Abstract
Background
Heart failure prediction after acute myocardial infarction may have important clinical implications.
Objective
To analyze the functional echocardiographic variables associated with heart failure in an infarction model in rats.
Methods
The animals were divided into two groups: control and infarction. Subsequently, the infarcted animals were divided into groups: with and without heart failure. The predictive values were assessed by logistic regression. The cutoff values predictive of heart failure were determined using ROC curves.
Results
Six months after surgery, 88 infarcted animals and 43 control animals were included in the study. Myocardial infarction increased left cavity diameters and the mass and wall thickness of the left ventricle. Additionally, myocardial infarction resulted in systolic and diastolic dysfunction, characterized by lower area variation fraction values, posterior wall shortening velocity, E-wave deceleration time, associated with higher values of E / A ratio and isovolumic relaxation time adjusted by heart rate. Among the infarcted animals, 54 (61%) developed heart failure. Rats with heart failure have higher left cavity mass index and diameter, associated with worsening of functional variables. The area variation fraction, the E/A ratio, E-wave deceleration time and isovolumic relaxation time adjusted by heart rate were functional variables predictors of heart failure. The cutoff values of functional variables associated with heart failure were: area variation fraction < 31.18%; E / A > 3.077; E-wave deceleration time < 42.11 and isovolumic relaxation time adjusted by heart rate < 69.08.
Conclusion
In rats followed for 6 months after myocardial infarction, the area variation fraction, E/A ratio, E-wave deceleration time and isovolumic relaxation time adjusted by heart rate are predictors of heart failure onset.
Keywords: Heart Failure / complications, Myocardial Infarction, Rats, Ventricular Dysfunction
Introduction
Heart failure syndrome is considered a public health problem with important prognostic implications. In this sense, around 50% of patients with cardiac dysfunction die within 5 years. In addition, 40% of patients die during the period of 1 year after the first hospitalization for heart failure, with many of the deaths occurring as sudden death.1,2
It is currently believed that the myocardial infarction (MI) is the main etiology of ventricular dysfunction. In this sense, epidemiological studies suggest that the signs and symptoms of heart failure are present in 25% of MI cases. Moreover, approximately 40% of MI cases are accompanied by systolic alterations in the left ventricle (LV). Recently, it was verified that 10% of MI patients have a restrictive pattern, suggesting severe diastolic dysfunction.3 Thus, the association between MI and ventricular dysfunction cannot be neglected.
One of the most often used strategies for the study of functional alterations caused by coronary occlusion is the use of the experimental infarction model in rats. Among other factors, this is due to the low cost and simplicity of handling these animals. The most important factor, however, refers to the similarity with the physiopathological changes that occur after an infarction in humans.4
Echocardiography has been widely used in the study of the morphological and functional alterations after coronary occlusion.5-18 However, there has been no consensus on which functional variables are predictive of heart failure in this model. Thus, our objective was to evaluate the functional variables associated with heart failure in the model. In addition, we intend to determine the critical values for heart failure prediction for each variable.
Methods
The experimental protocol of this study was approved by the Animal Experimentation Ethics Committee of our institution, complying with the Ethical Principies in Animal Experimentation adopted by the Brazilian College of Animal Experimentation.
Experimental infarction
Male Wistar rats, weighing between 200 and 250 g were studied. Acute myocardial infarction was produced according to the previously described method.19,20 Briefly, the rats were anesthetized with ketamine (70 mg/kg) and xylazine (5 mg/kg) and submitted to a left lateral thoracotomy. After exteriorization of the heart, the left atrium was moved away and the left coronary artery was ligated with a 5-0 monofilament nylon suture between the pulmonary artery outflow tract and the left atrium. Subsequently, the heart was returned to the chest, the lungs were inflated with positive pressure and the thorax closed using cotton 10 sutures. Coronary occlusion was not performed in 43 animals (Control Group).
The animals were kept in cages for recovery, fed standard commercial chow and had free access to water, with regular 12 hour light: dark cycles, at a temperature of approximately 25° C and controlled humidity.
Echocardiographic study
Echocardiography was performed 6 months after the infarction. The animals were anesthetized intramuscularly with ketamine (50 mg/kg) and xylazine (1 mg/kg), for the echocardiographic study. After trichotomy of the anterior chest region, the animals were positioned in the supine position in a specially designed grooves that allows slight left lateral rotation for the examination, using Philips equipment (HDI 5000 model) equipped with a multi-frequency electronic transducer up to 12 MH. All measurements were made in accordance with the recommendations of the American Society of Echocardiography/European Association of Echocardiography.21 Left ventricular cavity image was obtained by positioning the M-mode cursor between the papillary muscles, right below the mitral valve plane. The LV diastolic diameter (LVDD) and left ventricular septal thickness (LVST) were measured at the moment corresponding to the maximum cavity diameter. The LV Systolic diameter (LVSD) was measured at the maximum systolic excursion of the posterior wall of the cavity. Diastolic (DA) and systolic (SA) areas of LV were measured in two-dimensional mode, using planimetry at the parasternal plane of the smaller axis.
LV systolic function was assessed by calculating the area variation fraction (AVF = (DA-SA)/DA) and the posterior wall shortening velocity (PWSV). Diastolic function was assessed by the E/A ratio, the E-wave deceleration time (EDT) and the Isovolumic Relaxation Time Adjusted for Heart Rate (IVRT/HR).22,23
Histological analysis
After the echocardiographic study, the animals were euthanized and the hearts were removed and dissected. The right and left ventricles, including the interventricular septum, were separated. Cardiac tissue samples were fixed in a 10% formaldehyde solution for 48 hours, according to the previously described method.24,25
The histological sections were stained on slides with hematoxylin-eosin (HE) and Masson solution for assessment of the infarcted tissue, using a Leica DM LS microscope coupled to a video camera, which sends digital images to a computer with the Image Pro-Plus imaging analysis program (Media Cybernetics, Silver Spring, Maryland, USA).
The infarction size was determined in 5 to 6-mm sections from the apex, as the values in this region correspond to the mean values obtained from sections of the entire heart.24,25 To estimate the infarction size through histological analysis, the epicardial and endocardial circumferences of the infarcted and non-infarcted segments were determined. The infarction size is expressed as a percentage of the ventricular circumference measurements.
Heart failure criteria
The diagnosis of heart failure was made by thrombus detection in the left atrium, pleural effusion, ascites, and right ventricular hypertrophy, characterized by the ratio of the right ventricle weight adjusted for body weight > 0.8 mg/g, as previously described.4,26,27
Statistical analysis
The comparisons between the groups after 6 months were carried out using Student's t test when data showed normal distribution. When the data did not have normal distribution, comparisons between groups were performed using the Mann-Whitney U test. Data were expressed as mean ± standard deviation or median, with 25 and 75 percentiles. The predictive values were analyzed by logistic regression. In this analysis, the presence or absence of heart failure was used as the dependent variable. The cutoff values predictive of heart failure were determined using ROC curves. The significance level was set at 5%. Statistical analyses were performed using the SigmaPlot program for Windows, v.12.0 (Systat Software Inc., San Jose, CA, EUA).
Results
Six months after surgery, 88 animals with infarction (I) and 43 control animals (C) were included in the study.
Echocardiographic variables are shown in Table 1. As expected, the infarction increased the left cavity diameters, LV mass and wall thickness. Additionally, the MI resulted in systolic and diastolic dysfunction, characterized by lower values of the AVF, PWSV, EDT, associated with higher values of E/A ratio and IVRT/HR.
Table 1.
Echocardiographic study after six months of observation
| Variables | Control (n = 43) | AMI (n = 88) | p-value |
|---|---|---|---|
| LAD (mm) | 5.80 (5.55-6.10) | 7.72 (6.70-8.59) | < 0.001 |
| LVDD (mm) | 8.39 (8.12-8.80) | 10.96 (10.32-11.73) | < 0.001 |
| LVSD (mm) | 4.34 (4.08-4.66) | 8.70 (7.61-9.75) | < 0.001 |
| LVMI | 1.94(1.74-2.11) | 3.33 (2.79-4.09) | < 0.001 |
| E/A | 1.55 (1.40-1.69) | 1.68 (1.32-4.85) | 0.085 |
| IVRT/HR | 59.3 (5452-64.8) | 70.4 (61.4-77.9) | < 0.001 |
| EDT ( ms) | 45 (41-55) | 39 (33-48 | < 0.001 |
| AVF (%) | 67 (64-71) | 33 (35-36) | < 0.001 |
| PWSV (mm/s) | 37 (35-39) | 25 (20-28) | < 0.001 |
| PWDT | 1.49 (1.43-1.59) | 1.70 (1.59-1.87) | < 0.001 |
Data are expressed as median with 25 and 75 percentiles. AMI: animals with acute myocardial infarction; LAD: left atrium diameter; LVDD: left ventricular diastolic diameter; LVSD: left ventricular systolic diameter; LVMI: left ventricular mass index; E/A: E/A wave ratio; IVRT/HR: isovolumic relaxation time adjusted by heart rate; EDT: E-wave deceleration time; AVF: area variation fraction; PWSV: posterior wall shortening velocity; EDPP: posterior wall diastolic thickness.
Considering the infarcted animals, 54 animals (61%) developed heart failure. Animals with heart failure had larger infarctions (43.5 ± 7.5% vs. 40.0 ± 8.1%; p = 0.044), higher mass index and left cavity diameters, associated with worsening of functional variables, compared with the infarcted animals without heart failure (Table 2).
Table 2.
Echocardiographic study of infarcted animals after 6 months of observation
| Variables | Without HF (n = 34) | With HF (n = 54) | p-value |
|---|---|---|---|
| LAD (mm) | 6.90 ± 1.29 | 8.16 ± 1.30 | < 0.001 |
| LVDD (mm) | 10.6 ± 0.84 | 11.4 ± 1.05 | < 0.001 |
| LVSD (mm) | 8.17 ± 1.13 | 9.02 ± 1.39 | 0.002 |
| LVMI | 3.14 (2.81-3.59) | 3.64 (2.78-4.50) | 0.018 |
| E/A | 1.43 (1.24-1.70) | 3.91 (1.35-6.27) | 0.002 |
| IVRT/HR (ms) | 73.9 ± 12.1 | 66.7 ± 12.0 | 0.008 |
| EDT (ms) | 43.1 ± 8.8 | 38.3 ± 10.2 | 0.036 |
| AVF (%) | 33.3 ± 8.6 | 29.6 ± 7.6 | 0.040 |
| PWSV | 25.9 (22.6-28.4) | 24.2 (20.6-28.3) | 0.344 |
Data are expressed as mean ± standard deviation (for normal distribution) or median with 25 and 75 percentiles (for non-normal distribution). HF: heart failure; LAD: left atrium diameter; LVDD: left ventricular diastolic diameter; LVSD: left ventricular systolic diameter; LVMI: left ventricular mass index; E/A: E/A wave ratio; IVRT/HR: isovolumic relaxation time adjusted by heart rate; EDT: E-wave deceleration time; AVF: area variation fraction; PWSV: posterior wall shortening velocity.
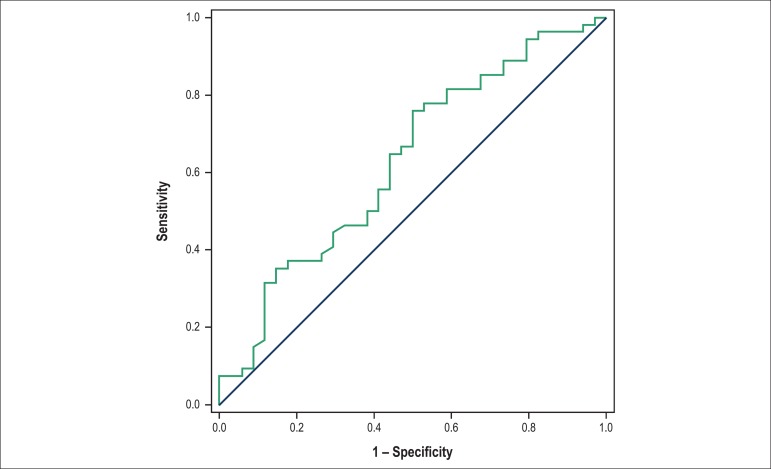
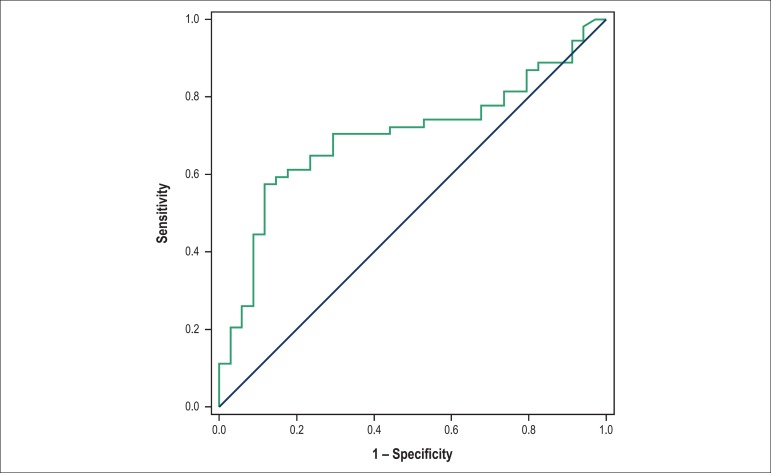
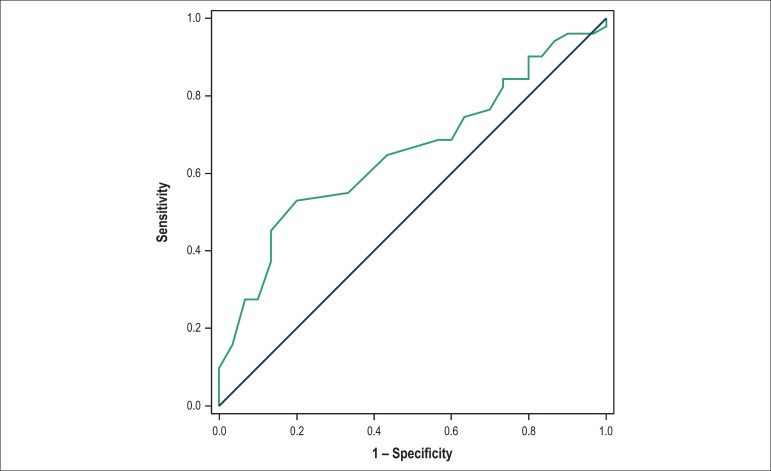
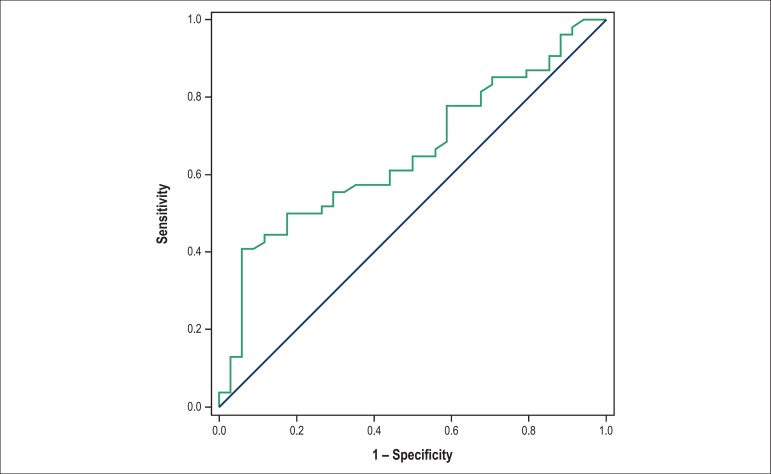
Table 3 shows the results of the regression analyses. AVF, E/A ratio, EDT and IVRT/HR were the functional variables predictors of heart failure. However, we found that the predictive value was low for the functional variables, suggesting the importance of other variables. Additionally, the cutoff values of the functional variables associated with heart failure were: AVF: < 31.18% (Figure 1); E/A ratio > 3.077 (Figure 2); EDT: < 42.11 ms (Figure 3) and IVRT/HR: < 69.08 (Figure 4).
Table 3.
Heart failure predictors 6 months after coronary occlusion
| Variables | OR | 95%CI | p-value |
|---|---|---|---|
| E/A | 1.529 | 1.183-1.976 | 0.001 |
| IVRT/HR | 0.949 | 0.911-0.989 | 0.013 |
| EDT (ms) | 0.951 | 0.906-0.998 | 0.040 |
| AVF (%) | 0.944 | 0.892-0.999 | 0.045 |
| PWSV (mm/s) | 0.077 | 0.905-1.055 | 0.554 |
OR: odds ratio; 95%CI: 95% confidence interval; E/A: E wave/A wave ratio; IVRT/HR, isovolumic relaxation time adjusted by heart rate; DTE: deceleration time of E wave; AVF: area variation fraction; PWSV: posterior wall shortening velocity.
Figure 1.
Cutoff value for the area variation fraction, as a heart failure predictor 6 months after infarction. Area under the curve: 0.6277; 95% confidence interval: 0.5066 to 0.7489; p-value: 0.044; cutoff < 31.18; sensitivity: 55.60%; specificity: 57.34%.
Figure 2.
Cutoff value for the E/A ratio as a heart failure predictor 6 months after infarction. Area under the curve: 0.6985; 95% confidence interval: 0.5875 to 0.8095, p-value: 0.0017; cutoff > 3,077; sensitivity: 57.93%o; specificity: 62.56%o.
Figure 3.
Cutoff value for the deceleration time of the E wave, as a heart failure predictor 6 months after infarction. Area under the curve: 0.6533; 95% confidence interval: 0.5341 to 0.7724; p-value: 0.0218; cutoff < 42.11; sensitivity: 59.77%; specificity: 51.85%.
Figure 4.
Cutoff value for the isovolumic relaxation time adjusted by heart rate as a heart failure predictor 6 months after infarction. Area under the curve: 0.6544; 95% confidence interval: 0.5398 to 0.7691; p-value: 0.01512; cutoff < 69.08; sensitivity: 55.10%; specificity: 60.49%.
Discussion
The aim of our study was to evaluate the functional variables associated with heart failure in an experimental MI model in rats. Our data suggested that AVF, E/A ratio, EDT and IVRT/HR are predictors of heart failure 6 months after infarction.
The first aspect to be considered is that in our study, most animals (61%) with infarction developed heart failure. In a previous study, we determined that infarctions affecting 40% of LV are required for the development of heart failure in this model.26 in accordance with this concept, in this study, the animals showed, on average, large infarcts. In this sense, we can infer that the coronary occlusion model in rats is appropriate for the study of the heart failure syndrome.
The second important aspect is related to the fact that, as expected, animals with heart failure had worsening of functional variables related to systolic function, when compared with animals without failure. However, in the regression analysis, the AVF, but not LVPW, was predictive of heart failure onset. It is believed that in models of regional LV akinesia, one-dimensional echocardiographic methods of functional assessment may be flawed. In this situation, it is recommended to use, for instance, the Simpson's method in humans. AVF is obtained through the analysis of the SA and DA, using the two-dimensional technique. However, the LVPW is obtained in single-dimensional mode. Therefore, our results emphasize that, in this model, similar to what occurs in humans, it is preferable to use systolic functional analysis with two-dimensional technique, such as AVF, for instance.
Another prominent aspect is related to the diastolic function. Unlike systolic function, the study of diastolic function through echocardiography in the rat model is not well standardized. Some of the main technical difficulties are the small size of the animal, with its implications on the transducer and heart rate of around 300 beats per minute. In our study, however, all assessed diastolic function variables were associated with heart failure onset. Thus, in this model, diastolic function assessed by E/A ratio, EDT and IVRT/HR were predictors of heart failure 6 months after coronary occlusion.
The most important aspect of our study is that heart failure prediction in the MI rat model has important implications. Although there is no consensus on the definition of cardiac dysfunction and heart failure, they are usually diagnosed by elevated end-diastolic pressure (PD2) of the LV (invasive hemodynamic method) and the presence of clinical signs assessed after death (RV hypertrophy, ascites, pleural effusion and left atrial thrombus), respectively. Therefore, our study suggests that echocardiography is a useful non-invasive tool for the prediction of this syndrome, as systolic function variables, as well the diastolic function parameters, little studied in this model, were associated with heart failure.
Previous studies have evaluated the association between echocardiographic parameters and heart failure. However, most studies have assessed the association between echocardiography and PD2, and not with heart failure clinical variables.6-10 Martinez et al.5 assessed the association between morphological and functional cardiac variables with the clinical manifestations of heart failure. However, the echocardiographic variables were studied through cluster analysis.5 Therefore, we believe that our study adds important information about the role of echocardiography as a predictor of long-term heart failure in this model.
Finally, it is already well-established that the major determinant of ventricular function, of the remodeling process and, consequently, of heart failure onset in this model, is the size of the infarction.4,26,28,29 In our study, however the difference in infarction size among animals with and without heart failure, although significant, was low (43 ± 7% vs. 40 ± 8%, respectively). Therefore, we conclude that other factors rather than the infarction size, are important determinants of heart failure onset in this model.
Among the possible candidates, we can include changes in ventricular cavity diameter, wall thickness alterations, changes in the normal LV configuration, from elliptical to the rounded shape, among others. Therefore, in some situations, changes in geometry alone could be responsible for ventricular global function impairment, by changing the load conditions to which the heart is submitted.
Conclusion
In rats followed for 6 months after MI, the area variation fraction, the E/A ratio, E-wave deceleration time and the isovolumic relaxation time adjusted by heart rate were predictors of heart failure onset.
Footnotes
Author contributions
Conception and design of the research and Writing of the manuscript: Polegato BF, Zornoff LAM; Acquisition of data: Polegato BF, Minicucci MF, Azevedo PS, Gonçalves AF, Lima AF, Martinez PF, Okoshi K; Analysis and interpretation of the data: Polegato BF, Minicucci MF, Azevedo PS, Gonçalves AF, Lima AF, Martinez PF, Okoshi MP Okoshi K, Zornoff LAM; Statistical analysis: Minicucci MF, Paiva SAR; Critical revision of the manuscript for intellectual content: Minicucci MF, Azevedo PS, Martinez PF, Okoshi MF, Okoshi K, Paiva SAR.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Liu L, Eisen HJ. Epidemiology of heart failure and scope of the problem. Cardiol Clin. 2014;32(1):1–8. doi: 10.1016/j.ccl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Pimentel M, Zimerman LI, Rohde LE. Stratification of the risk of sudden death in nonischemic heart failure. Arq Bras Cardiol. 2014;103(4):348–357. doi: 10.5935/abc.20140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Heart failure after myocardial infarction: clinical implications and treatment. Clin Cardiol. 2011;34(7):410–414. doi: 10.1002/clc.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zornoff LA, Paiva SA, Minicucci MF, Spadaro J. Experimental myocardium infarction in rats: analysis of the model. Arq Bras Cardiol. 2009;93(4):434-40, 426-32. doi: 10.1590/s0066-782x2009001000018. [DOI] [PubMed] [Google Scholar]
- 5.Martinez PF, Okoshi K, Zornoff LA, Oliveira SA, Jr, Campos DH, Lima AR, et al. Echocardiographic detection of congestive heart failure in postinfarction rats. J Appl Physiol (1985) 2011;111(2):543–551. doi: 10.1152/japplphysiol.01154.2010. [DOI] [PubMed] [Google Scholar]
- 6.Sjaastad I, Sejersted OM, Ilebekk A, Bjornerheim R. Echocardiographic criteria for detection of postinfarction congestive heart failure in rats. J Appl Physiol. 2000;89(4):1445–1454. doi: 10.1152/jappl.2000.89.4.1445. [DOI] [PubMed] [Google Scholar]
- 7.Jegger D, Jeanrenaud X, Nasratullah M, Chassot PG, Mallik A, Tevaearai H, et al. Noninvasive Doppler-derived myocardial performance index in rats with myocardial infarction: validation and correlation by conductance catheter. Am J Physiol Heart Circ Physiol. 2006;290(4):H1540–H1548. doi: 10.1152/ajpheart.00935.2005. [DOI] [PubMed] [Google Scholar]
- 8.Morgan EE, Faulx MD, McElfresh TA, Kung TA, Zawaneh MS, Stanley WC, et al. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287(5):H2049–H2053. doi: 10.1152/ajpheart.00393.2004. [DOI] [PubMed] [Google Scholar]
- 9.Prunier F, Gaertner R, Louedec L, Michel JB, Mercadier JJ, Escoubet B. Doppler echocardiographic estimation of left ventricular end-diastolic pressure after MI in rats. Am J Physiol Heart Circ Physiol. 2002;283(1):H346–H352. doi: 10.1152/ajpheart.01050.2001. [DOI] [PubMed] [Google Scholar]
- 10.Azevedo PS, Polegato BF, Minicucci MF, Pio SM, Silva IA, Santos PP, et al. Early echocardiographic predictors of increased left ventricular end-diastolic pressure three months after myocardial infarction in rats. Med Sci Monit. 2012;18(7):BR253–BR258. doi: 10.12659/MSM.883202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minicucci MF, Azevedo PS, Santos DF, Polegato BF, Santos PP, Okoshi K, et al. Echocardiographic predictors of ventricular remodeling after acute myocardial infarction in rats. Arq Bras Cardiol. 2011;97(6):502–506. doi: 10.1590/s0066-782x2011005000117. [DOI] [PubMed] [Google Scholar]
- 12.Kolseth SM, Rolim NP, Salvesen Ø, Nordhaug DO, Wahba A, Høydal MA. Levosimendan improves contractility in vivo and in vitro in a rodent model of post-myocardial infarction heart failure. Acta Physiol (Oxf.) 2014;210(4):865–874. doi: 10.1111/apha.12248. [DOI] [PubMed] [Google Scholar]
- 13.Vietta GG, Andrades ME, Dall'alba R, Schneider SI, Frick LM, Matte U, et al. Early use of cardiac troponin-I and echocardiography imaging for prediction of myocardial infarction size in Wistar rats. Life Sci. 2013;93(4):139–144. doi: 10.1016/j.lfs.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Babick A, Chapman D, Zieroth S, Elimban V, Dhalla NS. Reversal of subcellular remodelling by losartan in heart failure due to myocardial infarction. J Cell Mol Med. 2012;16(12):2958–2967. doi: 10.1111/j.1582-4934.2012.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grans CF, Feriani DJ, Abssamra ME, Rocha LY, Carrozzi NM, Mostarda C, et al. Resistance training after myocardial infarction in rats: its role on cardiac and autonomic function. Arq Bras Cardiol. 2014;103(1):60–68. doi: 10.5935/abc.20140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreira JB, Bechara LR, Bozi LH, Jannig PR, Monteiro AW, Dourado PM, et al. High-versus moderate-intensity aerobic exercise training effects on skeletal muscle of infarcted rats. J Appl Physiol (1985) 2013;114(8):1029–1041. doi: 10.1152/japplphysiol.00760.2012. [DOI] [PubMed] [Google Scholar]
- 17.Sofia RR, Serra AJ, Silva JA, Jr, Antonio EL, Manchini MT, Oliveira FA, et al. Gender-based differences in cardiac remodeling and ILK expression after myocardial infarction. Arq Bras Cardiol. 2014;103(2):124–130. doi: 10.5935/abc.20140113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litwin SE, Katz SE, Morgan JP, Douglas PS. Serial echocardiographic assessment of left ventricular geometry and function after large myocardial infarction in the rat. Circulation. 1994;89(1):345–354. doi: 10.1161/01.cir.89.1.345. [DOI] [PubMed] [Google Scholar]
- 19.Paiva SA, Novo R, Matsubara BB, Matsubara LS, Azevedo PS, Minicucci MF, et al. Beta-carotene attenuates the paradoxical effect of tobacco smoke on the mortality of rats after experimental myocardial infarction. J Nutr. 2005;135(9):2109–2113. doi: 10.1093/jn/135.9.2109. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer JM, Finn PV, Zornoff LA, Pfeffer MA. Endothelin-A receptor antagonism during acute myocardial infarction in rats. Cardiovasc Drugs Ther. 2000;14(6):579–587. doi: 10.1023/a:1007890126061. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereaux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Minicucci MF, dos Santos PP, Rafacho BP, Gonçalves AF, Silva RA, Chiuso-Minicucci F, et al. Mechanisms involved in the beneficial effects of spironolactone after myocardial infarction. PLoS One. 2013;8(9): doi: 10.1371/journal.pone.0076866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonçalves AF, Santos PP, Rafacho BP, Batista DF, Azevedo PS, Minicucci MF, et al. Vitamin D supplementation intensifies cardiac remodeling after experimental myocardial infarction. Int J Cardiol. 2014;176(3):1225–1226. doi: 10.1016/j.ijcard.2014.07.217. [DOI] [PubMed] [Google Scholar]
- 24.Minicucci MF, Azevedo PS, Oliveira SA, Jr, Martinez PF, Chiuso-Minicucci F, Polegato BF, et al. Tissue vitamin A insufficiency results in adverse ventricular remodeling after experimental myocardial infarction. Cell Physiol Biochem. 2010;26(4-5):523–530. doi: 10.1159/000322320. [DOI] [PubMed] [Google Scholar]
- 25.Gonçalves AF, Congio LH, dos Santos PP, Rafacho BP, Pereira BL, Claro RF, et al. Pamidronate attenuates diastolic dysfunction induced by myocardial infarction associated with changes in geometric patterning. Cell Physiol Biochem. 2015;35(1):259–269. doi: 10.1159/000369693. [DOI] [PubMed] [Google Scholar]
- 26.Minicucci MF, Azevedo PS, Martinez PF, Lima AR, Bonomo C, Guizoni DM, et al. Critical infarct size to induce ventricular remodeling, cardiac dysfunction and heart failure in rats. Int J Cardiol. 2011;151(2):242–243. doi: 10.1016/j.ijcard.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 27.Lima AR, Martinez PF, Okoshi K, Guizoni DM, Zornoff LA, Campos DH, et al. Myostatin and follistatin expression in skeletal muscles of rats with chronic heart failure. Int J Exp Pathol. 2010;91(1):54–62. doi: 10.1111/j.1365-2613.2009.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zornoff LA, Matsubara BB, Matsubara LS, Paiva SA, Spadaro J. Early rather than delayed administration of lisinopril protects the heart after myocardial infarction in rats. Basic Res Cardiol. 2000;95(3):208–214. doi: 10.1007/s003950050183. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, et al. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44(4):503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]