Abstract
After the existence of phlebotomine sand flies was first reported in China in 1910, the distribution of different species and their role in the transmission of visceral leishmaniasis (VL) have been extensively studied. Up until 2008, four species have been verified as vectors of VL, namely, Phlebotomus chinensis (Ph. sichuanensis), Ph. longiductus (Ph. chinensis longiductus), Ph. wui (Ph. major wui), and Ph. alexandri.
The sand fly species vary greatly depending on the natural environments in the different geographic areas where they are endemic. Ph. chinensis is euryecious and adaptable to different ecologies, and is thus distributed widely in the plain, mountainous, and Loess Plateau regions north of the Yangtze River. Ph. longiductus is mainly distributed in ancient oasis areas south of Mt. Tianshan in the Xinjiang Uygur autonomous region. Ph. wui is the predominant species in deserts with Populus diversifolia and Tamarix vegetation in Xinjiang and the western part of the Inner Mongolia autonomous region. Finally, Ph. alexandri is steroecious and found only in stony desert areas, such as at the foot of the mountains in Xinjiang and the western Hexi Corridor, in Gansu province. This review summarized the relationship between the geographic distribution pattern of the four sand fly species and their geographical landscape in order to foster research on disease distribution and sand fly control planning. Furthermore, some problems that remained to be solved about vectors of VL in China were discussed.
Electronic supplementary material
The online version of this article (doi:10.1186/s40249-016-0107-z) contains supplementary material, which is available to authorized users.
Keywords: Phlebotomus, Sand fly, Geographical distribution, Vector, Visceral leishmaniasis, Leishmania donovani, Leishmania infantum, Ph. chinensis, Ph. longiductus, Ph. wui, Ph. alexandri, Ph. mongolensis
Multilingual abstracts
Please see Additional file 1 for translation of the abstract into the six official working languages of the United Nations.
Introduction
After the existence of phlebotomine sand flies was first reported in China in 1910 [1], the Western scholars Young and Hertig conducted an experiment to understand the sand fly species Ph. chinensis and Ph. mongolensis and their transmission of Leishmania in Xuzhou, Jiangsu province in 1925. As a result, they pioneered research on vectors of visceral leishmaniasis (VL) in China [2]. Subsequently, scientists in the country have made substantial contributions to determining which sand fly species are vectors of VL. From 1936 to 1941, during World War II, scientists were for the first time able to determine, through field investigation and experimental research, that Ph. chinensis was a vector of VL in the plain areas of north China. Since 1964, the successors of these scientists have been able to prove that Ph. longiductus, Ph. wui, and Ph. alexandri are all vectors of VL in Xinjiang, Inner Mongolia, and other areas in China. The geographic distribution for the four sand fly species was mapped, facilitating research on disease distribution and sand fly control planning.
Review
Ph. chinensis
Geographic distribution
Ph. chinensis is widely distributed in China. As of 2011, it has been reported in 21 provinces, which includes 358 counties [3–5]. The species is prevalent as far north as Changchun, Jilin province (43°90′N, 125°50′E), as far south as Hekou, Yunnan province (23°40′N, 104°E), as far west as Zhangye, Gansu province (38°90′N, 100°40′E), and as far east as Jilin, Jilin province (43°80′N, 126°60′E). Ph. chinensis is the most predominant species in plain, mountainous, and Loess Plateau regions, in the area of 32 °– 43 ° N, 102 °–121 ° E (see Figs. 1 and 2), which is parallel to the geographical distribution of VL. According to vertical distribution surveys, Ph. chinensis is present at an altitude range of 10 m to 2,750 m [6], and is prevalent in the Jiangsu coastal plain and the high mountains and deep valleys of the northwestern Sichuan and southern Gansu provinces. The highest density of this species has been observed at an altitude of 1,300–1,900 m. Meanwhile, VL patients and dogs that have canine visceral leishmaniasis (CVL) were found in villages with altitudes of 1,980 m and 2,080 m, respectively [7]. Ph. chinensis is exophilic and widely scattered in mountainous areas and Loess Plateau [8, 9]. Due to the lack of effective measures to control wild Ph. chinensis, new cases of VL and CVL have frequently emerged in these terrains [10, 11].
Fig. 1.
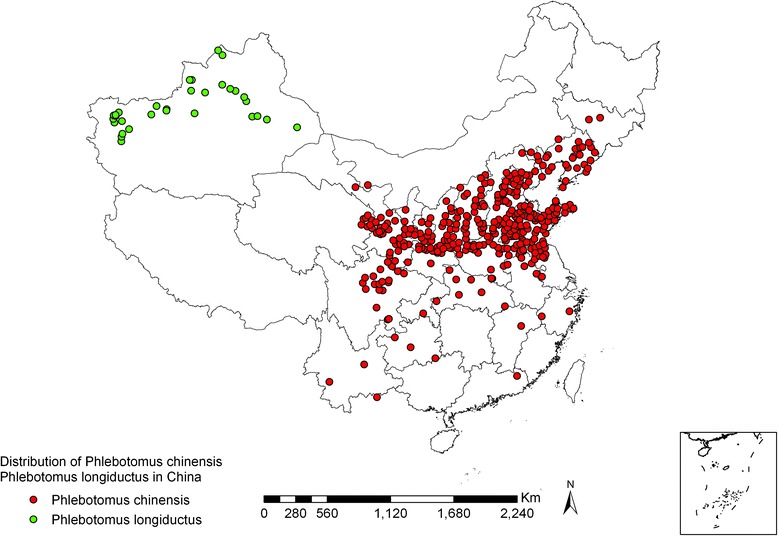
Geographic distribution of Ph. chinensis and Ph. longiductus sand flies in China
Fig. 2.

The Loess Plateau, covered with thick loess, was gradually eroded into a landscape with fragmented mountains and valleys due to long-term erosion
Vector incrimination
Leishmania obtained from VL patients was found to develop and reproduce in the alimentary tract of Ph. chinensis sand flies, with promastigotes migrating into the pharynx and proboscis
In 1925, Young and Hertig exposed hamsters (Cricetulus triton) intradermally infected with Leishmania isolated from VL patients to Ph. chinensis sand flies, and observed the development of the parasite in the sand flies. A total of 34 sand flies were fed with the hamsters’ blood. After three to eight days, the sand flies were dissected and examined; 29 (85.3 %) were found to be infected with promastigotes in the midgut and esophagus areas [2].
In the following year, Patton and Hindle performed a similar experiment in Jinan, Shandong province. After Ph. chinensis sand flies were fed with the blood of hamsters infected with VL or with the blood of human VL patients, the promastigotes infection rates were 77.7 % (122/157) and 4.9 % (5/102), respectively. In addition, it was observed that the promastigotes migrated to the pharynx and buccal cavity areas [12]. Since 1935, Chinese scholars had conducted this experiment in Qingjiangpu, Jiangsu province. Sun et al. fed 73 Ph. chinensis sand flies with the blood of VL patients. Six days later, five sand flies were found to be infected with promastigotes (6.9 %) [13].
The following year, Sun and Wu reproduced the experiment in Huai’an, Jiangsu province. The promastigote infection rates of sand flies fed on VL patients and VL hamsters were recorded as 19.3 % (26/135) and 56.3 % (36/64), respectively. Leishmania was seen to flourish in the stomach of the sand flies, and then migrating to the front-end of the alimentary tract [14]. Feng and Chung did a similar experiment, whereby they let Ph. chinensis sand flies bite four dogs with CVL in Peiping (now Beijing). Five days later, promastigotes were detected in the pharynx and proboscis areas of Ph. chinensis sand flies, with a high rate of infection of up to 85.8 % (103/120) [15].
Consequently, these findings indicate that Leishmania can develop and reproduce well in the alimentary tract of Ph. chinensis sand flies.
From 1958 to 1990, infection experiments were done in other areas, such as Tai’an, Lanzhou, and Jiuzhaigou, with all of them leading to the conclusion that Ph. chinensis is the ideal vector of Leishmania (see Table 1).
Table 1.
Experimental infection of Ph. chinensis sand flies with blood of VL-infected patients and CVL-infected dogs (Leishmania) from 1958 to 1990
| Year | Experimental sites (county) | Origin | No. dissected | No. infected (%) | Distribution of promastigotes in sand flies | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proboscis | Buccal cavity | Pharynx | Esophagus | Proventriculus | Midgut | Hindgut | Reference | |||||
| 1958 | Tai’an, Shandong province | VL patients | 177 | 62(35.0) | 2 | 1 | 2 | 0 | 38 | 61 | 13 | a |
| 1960 | Tai’an, Shandong province | VL patients | 228 | 90(39.5) | 1 | 1 | 4 | 5 | 71 | 87 | 13 | b |
| 1958 | Lanzhou, Gansu province | CVL dogs | 57 | 24(42.1) | 0 | 0 | 5 | 0 | 7 | 19 | 2 | a |
| 1980 | Lixian, Sichuan province | CVL dogs | 74 | 45(60.8) | 0 | 0 | 3 | 22 | 45 | 45 | 0 | [62] |
| 1990 | Jiuzhaigou, Sichuan province | VL patients | 331 | 229(69.2) | 5 | 0 | 40 | 104 | 193 | 229 | 45 | [20] |
aAnnual report, Institute of Parasitic Diseases, Chinese Academy of Medical Sciences, 1958, pp. 376–360
bAnnual report, Institute of Parasitic Diseases, Chinese Academy of Medical Sciences, 1960, pp. 426–436
Ph. chinensis sand flies naturally infected with VL in endemic areas, and experimental infection of hamsters (Cricetulus barabensis)
In 1935, Sun et al., collected 421 Ph. chinensis sand flies from patients’ houses in Wangshiguzhuang village, Huai’an, Jiangsu province, an area where VL was prevalent. They found that seven (1.7 %) sand flies were naturally infected with promastigotes in the midgut [13]. The following year, Sun and Wu collected 537 Ph. chinensis sand flies from two other VL-endemic villages in the same county, and 11 (2.05 %) sand flies were found to be naturally infected with promastigotes [16]. In the same year, 11 hamsters were inoculated with promastigotes from the midgut of six sand flies by intraperitoneal injection; VL was observed in four hamsters (36.4 %) after 193 to 293 days [17].
From 1939 to 1941, Feng and Chung collected and dissected groups of 16 and 57 Ph. chinensis sand flies from two CVL dog nests in Beijing, and found that two (12.5 %) and 34 (59.6 %) sand flies had promastigotes, respectively. After inoculation of one hamster, Leishmania parasites were detected in visceral smears 10 months later [18, 19].
In 1990, naturally infected Ph. chinensis sand flies were found in VL-endemic areas in Jiuzhaigou county, Sichuan province [20], and promastigotes isolated using immunological methods (dot-enzyme-linked immunosorbent assay using monoclonal antibody) were identified as L. donovani [21]. This provided further evidence for Ph. chinensis sand flies being transmitting vectors.
Transmission of Leishmania from CVL-infected dogs to hamsters via Ph. Chinensis bites
From 1940 to 1941, Feng and Chung let Ph. chinensis sand flies feed on the blood of dogs infected with CVL for three days, and then unleashed 82 fed Ph. chinensis sand flies on caged hamsters overnight. The animals had been anaesthetized by intraperitoneal injection of urethane and abdominal skins were shaven before exposure. One of the eight hamsters (12.5 %) developed VL after being bitten by a sand fly [22]. In 1941, Ho, Chu, and Yuan were able to reproduce the same experiment. They collected Ph. chinensis sand flies from the field and let them feed on VL-infected hamsters and CVL-infected dogs. A week later, the sand flies were unleashed in a cage with four normal hamsters. The hamsters were dissected six months later, with Leishmania amastigotes found in the spleen of one hamster (25 %) [23].
Since then, Chung, Feng, and Feng developed a method that can be used to obtain a large sample of Ph. chinensis sand flies infected with promastigotes for a transmission experiment. During transmission season, they tied a CVL-infected dog in an empty room, leaving the doors and windows open to let sand flies in at night. The next morning, the doors and windows were shut, and the sand flies were collected and fed with raisins, pears, or apples. Some of the sand flies survived for 15 days on such diets. Seventy-two normal hamsters were then exposed to these sand flies in batches. Forty-seven hamsters were dissected after 47 to 270 days, with eight of them developing VL. The result proved beyond doubt that VL is experimentally transmissible to hamsters via the bite of Ph. chinensis sand flies that fed on CVL-infected dogs [24].
Ph. chinensis sand flies are widely distributed in VL-endemic areas (except for Xinjiang), and the population density of sand flies is closely related to VL endemicity
In 1937, a survey showed that a high density of Ph. chinensis sand flies in the total sand fly population (ranging from 84.2 % to 92.9 %) was closely correlated with VL epidemics in Huai’an, Jiangsu province. In contrast, fewer VL cases were found in villages where Ph. chinensis sand flies accounted only for 3–18.8 % of the total sand fly population [25].
Since the founding of the People’s Republic of China, more attention has been paid to the control of Ph. chinensis sand flies. As a result, data which provides further support for the correlation between VL and population density of Ph. chinensis sand flies, has become increasingly available. By the end of 1959, researchers confirmed the existence of Ph. chinensis sand flies in 261 of the 270 VL-endemic counties and cities in 13 provinces/autonomous regions, except for in Xinjiang. Moreover, it was found that Ph. chinensis was the dominant species in these counties [3].
Ph. mongolensis
Geographic distribution
Ph. mongolensis is distributed in 240 counties/cities in 17 provinces (autonomous regions/municipalities) [3, 4, 26, 27]. These sand flies can be found as far north as Hebukesaier, Xinjiang (46°80′N, 85°70′E), as far south as Jingmen, Hubei province (31°N, 112°10′E), as far west as Huocheng, Xinjiang (44°N, 80°80′E), and as far east as Suizhong, Liaoning province (40°30′N, 120°30′E). Ph. mongolensis is distributed mainly in the northern China plain region (32°–40°N, 114°–120°′E), as well as desert areas covered with Chenopodiaceae plants in Western Inner Mongolia, the Hexi Corridor of Gansu province, and the Zhungeer basin north of Mt. Tianshan, Xinjiang. However, fewer Ph. mongolensis sand flies are found in the Loess Plateau (34°–40°N, 102°–114°′E). Ph. mongolensis is present at an altitude range of 10 m (Northern Jiangsu Coastal Plain) to 1,900 m (Dongxiang, Gansu province) vertically. Ph. mongolensis is the dominant species in desert areas, living in burrows of great gerbils (Rhombomys opimus) (see Figs. 3, 4, and 5) [28].
Fig. 3.
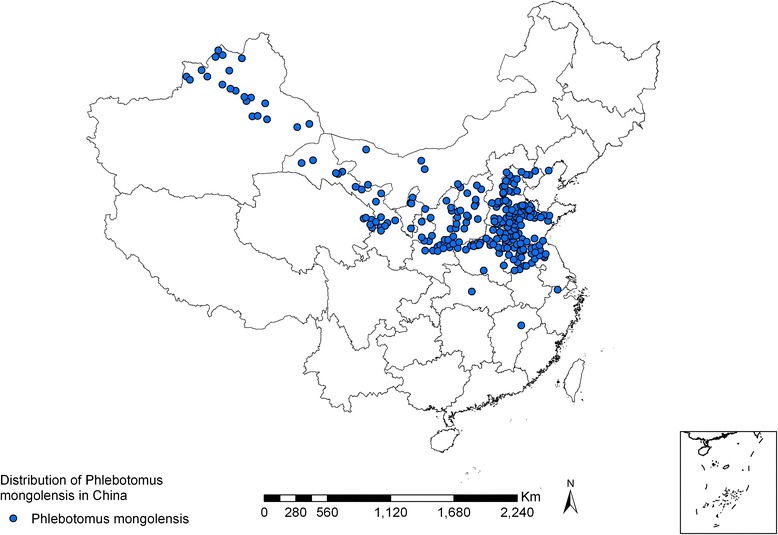
Geographic distribution of Ph. mongolensis sand flies in China
Fig. 4.

Sacsaou(Haloxylon ammodendron), a kind of shrub, belongs to the Chenopodiaceae family. Its developed root system is conducive to sand fixation and its leaves provide food for great gerbils (Rhombomys opimus). Great gerbils can climb up the branches and bite off twigs, then take the falling twigs into burrows for storage. Sacsaou-big gerbil-sand fly forms a food chain
Fig. 5.

The burrows of great gerbils (Rhombomys opimus), the habitat of Ph. mongolensis sand flies, and the habitat of Ph. andrejevi and Ph. caucasicus sand flies in desert areas
Vector incrimination
Besides Ph. chinensis, Ph. mongolensis was found to be a common anthropophilic sand fly species in Jiangsu, Shandong, and Beijing between 1925 and 1936. This sand fly species was thus examined for its role in the transmission of VL.
In 1958, Ph. mongolensis sand flies were experimentally infected with Leishmania in Lanzhou, Gansu and Huiming, Shandong. After feeding on the blood of VL patients, VL-infected hamsters, and CVL-infected dogs, the sand flies were checked for Leishmania promastigotes in the midgut. The results showed that the infection rate of Leishmania was much lower in this species than that of Ph. chinensis, with the infection generally limited to the midgut of the Ph. mongolensis sand flies. Promastigotes disappeared from the alimentary tract, as soon as the blood was digested (see Table 2).
Table 2.
Experimental infection of Ph. mongolensis sand flies with Leishmania obtained from VL patients or CVL-infected dogs
| Year | Experimental sites (county) | Source of Leishmania strain | No. dissected | No.infected (%) | Distribution of promastigotes in sand flies | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pharynx | Esophagus | Proventriculus | Midgut | Hindgut | Reference | |||||
| 1926 | Xuzhou, Jiangsu | VL patients | 14 | 0 | 0 | 0 | 0 | 0 | 0 | [2] |
| VL hamsters | 232 | 7(3.0) | 0 | 0 | 0 | 7 | 0 | |||
| 1927 | Jinan, Shandong | VL patients | 202 | 0 | 0 | 0 | 0 | 0 | 0 | [12] |
| VL hamsters | 1,170 | 430(36.8) | 0 | 0 | 0 | 430 | 0 | |||
| 1938 | Huai’an, Jiangsu | VL patients | 512 | 6 (1.17) | 0 | 0 | 0 | 6 | 0 | [14] |
| VL hamsters | 153 | 50(32.7) | 0 | 0 | 13 | 49 | 1 | |||
| 1939 | Beijing | CVL dogs | 449 | 56(12.5) | 0 | 0 | 0 | 56 | 8 | [15] |
| 1958 | Lanzhou, Gansu | CVL dogs | 449 | 58(12.9) | 0 | 0 | 3 | 53 | 15 | a |
| 1958 | Huiming, Shandong | VL hamsters | 125 | 14(11.2) | 0 | 0 | 0 | 14 | 0 | a |
aAnnual report, Institute of Parasitic Diseases, Chinese Academy of Medical Sciences, 1958, pp. 360–364
According to Feng’s observation in Beijing, after Ph. mongolensis fed on blood of CVL-infected dogs, promastigotes remained encased with blood in the peritrophic membrane and decreased in number as the peritrophic membrane shrank. Six to seven days later, residual blood and a small number of promastigotes in the membrane was discharged through the anus. Consequently, it can be inferred that Ph. mongolensis is not a permissive vector of transmitting VL in China [29].
Feng et al. also found the trapping of Leishmania in the peritrophic membrane of Ph. mongolensis sand flies after feeding on blood, in Lanzhou and Huiming in 1958 (Annual report, Institute of Parasitic Diseases, Chinese Academy of Medical Sciences, 1958).
On the other hand, Ph. mongolensis sand flies have been proven to be vectors of Leishmania gerbilli and L. turanica, which infect the subcutaneous tissue of Rhombomys opimus ears in the desert parts of western China [30–33]. After feeding on gerbil’s blood infected with L. turanica, about a quarter of Ph. mongolensis sand flies had their peritrophic membrane ruptured during blood meal digestion, and promastigotes continued to develop and reproduce in their midguts. Five days after feeding on blood, L. turanica promastigotes were found in the esophagus of the sand flies [34].
Ph. longiductus
Geographic distribution
The distribution of Ph. longiductus sand flies in China is limited to 30 counties (cities) in Xinjiang, covering an area as far north as Tacheng (46°45′N, 83°E), as far south as Yecheng (37°52′N, 77°24′E), as far west as Kashgar (37°50′N, 76°E), and as far east as Shanshan (42°90′N, 90°13′E) (see Fig. 1). The species has a wide distribution range in altitude, ranging from 90 m (Turfan) to 2,100 m (Wensu). Ph. longiductus is especially found in ancient oases, which have a history of hundreds of years, with some being more than 2,000 years old, at elevations of 1,000 to 1,500 m (see Figs. 1 and 6) in the western and northern rims of the Tarim Basin, south of Mt. Tianshan. There are few Ph. longiductus sand flies in the mountainous regions, but none are found in desert areas [5, 26].
Fig. 6.

Ancient oasis, with high density population, lush vegetation, and orchards in each household, is agricultural bases in Xinjiang (photo by Xiao-kun Ding)
Vector incrimination
Ph. longiductus has been the major vector of VL in endemic ancient oases, with an anthropophilic habit
Ph. longiductus accounts for most of the sand flies (82.3–100 %) when collected by means of tube aspirators and sticky papers at four different sites in ancient oases, followed by Ph. Wui, then Ph. alexandri and Sergentomyia sinkiangensis (see Table 3) [35–39].
Table 3.
Sand fly species and composition ratios in ancient oases of southern Xinjiang
| Year | Study sites (county) | No. collected | Sand fly species and composition ratio(%) | |||
|---|---|---|---|---|---|---|
| Ph. longiductus | Ph. wui | Ph. alexandri | S. sinkiangensis | |||
| 1959–1963 | Atushi | 5,631 | 4,634(82.3) | 971(17.2) | 21(0.4) | 5(0.1) |
| 1988–1990 | Kashgar | 8,382 | 6,947(82.9) | 1,435(17.1) | 0 | 0 |
| 1984 | Wensu | 4,978 | 4,605(92.5) | 346(7.0) | 17(0.3) | 10(0.5) |
| 2005 | Wushi | 328 | 328(100) | 0 | 0 | 0 |
Ph. longiductus, from the above sites, is anthropophilic, feeding on both humans and livestock. Female sand flies are present in different ovary development stages, nulliparous, or parous, when collected in households during the daytime [39–43].
The alimentary tract of Ph. longiductus sand flies is suitable for the development and reproduction of Leishmania
Batches of Ph. longiductus sand flies were dissected three to nine days after exposure to hamsters (C. barabensis) that were infected with Leishmania from patients in Xinjiang, Beijing, and Shandong. The results showed that Leishmania from all three regions developed and reproduced in the alimentary tract of Ph. longiductus sand flies. The sand fly infection rate was related to the intensity of VL infection in hamsters. In infected sand flies, promastigotes were observed to migrate from the midgut into the pharynx (see Table 4), suggesting that Ph. longiductus sand flies are vectors of VL in Xinjiang [38, 40].
Table 4.
Experimental infection of Ph. longiductus sand flies with Leishmania from VL patients
| Experimental sites (county) | Source of Leishmania strain | No. dissected | No. infected (%) | Distribution of promastigotes in sand flies | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proboscis | Pharynx | Esophagus | Proventriculus | Midgut | Hindgut | |||||
| Atushi | Xinjiang | 40 | 7(17.5) | 0 | 1 | 2 | 4 | 6 | 0 | [40] |
| Wensu | Xinjiang | 45 | 28(62.2) | 1 | 7 | 21 | 28 | 28 | 0 | [38] |
| Atushi | Beijing | 32 | 31(96.8) | 0 | 3 | 25 | 27 | 30 | 8 | [40] |
| Atushi | Shandong | 83 | 46(55.4) | 0 | 3 | 32 | 43 | 46 | 9 | [40] |
In Kazakhstan, which neighbors Xinjiang, Dergacheva and Strelkova (1985) reported infection in hamsters bitten by Ph. longiductus sand flies infected with L. donovani [41]. In 1990, the World Health Organization (WHO) listed Ph. longiductus as a proven vector of VL in Kazakhstan [42], although Ph. longiductus sand flies naturally infected with Leishmania have not yet been found, pending further investigation in Xinjiang.
Ph. wui
Geographic distribution
Ph. wui sand flies are found in 37 counties (banners) in Xinjiang, Gansu, and Inner Mongolia, including in the area as far north as Tacheng, Xinjiang (46°45′N, 83°E), as far south as Minfeng, Xinjiang (37°04′N, 82°41′E), as far west as Shufu, Xinjiang (39°50′N, 75°85′E), and as far east as Eji’naqi, Inner Mongolia (41°57′N, 77°50′E) (see Fig. 7). The vertical distribution spreads from 90 m (Taoergou, Turfan, Xinjiang) to 1,500 m (Atushi, Xinjiang). Ph. wui is distributed at its highest density in desert areas at the edge of the Tarim Basin in south Xinjiang and western Inner Mongolia. The ecological habitats are mainly sites several kilometers away from rivers with lower water tables and sparse vegetation, of the Poplar diversifolia and Tamarix taklamakanensis varieties (see Figs. 8 and 9), and also in wild animal burrows, half-buried underground structures, surface collapsed pits, tree holes, and outer wall cracks of houses surrounding villages. Ph. wui enters rooms attracted by light at night and leaves at dawn [44–48]. When Poplar diversifolia dies due to river drying and desertification, Ph. wui sand flies become scarce or disappear completely, as is also the case in places far from rivers and without Poplar diversifolia vegetation [49, 50].
Fig. 7.
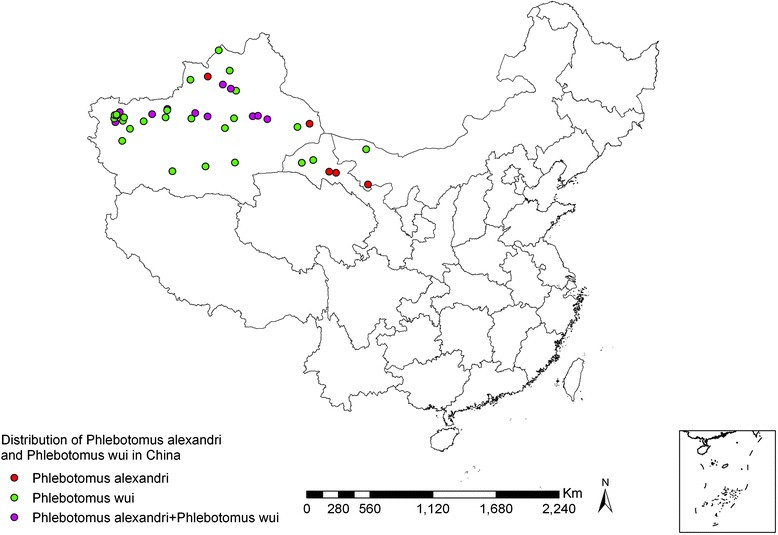
Geographic distribution of Ph. alexandri and Ph. wui sand flies in China
Fig. 8.

Diversiform-leaved poplar Populus diversifolia, old branches with cordate leaves, epicormic branches with long, narrow leaves
Fig. 9.

Tamarix is a kind of shrub with small leaves and developed roots, blooming small red flowers in the summer
In general, Ph. wui accounts for up to 17.2 % of the total sand fly population in ancient oases, while it is rarely found in mountainous areas and not found in stony desert areas [49].
Vector incrimination
Surveys have shown that Ph. wui was the dominant species in desert areas with vegetation of P. diversifolia and T. taklamakanensis
Surveys done in many places have indicated that only two species, namely Ph. wui and S. sinkiangensis, are present in VL-endemic deserts with P. diversifolia and T. taklamakanensis vegetation. Ph. wui is the dominant species, accounting for 76.4–99.9 % of total sand fly population (see Table 5).
Table 5.
Sand fly species and composition ratios in desert areas with P. diversifolia and T. taklamakanensis vegetation, in Xinjiang and Inner Mongolia
| Year | Survey sites (county) | No. dissected | Sand fly species and composition ratio(%) | Reference | |
|---|---|---|---|---|---|
| Ph. wui | S. sinkiangensis | ||||
| 1963–1966 | Alar, Xinjiang | 29,202 | 22,666(77.6) | 6,536(22.4) | [44] |
| 1972 | Eji’naqi, Inner Mongolia | 4,492 | 4,429(98.6) | 63(1.4) | [43] |
| 1975–1980 | Bachu, Xinjiang | 683 | 522(76.4) | 166(23.6) | [45] |
| 2007 | Minfeng, Xinjiang | 1,210 | 1,200(99.2) | 10(0.8) | [46] |
| 2010 | Jiashi, Xinjiang | 4,540 | 4,535(99.9) | 5(0.1) | [47] |
Ph. wui sand flies feed mainly on human and other homeothermic animal blood. They frequently bite humans outdoors in the evening and also enter houses to feed on human blood. S. sinkiangensis is another species, which primarily feeds on lizards, with a feeding rate of 74.9 % (161/215) on lizards, and only very occasionally (1.6 %, 2/122) on humans and (0.8 %, 1/123) rats [40]. As a result, S. sinkiangensis is not considered to be a vector of VL [40].
After Ph. wui was artificially infected with Leishmania obtained from VL patients, promastigotes were observed to migrate into the pharynx
Batches of Ph. wui sand flies were dissected after exposure to hamsters infected with Leishmania obtained from VL patients in Xinjiang and being kept for three to seven days at a temperature of 22–26 °C. The promastigote infection rate was 85.1 % (211/248). In addition to the midgut, promastigotes were found in the esophagus (35.1 %, 74/211) and the pharynx (6.6 %, 14/211) of the infected sand flies on the fourth or fifth day. Moreover, they were also present in the esophagus typhlosolis and Malpighian tubules of heavily infected sand flies. The results showed that the alimentary tract of Ph. wui sand flies is extremely favorable for the development and reproduction of Leishmania obtained from VL patients [40].
Leishmania from naturally-infected Ph. wui sand flies is homologous to L. infantum
Female sand flies were collected by human-baited traps and light traps set up inside and outside of human dwellings in desert areas at night. Parous and >1/2 blood digestion sand flies were dissected and examined microscopically, giving the natural infection rate of 0.4–5.7 % [40, 43, 45, 46] (see Table 6). The distribution of promastigotes in the alimentary tract of Ph. wui sand flies is similar to that of experimentally infected sand flies.
Table 6.
Promastigote natural infection in Ph. wui sand flies in desert areas with vegetation of P. diversifolia and T. taklamakanensis in Xinjiang and Inner Mongolia
| Year | Survey sites (county) | No. dissected | No. infected (%) | Distribution of promastigotes in sand flies | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proboscis | Pharynx | Esophagus | Proventriculus | Midgut | Hindgut | Reference | ||||
| 1964 | Alar, Xinjiang | 300 | 17(5.7) | 0 | 4 | 12 | 15 | 16 | 5 | [40] |
| 1972 | Eji’naqi, Inner Mongolia | 1,998 | 34(1.7) | 0 | 2 | 16 | 31 | 34 | 2 | [43] |
| 1977 | Bachu, Xinjiang | 453 | 4(0.9) | 2 | 0 | 3 | 4 | 4 | 0 | [45] |
| 2007 | Minfeng, Xinjiang | 523 | 2(0.4) | 0 | 0 | 1 | 2 | 2 | 2 | [46] |
Fourteen isolates of promastigotes were taken from naturally-infected sand flies by cultivation in Novy-MacNeal-Nicolle medium. Except for the Bachu isolate [PHL(IWUI)/CN/77/771], the remaining 13 were then inoculated into the abdominal cavity and subcutaneous tissue of hamsters. Two to four months later, all infected animals suffered from serious VL, with their livers and spleens getting heavily infected [40, 43].
Genotype heterogeneity analysis of nDNA and kDNA from the Bachu isolate revealed that it is homologous to L. infantum [51], identical to the Leishmania from VL patients in similar areas [48]. Ph. wui is thus confirmed as a vector of VL in desert areas.
Ph. alexandri
Geographic distribution
Ph. alexandri sand flies are distributed in 17 counties in Xinjiang, Gansu, and Inner Mongolia regions, as shown in Fig. 7. They are found in areas as far north as Guertu, Wusu, Xinjiang (44°50′N, 76°10′E), as far south as Yingjisha, Xinjiang (39°10′N, 76°10′E), as far west as Atushi, Xinjiang (39°70′N, 76°10′E), and as far east as Yabulai Mountains, Alxa Youqi, Inner Mongolia (39°40′N, 103°10′E) (see Fig. 7). Ph. alexandri is often present in areas of piedmont and stony desert at an altitude of 500 to 1,750 m [5]. Since stony deserts lack natural vegetation, except for small shrubs, areas get eroded due to rainstorms in summer (see Fig. 10). Ph. alexandri sand flies inhabit various kinds of burrows in such landscapes [52], but can also be found in dried wells at the foot of mountains [53]. Ph. alexandri sand flies enter residential areas at night, but rarely stays long indoors during the daytime. They frequently dwell in large caves bite to humans who enter the caves in the daytime [52]. This species is rarely found in mountainous areas and is occasionally found in oases [49].
Fig. 10.

Erosion gully at the foot of stony desert terrain. In summer, the temperature can go over 50 °C during the day, and drop quickly after sunset. A large number of Ph. alexandri sand flies can be captured with human-baited traps in the groove
Vector incrimination
Ph. alexandri is the principal species in VL-endemic areas at the junction of the foot of Mt. Tianshan and the stony desert in Xinjiang
In 1983, a total of 8,843 sand flies were captured in Meiyaogou Turfan, among which Ph. alexandri was the dominant species, accounting for 81.1 % (7,176/8,843) of the sand flies [54]. The following year, similar results were obtained in a similar investigation in Damazha, Wensu, Xinjiang. Ph. alexandri sand flies constitute 91.5 % (1,704/1,863) of the total sand fly population that is caught and examined [38].
Ph. alexandri is markedly anthropophilic
Sand flies were collected for a study in Meiyaogou, providing evidence of the anthropophilicity of Ph. alexandri. Using a human as bait, 835 of the 837 (99.8 %) sand flies caught indoors and in caves belonged to the Ph. alexandri species. Another survey also indoors and in caves, all of the 213 sand flies caught that were sucking blood were identified as Ph. alexandri [54]. In the Damazha in Wensu counties, 15 of the 1,704 (0.9 %) Ph. alexandri sand flies were found in caves, with the remaining 1,689 found in stony desert areas at night. In the Meiyaogou, when 96 Ph. alexandri sand flies were kept in the same cage with two anesthetized lizards overnight, it was found that they refused the lizards’ blood [54].
Ph. alexandri sand flies are susceptible to Leishmania infection obtained from VL patients
Ph. alexandri were dissected four to twelve days after exposure to hamsters that were infected with Leishmania obtained from VL patients in Xinjiang. Promastigotes invaded the esophagus and pharynx on days four to six, and the proboscis on the ninth day. Promastigotes formed rosettes in the junction of the hindgut and Malpighian tubes in some of the heavily infected sand flies. This showed that the Leishmania infection is extremely adaptable to the alimentary tract of this species (see Table 7). Furthermore, it was reported that Ph. alexandri sand flies were also susceptible to Leishmania from VL patients in Henan and Gansu provinces. After feeding on hamsters infected with Leishmania, promastigotes flourished in the midgut of this species, and invaded the pharynx on the eighth day [55].
Table 7.
Experimental infection of Ph. alexandri sand flies with Leishmania obtained from VL patients
Natural infection of Ph. alexandri with promastigotes
A total of 643 and 386 Ph. alexandri sand flies were collected outdoors and indoors in Meiyaogou and Damazha, respectively. They were dissected after complete blood digestion for microscopic examination. Thirteen (2.02 %) and four (1.04 %) sand flies from Meiyaogou and Damazha, respectively, were found to be infected with promastigotes. Six heavily infected sand flies showed promastigotes congested in the midgut, the pharynx, the buccal cavity, and the proboscis. The results were consistent with that of an artificial infection [38, 54]. Promastigotes from seven sand flies were isolated and inoculated into the peritoneal cavity and skin of seven normal hamsters. The hamsters were examined 88–97 days later, with infection by amastigotes observed in all of them [54].
Promastigotes isolated from the sand fly midgut proliferate in the Novy-MacNeal-Nicolle medium. One isolate (IALE/CN/88/Turfan10) was identified as L. donovani (zymodeme: MON-138) using isozyme electrophoresis detection (J.A. Rioux, 1991, unpublished data) by Laboratoire d’Ecologie médicale et de Pathologie parasitaire in Montpellier, France. At the same location, another isolate (IALE/CN/87/self-1) was identified as L. infantum through nDNA and kDNA genotype analysis [51], which was highly homologous to the WHO reference strain L. donovani (MHOM/IN/80/DD8) through repetitive DNA sequence homology analysis [56]. The difference may be attributed to the different methods used.
Experimental transmission of Leishmania to normal hamsters via the bite of Ph. alexandri sand flies, infected with Leishmania obtained from VL patients
Two batches of sand flies (one with 18 and the other with 23 sand flies) fed on a hamster that had been infected with Leishmania from VL patients. Eleven to twelve days later, two normal hamsters were exposed to these sand flies. Only three sand flies fed on the blood from hamster no. 1 and seven fed on hamster no. 2. These 10 fed sand flies were dissected, with promastigotes found in six of them. Among them, five sand flies were heavily infected with promastigotes in the proventriculus, and even in the pharynx and proboscis. Hamster no. 1 was dissected on the 147th day and hamster no. 2 hamster on the 145th day. Amastigotes were found in the smears of the liver, spleen, and lymphonodus of both hamsters, demonstrating that both developed VL [54]. These results suggest the capacity of Ph. alexandri sand flies as vectors of VL.
Conclusions
Past investigations have shown that vectors of VL vary in different geographic landscapes in VL-endemic areas of China. Ph. chinensis sand flies are the dominant and the most important vectors in mid-east plain, mountainous, and Loess Plateau areas in China. In the vast region stretching from western Inner Mongolia to Xinjiang, Ph. longiductus sand flies prevalent in ancient oases are a major vector of VL. Ph. wui and Ph. alexandri sand flies are vectors of VL in deserts with vegetation of P. diversifolia and T. taklamakanensis, and at the foot of mountains in stony deserts, respectively.
Ph. mongolensis sand flies are widely distributed in central and eastern plains of the country. Leishmania isolated from VL patients replicates, but fails to break free from encapsulation of the peritrophic membrane, when human blood is digested completely in the midgut of this species. Ph. mongolensis sand flies are thus not vectors of human leishmaniasis. However, the species are a proven vector transmitting L. gerbilli and L. turanica, which parasitize subcutaneous tissues to produce ear lesions in the great gerbil (Rhombomys opimus) [30, 31, 33]. It has been elucidated which sand fly species are vectors of VL in major epidemic areas in China, but this is less definitive in areas with sporadic VL cases, such as the ones in deep valleys in Mt. Tianshan, Xinjiang, and areas in Dunhuang and Guazhou, Gansu. These areas need to be investigated further.
The four sand fly species that are vectors of VL in China are distributed in different geographic areas that have different ecological features. Further studies that elucidate the relationship between the geographic distribution pattern of sand flies and their natural environment—climate, physical and chemical properties of soil, vegetation, ground temperature, and annual precipitation—are needed in order to foster VL control and surveillance.
In addition, cutaneous leishmaniasis (CL) has often been diagnosed among workers who returned from the Middle East and North Africa [57, 58]. Cases of VL and CL have also been reported among visitors from Western Europe and South America [59, 60]. Research should be conducted on whether Leishmania brought by these imported cases to China can be transmitted by indigenous sand fly vectors. In recent years, because the electric light has become more widespread, a large number of Ph. wui sand flies with strong phototaxis enter dwellings at night, attracted by light, from the surrounding deserts in ancient Kashgar oasis, south of Xinjiang [61]. Further studies on the role of Ph. wui sand flies in the transmission of VL in these regions are thus needed.
Acknowledgements
We express our gratitude to Sen-Hai Yu and Xiao-Nong Zhou (both from the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention) for reviewing the manuscript. We also thank Dr. Shan Lv for drawing the geographic distribution map of sand flies. In addition, we are grateful to Professor J.A. Rioux (from Laboratoire d’Ecologie médicale et de Pathologie parasitaire, Montpellier, France) for identifying the Leishmania parasites isolated from Ph. alexandri sand flies. This project was supported by the 12th Five-Year Plan for the National Major Program (grant no. 2012ZX10004219) and the National S &T Major Program (grant no. 2012ZX10004220).
Abbreviations
- CL
Cutaneous leishmaniasis
- CVL
Canine visceral leishmaniasis
- VL
Visceral leishmaniasis
- WHO
World health organization
Additional file
Multilingual abstracts in the six official working languages of the United Nation. (PDF 384 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LRG and ZBZ conceived the review, carried out the literature search, analyzed the data, and wrote the paper. CFJ, QF, and JJC participated in the data collection and gave suggestions on the content and structure of this paper. All authors read and approved the final version of the manuscript before submission to Infectious Diseases of Poverty.
Authors’ information
Li-Ren Guan and Zheng-Bin Zhou are the co-first authors, and both of them are from the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention.
Contributor Information
Li-Ren Guan, Email: guanliren@163.com.
Zheng-Bin Zhou, Email: ytuzhouzhengbin@163.com.
Chang-Fa Jin, Email: kingchf@126.com.
Qing Fu, Email: fuqingcdc@163.com.
Jun-Jie Chai, Email: chaijunjie2004@163.com.
References
- 1.Bolt RA. Sandflies (Phlebotomus) in China and their relation to disease. Chin Med J. 1915;29:78–86. [Google Scholar]
- 2.Young CW, Hertig M. The development of flagellates in Chinese sandflies (Phlebotomus) fed on hamsters infected with Leishmania donovani. Proc Soc Exp Biol Med. 1926;23:611–5. doi: 10.3181/00379727-23-3082. [DOI] [Google Scholar]
- 3.Ho KZ, Wang J, Huang CM. Collected papers on the research of kala-azar (1950–1959) Shanghai: Shanghai Publishing House of Science and Technology; 1959. [Google Scholar]
- 4.Leng YJ, Zhang LM. Zoogeographical distribution of phlebotomine sandflies in China (Diptera: Psychodidae) Contr Blood-sucking Dip Ins. 1991;3:86–102. [Google Scholar]
- 5.Tang LH, Xu LQ, Chen YD. Phlebotominae sandflies.// Parasitic Diseases Control and Research in China. Beijing: Beijing Publishing House of Science and Technology; 2012. [Google Scholar]
- 6.Wu YX, Li GR, Liao QY, Chen YF, Gao B. Epidemiology and control of kala-azar in Sichuan Province. End Dis Bull. 1995;10(4):25–9. [Google Scholar]
- 7.Yin ZC, Wu YX, Liu PZ, Guan LR. Study on the vertical distribution of canine visceral leishmaniasis in Wenchuan and Nanping counties, Sichuan Provinces. End Dis Bull. 1990;5(3):25–40. [Google Scholar]
- 8.Guan LR, Wang J, Liu PZ, Zhang ZG. The bionomics of Phlebotomus chinensis in the mountainous regions of southern Kansu and the loess plateau of northern Shansi. Acta Entomol Sinica. 1980;23(1):25–31. [Google Scholar]
- 9.Guan LR. A supplement to the research on sandfly biology in China. Int J Med Parasit Dis. 2010;37(2):65–7. [Google Scholar]
- 10.Guan LR. Present situation of visceral leishmaniasis and prospect for its control in China. Chin J Parasitol Parasit Dis. 2009;27(5):394–7. [PubMed] [Google Scholar]
- 11.Tian T, Wu WP, Wang LY, Chen HT. Epidemiological analysis on visceral leishmaniasis in China during 2008–2011. Int J Med Parasit Dis. 2012;39(4):223–6. [Google Scholar]
- 12.Patton WS, Hindle E. The development of Chinese Leishmania in Phlebotomus major var. chinensis and Phlebotomus sergenti var. Proc Roy Soc. 1927;Ser B 101(B710):369–90. doi: 10.1098/rspb.1927.0021. [DOI] [Google Scholar]
- 13.Sun CJ, Yao YT, Chu HJ, Wu CC. Natural infection of Phlebotomus chinensis with flagellates morphologically indistinguishable from those of L. donovani. Chin Med J. 1936;50:911. [Google Scholar]
- 14.Wu CC, Sun CJ. Notes on the study of kala-azar transmission. Part III. Experimental infection of Chinese sandflies of the erect-haired division fed on kala-azar patients and infected Chinese hamsters. Chin Med J. 1938;52(Suppl.2):579–91.
- 15.Feng LC, Chung HL. The development of Leishmania in Chinese sandflies fed on dogs with canine leishmaniasis. Chin Med J. 1939;56:35–46. [Google Scholar]
- 16.Sun CJ, Wu CC. Notes on the study of kala-azar transmission. Part II. Further observations on the natural infection of Phlebotomus chinensis with Leptomonas donovani. Chin Med J. 1937;52(5):665. [Google Scholar]
- 17.Yao YT, Wu CC. The finding of Phlebotomus chinensis Newstead from Yunnan and its bearing on the transmission of kala-azar in South China, with remarks on the success in infecting Chinese hamsters with flagellates from naturally infected P. chinensis found in Tsingkiangpu. Chin Med J. 1941;60(3):232–40. [Google Scholar]
- 18.Chung HL, Feng LC. Natural infection of Phlebotomus chinensis in Peiping with Leishmania flagellates. Chin Med J. 1939;56(1):47–51. [Google Scholar]
- 19.Chung HL, Feng LC. Further observations on the natural infection of Phlebotomus chinensis in Peiping with Leishmania flagellates. Chin Med J. 1941;59(6):540–2. [Google Scholar]
- 20.Xiong GH, Jin CF. Studies on the longitudinal distribution of sandfly Phlebotomus chinensis and its relation to kala-azar in Southern Gansu and Northern Sichuan. End Dis Bull. 1989;4(4):15–22. [Google Scholar]
- 21.Qu JQ, Xu YX, Guan LR, Bao YF. Identification on L. donovani promastigotes from Phlebotomus by monoclonal antibody. Chin J Parasitol Parasit Dis. 1990;8(4):274–6. [PubMed] [Google Scholar]
- 22.Feng LC, Chung HL. Experiment on the transmission of kala-azar from dogs to hamsters by Chinese sandflies. Chin Med J. 1941;60:489–96. [Google Scholar]
- 23.Ho EA, Chu HJ, Yuan IC. Transmission of Leishmaniasis to the Chinese hamsters (C. griseus) by the bite of Chinese sandflies (P. chinensis) Chin Med J. 1943;62:207–9. [Google Scholar]
- 24.Chung HL, Feng LC, Feng SL. Observations concerning the successful transmission of kala-azar in North China by the bite of naturally infected Ph. chinensis. Peking Nat Hist Bull. 1950;19(2–3):301–26. [Google Scholar]
- 25.Wang CJ, Wu CC. Kala-azar. Beijing: People’s Medical Publishing House; 1956. [Google Scholar]
- 26.Zuo XP, Chai JJ, Guan LR. Forty-year research of Phlebotominae sandflies in Xinjiang, China (1958–1998) End Dis Bull. 1998;13(4):97–102. [Google Scholar]
- 27.Chen SB, Guan LR, Li F, Liu PZ, Feng Y, Yang CM. The fauna of Phlebotomus sandflies in Gansu Province. Chin J Parasitol Parasit Dis. 2003;21(5):314–5. [PubMed] [Google Scholar]
- 28.Wang J, Xiong GH, Liu PZ. A bionomics study of Phlebotomus mongolensis in the desert area of Kansu Province, Northwest China. Acta Entomol Sinica. 1963;12(5–6):679–87. [Google Scholar]
- 29.Feng LC. The role of the peritrophic membrane in Leishmania and Trypanosoma infections of sandflies. Peking Nat Hist Bull. 1950;19(2–3):327–34. [Google Scholar]
- 30.Wang J, Qu JQ, Guan LR. A study on the Leishmania parasite of the big gerbil in northwest China. Acta parasitologica sinica. 1964;1:105–14. [Google Scholar]
- 31.Liu PZ, Guan LR, Li SX, Yu JG, Li TK, Cao HX, et al. Leishmania gerbilli in desert area of Hexi Zoulang of Gansu Province. Chin J Epidemiol. 1982;3(5):304–5. [Google Scholar]
- 32.Guan LR, Xu YX, Jia JX, Zuo XP, Wang G, Hou YY. Leishmaniasis in Karamay V. Observation on the bionomics of sandflies in Karamay, Xinjiang, China. End Dis Bull. 1991;6(2):55–61. [Google Scholar]
- 33.Guan LR, Yang YQ, Qu JQ, Shen WX. Discovery and study of Leishmania turanica for the first time in China. Bull WHO. 1995;73(5):667–72. [PMC free article] [PubMed] [Google Scholar]
- 34.Guan LR, Xu YX, Zuo XP, Wang G. Leishmaniasis in Karamay VII.Experimental infection of three species of sandflies with Leishmania from big gerbil. End Dis Bull. 1992;7(2):64–8. [Google Scholar]
- 35.Chai JJ, Guo YK. Epidemiological investigation on kala-azar in Atushi of Xinjiang. Chin J Hygiene. 1963;8(2):100–2. [Google Scholar]
- 36.Xiong GH, Guan LR, Chai JJ, Guo YK. Observation on the bionomics of sandflies in Atushi, Sinkiang. Acta Parasitologica Sinica. 1964;1(2):195–200. [Google Scholar]
- 37.Wang G, Wang J, Chai JJ, Li JS, Fu YF, Hailaiti, et al. A study on the bionomics of sandflies in oasis area of Kashgar, Xinjiang. End Dis Bull. 1992;7(1):72–6. [Google Scholar]
- 38.Guan LR, Xu YX, Maoyiding K, Wang W. The sandfly fauna and its role in transmission of kala-azar in four landscape zones of Aksu region, Xinjiang. J Parasitol Parasit Dis. 1986;4(3):169–72. [PubMed] [Google Scholar]
- 39.Zuo XP, Deng ZB, Alimu, Zhang S. Study of the prevalence of kala-azar in Wushi county of Xinjiang. End Dis Bull. 2005;20(1):33–4. [Google Scholar]
- 40.Xiong GH, Guan LR, Guo YK. A study of the kala-azar vectors in Xinjiang. J Control Res Epidemic Dis. 1974;4:327–34. [Google Scholar]
- 41.Dergacheva TI, Strelkova MV. Epidemiological role of sandflies Phlebotomus smirnovi Perfiliev, 1941 and P. longiductus Parrot, 1928 in visceral leishmaniasis foci in the Kazakh SSR. Trans Roy Soc Trop Med Hyg. 1985;79(1):34–6. doi: 10.1016/0035-9203(85)90227-5. [DOI] [PubMed] [Google Scholar]
- 42.WHO . Control of the Leishmaniasis-Report of a WHO Expert Committee. Geneva: WHO Technical Report Series 793; 1990. [Google Scholar]
- 43.Xiong GH, Hu YD, Guan LR, Wang J. The natural nidus of kala-azar in northwest China. J Control Res Epid Dis. 1976;1:63–8. [Google Scholar]
- 44.Xiong GH, Guan LR, Wang J, Hu YD, Qu JQ, Jin CF, et al. The bionomics of Phlebotomus major wui Yang & Hsiung, 1965, and its control in desert area of Sinkiang. Acta Entomol Sin. 1979;22(4):428–36. [Google Scholar]
- 45.Zhao EM, Chai JJ, Li BS, Yimanmu A, Jiao LE. Epidemiologic survey of Kala-azar in Bachu reclamation area, Xinjiang. J Xinjiang Med College. 1985;8(3):259–62. [Google Scholar]
- 46.Jin CF, Zuo XP, Gu DA, Yisilayin O, Lan QX, Zhang Y, et al. A newly identified endemic area of visceral leishmaniasis in Minfeng county of south Xinjiang II. Investigation on phlebotomine vectors. Chin Parasitol Parasit Dis. 2008;26(2):132–5. [PubMed] [Google Scholar]
- 47.Gu DA, Zuo XP, Yisilayin O, Lan QX, Jin CF, Zhou XJ, et al. Investigation on transmitting vectors of visceral leishmaniasis in Jiashi country, Xinjiang. Chin J Parasitol Parasit Dis. 2010;28(4):280–3. [PubMed] [Google Scholar]
- 48.Wang JY, Gao CH, Yang YT, Chen HT, Zhu XH, Lv S, et al. An outbreak of the desert sub-type of zoonotic visceral leishmaniasis in Jiashi, Xinjiang Uygur Autonomous Region, People’s Republic of China. Parasitol Int. 2010;59(3):331–7. doi: 10.1016/j.parint.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Guan LR, Chai JJ, Yang LP. Relationship between geographical distribution of sandflies and landscape features in Xinjiang Uyger Autonomous Region of China. Chin Parasitol Parasit Dis. 1996;14(1):26–32. [Google Scholar]
- 50.Guan LR. The biology of phlebotominae and relation to leishmaniasis. End Dis Bull. 1988;3(2):78–84. [Google Scholar]
- 51.Lu HG, Zhong L, Guan LR, Qu JQ, Hu XS, Chai JJ, et al. Separation of Chinese Leishmania isolates into five genotypes by kinetoplast and chromosomal DNA heterogeneity. Amer Soc Trop Med Hyg. 1994;50(6):763–70. doi: 10.4269/ajtmh.1994.50.763. [DOI] [PubMed] [Google Scholar]
- 52.Guan LR, Xu YX, Jia JX, Wang G, Zuo XP. Observation on the bionomics of Phlebotomus alexandri in Turfan basin, Xinjiang. End Dis Bull. 1987;2(1):36–43. [Google Scholar]
- 53.Xiong GH, Wang J, Hu YD, Liu PZ. Studies on the bionomics of Phlebotomus alexandri in Gansu province. Acta Entomol Sin. 1963;12(4):458–62. [Google Scholar]
- 54.Guan LR, Xu YX, Li BS, Dong J. The role of Phlebotomus alexandri Sinton, 1928 in the transmission of kala-azar. Bull WHO. 1986;64(1):107–12. [PMC free article] [PubMed] [Google Scholar]
- 55.Guan LR, Xiang XY, Wang G, Jia JX. Observation on the infectivity of Leishmania donovani from different areas to Phlebotomus alexandri from Xinjiang. Chin J Parasitol Parasit Dis. 1990;8(2):104–7. [PubMed] [Google Scholar]
- 56.Wang JY, Gao CH, Yang YT, Bao YF. Evaluation of a repetitive DNA sequence of Leishmania on the identification of Leishmania isolates in China. Chin J Zoonoses. 2005;21(4):304–8. [Google Scholar]
- 57.Chen YD. Report on eight Cutaneous leishmaniasis cases. Chin J Parasitol Parasit Dis. 1994;12(1):74. [Google Scholar]
- 58.Yang YT, Zhang M, Gao CH, Shi F, Guan LR, Wang JY. Detection and species identification of two imported cases of cutaneous leishmaniasis. Chin J Parasitol Parasit Dis. 2011;29(6):461–4. [PubMed] [Google Scholar]
- 59.Hu ZH, Gao Y, Shao JB, Teng GL, Jiang LC. Report on a case of imported Kala-aza. J Clinical Pediatrics. 2006;24(2):92–4. [Google Scholar]
- 60.Yang YT, Guan LR, Qu JQ, Bao YF. A case of American leishmaniasis imported from Venezuela. Chin J Parasitol Parasit Dis. 2001;19(5):307. [PubMed] [Google Scholar]
- 61.Zhou ZB, Gu DA, Guan LR, Kaisaier, Lv S, Zhang Y. Investigation on fluctuation of main sandfly species composition ratio and its relationship with visceral leishmaniasis in Kaxgar oasis, Xinjiang. Int J Med Parasit Dis. 2015;42(2):82–5. [Google Scholar]
- 62.Yin ZC, Yu FQ. Experimental infection of Phlebotomus chinensis from mountainous area with Leishmania donovani from dogs. Bull Control Res Parasit Dis. 1981;9(1):49–50. [Google Scholar]


