Abstract
Background
Patients with type 2 diabetes (T2D) have a substantial increased risk for cardiovascular (CV) disease and associated mortality than those without diabetes. Dulaglutide is a once-weekly glucagon-like peptide-1 receptor agonist that is approved for treatment of T2D.
Methods
This meta-analysis evaluates the CV risk in patients with T2D treated with dulaglutide in 9 randomized safety and efficacy trials. Mean (median) treatment duration was 333 (358) days. Reported CV events were independently adjudicated by a treatment-blinded clinical endpoint committee. The primary measure was a 4-component major adverse CV event (4-component MACE) composite endpoint of death due to CV causes, nonfatal myocardial infarction (MI), nonfatal stroke, or hospitalization for unstable angina. Additional pre-specified endpoints included adjudicated coronary revascularizations, hospitalization for heart failure, and all-cause mortality. A Cox proportional hazards regression model (stratified by study) was used to estimate the hazard ratio (HR) and confidence interval (CI). Tests of treatment effects for the primary endpoint were conducted at a 2-sided alpha level of 0.0198 and a corresponding 98.02 % CI was calculated. Statistical heterogeneity between the strata (studies) was tested by including in the Cox model an interaction term between treatment and strata.
Results
The analysis included 6010 randomized patients [dulaglutide: 3885; comparator therapy (active or placebo): 2125]; cumulative exposure to dulaglutide or comparator therapy was 3941 and 2223 patient-years, respectively. The demographic and baseline CV disease characteristics were similar across groups. Twenty-six (0.67 %) patients in the dulaglutide group versus 25 (1.18 %) in the comparator group experienced a primary 4-component MACE (HR 0.57; adjusted 98.02 % CI 0.30, 1.10). Results for the 3-component MACE (composite endpoint of death due to CV causes, nonfatal MI or stroke), 6-component MACE (composite endpoint of death due to CV causes, nonfatal MI or stroke, hospitalization for unstable angina or heart failure, or coronary revascularizations) and all-cause mortality were consistent with the primary analysis (HR < 1.0 for all).
Conclusions
These results suggest that dulaglutide does not increase the risk of major CV events in T2D patients. The ongoing CV outcomes study, Researching CV Events with a Weekly Incretin in Diabetes (REWIND), will further assess CV safety of dulaglutide.
Electronic supplementary material
The online version of this article (doi:10.1186/s12933-016-0355-z) contains supplementary material, which is available to authorized users.
Keywords: Type 2 diabetes, Meta-analysis, Glucagon-like peptide-1 (GLP-1), Incretin, Cardiovascular events, MACE
Background
Patients with type 2 diabetes (T2D) have a substantially increased lifetime risk for cardiovascular (CV) disease and associated death; older persons with T2D may have twofold to fourfold greater risk than those without diabetes [1–5]. Concerns have been raised regarding the effects of different anti-diabetic medications on CV safety [6], therefore, regulatory agencies now require that the CV safety of anti-diabetic medications be thoroughly studied prior to regulatory review [Food and Drug Administration (FDA) 2008; Committee for Medicinal Products for Human Use (CHMP) 2012] [7, 8]. It is advised to perform a meta-analysis of the clinical development data to demonstrate that the upper bound of the 95 % confidence interval (CI) of the hazard ratio (HR) for major adverse CV events in the treatment group is <1.8 compared to the comparator therapy (control) group. Additionally, it is advised to demonstrate that the upper bound of the 95 % CI of the HR is <1.3 in a dedicated CV outcomes study.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) significantly decrease hemoglobin A1c (HbA1c) via stimulating glucose-dependent insulin secretion, suppressing glucagon, delaying gastric emptying, and reducing appetite and food intake [9]. Early long-term glycemic control may decrease macrovascular complications in T2D [10, 11], therefore, treatment with GLP-1 RAs may modify CV risk. In addition to glycemic control, treatment with GLP-1 RAs exerts additional effects that may potentially alter the CV risk, including a decrease in body weight and a small decrease in systolic blood pressure (SBP), but with a small increase in heart rate.
Dulaglutide, a once-weekly long-acting GLP-1 RA, exhibits GLP-1 mediated effects [12, 13]. The Assessment of Weekly AdministRation of dulaglutide in Diabetes (AWARD) clinical development program demonstrated that treatment with dulaglutide improved glycemic control and caused significant decreases in HbA1c when compared to placebo or active comparators [14–23]. Treatment with dulaglutide was also associated with effects that may potentially alter CV risk including weight loss (or attenuation of weight gain) [14–23] and a small decrease in SBP, but with a small increase in heart rate [17]. This pre-specified CV meta-analysis evaluated CV risk in the dulaglutide clinical development program and included prospectively blinded adjudicated CV events from 9 controlled clinical studies, using different comparators and background medications, and encompassing a broad spectrum of the T2D population.
Methods
Studies
This CV meta-analysis included adjudicated CV events from a total of 9 clinical trials [14–23]; 4 Phase 2 studies (study durations: 12–26 weeks) and 5 Phase 3 studies (study durations: 52–104 weeks) (Table 1). A total of 3885 patients randomized to dulaglutide and a total of 2125 patients randomized to comparator therapy (placebo or active comparator) are included in this analysis. The dose of dulaglutide in the Phase 2 studies and the dose-finding portion of AWARD-5 ranged from 0.1 to 3 mg and it was 0.75 and 1.5 mg in the Phase 3 studies. While all 4 Phase 2 studies were placebo-controlled, comparator agents in the 5 Phase 3 studies included placebo, sitagliptin, exenatide, insulin glargine, or metformin. After completing the study treatment period, patients in these studies discontinued the assigned therapies and did not continue treatment in safety follow-up periods or in separate protocols.
Table 1.
Phase 2 and Phase 3 dulaglutide studies included in cardiovascular meta-analysis
| Study | Reference | Patients randomized | Dulaglutide doses (mg) | Treatment duration (weeks) | Comparators | Background therapy | |
|---|---|---|---|---|---|---|---|
| All (N) | Dulaglutide (n) | ||||||
| Phase 2 studies | |||||||
| Dose titrationa | [14] | 262 | 196 | 0.5 → 1.0, 1.0, 1.0 → 2.0 | 16 | Placebo | 2 OAMs |
| Monotherapya | [15] | 167 | 135 | 0.1, 0.5, 1.0, 1.5 | 12 | Placebo | None |
| Japanese studya | [16] | 145 | 108 | 0.25, 0.5, 0.75 | 12 | Placebo | None |
| ABPM studya | [17] | 755 | 505 | 0.75, 1.5 | 26 | Placebo | ≥1 OAM |
| Phase 3 studies | |||||||
| AWARD-1b | [18] | 978 | 559 | 0.75, 1.5 | 52 | Placebo, exenatide | Metformin + pioglitazone |
| AWARD-2b | [19] | 810 | 545 | 0.75, 1.5 | 78 | Glargine | Metformin + glimepiride |
| AWARD-3a | [20] | 807 | 539 | 0.75, 1.5 | 52 | Metformin | None |
| AWARD-4b | [21] | 884 | 588 | 0.75, 1.5 | 52 | Glargine | Lispro ± metformin |
| AWARD-5a | [22, 23] | 1202 | 710 | Stage 2: 0.75, 1.5 | 104 | Placebo, sitagliptin | Metformin |
ABPM ambulatory blood pressure monitoring, AWARD Assessment of Weekly AdministrRation of LY2189265 (dulaglutide) in Diabetes, OAM oral anti-diabetic medication
aDouble-blind studies
bOpen-label studies
As previously indicated in original publications for each clinical trial, all patients provided written informed consent before initiation of study procedures. Institutional review boards of all participating sites approved the protocol. Trials were conducted in compliance with Good Clinical Practice guidelines and the ethical principles stated in the Declaration of Helsinki [14–23].
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria for reporting meta-analyses [24] were followed; however, this meta-analysis was based on a pre-specified meta-analysis plan specifying that the analysis would include Phase 2 and Phase 3 studies from the dulaglutide clinical development program. Therefore, many of the PRISMA criteria related to reporting literature search strategy and criteria used for inclusion or exclusion of studies did not apply for this pre-specified meta-analysis.
This meta-analysis was performed on patient-level data from the included studies and all CV events were prospectively collected and adjudicated based on a common charter and based on the same event definitions. As prospectively defined, data from dulaglutide clinical pharmacology studies were not included in the meta-analysis due to their shorter durations of exposure (<6 weeks). There were no investigator-reported events of CV-related death, non-fatal stroke, non-fatal MI, or hospitalization for unstable angina in these studies.
Analysis population
Common inclusion criteria in the Phase 3 trials included a diagnosis of inadequately controlled T2D, age ≥18 years, a baseline HbA1c of 6.5–11.0 % (depending on specific study), previous treatment with diet and exercise alone or ongoing treatment with one or more oral anti-diabetic medications (OAM), stable weight (±5 %) for at least 3 months prior to screening, and a written informed consent. Females of childbearing potential were required to agree to use a reliable method of birth control during the study. Patients with recent CV events were generally excluded [for example, myocardial infarction (MI), stroke, or heart failure within 2–6 months]; Phase 2 studies and AWARD-5 study excluded patients with heart failure; AWARD-2 and AWARD-4 only excluded New York Heart Association (NYHA) class III or class IV heart failure; in addition, NYHA class II heart failure was also excluded in AWARD-1 due to concomitant administration of pioglitazone; and AWARD-3 only excluded NYHA class IV heart failure. The majority of studies excluded patients with serum creatinine ≥1.5 mg/dL (male) or ≥1.4 mg/dL (female), or creatinine clearance <60 mL/min. However, 3 studies included patients with higher serum creatinine levels or a lower estimated glomerular filtration rate (eGFR) if not contraindicated with concomitant medications; therefore, some patients with a eGFR < 60 mL/min/1.73 m2 were included in the meta-analysis [14, 17, 21]. Entry criteria for each study have been previously published [14–23]. The analysis population included all randomized patients [intent-to-treat (ITT) population].
Adjudication
An expert panel, blinded to treatment, [Duke Clinical Research Institute (DCRI), Duke University Medical Center] adjudicated the following CV events: death (CV and non-CV), acute coronary syndromes (MI and hospitalization for unstable angina), cerebrovascular events (stroke and transient ischemic attack), coronary revascularization procedures (coronary artery bypass grafting and percutaneous coronary interventions), and hospitalization for heart failure. CV events were initially identified in the 9 included studies by site investigators or study personnel during study conduct. Clinical sites were responsible for reporting applicable adverse events (AEs) and serious adverse events (SAEs), and completing a CV event-specific electronic case report form (eCRF). In addition, clinical trial databases were queried for specific events based on reported AE terms (i.e., MedDRA Preferred Terms) to identify potentially unreported events. CV events were also reported if potentially unreported events were identified by monitoring personnel or adjudicators. All identified CV events were sent to the DCRI clinical event classification (CEC) committee for adjudication. The CEC committee prospectively adjudicated all identified CV events in a blinded manner throughout the course of the studies based on pre-specified criteria and a pre-defined adjudication process.
Objectives
The primary objective was to compare, for all randomized patients (ITT population), the time from randomization to first occurrence of the composite endpoint of death due to CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina [4-component Major Adverse CV Event (MACE)] between patients receiving any dose of dulaglutide and those receiving comparator therapy (placebo or active comparator).
Additional objectives evaluated in this study included:
Time from randomization to first occurrence of CV events observed with once-weekly dulaglutide versus comparator group for each individual component of the composite primary endpoint.
Time from randomization to first occurrence of the 3-component MACE endpoint (composite of death due to CV causes, nonfatal MI or stroke).
Time from randomization to first occurrence of the 6-component MACE endpoint (composite of death due to CV causes, nonfatal MI or stroke, hospitalization for unstable angina or heart failure, or coronary revascularizations).
Time from randomization to first occurrence of hospitalization for heart failure, coronary revascularizations, and all-cause mortality.
All analyses including the subgroup analysis were conducted on all randomized patients (ITT population). Comparisons of dulaglutide versus placebo or active comparators, and dulaglutide dose (0.75 or 1.5 mg) versus placebo or all comparators, were also conducted. Two sensitivity analyses were conducted for the per-protocol (PP) and completers populations. The PP population was defined as all randomized patients who had not discontinued study drug or discontinued from the study, had an overall compliance of ≥75 %, and had no important protocol deviations. The completers population was defined as all randomized patients who completed a given study regardless of compliance with the protocol.
Statistical analysis
The primary analysis was a Cox proportional hazards regression model stratified by study (stratum). The model included treatment as a fixed effect with only 2 levels for the factor (dulaglutide or comparator groups). Stratification by study was used to account for potential differences in the HRs due to differing durations of the Phase 2 and 3 trials and differing lengths of follow-up periods. Thus, all Phase 2 studies were combined into one stratum; each of the Phase 3 AWARD studies (Table 1) formed an individual stratum. Since there were no patients with an adjudicated primary CV endpoint event in the comparator therapy arm of AWARD-3, it was combined with AWARD-5 into a single stratum. Statistical heterogeneity between the strata was tested by including in the primary analysis model an interaction term between treatment and strata.
All adjudicated CV events during the treatment periods and up to 30 days after treatment discontinuation were included in the analyses. Unless otherwise noted, all tests of treatment effects were conducted at a 2-sided alpha level of 0.05 and the CI was calculated at a 2-sided 95 % level. The pre‐specified meta‐analysis plan included a second potential meta‐analysis in case the upper bound of the CI for the HR was not <1.8 in the first meta‐analysis; adjustment for multiplicity was performed for the primary endpoint using the Pocock alpha-spending function to control for type 1 error. Tests of treatment effects for the primary endpoint were conducted at a 2-sided alpha level of 0.0198 and the CI was calculated at a 2-sided 98.02 % level. The second meta‐analysis was not performed since this first meta-analysis showed the upper bound of the 98.02 % CI for the HR was <1.8. All tests of interactions between treatment groups and other factors were conducted at a 2-sided alpha level of 0.10.
Counts and proportions of patients who experienced a primary endpoint and person-years of follow-up for the primary endpoint and the incidence rates were calculated. The incidence rate was calculated by dividing the number of patients who developed the event during the study period by the event-specific person-years of follow-up. In addition, Kaplan–Meier plots were reported for the primary endpoint, as well as Forest plots of the results by study (stratum).
Sensitivity analyses consisted of a re-evaluation of the primary endpoint for the PP and completers populations. Subgroup analysis, using the Cox proportional hazards regression model, included the subgroups of sex, age (≤65, >65 years), duration of diabetes (<5, [5–10], >10 years), prior history of CV disease, prior history of hypertension, prior history of hyperlipidemia, body mass index (BMI; <30, ≥30 kg/m2), baseline HbA1c [<8 % (<63.9 mmol/mol), ≥8 % (≥63.9 mmol/mol)], race (White, non-White), and geography (North America, South America, Europe, Asia Pacific, other). As a sensitivity analysis, MedDRA preferred terms were used to categorize AE reflecting CV specific events, regardless of their adjudication outcome. All analyses were implemented using SAS® Version 8.2 or higher.
Results
Demographic and baseline disease characteristics
This CV meta-analysis included a total of 6010 patients: 3885 treated with dulaglutide and 2125 treated with comparator therapy (placebo or an active) (Table 2). The mean age of patients was 56.1 years and the majority of patients were <65 years of age (81.6 %) and over two-thirds were White (68.3 %); 31.1 % of the patients were Hispanic or Latino. More than half (55.7 %) were enrolled in North America; 21.9 % were enrolled in the European Union (EU), 10.1 % in South America, 6.7 % in Asia Pacific, and 5.6 % in other regions. The overall proportions of males (51.2 %) and females (48.8 %) and mean BMI (32.3 kg/m2) were similar in the dulaglutide and comparator groups.
Table 2.
Demographic and other baseline characteristics of patients included in the cardiovascular meta-analysis—all randomized patients (ITT population)
| Variable | All comparators (N = 2125) |
All dulaglutide (N = 3885) |
Total (N = 6010) |
p valuea |
|---|---|---|---|---|
| Mean age (years) | 56.0 | 56.2 | 56.1 | 0.78 |
| Age group [n (%)] | – | – | – | 0.30 |
| <65 years | 1724 (81.1) | 3177 (81.8) | 4901 (81.6) | |
| ≥65 years | 401 (18.9) | 708 (18.2) | 1109 (18.5) | |
| Sex [n (%)] | – | – | – | 0.27 |
| Female | 1016 (47.8) | 1916 (49.3) | 2932 (48.8) | |
| Male | 1109 (52.2) | 1969 (50.7) | 3078 (51.2) | |
| Ethnicity [n (%)] | – | – | – | 0.23 |
| Hispanic or Latino | 660 (31.1) | 1209 (31.1) | 1869 (31.1) | |
| Not Hispanic or Latino | 1463 (68.9) | 2676 (68.9) | 4139 (68.9) | |
| Raceb [n (%)] | – | – | – | 0.27 |
| American Indian or Alaska Native | 135 (6.4) | 231 (6.0) | 366 (6.1) | – |
| Asian | 271 (12.8) | 509 (13.1) | 780 (13.0) | – |
| Black or African American | 113 (5.3) | 245 (6.3) | 358 (6.0) | – |
| White | 1446 (68.1) | 2656 (68.4) | 4102 (68.3) | – |
| Mean duration of diabetes (years) | 8.0 | 7.9 | 7.9 | 0.22 |
| Duration of diabetes | – | – | – | 0.01 |
| <5 years | 765 (36.0) | 1494 (38.5) | 2259 (37.6) | |
| ≥5 and <10 years | 659 (31.0) | 1088 (28.0) | 1747 (29.1) | |
| ≥10 years | 701 (33.0) | 1303 (33.5) | 2004 (33.3) | |
| Mean BMI (kg/m2) | 32.4 | 32.3 | 32.3 | 0.51 |
| Fasting plasma glucose | ||||
| Mean (mmol/L) | 9.07 | 9.01 | 9.04 | 0.69 |
| <5.6 mmol/L [n (%)] | 109 (5.2) | 200 (5.2) | 309 (5.2) | 0.79 |
| ≥5.6 mmol/L [n (%)] | 1994 (94.8) | 3630 (94.8) | 5624 (94.8) | |
| HbA1c | ||||
| Mean (%) | 8.1 | 8.1 | 8.1 | 0.87 |
| Mean (mmol/mol) | 65.0 | 65.0 | 65.0 | |
| <8 % (<63.9 mmol/mol) [n (%)] | 1113 (52.6) | 2039 (52.6) | 3152 (52.6) | 0.62 |
| ≥8 % (≥63.9 mmol/mol) [n (%)] | 1005 (47.5) | 1838 (47.4) | 2843 (47.4) | |
BMI body mass index, HbA1c glycosylated hemoglobin A1c
aCategorical variables were compared between treatments by Cochran–Mantel–Haenszel test. Continuous variables were compared between treatments by ANOVA model adjusting for strata: variable = treatment + stratum
bCategories omitted from the table: Native Hawaiian or Other Pacific Islander (0.1 % total), multiple (0.9 % total), and unknown (5.7 % total)
The 2 treatment groups were similar with respect to most baseline characteristics including mean HbA1c (8.1 %), fasting plasma glucose (FPG; 9.04 mmol/L), and duration of diabetes (7.9 years) (Table 2). There was a small, but significant difference between treatment groups with respect to subgroups defined by baseline duration of diabetes (i.e., <5 years for 38.5 % dulaglutide vs. 36.0 % comparators; ≥5 to <10 years for 28 % dulaglutide vs. 31 % comparators; p = 0.012).
In addition, baseline CV risk factors or prior CV disease including history of smoking, hypertension, hyperlipidemia, prior stroke/transient ischemic attack, and multiple other CV interventions, as well as renal function were similar between the two treatment groups (Table 3). However, prior MI at baseline was slightly higher for the dulaglutide group compared to the comparator group (3.4 vs. 2.4 %, p = 0.049) (Table 3).
Table 3.
Comparison of cardiovascular risk factors at baseline—all randomized patients (ITT population)
| Variable | All comparators (N = 2125) n (%) |
All dulaglutide (N = 3885) n (%) |
Total (N = 6010) n (%) |
p valuea |
|---|---|---|---|---|
| Prior MI | 51 (2.4) | 132 (3.4) | 183 (3.0) | 0.049 |
| History of unstable angina | 34 (1.6) | 55 (1.4) | 89 (1.5) | 0.632 |
| Prior coronary revascularization | 65 (3.1) | 115 (3.0) | 180 (3.0) | 0.738 |
| History of stroke or TIA | 35 (1.7) | 63 (1.6) | 98 (1.6) | 0.979 |
| History of heart failure | 12 (0.6) | 16 (0.4) | 28 (0.5) | 0.284 |
| History of (documented) coronary artery disease | 86 (4.1) | 189 (4.9) | 275 (4.6) | 0.163 |
| Has hypertension | 1357 (63.9) | 2451 (63.1) | 3808 (63.4) | 0.504 |
| Has hyperlipidemiab | 1176 (55.3) | 2116 (54.5) | 3292 (54.8) | 0.296 |
| History of carotid revascularizationc | 4 (0.2) | 8 (0.2) | 12 (0.2) | 0.863 |
| History of lower extremity arterial revascularizationc | 7 (0.5) | 8 (0.3) | 15 (0.3) | 0.380 |
| History of peripheral vascular diseasec | 30 (1.4) | 57 (1.5) | 87 (1.5) | 0.962 |
| History of atrial fibrillationc | 30 (1.4) | 38 (1.0) | 68 (1.1) | 0.173 |
| Current smoker | 335 (15.9) | 551 (14.3) | 886 (14.8) | 0.101 |
| Current smoker with hypertension and hyperlipidemia | 138 (6.5) | 217 (5.6) | 355 (5.9) | 0.161 |
| Kidney function group by eGFR | – | – | – | 0.899 |
| <30 mL/min/1.73 m2 | 1 (0.1) | 2 (0.1) | 3 (0.1) | |
| 30 ≤ eGFR < 60 mL/min/1.73 m2 | 126 (5.9) | 228 (5.9) | 354 (5.9) | |
| ≥60 mL/min/1.73 m2 | 1998 (94.0) | 3654 (94.1) | 5652 (94.1) | |
| Albuminuria group by urine ACRd | – | – | – | 0.433 |
| <30 mg/g | 1566 (76.8) | 2811 (77.1) | 4377 (77.0) | |
| 30 ≤ Urine ACR ≤ 300 mg/g | 395 (19.4) | 713 (19.6) | 1108 (19.5) | |
| >300 mg/g | 79 (3.9) | 121 (3.3) | 200 (3.5) |
eGFR estimated glomerular filtration rate, HDL high density lipoprotein, LDL low density lipoprotein, MI myocardial infarction, TIA transient ischemic attack, urine ACR urinary albumin/creatinine ratio
aTreatments were compared by Cochran–Mantel–Haenszel test. Strata = studies
bHaving hyperlipidemia or taking lipid lowering drugs at baseline or having LDL-C ≥160 mg/dL or HDL-C <40 mg/dL or triglycerides ≥200 mg/dL. Missing value was set to category ‘No’
cCV risk factors were either collected directly or were identified by searching historical events and pre-existing events at baseline with relevant preferred terms. Missing value is set to category ‘No’. History of lower extremity arterial revascularization was not collected, could not be defined, and is missing for the dose titration study, the monotherapy study, and AWARD-5 study
dUrine ACR was not collected in the dose titration study
Effect on primary 4-component MACE and individual components
Results of the meta-analysis showed that 26 (0.67 %) patients in the dulaglutide group and 25 (1.18 %) patients in the comparator group experienced at least 1 of the 4 major CV events (death due to CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina) (Table 4). The estimated HR for the comparison was 0.57 with an adjusted 98.02 % CI of 0.30, 1.10 (p = 0.046), indicating that there was no significant difference between treatment groups. These data suggest that treatment with dulaglutide was not associated with an increase in the risk of experiencing a 4-component MACE endpoint versus comparator therapy. In addition, the upper bound of the adjusted 2-sided 98.02 % CI for the HR (1.10) was less than the FDA-stipulated limit of 1.8. The treatment difference was also consistent across the strata (studies); there was no significant interaction between treatment and strata (interaction p = 0.598).
Table 4.
Time-to-event analysis of the primary cardiovascular (CV) endpoint and individual components—all randomized patients (ITT population)
| Endpoint component | All comparators (N = 2125) n (%) |
All dulaglutide (N = 3885) n (%) |
HRa
(adj. 98.02 % CI) |
p valuea |
|---|---|---|---|---|
| Primary 4-component MACE endpoint | 25 (1.18) | 26 (0.67) | 0.57 (0.30, 1.10) | 0.046 |
| Death from CV causesb | 5 (0.24) | 3 (0.08) | 0.35 (0.07, 1.87) | 0.119 |
| Nonfatal MI | 14 (0.66) | 9 (0.23) | 0.35 (0.13, 0.95) | 0.014 |
| Nonfatal stroke | 4 (0.19) | 12 (0.31) | 1.61 (0.42, 6.20) | 0.411 |
| Hospitalization for unstable angina | 6 (0.28) | 3 (0.08) | 0.28 (0.05, 1.46) | 0.054 |
| Exposure (event specific person-years follow-up) | 2211.31 | 3926.90 | – | – |
| Incidence rate per 100 person-years | 1.13 | 0.66 | – | – |
AWARD Assessment of Weekly AdministrRation of LY2189265 (dulaglutide) in Diabetes, CV cardioavascular, HR hazard ratio, MACE major adverse CV event, MI myocardial infarction
aCalculated from a stratified Cox Proportional Hazards regression model: response = treatment. Strata = studies; all Phase 2 studies formed one stratum, AWARD-3 and AWARD-5 formed one stratum. 2-sided p value to be compared to an alpha level of 0.0198 for test of superiority
bDeath from CV causes is defined as a death resulting from an acute MI, sudden cardiac death, death due to heart failure, death due to stroke, and death due to other CV causes
The Kaplan–Meier curves for the estimated cumulative incidence of the time to first occurrence of the 4-component MACE appear to start separating early, with the earliest divergence appearing around 28 weeks of exposure to treatment (Fig. 1). A Forest plot of the primary 4-component MACE comparing HR values with 98.02 % CIs by stratum (study) shows that the HRs for dulaglutide compared to comparator therapy are generally <1.0 which is consistent with the overall result (Fig. 2).
Fig. 1.
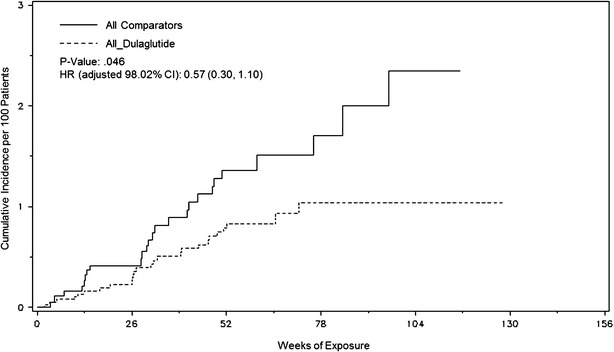
Time to first primary 4-component MACE. A Kaplan–Meier plot (with estimated HR [98.02 % CI] and p value) illustrating the time in weeks from randomization to the first occurrence of any of the 4 components of the primary endpoint measure
Fig. 2.
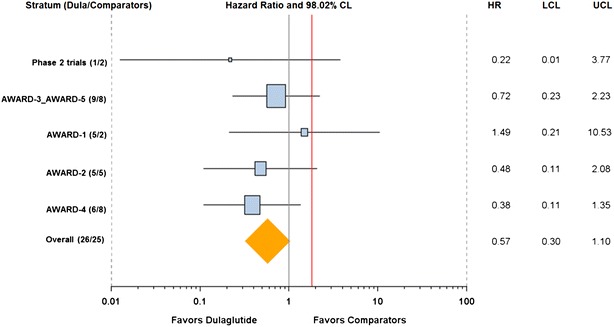
Forest plot of the primary 4-component MACE endpoint by stratum. A comparison of the primary analysis results (HR [98.02 % CI]) in each stratum (study or combinations of studies by which the primary analysis was stratified) with the overall result. Numbers of CV events per each treatment group (Dula/Comparators) are indicated in the parentheses in the y-axis under Stratum
There was no significant difference between the two treatment groups for the risk of death due to CV causes, nonfatal stroke, or hospitalization for unstable angina. However, the relative risk of experiencing a nonfatal MI was significantly lower in the dulaglutide group compared with the comparator group (estimated HR 0.35; adjusted 98.02 % CI [0.13, 0.95; p = 0.014]) (Table 4).
Results of the sensitivity analyses for the primary 4-component MACE endpoint
Time-to-event sensitivity analysis results for the PP and completers populations (Additional file 1: Tables S1 and S2, respectively) showed no significant differences between the 2 treatment groups with respect to the composite 4-component MACE endpoint. A total of 24 primary 4-component MACE events were reported in the PP population; 13 occurred in the dulaglutide group and 11 in the comparator group, with no significant difference between the 2 groups (HR 0.63; 98.02 % CI 0.24, 1.63; p = 0.26). Results in the completers population were consistent with the PP analysis.
Consistent with the primary comparison, there was no significant difference in the time to occurrence of the 4-component MACE by dulaglutide (all doses) versus active comparators or dulaglutide (all doses) versus placebo (Additional file 1: Table S3). Furthermore, no significant differences were observed when comparing dulaglutide dose (1.5 or 0.75 mg) versus placebo or dulaglutide dose versus all comparator therapy (active and placebo) (Additional file 1: Table S3).
Results of additional endpoints analyses
No significant differences were observed between the two treatment groups for the risk of experiencing all-cause mortality (HR 0.50; 95 % CI 0.18, 1.38), composite 3-component MACE, or heart failure requiring hospitalization (Table 5). However, there was a significant difference between the treatment groups for the 6-component MACE endpoint (HR 0.57; 95 % CI 0.37, 0.90; p = 0.016) and the time to coronary revascularization endpoint (HR 0.44; 95 % CI 0.21, 0.92; p = 0.029). No other significant differences were observed between the 2 treatment groups.
Table 5.
Time-to-event analysis of other cardiovascular (CV) endpoints—all randomized patients (ITT population)
| Endpoint component | All comparators (N = 2125) n (%) |
All dulaglutide (N = 3885) n (%) |
HRa
Est. (95 % CI) |
p valuea |
|---|---|---|---|---|
| All cause mortality | 8 (0.38) | 7 (0.18) | 0.50 (0.18, 1.38) | 0.181 |
| 3-Component MACE endpointb | 21 (0.99) | 23 (0.59) | 0.60 (0.33, 1.08) | 0.090 |
| 6-Component MACE endpointc | 37 (1.74) | 39 (1.00) | 0.57 (0.37, 0.90) | 0.016 |
| Heart failure requiring hospitalization | 2 (0.09) | 7 (0.18) | 2.02 (0.41, 9.88) | 0.378 |
| Coronary revascularization | 16 (0.75) | 13 (0.33) | 0.44 (0.21, 0.92) | 0.029 |
| Percutaneous coronary intervention | 14 (0.66) | 11 (0.28) | 0.43 (0.19, 0.95) | 0.036 |
| Coronary artery bypass grafting | 2 (0.09) | 2 (0.05) |
AWARD Assessment of Weekly AdministrRation of LY2189265 (dulaglutide) in Diabetes, CV cardioavascular, HR Est estimated hazard ratio, MACE major adverse CV event, MI myocardial infarction
aCalculated from a stratified Cox Proportional Hazards regression model: response = treatment. Strata = studies. All Phase 2 studies form one stratum, AWARD-3 and AWARD-5 form one stratum. When the total number of outcomes is <10, survival analysis is not performed. Instead when the total number of outcomes is <10 and ≥5, Mantel–Haenszel odds ratio and p value by Cochran–Mantel–Haenszel test are reported; When the total number of outcomes is <5, ratio and p-value are not reported
bComposite endpoint of death from CV causes, nonfatal MI, or nonfatal stroke
cComposite endpoint of death from CV causes, nonfatal MI, nonfatal stroke, hospitalization for unstable angina, coronary revascularization, or heart failure requiring hospitalization
Subgroup analysis
The results of the subgroup analysis for the ITT population indicated that there was no treatment by subgroup interaction and use of dulaglutide resulted in a consistent effect across subgroups (data not shown). The HR point estimate was <1.0 for all subgroups tested (favoring dulaglutide) except for tobacco use (HR 1.38), however, this effect was not significant (95 % CI 0.41, 4.58).
Overall CV adverse events post-baseline (regardless of adjudication)
As a sensitivity analysis, AE preferred terms were used to identify certain categories of CV AEs including coronary artery disease, angina, atrial fibrillation, MI, stroke or transient ischemic attack, heart failure, coronary revascularization, and carotid revascularization. Proportions of patients with ≥1 CV AE, reported post-baseline regardless of adjudication, were comparable between the dulaglutide (8.6 %) and the comparators (9.1 %) groups. No significant differences were observed in any category between the two groups (data not shown).
Discussion
In consideration of the substantial risk for CV events in persons with diabetes, regulatory agencies have required rigorous assessment of CV safety for new diabetic medications [7, 8]. Although, data from final CV outcome studies are still limited for the GLP-1 class, retrospective and pre-specified CV meta-analyses support continued clinical use of this class while awaiting confirmatory CV outcome data. Currently, only one GLP-1 RA (lixisenatide) has reported CV outcome study (ELIXA) results. This study showed that treatment of patients with a prior CV event with lixisenatide does not alter the risk for CV events [25].
CV meta-analysis results for dulaglutide and other GLP-1RAs
This CV meta-analysis included data from 9 controlled dulaglutide clinical studies with different comparators, background medications, and covering a broad spectrum of the T2D population. The baseline demographics and CV risk characteristics were comparable between the dulaglutide and comparator group. However, prior MI at baseline was slightly higher for the dulaglutide group compared to the comparator group (3.4 vs. 2.4 %, p = 0.049), an effect that would not be expected to introduce favorable bias for dulaglutide.
Overall, this CV meta-analysis suggests that treatment with dulaglutide was not associated with an increase in the risk of experiencing a MACE endpoint compared with comparator therapies. The incidence of the 4-component MACE in the dulaglutide group over time was consistently lower than the comparator group (Fig. 1). The results of the dulaglutide CV meta-analysis are consistent with other GLP-1 RAs CV meta-analyses data. Retrospective post hoc CV meta-analyses demonstrated that treatment with shorter-acting GLP-1 RAs (liraglutide or exenatide) did not increase the risk of MACE in patients with T2D [26, 27]. Recent pre-specified CV meta-analyses of prospectively adjudicated events also suggest that treatment with the longer-acting GLP-1 RAs, albiglutide and taspoglutide, does not increase the risk of MACE in patients with T2D [28, 29].
Evaluation of individual component endpoints demonstrated no significant difference between dulaglutide and comparators for risk of death from CV causes, non-fatal stroke, or hospitalization for unstable angina. The incidence for non-fatal stroke was numerically higher in the dulaglutide group compared to the comparator group, but with a HR 98.02 % CI that ranged from 0.42 to 6.20. On the other hand, the relative risk of experiencing a nonfatal MI was significantly lower with the dulaglutide group compared with the comparator group (estimated HR: 0.35; adjusted 98.02 % CI [0.13, 0.95; p = 0.014]). However, the total number of events was small and therefore, the interpretation of these results should be done with caution.
Additional endpoints including the 3-component MACE and the 6-component MACE also show results consistent with results of the primary measure, indicating that treatment with dulaglutide does not increase the risk for MACE whether defined narrowly (3-component MACE) or broadly (6-component MACE). Evaluation of individual component endpoints showed a significant decrease in the risk for the combined coronary revascularization endpoint (HR 0.44; 95 % CI [0.21, 0.92]). No significant difference was observed for the heart failure requiring hospitalization endpoint wherein the estimated HR was 2.02 and the 95 % CI was [0.41, 9.88]. Furthermore, additional analyses conducted to examine the primary 4-component MACE endpoint in the PP and completers population, different dulaglutide doses (0.75 and 1.5 mg), and subgroup analyses are all consistent with the outcome of the primary analysis observed in all randomized patients.
The incidence for major CV events (3-component, 4-component, and 6-component MACE) ranged from 0.59 to 1.00 % in the dulaglutide group compared to 0.99–1.74 % in the comparator group. This incidence rate of MACE events is consistent with rates observed in recent CV meta-analyses, which evaluated liraglutide, albiglutide, taspoglutide, saxagliptin, and vildagliptin [26, 28–31]. Incidence rates per 100 person-years for all major CV endpoints (the primary 4-component MACE) were lower in the dulaglutide group compared with the comparators group (0.66 vs. 1.13, respectively).
Cardiovascular effects of GLP-1 RAs
Pre-clinical and recent human data indicate that GLP-1 RAs may play a cardioprotective role via reduction in myocardium infarct size, improving endothelial function, and vasodilation [32–35]. However, another study showed that chronic treatment with liraglutide for 14 weeks did not affect endothelial function [36] indicating that this may be limited to acute effect. Recent clinical data indicate that GLP-1 RAs may alter other modifiable CV risk factors such as body weight, blood pressure, or lipids [32, 37] potentially leading to reduction in macrovascular risk [38]. It is unknown if these effects would prevent CV events, this hypothesis remains to be confirmed in long-term CV outcomes studies.
Potential alteration of CV risk factors by dulaglutide or other anti-hyperglycemic treatments, effects of long-term glycemic control, and the need for long-term data
In all of the 9 Phase 2 and Phase 3 studies included in this meta-analysis, treatment with dulaglutide improved glycemic control compared to placebo or active comparators [14–23]. Consistent with the GLP-1 RAs’ mechanism of action, treatment with dulaglutide also has been associated with alterations of other additional parameters that may be implicated in modifying CV risk including lower risk of hypoglycemia [39], body weight loss [39], and a small decrease in SBP [17]. Although T2D is an established risk factor for macrovascular disease, several studies demonstrated that intensive glycemic control only resulted in an insignificant slight decrease in CV endpoints [40, 41]. However, a recent placebo-controlled study in patients with T2D at high risk for CV events demonstrated that a lower rate of 3-component MACE was observed when empagliflozin was added to standard care compared to placebo [42]. Although rates of MI and stroke were similar between the two treatment groups, empagliflozin-treated patients had significantly lower rates of CV-related mortality (3.7 vs. 5.9 % in the placebo group; 38 % relative risk reduction), hospitalization for heart failure (2.7 and 4.1 %, respectively; 35 % relative risk reduction), and all-cause mortality (5.7 and 8.3 %, respectively; 32 % relative risk reduction). In addition to glycemic efficacy, treatment with empagliflozin has been also associated with body weight loss, diuretic effect, and a decrease in blood pressure [42], effects that may lead to an overall lowering of CV risk. Furthermore, long-term follow-up data after intensive glycemic control in patients at early stage of T2D showed significant decrease in MI and all-cause mortality [10], indicating that early glycemic control may play a significant role in modulating long-term CV outcomes and delaying diabetes-related macrovascular complications. The recent 10-year long-term follow-up from the Veteran Affairs Diabetes Trial (VADT) study provides additional evidence that the CV benefits from the initial intensive glucose control (median of 5.6 years) is also observed for patients with a long duration of T2D (mean of 11.6–12 years) [43]. After nearly 10 years of follow-up, patients in the VADT trial who were randomized to 5.6 years of intensive glucose control had a significantly lower risk (17 % reduction; p = 0.04) of having a major CV event than those given standard therapy. Therefore, early treatment and long-term follow up data may be helpful in the evaluation of the potential CV benefit related to the glycemic effects of GLP-1 RAs.
Strengths and limitations of this CV meta-analysis
Among strengths of this CV meta-analysis is the inclusion of a broad population that is representative of the general T2D population. This meta-analysis included patients at different stages of T2D, with a mean duration of diabetes ranging from 2.6 to 12.7 years and with multiple CV risk factors [14–23]. Although this CV meta-analysis suggests that dulaglutide treatment is not associated with excess CV risk, limitations of CV meta-analyses in general include the relatively short treatment duration. In addition, inclusion of a diverse population at different stages of T2D, while appropriate for evaluation of CV safety in patients with different CV risk factors, may not enable evaluation of potential CV benefits due to the limited number of patients at earlier stages of T2D and the relatively short-term follow up. It is also important to note that patients with a recent history of clinically significant and potentially unstable CV disease were excluded from the dulaglutide clinical trial program. For example, patients with congestive heart failure (mainly NYHA class III or IV), or certain recent CV events including MI or stroke, were excluded from most studies. In addition, patients with eGFR < 60 mL/min/1.73 m2 were also excluded from most studies. Therefore, certain populations with high CV risk may have been excluded from the overall population included in this CV meta-analysis, which is consistent with most other development programs [26–29]. Accordingly, the low rate of CV events observed in the Phase 2 and 3 T2D populations is a common limitation for these CV meta-analyses in general.
Conclusions
This CV meta-analysis suggests that treatment of patients with T2D with once-weekly dulaglutide for up to 104 weeks does not increase the risk for CV events. In addition, all sensitivity analyses and additional endpoints consistently suggest that dulaglutide does not increase the risk for CV events. Definitive data regarding the assessment of effects of long-term treatment with dulaglutide on CV risk will be provided by the currently ongoing, large CV outcome Researching CV Events with a Weekly Incretin in Diabetes (REWIND) study.
Authors’ contributions
FTB, CMA, and PS participated in the analysis and assessment of data included in this manuscript. All authors helped in the interpretation of data, drafting and revising the various drafts of the manuscript, and have approved the final version. Each author is also accountable for the accuracy and integrity of this final version. All authors read and approved the final manuscript.
Acknowledgements
The study was funded by Eli Lilly and Company. Writing support was provided by Teresa Tartaglione, PharmD and Sesha Reddigari, PhD of ClinGenuity, LLC and funded by Eli Lilly and Company.
Competing interests
Dr. Ferdinand is a consultant/advisor for Eli Lilly and Company, Amgen, Sanofi, and Boehringer Ingelheim. Dr. Sager is a safety consultant (member of Diabetes or Safety Monitoring Board, Cardiovascular Disease End Point committee, consultant, or advisory board) to Eli Lilly and Company and NDA Partners LLC. He is the chair of the FDA Cardiovascular and Renal Drugs Advisory Committee and a member of the CSRC Executive Committee and serves on the Board of Directors of Anthera Pharmaceuticals, Inc. Dr. Fady Botros and Dr. Charles Atisso are employees and stockholders of Eli Lilly and Company.
Abbreviations
- AE
adverse events
- AWARD
Assessment of Weekly AdministRation of LY2189265 (dulaglutide) in Diabetes
- BMI
body mass index
- CEC
clinical event classification
- CHMP
Committee for Medicinal Products for Human Use
- CI
confidence interval
- CV
cardiovascular
- DCRI
Duke Clinical Research Institute
- eCRF
electronic case report form
- eGFR
estimated glomerular filtration rate
- EU
European Union
- FDA
Food and Drug Administration
- FPG
fasting plasma glucose
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HR
hazard ratio
- ITT
intent-to-treat
- MACE
major adverse cardiovascular event
- MI
myocardial infarction
- NYHA
New York Heart Association
- OAM
oral anti-diabetic medications
- PP
per protocol
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- REWIND
Researching Cardiovascular Events with a Weekly Incretin in Diabetes
- SAE
serious adverse events
- SBP
systolic blood pressure
- T2D
type 2 diabetes
- VADT
Veteran Affairs Diabetes Trial
Additional file
10.1186/s12933-016-0355-z Supplementary tables.
Contributor Information
Keith C. Ferdinand, Email: kferdina@tulane.edu
Fady T. Botros, Email: botros_fady_t@lilly.com
Charles M. Atisso, Email: charles.atisso@lilly.com
Philip T. Sager, Email: Psager@Stanford.edu
References
- 1.Sarwar N, Gao P, Seshasai SR, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 3.National diabetes fact sheet (CDC). National estimates and general information on diabetes and prediabetes in the United States. http://www.familydocs.org/f/CDC%20Diabetes%20fact%20sheet-2011.pdf.
- 4.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D’Agostino RB, Sr, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 5.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 6.Hirshberg B, Katz A. Insights from cardiovascular outcome trials with novel antidiabetes agents: what have we learned? An industry perspective. Curr Diab Rep. 2015;15:87. doi: 10.1007/s11892-015-0663-9. [DOI] [PubMed] [Google Scholar]
- 7.United States Food and Drug Administration Guidance for Industry, Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008 FDA guidance document. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf.
- 8.Guideline on Clinical Investigation in Treatment and Prevention of Diabetes Mellitus. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf.
- 9.Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005;21:91–117. doi: 10.1002/dmrr.538. [DOI] [PubMed] [Google Scholar]
- 10.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 12.Glaesner W, Mark Vick A, Millican R, Ellis B, Tschang SH, Tian Y, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26:287–296. doi: 10.1002/dmrr.1080. [DOI] [PubMed] [Google Scholar]
- 13.Kuritzky L, Umpierrez G, Ekoe JM, Mancillas-Adame L, Lando LF. Safety and efficacy of dulaglutide, a once weekly GLP-1 receptor agonist, for the management of type 2 diabetes. Postgrad Med. 2014;126:60–72. doi: 10.3810/pgm.2014.10.2821. [DOI] [PubMed] [Google Scholar]
- 14.Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ, 3rd, Group EGOS The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab. 2011;13:418–425. doi: 10.1111/j.1463-1326.2011.01366.x. [DOI] [PubMed] [Google Scholar]
- 15.Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet Med. 2012;29:1260–1267. doi: 10.1111/j.1464-5491.2012.03745.x. [DOI] [PubMed] [Google Scholar]
- 16.Terauchi Y, Satoi Y, Takeuchi M, Imaoka T. Monotherapy with the once weekly GLP-1 receptor agonist dulaglutide for 12 weeks in Japanese patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomised, double-blind, placebo-controlled study. Endocr J. 2014;61:949–959. doi: 10.1507/endocrj.EJ14-0147. [DOI] [PubMed] [Google Scholar]
- 17.Ferdinand KC, White WB, Calhoun DA, Lonn EM, Sager PT, Brunelle R, et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731–737. doi: 10.1161/HYPERTENSIONAHA.114.03062. [DOI] [PubMed] [Google Scholar]
- 18.Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1) Diabetes Care. 2014;37:2159–2167. doi: 10.2337/dc13-2760. [DOI] [PubMed] [Google Scholar]
- 19.Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide vs. insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2) Diabetes Care. 2015;38:2241–2249. doi: 10.2337/dc14-1625. [DOI] [PubMed] [Google Scholar]
- 20.Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3) Diabetes Care. 2014;37:2168–2176. doi: 10.2337/dc13-2759. [DOI] [PubMed] [Google Scholar]
- 21.Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385:2057–2066. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 22.Skrivanek Z, Gaydos BL, Chien JY, Geiger MJ, Heathman MA, Berry S, et al. Dose-finding results in an adaptive, seamless, randomized trial of once-weekly dulaglutide combined with metformin in type 2 diabetes patients (AWARD-5) Diabetes Obes Metab. 2014;16:748–756. doi: 10.1111/dom.12305. [DOI] [PubMed] [Google Scholar]
- 23.Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17:849–858. doi: 10.1111/dom.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober KV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 26.Marso SP, Lindsey JB, Stolker JM, House JA, Martinez Ravn G, Kennedy KF, et al. Cardiovascular safety of liraglutide assessed in a patient-level pooled analysis of phase 2: 3 liraglutide clinical development studies. Diab Vasc Dis Res. 2011;8:237–240. doi: 10.1177/1479164111408937. [DOI] [PubMed] [Google Scholar]
- 27.Ratner R, Han J, Nicewarner D, Yushmanova I, Hoogwerf BJ, Shen L. Cardiovascular safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc Diabetol. 2011;10:22. doi: 10.1186/1475-2840-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher M, Petrie MC, Ambery PD, Donaldson J, Ye J, McMurray JJV. Cardiovascular safety of albiglutide in the Harmony programme: a meta-analysis. Lancet Diabetes Endocrinol. 2015;3:697–703. doi: 10.1016/S2213-8587(15)00233-8. [DOI] [PubMed] [Google Scholar]
- 29.Seshasai SR, Bennett RL, Petrie JR, Bengus M, Ekman S, Dixon M, et al. Cardiovascular safety of the glucagon-like peptide-1 receptor agonist taspoglutide in people with type 2 diabetes: an individual participant data meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2015;17:505–510. doi: 10.1111/dom.12448. [DOI] [PubMed] [Google Scholar]
- 30.Cobble ME, Frederich R. Saxagliptin for the treatment of type 2 diabetes mellitus: assessing cardiovascular data. Cardiovasc Diabetol. 2012;11:6. doi: 10.1186/1475-2840-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweizer A, Dejager S, Foley JE, Couturier A, Ligueros-Saylan M, Kothny W. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large Phase III type 2 diabetes population. Diabetes Obes Metab. 2010;12:485–494. doi: 10.1111/j.1463-1326.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 32.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114:1788–1803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 33.Anagnostis P, Athyros VG, Adamidou F, Panagiotou A, Kita M, Karagiannis A, et al. Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes Metab. 2011;13:302–312. doi: 10.1111/j.1463-1326.2010.01345.x. [DOI] [PubMed] [Google Scholar]
- 34.Davidson MH. Cardiovascular effects of glucagonlike peptide-1 agonists. Am J Cardiol. 2011;108(3 Suppl):33B–41B. doi: 10.1016/j.amjcard.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 35.Torimoto K, Okada Y, Mori H, Otsuka T, Kawaguchi M, Matsuda M, et al. Effects of exenatide on postprandial vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14:25. doi: 10.1186/s12933-015-0188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomoto H, Miyoshi H, Furumoto T, Oba K, Tsutsui H, Miyoshi A, et al. A comparison of the effects of the GLP-1 analogue liraglutide and insulin glargine on endothelial function and metabolic parameters: a randomized, controlled trial Sapporo Athero-Incretin Study 2 (SAIS2) PLoS One. 2015;10(8):e0135854. doi: 10.1371/journal.pone.0135854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simó R, Guerci B, Schernthaner G, Gallwitz B, Rosas-Guzmàn J, Dotta F, et al. Long-term changes in cardiovascular risk markers during administration of exenatide twice daily or glimepiride: results from the European exenatide study. Cardiovasc Diabetol. 2015;14:116. doi: 10.1186/s12933-015-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul SK, Klein K, Maggs D, Best JH. The association of the treatment with glucagon-like peptide-1 receptor agonist exenatide or insulin with cardiovascular outcomes in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol. 2015;14:10. doi: 10.1186/s12933-015-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurung T, Shyangdan DS, O’Hare JP, Waugh N. A novel, long-acting glucagon-like peptide receptor-agonist: dulaglutide. Diabetes Metab Syndr Obes. 2015;10(8):363–386. doi: 10.2147/DMSO.S34418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schernthaner G. Diabetes and cardiovascular disease: is intensive glucose control beneficial or deadly? Lessons from ACCORD, ADVANCE, VADT, UKPDS, PROactive, and NICE-SUGAR. Wien Med Wochenschr. 2010;160:8–19. doi: 10.1007/s10354-010-0748-7. [DOI] [PubMed] [Google Scholar]
- 41.Ray KK, Seshasai SRK, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 42.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 43.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]


