodruff JJ. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974;113:1726–1734. [PubMed] [Google Scholar]
- 6.Huber SA, Job LP, Auld KR. Influence of sex hormones on Coxsackie B-3 virus infection in Balb/c mice. Cell Immunol. 1982;67:173–179. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- 7.Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber SA, Kupperman J, Newell MK. Hormonal regulation of CD4(+) T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyden DC, Olszewski J, Feran M, Job LP, Huber SA. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–438. [PMC free article] [PubMed] [Google Scholar]
- 10.Lyden DC, Huber SA. Aggravation of coxsackievirus, group B, type 3-induced myocarditis and increase in cellular immunity to myocyte antigens in pregnant Balb/c mice and animals treated with progesterone. Cell Immunol. 1984;87:462–472. doi: 10.1016/0008-8749(84)90015-7. [DOI] [PubMed] [Google Scholar]
- 11.Huber SA, Job LP, Auld KR. Influence of sex hormones on Coxsackie B-3 virus infection in Balb/c mice. Cell Immunol. 1982;67:173–179. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- 12.Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber SA, Polgar J, Schultheiss P, Schwimmbeck P. Augmentation of pathogenesis of coxsackievirus B3 infections in mice by exogenous administration of interleukin-1 and interleukin-2. J Virol. 1994;68:195–206. doi: 10.1128/jvi.68.1.195-206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber SA, Kupperman J, Newell MK. Hormonal regulation of CD4(+) T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161:27–34. [PubMed] [Google Scholar]
- 16.Islander U, Erlandsson MC, Hasséus B, Jonsson CA, Ohlsson C, et al. Influence of oestrogen receptor alpha and beta on the immune system in aged female mice. Immunology. 2003;110:149–157. doi: 10.1046/j.1365-2567.2003.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- 18.Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, et al. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167:542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- 19.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpuzoglu-Sahin E, Hissong BD, Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52:113–127. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- 21.Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, et al. Estrogen receptor alpha (ERalpha) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17beta-estradiol acts through ERalpha to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol. 2005;175:5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 22.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Tai P, Wang J, Jin H, Song X, Yan J, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 25.Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, et al. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002;70:238–248. doi: 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- 26.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 27.Scariano JK, Emery-Cohen AJ, Pickett GG, Morgan M, Simons PC, et al. Estrogen receptors alpha (ESR1) and beta (ESR2) are expressed in circulating human lymphocytes. J Recept Signal Transduct Res. 2008;28:285–293. doi: 10.1080/10799890802084614. [DOI] [PubMed] [Google Scholar]
- 28.Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, et al. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol. 2008;180:727–738. doi: 10.4049/jimmunol.180.2.727. [DOI] [PubMed] [Google Scholar]
- 29.Douin-Echinard V, Laffont S, Seillet C, Delpy L, Krust A, et al. Estrogen receptor alpha, but not beta, is required for optimal dendritic cell differentiation and [corrected] CD40-induced cytokine production. J Immunol. 2008;180:3661–3669. doi: 10.4049/jimmunol.180.6.3661. [DOI] [PubMed] [Google Scholar]
- 30.Tsutsumi S, Zhang X, Takata K, Takahashi K, Karas RH, et al. Differential regulation of the inducible nitric oxide synthase gene by estrogen receptors 1 and 2. J Endocrinol. 2008;199:267–273. doi: 10.1677/JOE-07-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 32.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 33.Huber SA. Coxsackievirus B3-induced myocarditis: infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology. 2008;378:292–298. doi: 10.1016/j.virol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- 35.Cui J, Shin T, Kawano T, Sato H, Kondo E, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 36.Knowlton KU, Jeon ES, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70:7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Houten N, Bouchard PE, Moraska A, Huber SA. Selection of an attenuated Coxsackievirus B3 variant, using a monoclonal antibody reactive to myocyte antigen. J Virol. 1991;65:1286–1290. doi: 10.1128/jvi.65.3.1286-1290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, McMurray RW. Effects of estrogen receptor subtype-selective agonists on immune functions in ovariectomized mice. Int Immunopharmacol. 2006;6:1413–1423. doi: 10.1016/j.intimp.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Huber SA. Gammadelta T lymphocytes kill T regulatory cells through CD1d. Immunology. 2010;131:202–209. doi: 10.1111/j.1365-2567.2010.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber SA, Graveline D, Born WK, O'Brien RL. Cytokine production by Vgamma(+)-T-cell subsets is an important factor determining CD4(+)-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J Virol. 2001;75:5860–5869. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber SA. Depletion of gammadelta+ T cells increases CD4+ FoxP3 (T regulatory) cell response in coxsackievirus B3-induced myocarditis. Immunology. 2009;127:567–576. doi: 10.1111/j.1365-2567.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W, Moussawi M, Roberts B, Boyson JE, Huber SA. Cross-regulation of T regulatory-cell response after coxsackievirus B3 infection by NKT and γδ T cells in the mouse. Am J Pathol. 2013;183:441–449. doi: 10.1016/j.ajpath.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W, Huber SA. Cross-talk between cd1d-restricted nkt cells and γδ cells in t regulatory cell response. Virol J. 2011;8:32. doi: 10.1186/1743-422X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Job LP, Lyden DC, Huber SA. Demonstration of suppressor cells in coxsackievirus group B, type 3 infected female Balb/c mice which prevent myocarditis. Cell Immunol. 1986;98:104–113. doi: 10.1016/0008-8749(86)90271-6. [DOI] [PubMed] [Google Scholar]
- 45.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 46.Soltysik K, Czekaj P. Membrane estrogen receptors - is it an alternative way of estrogen action? J Physiol Pharmacol. 2013;64:129–142. [PubMed] [Google Scholar]
- 47.Yakimchuk K, Jondal M, Okret S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Mol Cell Endocrinol. 2013;375:121–129. doi: 10.1016/j.mce.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Aristimuno C, Teijeiro R, Valor L, Alonso B, Tejera-Alhambra M, et al. Sex-hormone receptors pattern on regulatory T-cells: clinical implications for multiple sclerosis. Clin Exp Med. 2012;12:247–255. doi: 10.1007/s10238-011-0172-3. [DOI] [PubMed] [Google Scholar]
- 49.Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Med Microbiol Immunol. 2005;194:121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- 50.Coquet JM, Rausch L, Borst J. The importance of co-stimulation in the orchestration of T helper cell differentiation. Immunol Cell Biol. 2015;93:780–788. doi: 10.1038/icb.2015.45. [DOI] [PubMed] [Google Scholar]
- 51.Kanno Y, Vahedi G, Hirahara K, Singleton K, O'Shea JJ. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol. 2012;30:707–731. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]