Abstract
The receptor protein tyrosine phosphatases (RPTPs) exhibit a wide repertoire of cellular signalling functions. In particular, type IIa RPTP family members have recently been highlighted as hubs for extracellular interactions in neurons, regulating neuronal extension and guidance, as well as synaptic organisation. In this review, we will discuss the recent progress of structural biology investigations into the architecture of type IIa RPTP ectodomains and their interactions with extracellular ligands. Structural insights, in combination with biophysical and cellular studies, allow us to begin to piece together molecular mechanisms for the transduction and integration of type IIa RPTP signals and to propose hypotheses for future experimental validation.
Keywords: Receptor protein tyrosine phosphatase, Cell surface receptor, Cell surface signalling, Crystal structure, Neuronal extension, Neuronal synapse
1. Introduction
The receptor protein tyrosine phosphatases (RPTPs) are important mediators of signal transduction at the plasma membrane, featuring in events as diverse as cell growth and proliferation (tumour suppressors), neural development (axon guidance molecules) and the immune response [1–5]. The 21 mammalian family members are type I membrane proteins, containing either single or a tandem of highly conserved intracellular phosphotyrosine-specific phosphatase domains (Fig. 1a) [1]. For those RPTPs possessing tandem catalytic domains, with the exception of RPTPα [6], only the membrane proximal domain (D1) is active on known phosphotyrosine substrates, while the membrane-distal domain (D2) seems to be required for optimal D1 activity [7–11].
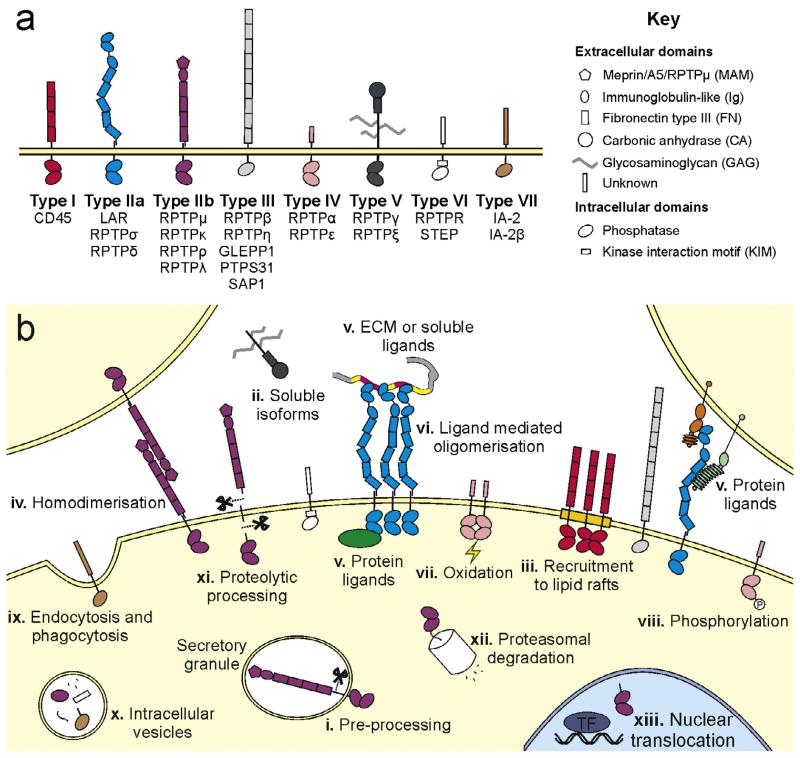
Fig. 1.
RPTP family classification, cellular localisation and regulation. (a) Classification of the human RPTPs, based on [1]. (b) Schematic depicting RPTP cellular location and modes of RPTP regulation: (i) the pro-proteins of both type IIa and IIb RPTPs are processed by subtilisin/kexin-like endoproteases in the trans-Golgi and subsequently presented at the cell surface as two non-covalently bonded subunits [35,36,131–134]. (ii) Alternative splicing occurs across the RPTP families, yielding multiple receptor isoforms [1]. The type V RPTP, RPTPζ, can also be expressed as a soluble isoform, more commonly known as phosphacan [135]. (iii) At the plasma membrane of activated T cells, the type I RPTP CD45, is recruited to lipid rafts [136]. (iv) Trans-homodimerisation of the type IIb RPTPs mediates the stability of adherens junctions [14,137]. (v) Although many of the RPTPs remain orphan receptors, the type IIa RPTPs have been shown to bind extracellular matrix (ECM) or soluble ligands [18,25,26] and cell surface proteins [27–29]. (vi) Ligand binding can potentially modulate clustering of RPTPs, for instance heparan sulphate proteoglycans (HSPGs) induce oligomerisation of type IIa RPTPs [58]. (vii) The generally inactive, membrane-distal phosphatase domain (D2) is thought to function as a redox sensor; oxidation of the D2 catalytic site cysteine regulates RPTPα (a type IV RPTP) dimerisation and phosphatase activity [138]. (viii) RPTPs may themselves be phosphorylated. For example Tyr and Ser phosphorylation mediates the activity of RPTPα and its interaction with intracellular signalling proteins [139,140]. (ix) RPTPs can be retrieved from the plasma membrane by endocytosis; the type VIII (pseudo-phosphatase) RPTP IA-2β contains a YXXφ motif within the cytoplasmic tail, targeting the receptor for clathrin-mediated endocytosis [141]. Alternatively, the type IIa RPTP, RPTPσ has been found to regulate autophagy and (x) to be located in autophagic vesicles targeted for lysosomal degradation [142]. (xi) Mature types IIa and IIb RPTPs at the cell surface can undergo proteolytic processing events to shed ectodomain fragments into the extracellular environment, followed by release of the intracellular tandem phosphatase domains [133,143]. (xii) This intracellular fragment may subsequently be targeted for proteosomal degradation. (xiii) However, it has been demonstrated that the intracellular fragment of RPTPκ and RPTPρ may also be imported into the nucleus and regulate transcription via dephosphorylation of transcriptional activators (TF) such as β-catenin and STAT3 [133,144].
Numerous mechanisms have been reported for the regulation of RPTP phosphatase activity, including alternative splicing, post-translational modifications, oligomerisation, ligand binding and proteolysis (summarised in Fig. 1b and discussed in greater detail elsewhere [2,3,5]). Structural analysis has revealed many details of the catalytic mechanism and substrate specificity of the RPTP phosphatase domains [12]. However, we have a far less complete understanding of the architecture and functions of the diverse extracellular regions. Many RPTPs contain domains typically associated with cell adhesion molecules (CAMs), such as immunoglobulin-like (Ig) and fibronectin type III (FN) units, and are involved in cell–cell or cell–matrix contacts (Fig. 1) [13–22]. The first extracellular RPTP structure, of the type IIb family member RPTPμ, revealed a tight and rigid trans dimer, able to gauge inter-membrane distances at cell-to-cell contacts and thus position the phosphatase to the appropriate functional location at the adherens junction [14]. The first extracellular RPTP-ligand complex structure, of the type V RPTPγ bound to contactin-4, provided a rationale for the selectivity of type V RPTPs with contactin ligands 1–6, important for recognition events in neuronal wiring [17]. In general, most RPTP family members are still classified as orphan receptors, with no extracellular ligands known to date [23]. The type IIa RPTPs, however, are remarkable in that a large collection of protein ligands have recently been described. These ligands are particularly relevant within the nervous system [18,19,24–34], offering extensive scope for investigation into neuronal RPTP function. As a result, this review will focus on recent progress in the structural analysis of extracellular type IIa RPTPs and their ligand interactions.
2. Type IIa RPTP domain organisation and structure
The type IIa RPTP family members share a common domain architecture: an extracellular region containing up to three Ig domains and nine FN repeats, modified by alternative splicing, a single transmembrane helix and tandem intracellular phosphatase domains (Fig. 2a). The receptors are produced as pro-proteins, processed intracellularly, at a dibasic amino acid site located within the FN9 repeat, by a subtilisin-like endoprotease and subsequently expressed on the cell surface as two non-covalently bonded subunits (Fig. 1b) [35,36]. Type IIa RPTPs have early ancestors, however they only acquired the domain architecture of the modern vertebrate orthologues from Cnidaria onwards, which coincided with the evolution of the first centralised bilateral nervous system [3]. The family has gradually diversified in evolution, with one member in Caenorhabditis elegans (PTP-3), two in Drosophila (DLAR and possibly PTP69D) and three in vertebrates (RPTPσ, RPTPδ and LAR), presumably to offer additional functionality and complexity. Variation of type IIa RPTPs is also generated via alternative splicing, in particular within the ectodomain, which yields multiple receptor isoforms (Fig. 2a) [37–41]. In addition to possible splicing of whole domains (Ig3, FN5 and FN4-7), four micro-exon inserts define type IIa RPTP isoforms. In humans, the longest isoforms share 66–72% overall sequence identity (83–87% between intracellular regions, 58–64% between extracellular regions). They have overlapping, though distinct expression patterns, predominantly in the developing and adult nervous systems [35,36,39,42–50].
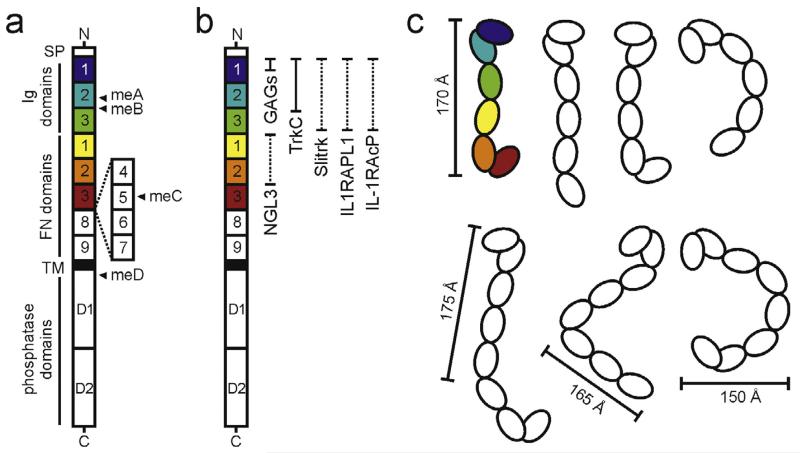
Fig. 2.
Architecture and flexibility of the type IIa RPTP ectodomain. (a) Domain organisation of the type IIa RPTPs. N, amino-terminus (extracellular); SP, secretion signal peptide; TM, transmembrane; C, C-terminus (intracellular); Ig, immunoglobulin-like; FN, fibronectin type-III. Alternative splicing of the type IIa RPTPs by insertion of FN domains 4–7 and mini exons A–D (closed arrowheads) generates many isoforms, which exhibit distinct expression patterns [35,39]. Additional isoforms, lacking Ig3 (RPTPσ) or FN5 (LAR) domains, have also been reported [37,41]. (b) Type IIa RPTP domains required for binding to the listed extracellular ligands are illustrated by filled or dashed vertical bars (structural information currently available or unavailable respectively). (c) Cartoon illustrating the flexibility revealed by combined crystallographic, electron microscopy and small-angle X-ray scattering analyses of the human RPTPσ ectodomain. Conformations representative of Ig1-FN3 (top) and short ectodomain (bottom) molecules are depicted [57]. The conformation corresponding to the human RPTPσ Ig1-FN3 crystal structure (PDB accession code 4PBX) is coloured. Approximate molecular dimensions are indicated.
The initial focus of structural efforts for the RPTPs was directed towards the intracellular catalytic domains [51,52]. Crystal structures of the tandem phosphatase domains (D1D2) of both human RPTPσ and LAR have been determined [7,53] and, based on these, RPTPδ D1D2 has been modelled [12]. These studies suggest that similar to D1D2 modules across other RPTPs, with the notable exception of RPTPγ, the type IIa RPTP tandem phosphatase domains are monomeric in solution [12]. Several experimental studies demonstrate that cis-dimerisation or oligomerisation of receptor protein tyrosine phosphatases (RPTPs) negatively regulates their catalytic activity [16,54–56], although this has not been demonstrated definitively for all RPTPs. Evidence against the early ‘inhibitory wedge’ model [51] as a general mechanism for this inactivation has gradually accumulated (discussed in detail in [5,12]). The relative orientation of type IIa RPTP D1D2 domains upon ligand-mediated receptor clustering, and its impact on the catalytic function and downstream signalling properties of the receptors, therefore remain outstanding questions.
Structural work on type IIa RPTP extracellular regions is now emerging, revealing surprising flexibility, in contrast to the observed rigidity of the RPTPμ ectodomain [14] and the linear rods typically used to depict RPTP ectodomains in schematics (Fig. 2b) [57]. The two N-terminal Ig1-2 domains consistently adopt a rigid V-shaped arrangement, stabilised by extensive interdomain contacts [57–59], but essentially every other inter-domain linker appears to represent a flexible hinge point [57]. The Ig2–Ig3 linker, a site for alternative splicing, is particularly noteworthy. In receptor isoforms containing the meB exon, this hinge point is extended by a further four amino acids (ELRE inserted between R227 and R228; numbering corresponds to chicken RPTPσ), potentially introducing additional flexibility. The structural rigidity of elongated, multi-domain receptors such as RPTPμ, cadherins and SYG1/SYG2 may facilitate close receptor packing to enhance cell–cell adhesion and/or signalling, enable tight regulation of cell–cell contact distances and allow efficient force transduction between cells [14,60–62]. However, flexibility may be a valuable feature for synaptic signalling hubs such as the type IIa RPTPs and α-neurexin [57,63–66], for instance, to allow larger molecules, with potentially broader functionality, to fit into narrow cell–cell junctions (approximately 240 Å for mammalian excitatory synapses [67]).
3. Interaction of type IIa RPTPs with proteoglycans at neuronal growth cones
Two heparan sulphate proteoglycans (HSPGs) within the retinal basal membrane, agrin and collagen XVIII, were the first type IIa RPTP ligands to be identified [18]. Additional HSPGs, membrane-spanning syndecan (Sdc) and glycophosphatidylinositol (GPI)-membrane anchored dallylike (Dlp), were reported to bind DLAR in the context of the Drosophila neuromuscular junction [19,24]. More recently, secreted extracellular matrix chondroitin sulphate proteoglycans (CSPGs), major components of the reactive glial scar following injury, were also shown to bind to type IIa RPTPs in vertebrate central nervous system (CNS) neurons [25,26,68]. Intriguingly, interactions of type IIa RPTPs with HSPGs versus CSPGs, elicit contrasting functional consequences: while HSPG binding to RPTPσ promotes neurite outgrowth [18,69], CSPG binding inhibits neuronal extension and nerve regeneration [25,68].
Proteoglycans are composed of a core protein module to which a variable number of glycosaminoglycan (GAG) chains are covalently attached [70]. GAGs are linear polymers of repeating disaccharide units; alternating N-acetylated glucosamine (GlcNAc) and uronic acid residues (GlcA or IdoA) for heparan sulphate (HS) or N-acetylated galactosamine (GalNAc) and glucuronic acid (GlcA) for chondroitin sulphate (CS) [70]. Further GAG diversity is generated by sulphation at defined positions along the chains [70]. Enzymatic digestion of the GAG chains abolished binding of type IIa RPTPs to both HSPGs and CSPGs [18,25], demonstrating that the interaction is via the negatively charged proteoglycan HS or CS chains. On the type IIa RPTPs, positively charged residues (K67, K68, K70, K71, R96 and R99, residue numbering corresponding to chicken RPTPσ) within the N-terminal Ig domain were shown to be required [18,25]. Crystal structures of the two N-terminal Ig domains (Ig1-2) across type IIa RPTP family members and different species, revealed that these basic residues lie on loops between Ig1 β strands C–D and E–F and create an extended positively charged surface (Fig. 3a) [58,59]. An additional crystal structure of human LAR Ig1-2 in complex with the small molecule GAG-mimetic sucrose octasulphate (SOS), not only confirmed the site of GAG binding, but also demonstrated mobility of the C–D loop containing the four basic lysine residues [58].
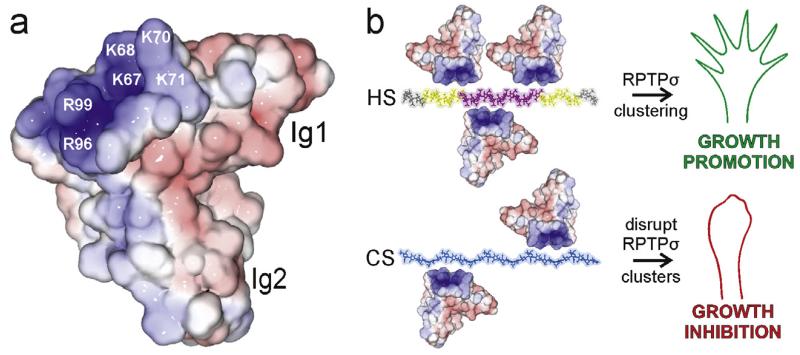
Fig. 3.
Structural and functional consequences of type IIa RPTP-proteoglycan binding. (a) Solvent accessible surface of human RPTPσ Ig1-2 (2YD3 [58]) coloured by electrostatic potential from red (−8 kT/e) to blue (+8 kT/e). Labelled residues are important for proteoglycan binding (numbering corresponds to chicken RPTPσ). (b) Model for HS-induced clustering of the type IIa RPTPs believed to result in neuronal extension (top). Disruption of type IIa RPTP clusters through interaction with CS results in growth inhibition (bottom). The heparin 30-mer is coloured to indicate zones of differing sulphation level in HS: grey (low), intermediate (yellow) and high (pink). GAG chains were built from the following PDB accession numbers: heparin (1HPN [145]) and chondroitin-4 sulphate (1C4S [146]).
Highly sulphated proteoglycan GAG chains carry a strong negative charge and bind to a wide range of proteins at the cell surface, playing roles in the assembly of various cell surface protein–protein complexes [71,72]. For example, HSPGs are crucial for the regulation of axon guidance molecules such as Robo, Slit and DCC [73,74] and for assembling key protein–protein complexes in both the FGF and hedgehog signalling pathways [75–77]. Therefore, the specificity of protein-GAG interactions is an important question [72,78–81]. The flexible, solvent-exposed nature of the Ig1 binding surface, and the observation that this site can bind to both HSPGs and CSPGs, would suggest that the type IIa RPTPs are likely to exhibit limited GAG binding specificity. However, amongst chondroitin sulphate isoforms, RPTPσ does demonstrate preferential binding to CS-E [82].
Given that HSPGs and CSPGs bind to the same type IIa RPTP interaction site, with approximately the same binding affinity (Kd 10–20 nM) [58], how then can these binding events produce such different functional outcomes? Solution studies illustrated that HS, but not CS, is able to promote clustering of the type IIa RPTPs [58]. For HS chains, regions of high sulphation (typically three sulphate groups per disaccharide) lie adjacent to intermediately modified sections and are interspaced by stretches largely lacking sulphation [83], whereas CS is sulphated at lower levels (usually one (CS-A and CS-C) or two (CS-D and CS-E) groups per disaccharide) [81,84–86]. Therefore, it has been proposed that the islands of higher sulphation present in HS but not CS are responsible for the observed oligomerisation in solution and may promote close packing of type IIa RPTP molecules at the cell surface (Fig. 3b) [58]. Receptor clustering would lead to an uneven distribution of tyrosine phosphatase activity at the plasma membrane, regardless of whether the clustered RPTPs themselves become deactivated. The consequent existence of transient zones with reduced phosphatase activity is expected to increase the localised levels of phosphorylated proteins involved in signalling pathways that stimulate neuronal extension [87,88]. Conversely, secreted CSPGs present in the post-traumatic glial scars could compete with HSPGs for type IIa RPTP binding, disrupting receptor clusters to produce a more uniform distribution of the phosphatase activity at the cell surface and ultimately inhibit axon growth.
The contrasting consequences of type IIa RPTP interaction with HSPGs versus CSPGs have striking similarities to their ability to functionally modulate the axon guidance molecule Sema5A [89]. The interaction of Sema5A with either HSPGs or CSPGs renders Sema5A a permissive or a repulsive axon guidance signal, respectively [89]. Importantly, Nogo receptors NgR1 and NgR3 also bind CSPGs with inhibitory consequences upon neuronal regeneration and simultaneous ablation of RPTPσ, NgR1 and NgR3 leads to enhanced optic nerve regeneration following crush injury relative to single RPTPσ or dual NgR1/NgR3 loss [82]. Candidate type IIa RPTP substrates for involvement in growth-promoting signalling pathways in the axonal growth cone are also present at the synapse and will be discussed further in the following section.
4. Extracellular interactions of type IIa RPTPs at neuronal synapses
A variety of CAMs are believed to play important roles in the establishment and maintenance of chemical synapses [90,91]. Initially, the specificity of axon-dendrite contacts is mediated by an array of cell adhesion molecules such as members of the side-kick and Dscam families [92]. Subsequent adhesive interactions are required to recruit signalling molecules and ion channels, to promote the specialisation of both pre- and postsynaptic sites and to maintain the structural integrity of the synapse [90,91]. Such functions are performed by a variety of homophilic and heterophilic molecular systems, including neurexin/neuroligin, SynCAMs, ephrin/Eph receptors and netrin-G/Netrin-G ligand (NGL), which establish bidirectional trans-synaptic signalling complexes [91,93–96]. The majority of the early in vivo work on the type IIa RPTPs focussed upon their role either in axon outgrowth and guidance [8,45,69,97] or at the presynaptic terminals of C. elegans and Drosophila neuromuscular junctions [19,40,98] and more recent studies have additionally highlighted their presynaptic functions in vertebrate systems [27–34,66,99]. Presynaptic type IIa RPTPs are also able to mediate both presynaptic differentiation and recruitment of the postsynaptic density components, through interaction with their postsynaptic ligands [27–34]. It should be noted that, while we will focus here on their presynaptic function, these proteins have also been reported to play important roles on the postsynaptic side [100,101].
In vertebrates, the catalogue of postsynaptic protein ligands for the three type IIa RPTPs has been rapidly expanding in recent years, and it currently includes: Netrin-G ligand-3 (NGL-3) [27,28], TrkC receptor protein tyrosine kinase [29], Slit- and Trk-like receptors 1–6 (Slitrk1-6) [30,31], interleukin-1 receptor accessory protein (IL-1RacP) [32] and IL-1-receptor accessory protein-like 1 (IL1RAPL1) [33,34]. In practice, the large number of potential complexes is restricted by protein expression patterns, for example RPTPδ-Slitrk3 interaction appears to be specific for inhibitory synapses [30,31]. Several of the postsynaptic ligands also demonstrate a preference amongst the type IIa RPTPs, for instance TrkC selectively binds RPTPσ [29,57]. The specificity or strength of all type IIa RPTP-ligand interactions listed above, with the exception of RPTP-NGL3, are additionally regulated through alternative splicing at the RPTP meA and meB sites [29,30,32,33], a striking similarity to the binding specificity generated via alternative splicing in the neurexin–neuroligin system [66].
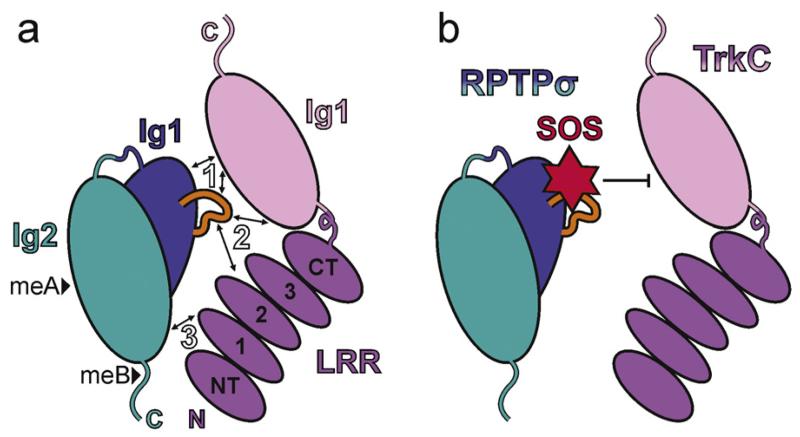
Structural analyses of type IIa RPTP-ligand complexes are now revealing the molecular basis for these interaction specificities. Crystal structures of the N-terminal leucine rich repeat (LRR) and Ig-domain of TrkC bound to RPTPσ Ig1-2, the minimal units required for the binding and synaptogenic activity of this trans-synaptic pair [29,57], display a 1:1 complex, the orientation of which is consistent with a trans RPTPσ:TrkC complex spanning the synaptic cleft (Fig. 4a) [57]. Biophysical and cell based assays have demonstrated that three major binding sites along the extended protein–protein interface mediate RPTPσ:TrkC binding [57]. A small number of residue differences between type IIa RPTPs and Trk receptors at these three sites provide satisfying explanations for reported interaction specificities [29,57]. While the meA mini-exon insertion site on RPTPσ Ig2 lies remotely from the RPTPσ-TrkC interface (Fig. 4a), insertion of the meB mini-exon reduces RPTPσ:TrkC binding [29,57] and is predicted to disrupt a putative fourth accessory RPTPσ-TrkC binding site.
Fig. 4.
Synaptic type IIa RPTP-ligand complexes. (a) Cartoon illustrating the trans interaction mode observed in the chicken RPTPσ Ig12:TrkC LRRIg1 crystal structure (PDB accession code 4PBW) [57]. The three major binding sites are indicated by arrows and white labels. Protein features labelled as follows: Ig, immunoglobulin-like domain; LRR, leucine rich repeat domain (containing NT, amino-terminal cap; 1–3, leucine rich repeats; CT, carboxy-terminal cap); N, amino-terminus; C, carboxy-terminus; black arrowheads, potential meA and meB insertions; orange loop, RPTPσ Lys-loop. (b) Cartoon to illustrate the overlap of the sucrose octasulphate binding site on LAR (based on PDB accession code 2YD8 [58]) with the RPTPσ:TrkC interface. HS and heparin oligosaccharides have been shown to compete with TrkC for RPTPσ binding [57].
The list of synaptic extracellular type IIa RPTP ligands also includes proteoglycans [19]. HSPGs are particularly abundant, either as membrane spanning or GPI-anchored proteins at the pre- and postsynapse or as molecules secreted into the extracellular milieu [71,73]. The importance of synaptic ligand location in type IIa RPTP regulation is illustrated by events at the Drosophila neuromuscular junction. While the interaction of type IIa RPTP dLAR with presynaptic HSPG dSyndecan promotes bouton growth, the postsynaptic HSPG dDallylike competes with dSyndecan for dLAR binding, leading to an inhibition of growth and active zone stabilisation [19]. There is strong evidence for the importance of HSPGs for correct mammalian glutamatergic synaptic function [102,103] and for the interaction of HSPGs with specific synaptic cell surface receptors [104,105].
Given the diversity of possible interactions with the type IIa RPTPs, a key question to address, is the competitive or cooperative nature of different ligand combinations. Recent analyses are beginning to shed light on this. For example, two RPTPσ residues essential for TrkC interaction (R96 and R99), form part of the extended positively charged surface on RPTPσ Ig1 (Fig. 3a) [57,58] and are also absolutely required for RPTPσ interactions with HSPGs [18,58]. Indeed, HS and heparin (a highly sulphated HS analogue) oligomers were able to directly compete with TrkC for RPTPσ binding in both biophysical and cell based assays (Fig. 4b) [57]. In contrast to TrkC, the interaction of RPTPσ with another trans-synaptic protein ligand, NGL-3, reported to engage the FN1-2 domains [27], appeared insensitive to proteoglycans in both experimental settings [57].
Which extracellular features of the type IIa RPTPs may equip them to function as interaction hubs? The length of the type IIa RPTP ectodomain may be important to extend the HSPG binding site beyond a saturating layer of cis interactions, analogous to the use of a longer ectodomain by Sialoadhesin, a member of the sialic acid-binding Siglec family of cell surface receptors, to ‘escape’ from the inhibitory glycocalyx on the same cell surface [106]. The flexibility of the type IIa RPTP ectodomains may also be a functionally important characteristic, allowing a range of conformations at the plasma membrane of both the growth cone and the presynaptic terminals (Fig. 5) [57]. At the transition from axonal extension to synaptogenesis, an array of additional RPTPσ ligands are presented by the post-synaptic neuronal surface [66,99], which compete with each other, the presynaptic ligands and soluble HSPGs for type IIa RPTP binding. For example, during synapse formation and stabilisation, the post-synaptic TrkC ligand must out-compete proteoglycans for RPTPσ binding, providing an adhesive trans interaction that may also disrupt HSPG-mediated RPTPσ clusters (Fig. 5) [57]. Binding of RPTPσ to a first postsynaptic ligand, will limit the conformational freedom of the flexible receptor ectodomain and reduce the entropic penalty for ligand binding at other RPTPσ sites, potentially facilitating the formation of higher order cell surface assemblies [107]. Soluble factors such as the NT3 neurotrophin and the astrocyte-derived HSPGs glypican-4 and glypican-6, may additionally regulate this system (Fig. 5) [108]. Proteolytic cleavage events add a further level of complexity. Cleavage of the whole type IIa RPTP ectodomain [36] and potentially of Ig1-2 fragments [57,58] would firstly decouple the receptor phosphatase activity from regulation via ligand binding to these regions. Secondly, the released soluble RPTPσ fragments may also be able to compete with the remaining intact receptors for binding to extracellular ligands.
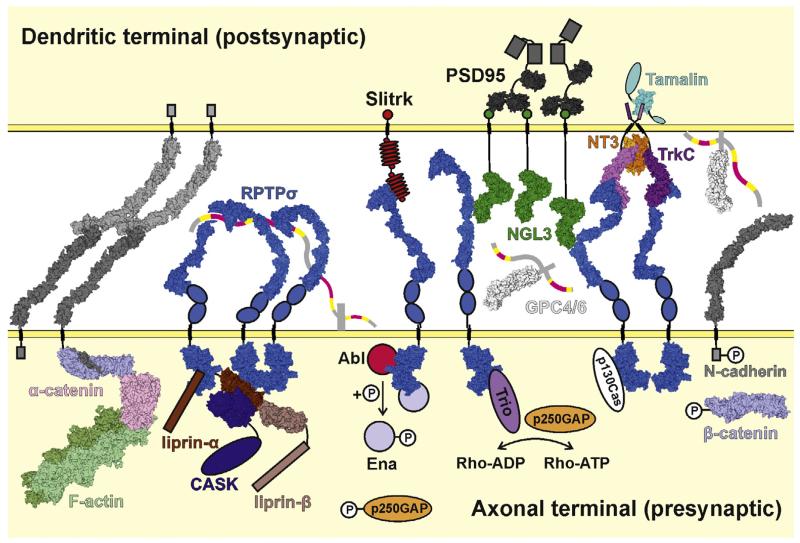
Fig. 5.
Model depicting synaptic interactions and signalling of type IIa RPTPs. The type IIa RPTP (RPTPσ illustrated) flexible ectodomains are able to adopt a variety of conformations [57]. For the RPTPσ N-terminal Ig domain to interact with presynaptic proteoglycans such as syndecan, more bent forms must be adopted (left), whereas to bind postsynaptic ligands such as TrkC, NGL-3, Slitrk and glypican, more extended conformations are required (right). HSPGs and soluble NT3 produce higher order RPTPσ arrays. RPTPσ can bind to presynaptic scaffolding proteins including liprin-α. Presynaptic RPTPσ signalling may also occur through dephosphorylation of N-cadherin and β-catenin and the stabilisation of adhesive N-cadherin:β-catenin:α-catenin:F-actin complexes or through several indicated cascades which culminate in activation of the Rho-GTPases and subsequent cytoskeletal remodelling. The same trans-synaptic interactions also lead to recruitment of postsynaptic density components such as PSD-95. Space filling models are derived from the following PDB accession codes and studies: human RPTPσ Ig1-2 (2YD3 [58]), Ig1-3 (2YD9 [58]), Ig1-FN3 (4PBX [57]; crystal structure used in full and divided into fragments to generate full range of ectodomain conformations; blue ovals represent FN4 and FN5 domains) and D1D2 (2FH7 [53]), mouse NGL-3 LRRIg1 (3ZYO [147]; green circles represent PDZ domain binding motifs), human RPTPσ Ig1-2:mouse TrkC LRRIg1 complex (4PBW [57]; aligned to 2IFG to generate model of RPTPσ:TrkC:NT3 complex), human TrkA LRR-Ig2:NGF complex (2IFG [148]; used to represent TrkC LRR-Ig2:NT3), rat tamalin PDZ domain (2EGO [149]; cyan ovals represent additional tamalin protein domains), human glypican-1 core protein (4AD7 [150]; heparan sulphate chains represented by ribbons and coloured as in Fig. 3b), mouse N-cadherin ectodomain (3Q2W [60]; grey boxes represent unbound N-cadherin intracellular tails, for clarity only one N-cadherin:α-catenin:β-catenin complex is shown), human α-catenin (4IGG [151]), zebrafish β-catenin (2Z6G [152]), mouse α-catenin:mouse β-catenin complex (4ONS [153], 4IGG and 2Z6G aligned to this crystal structure to obtain a more complete complex model), mouse E-caherin:β-catenin (1I7X [154], used to generate model of N-caderin intracellular domain with β-catenin), rabbit F-actin filament (3MFP [155]), rat PSD-95 PDZ-binding domains (3GSL [156] and 1TQ3), human liprin-α2 SAM1-3:mouse liprin-β1 SAM1-3 complex (3TAD [157]; brown boxes represent coiled-coil regions) and human CASK kinase domain:human liprin-α2 SAM1-3 complex (3TAC [157]; aligned with 3TAD to generate CASK:liprin-α:liprin-β model; blue oval represents remainder of CASK molecule). Structural information is currently lacking for Slitrk (red ovals, LRR repeats; small red circles, cysteine-rich caps; intracellular red circle, PDZ domain binding motif) and many HSPGs (grey stalk, protein core; heparan sulphate chains coloured as for glypican-1). Intracellular proteins signalling downstream of RPTPσ are represented as simple ovals or circles. Key phosphorylation sites are indicated by small white circles labelled ‘P’.
5. Intracellular interactions of type IIa RPTPs at neuronal synapses
Trans-synaptic interactions between type IIa RPTP ectodomains and their ligands trigger bi-directional intracellular signals. These lead to both presynaptic differentiation, including the accumulation of synaptic vesicles, and clustering of postsynaptic density proteins [27–34,109]. The exact mechanisms of signal transduction across the plasma membranes remain to be elucidated, and may vary on a case-by-case basis, but interactions spanning the synaptic cleft are likely to impact on the localisation, trafficking/turnover rate, relative arrangement and possibly conformation of the cell surface proteins involved, all of which can modulate downstream signalling. On the intracellular side of the membrane, type IIa RPTPs interact directly with liprin-α [39,98,110–112] a key component of the active zone scaffolding array, which stabilises the receptors at the synapse and links them to other components of the presynaptic machinery [113]. On the opposing cell surface, postsynaptic ligands for the type IIa RPTPs contain intracellular PDZ domain binding motifs, facilitating the recruitment of scaffolding proteins such as PSD-95 (Fig. 5). TrkC does not contain a typical PDZ domain binding motif, but is able to bind to the scaffolding protein tamalin [114]. Ligands containing intracellular catalytic domains such as the TrkC isoform TrkCTK+, may provide additional signalling routes, although this requires further investigation. So far, it has been shown that kinase activity is not required for the RPTPσ-mediated synaptogenic function of TrkC [29].
A recent screen of RPTP family members against an array of phosphopeptides revealed that their substrate selectivity varies significantly [12]. While the type I RPTP CD45 displayed a high enzymatic activity for almost all substrates, RPTPσ was only able to dephosphorylate a peptide derived from its physiological substrate N-cadherin [12]. This suggests that the type IIa RPTPs may regulate quite specific downstream signalling pathways, rather than gross phosphotyrosine levels, both in the growth cone and at the synapse. The currently known type IIa RPTP substrates are depicted in Fig. 5. Assembly of dephosphorylated N-cadherin and β-catenin [115–117] with α-catenin to form adhesive complexes linked to the actin cytoskeleton, would likely increase synaptic stability [117]. However, upon phosphorylation of N-cadherin or β-catenin, formation of these adhesion sites will likely be disrupted. Therefore, a reduction in the enzymatic activity of type IIa RPTPs would be predicted to disrupt synaptic stability (Fig. 5) [117]. Other potential type IIa RPTP signalling mechanisms also converge on the actin cytoskeleton. For example, Ableson kinase (Abl) and its substrate Enabled (Ena), a facilitator of actin polymerisation [118], are both type IIa RPTP ligands and substrates [8]. In addition, the RPTP intracellular binding partner Trio, as well as p250GAP, a type IIa RPTP substrate, are both Rho family GTPases, important regulators of actin dynamics (Fig. 5) [119–122]. Therefore, the phosphatase activity of the type IIa RPTPs appears to contribute to synaptic organisation and stability by transmitting multiple signals that modulate actin cytoskeleton organisation.
6. Conclusions and future directions
Numerous novel synaptic ligands have been identified for the type IIa RPTPs over recent years and structural work characterising their modes of interaction is progressing at pace. The structural and biophysical characterisation of RPTPσ binding to either proteoglycan GAGs or protein ligands have revealed specific binding modes for each interaction, as well as overlaps in binding sites. These observations raise important new questions for future investigation. Which ligands compete for type IIa RPTP binding? Which combination of ligands might bind in a complementary manner? Is it possible to modulate such interactions in vivo, and thus provide novel strategies for nerve regeneration? Chemical synapses and their many components exist in a highly dynamic state [123,124]. What is the timing of the different interactions during the processes of axon-dendrite recognition, presynaptic differentiation, recruitment of the postsynaptic machinery, and at later stages during synaptic stabilisation and maintenance?
The type IIa RPTPs have been described as synaptic signalling hubs, important organising modules linking signals from the synaptic cleft to the scaffolding networks of both the pre- and postsynapse [66,99]. Proteomics studies have identified RPTPσ and RPTPδ in voltage-gated Ca2+ channel nano-environments [125] and shown RPTPσ to be associated with GluRδ2-centric protein complexes [109]. Therefore are these type IIa RPTP signalling hubs actually an important component of much larger, localised multi-component synaptic structures? Key synaptic signal transduction molecules, including postsynaptic NMDA receptors, have been reported to be part of such ‘supercomplexes’ [126]. While it is well established that voltage-gated Ca2+ channel nano-environments exist at the pre- and postsynapse to confine the transient increase in intracellular Ca2+ concentration to very local spatiotemporal domains [127], the concept of ‘nanodomains’ has only more recently been extended to neurotransmitter receptors. Superresolution light microscopy studies have identified that postsynaptic AMPA receptors are also clustered in dynamic nanodomains, as are postsynaptic scaffolding proteins including PSD95 [128,129]. The type IIa RPTPs have previously been linked to AMPA receptor synaptic recruitment [100,130] and their potential role in these localised, dynamic signalling domains remains to be determined.
Future structural studies on full length type IIa RPTPs will be crucial to demonstrate the impact of ectodomain conformation, receptor oligomerisation and transmembrane interactions upon the arrangement, ligand interactions and activity of the intracellular phosphatase domains. The determination of type IIa RPTP structures in the context of larger protein–protein complex arrays, initially reconstituted in vitro and ultimately within intact synapses, would provide essential details about the signalling system in progressively more realistic, close to physiological settings. Finally, the development of imaging strategies to monitor downstream signalling of synaptic type IIa RPTPs in primary neuronal cell cultures, and ideally also in vivo, will be essential to properly appreciate their enzymatic in addition to their organisational functionality. A comprehensive mechanistic understanding of type IIa RPTP synaptic function, guided by structural biology, could potentially shed new light on neurological disorders linked to dysfunction of synaptic signalling hubs and inspire new strategies for nerve regeneration.
Acknowledgements
This work was funded by the UK Medical Research Council (MRC, G0700232 and L009609 to A.R.A. and G9900061 to E.Y.J.) and Cancer Research UK (A10976 to E.Y.J.). The Wellcome Trust Centre for Human Genetics is supported by Wellcome Trust grant 090532/Z/09/Z. C.H.C. is a Human Frontiers Science Program long-term Postdoctoral Fellow (LT000100/2013). A.R.A. is an MRC Senior Research Fellow.
References
- [1].Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- [2].Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- [3].Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- [4].Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–20. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- [5].Nikolaienko RM, Agyekum B, Bouyain S. Receptor protein tyrosine phosphatases and cancer: new insights from structural biology. Cell Adhes Migr. 2012;6:356–64. doi: 10.4161/cam.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Y, Pallen C. The receptor-like protein tyrosine phosphatase HPTP alpha has two active catalytic domains with distinct substrate specificities. EMBO J. 1991;10:3231–7. doi: 10.1002/j.1460-2075.1991.tb04886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nam H-J, Poy F, Krueger N, Saito H, Frederick C. Crystal structure of the tandem phosphatase domains of RPTP LAR. Cell. 1999;97:449–57. doi: 10.1016/s0092-8674(00)80755-2. [DOI] [PubMed] [Google Scholar]
- [8].Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–12. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- [9].Krueger NX, Reddy RS, Johnson K, Bateman J, Kaufmann N, Scalice D, et al. Functions of the ectodomain and cytoplasmic tyrosine phosphatase domains of receptor protein tyrosine phosphatase Dlar in vivo. Mol Cell Biol. 2003;23:6909–21. doi: 10.1128/MCB.23.19.6909-6921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Felberg J, Lefebvre DC, Lam M, Wang Y, Ng DHW, Birkenhead D, et al. Subdomain X of the kinase domain of Lck binds CD45 and facilitates dephosphorylation. J Biol Chem. 2004;279:3455–62. doi: 10.1074/jbc.M309537200. [DOI] [PubMed] [Google Scholar]
- [11].Nam H-J, Poy F, Saito H, Frederick CA. Structural basis for the function and regulation of the receptor protein tyrosine phosphatase CD45. J Exp Med. 2005;201:441–52. doi: 10.1084/jem.20041890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barr AJ, Ugochukwu E, Lee WH, King ONF, Filippakopoulos P, Alfano I, et al. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–63. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brady-Kalnay SM, Tonks NK. Protein tyrosine phosphatases as adhesion receptors. Curr Opin Cell Biol. 1995;7:650–7. doi: 10.1016/0955-0674(95)80106-5. [DOI] [PubMed] [Google Scholar]
- [14].Aricescu AR, Siebold C, Choudhuri K, Chang VT, Lu W, Davis SJ, et al. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–20. doi: 10.1126/science.1144646. [DOI] [PubMed] [Google Scholar]
- [15].Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Levt S, et al. The carbonic anhydrase domain of receptor tyrosine phosphatase β is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–60. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- [16].Fukada M, Fujikawa A, Chow JPH, Ikematsu S, Sakuma S, Noda M. Protein tyrosine phosphatase receptor type Z is inactivated by ligand-induced oligomerization. FEBS Lett. 2006;580:4051–6. doi: 10.1016/j.febslet.2006.06.041. [DOI] [PubMed] [Google Scholar]
- [17].Bouyain S, Watkins DJ. The protein tyrosine phosphatases PTPRZ and PTPRG bind to distinct members of the contactin family of neural recognition molecules. Proc Natl Acad Sci U S A. 2010;107:2443–8. doi: 10.1073/pnas.0911235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase σ. Mol Cell Biol. 2002;22:1881–92. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–31. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- [20].Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, et al. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–95. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Whiteford JR, Xian X, Chaussade C, Vanhaesebroeck B, Nourshargh S, Couchman JR. Syndecan-2 is a novel ligand for the protein tyrosine phosphatase receptor CD148. Mol Biol Cell. 2011;22:3609–24. doi: 10.1091/mbc.E11-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee H-K, Cording A, Vielmetter J, Zinn K. Interactions between a receptor tyrosine phosphatase and a cell surface ligand regulate axon guidance and glial–neuronal communication. Neuron. 2013;78:813–26. doi: 10.1016/j.neuron.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mohebiany AN, Nikolaienko RM, Bouyain S, Harroch S. Receptor-type tyrosine phosphatase ligands: looking for the needle in the haystack. FEBS J. 2013;280:388–400. doi: 10.1111/j.1742-4658.2012.08653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fox AN, Zinn K. The heparan sulfate proteoglycan Syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol. 2005;15:1701–11. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- [25].Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, et al. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–6. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–66. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kwon SK, Woo J, Kim SY, Kim H, Kim E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. J Biol Chem. 2010;285:13966–78. doi: 10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Woo J, Kwon S-K, Choi S, Kim S, Lee J-R, Dunah AW, et al. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–37. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- [29].Takahashi H, Arstikaitis P, Prasad T, Bartlett TE, Wang YT, Murphy TH, et al. Postsynaptic TrkC and presynaptic PTPσ function as a bidirectional excitatory synaptic organizing complex. Neuron. 2011;69:287–303. doi: 10.1016/j.neuron.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Takahashi H, Katayama K, Sohya K, Miyamoto H, Prasad T, Matsumoto Y, et al. Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nat Neurosci. 2012;15:389–98. doi: 10.1038/nn.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yim YS, Kwon Y, Nam J, Yoon HI, Lee K, Kim DG, et al. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2013;110:4057–62. doi: 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yoshida T, Shiroshima T, Lee S-J, Yasumura M, Uemura T, Chen X, et al. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J Neurosci. 2012;32:2588–600. doi: 10.1523/JNEUROSCI.4637-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yoshida T, Yasumura M, Uemura T, Lee S-J, Ra M, Taguchi R, et al. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase δ. J Neurosci. 2011;31:13485–99. doi: 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Valnegri P, Montrasio C, Brambilla D, Ko J, Passafaro M, Sala C. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPδ and RhoGAP2. Hum Mol Genet. 2011;20:4797–809. doi: 10.1093/hmg/ddr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pulido R, Krueger NX, Serra-Pagès C, Saito H, Streuli M. Molecular characterization of the human transmembrane protein-tyrosine phosphatase: evidence for tissue-specific expression of alternative human transmembrane protein-tyrosine phosphatase isoforms. J Biol Chem. 1995;270:6722–8. doi: 10.1074/jbc.270.12.6722. [DOI] [PubMed] [Google Scholar]
- [36].Streuli M, Krueger N, Ariniello P, Tang M, Munro J, Blattler W, et al. Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region. EMBO J. 1992;11:897–907. doi: 10.1002/j.1460-2075.1992.tb05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].O’Grady P, Krueger NX, Streuli M, Saito H. Genomic organization of the human LAR protein tyrosine phosphatase gene and alternative splicing in the extracellular fibronectin type-III domains. J Biol Chem. 1994;269:25193–9. [PubMed] [Google Scholar]
- [38].Stoker AW. Isoforms of a novel cell adhesion molecule-like protein tyrosine phosphatase are implicated in neural development. Mech Dev. 1994;46:201–17. doi: 10.1016/0925-4773(94)90071-x. [DOI] [PubMed] [Google Scholar]
- [39].Pulido R, Serra-Pagès C, Tang M, Streuli M. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc Natl Acad Sci U S A. 1995;92:11686–90. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ackley BD, Harrington RJ, Hudson ML, Williams L, Kenyon CJ, Chisholm AD, et al. The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J Neurosci. 2005;25:7517–28. doi: 10.1523/JNEUROSCI.2010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Muise AM, Walters T, Wine E, Griffiths AM, Turner D, Duerr RH, et al. Protein-tyrosine phosphatase sigma is associated with ulcerative colitis. Curr Biol. 2007;17:1212–8. doi: 10.1016/j.cub.2007.06.013. [DOI] [PubMed] [Google Scholar]
- [42].Yan H, Grossman A, Wang H, D’Eustachio P, Mossie K, Musacchio JM, et al. A novel receptor tyrosine phosphatase-sigma that is highly expressed in the nervous system. J Biol Chem. 1993;268:24880–6. [PubMed] [Google Scholar]
- [43].Mizuno K, Hasegawa K, Katagiri T, Ogimoto M, Ichikawa T, Yakura H. MPTP delta, a putative murine homolog of HPTP delta, is expressed in specialized regions of the brain and in the B-cell lineage. Mol Cell Biol. 1993;13:5513–23. doi: 10.1128/mcb.13.9.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang JS, Longo FM. LAR tyrosine phosphatase receptor: alternative splicing is preferential to the nervous system, coordinated with cell growth and generates novel isoforms containing extensive CAG repeats. J Cell Biol. 1995;128:415–31. doi: 10.1083/jcb.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stoker AW, Gehrig B, Haj F, Bay BH. Axonal localisation of the CAM-like tyrosine phosphatase CRYP alpha: a signalling molecule of embryonic growth cones. Development. 1995;121:1833–44. doi: 10.1242/dev.121.6.1833. [DOI] [PubMed] [Google Scholar]
- [46].Wang H, Yan H, Canoll PD, Silvennoinen O, Schlessinger J, Musacchio JM. Expression of receptor protein tyrosine phosphatase-σ (RPTP-σ) in the nervous system of the developing and adult rat. J Neurosci Res. 1995;41:297–310. doi: 10.1002/jnr.490410303. [DOI] [PubMed] [Google Scholar]
- [47].Naoto E, Su Jane R, Evan EO, Robert V, Gideon AR, Azriel S. Human protein tyrosine phosphatase-sigma: alternative splicing and inhibition by bisphosphonates. J Bone Miner Res. 1996;11:535–43. doi: 10.1002/jbmr.5650110415. [DOI] [PubMed] [Google Scholar]
- [48].Schaapveld RQJ, Schepens JTG, Bächner D, Attema J, Wieringa B, Jap PHK, et al. Developmental expression of the cell adhesion molecule-like protein tyrosine phosphatases LAR, RPTPδ and RPTPσ in the mouse. Mech Dev. 1998;77:59–62. doi: 10.1016/s0925-4773(98)00119-1. [DOI] [PubMed] [Google Scholar]
- [49].Johnson KG, Holt CE. Expression of CRYP-α, LAR, PTP-δ, and PTP-ρ in the developing Xenopus visual system. Mech Dev. 2000;92:291–4. doi: 10.1016/s0925-4773(99)00345-7. [DOI] [PubMed] [Google Scholar]
- [50].Stoker AW. RPTPs in axons, synapses and neurology. Semin Cell Dev Biol. 2015;37:90–7. doi: 10.1016/j.semcdb.2014.09.006. [DOI] [PubMed] [Google Scholar]
- [51].Bilwes AM, den Hertog J, Hunter T, Noel JP. Structural basis for inhibition of receptor protein-tyrosine phosphatase-α by dimerization. Nature. 1996;382:555–9. doi: 10.1038/382555a0. [DOI] [PubMed] [Google Scholar]
- [52].Hoffmann KMV, Tonks NK, Barford D. The crystal structure of domain 1 of receptor protein-tyrosine phosphatase μ. J Biol Chem. 1997;272:27505–8. doi: 10.1074/jbc.272.44.27505. [DOI] [PubMed] [Google Scholar]
- [53].Almo S, Bonanno J, Sauder J, Emtage S, Dilorenzo T, Malashkevich V, et al. Structural genomics of protein phosphatases. J Struct Funct Genomics. 2007;8:121–40. doi: 10.1007/s10969-007-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Desai DM, Sap J, Schlessinger J, Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73:541–54. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- [55].Jiang G, den Hertog J, Su J, Noel J, Sap J, Hunter T. Dimerization inhibits the activity of receptor-like protein-tyrosine phosphatase-α. Nature. 1999;401:606–10. doi: 10.1038/44170. [DOI] [PubMed] [Google Scholar]
- [56].den Hertog J, van der Wijk T, Tertoolen LGJ, Blanchetot C. Receptor protein-tyrosine phosphatase dimerization. Methods Enzymol. 2003;366:224–40. doi: 10.1016/s0076-6879(03)66018-0. [DOI] [PubMed] [Google Scholar]
- [57].Coles CH, Mitakidis N, Zhang P, Elegheert J, Lu W, Stoker AW, et al. Structural basis for extracellular cis and trans RPTPσ signal competition in synaptogenesis. Nat Commun. 2014 doi: 10.1038/ncomms6209. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, et al. Proteoglycanspecific molecular switch for RPTPσ clustering and neuronal extension. Science. 2011;332:484–8. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Biersmith BH, Hammel M, Geisbrecht ER, Bouyain S. The immunoglobulin-like domains 1 and 2 of the protein tyrosine phosphatase LAR adopt an unusual horseshoe-like conformation. J Mol Biol. 2011;408:616–27. doi: 10.1016/j.jmb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19:244–56. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature. 2012;492:128–32. doi: 10.1038/nature11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ozkan E, Chia PH, Wang RR, Goriatcheva N, Borek D, Otwinowski Z, et al. Extracellular architecture of the SYG-1/SYG-2 adhesion complex instructs synaptogenesis. Cell. 2014;156:482–94. doi: 10.1016/j.cell.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Comoletti D, Miller MT, Jeffries CM, Wilson J, Demeler B, Taylor P, et al. The macromolecular architecture of extracellular domain of αNRXN1: domain organization, flexibility, and insights into trans-synaptic disposition. Structure. 2010;18:1044–53. doi: 10.1016/j.str.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chen F, Venugopal V, Murray B, Rudenko G. The structure of neurexin 1α reveals features promoting a role as synaptic organizer. Structure. 2011;19:779–89. doi: 10.1016/j.str.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Miller MT, Mileni M, Comoletti D, Stevens RC, Harel M, Taylor P. The crystal structure of the α-neurexin-1 extracellular region reveals a hinge point for mediating synaptic adhesion and function. Structure. 2011;19:767–78. doi: 10.1016/j.str.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Takahashi H, Craig A. Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 2013;36:522–34. doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lucić V, Yang T, Schweikert G, Förster F, Baumeister W. Morphological characterization of molecular complexes present in the synaptic cleft. Structure. 2005;13:423–34. doi: 10.1016/j.str.2005.02.005. [DOI] [PubMed] [Google Scholar]
- [68].Fry EJ, Chagnon MJ, López-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58:423–33. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- [69].Ledig MM, Haj F, Bixby JL, Stoker AW, Mueller BK. The receptor tyrosine phosphatase Crypα promotes intraretinal axon growth. J Cell Biol. 1999;147:375–88. doi: 10.1083/jcb.147.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Esko JD, Kimata K, Lindahl U. Chapter 16: proteoglycans and sulfated glycosaminoglycans. 2nd ed. Cold Spring Harbor; NY: 2009. [PubMed] [Google Scholar]
- [71].Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- [72].Xu D, Esko JD. Demystifying heparan sulfate–protein interactions. Annu Rev Biochem. 2014;83:129–57. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- [74].Lee J-S, Chien C-B. When sugars guide axons: insights from heparan sulphate proteoglycan mutants. Nat Rev Genet. 2004;5:923–35. doi: 10.1038/nrg1490. [DOI] [PubMed] [Google Scholar]
- [75].Harmer NJ, Ilag LL, Mulloy B, Pellegrini L, Robinson CV, Blundell TL. Towards a resolution of the stoichiometry of the fibroblast growth factor (FGF)-FGF receptor-heparin complex. J Mol Biol. 2004;339:821–34. doi: 10.1016/j.jmb.2004.04.031. [DOI] [PubMed] [Google Scholar]
- [76].Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–37. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- [77].Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24:2001–12. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bülow HE, Hobert O. Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron. 2004;41:723–36. doi: 10.1016/s0896-6273(04)00084-4. [DOI] [PubMed] [Google Scholar]
- [79].Holt CE, Dickson BJ. Sugar codes for axons. Neuron. 2005;46:169–72. doi: 10.1016/j.neuron.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–73. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- [81].Maeda N, Ishii M, Nishimura K, Kamimura K. Functions of chondroitin sulfate and heparan sulfate in the developing brain. Neurochem Res. 2011;36:1228–40. doi: 10.1007/s11064-010-0324-y. [DOI] [PubMed] [Google Scholar]
- [82].Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–12. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Murphy KJ, Merry CLR, Lyon M, Thompson JE, Roberts IS, Gallagher JT. A new model for the domain structure of heparan sulfate based on the novel specificity of K5 lyase. J Biol Chem. 2004;279:27239–45. doi: 10.1074/jbc.M401774200. [DOI] [PubMed] [Google Scholar]
- [84].Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol. 2007;17:536–45. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- [85].Kwok JCF, Warren P, Fawcett JW. Chondroitin sulfate: a key molecule in the brain matrix. Int J Biochem Cell Biol. 2012;44:582–6. doi: 10.1016/j.biocel.2012.01.004. [DOI] [PubMed] [Google Scholar]
- [86].Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15:414–22. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- [87].Wu DY, Goldberg DJ. Regulated tyrosine phosphorylation at the tips of growth cone filopodia. J Cell Biol. 1993;123:653–64. doi: 10.1083/jcb.123.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Robles E, Woo S, Gomez TM. Src-dependent tyrosine phosphorylation at the tips of growth cone filopodia promotes extension. J Neurosci. 2005;25:7669–81. doi: 10.1523/JNEUROSCI.2680-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–75. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [90].Missler M, Sudhof TC, Biederer T. Synaptic cell adhesion. Cold Spring Harb Perspect Biol. 2012;4:a005694. doi: 10.1101/cshperspect.a005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–20. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–9. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- [93].Craig AM, Kang Y. Neurexin–neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, et al. Syn-CAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–31. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- [95].Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–64. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kim S, Burette A, Chung HS, Kwon S-K, Woo J, Lee HW, et al. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- [97].Rashid-Doubell F, McKinnell I, Aricescu AR, Sajnani G, Stoker A. Chick PTP-sigma regulates the targeting of retinal axons within the optic tectum. J Neurosci. 2002;22:5024–33. doi: 10.1523/JNEUROSCI.22-12-05024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-α and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- [99].Um JW, Ko J. LAR-RPTPs: synaptic adhesion molecules that shape synapse development. Trends Cell Biol. 2013;23:465–75. doi: 10.1016/j.tcb.2013.07.004. [DOI] [PubMed] [Google Scholar]
- [100].Dunah AW, Hueske E, Wyszynski M, Hoogenraad CC, Jaworski J, Pak DT, et al. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8:458–67. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- [101].Hoogenraad CC, Feliu-Mojer MI, Spangler SA, Milstein AD, Dunah AW, Hung AY, et al. Liprinα1 degradation by calcium/calmodulin-dependent protein kinase II regulates LAR receptor tyrosine phosphatase distribution and dendrite development. Dev Cell. 2007;12:587–602. doi: 10.1016/j.devcel.2007.02.006. [DOI] [PubMed] [Google Scholar]
- [102].Lauri SE, Kaukinen S, Kinnunen T, Ylinen A, Imai S, Kaila K, et al. Regulatory role and molecular interactions of a cell-surface heparan sulfate proteoglycan (N-syndecan) in hippocampal long-term potentiation. J Neurosci. 1999;19:1226–35. doi: 10.1523/JNEUROSCI.19-04-01226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Irie F, Badie-Mahdavi H, Yamaguchi Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc Natl Acad Sci U S A. 2012;109:5052–6. doi: 10.1073/pnas.1117881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].de Wit J, O’Sullivan ML, Savas JN, Condomitti G, Caccese MC, Vennekens KM, et al. Unbiased discovery of glypican as a receptor for LRRTM4 in regulating excitatory synapse development. Neuron. 2013;79:696–711. doi: 10.1016/j.neuron.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Siddiqui TJ, Tari PK, Connor SA, Zhang P, Dobie FA, She K, et al. An LRRTM4-HSPG complex mediates excitatory synapse development on dentate gyrus granule cells. Neuron. 2013;79:680–95. doi: 10.1016/j.neuron.2013.06.029. [DOI] [PubMed] [Google Scholar]
- [106].Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci U S A. 1998;95:7469–74. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature. 2011;475:510–3. doi: 10.1038/nature10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Allen N, Bennett M, Foo L, Wang G, Chakraborty C, Smith S, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–4. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Uemura T, Lee S-J, Yasumura M, Takeuchi T, Yoshida T, Ra M, et al. Trans-synaptic interaction of GluRdelta2 and neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–79. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- [110].Serra-Pagès C, Medley QG, Tang M, Hart A, Streuli M. Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins. J Biol Chem. 1998;273:15611–20. doi: 10.1074/jbc.273.25.15611. [DOI] [PubMed] [Google Scholar]
- [111].Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–5. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]
- [112].Stryker E, Johnson KG. LAR, liprinα and the regulation of active zone morphogenesis. J Cell Sci. 2007;120:3723–8. doi: 10.1242/jcs.03491. [DOI] [PubMed] [Google Scholar]
- [113].Südhof Thomas C. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Esteban PF, Yoon HY, Becker J, Dorsey SG, Caprari P, Palko ME, et al. A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J Cell Biol. 2006;173:291–9. doi: 10.1083/jcb.200512013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin–catenin complex. J Cell Biol. 1996;134:1519–29. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Muller T, Choidas A, Reichmann E, Ullrich A. Phosphorylation and free pool of β-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem. 1999;274:10173–83. doi: 10.1074/jbc.274.15.10173. [DOI] [PubMed] [Google Scholar]
- [117].Siu R, Fladd C, Rotin D. N-Cadherin is an in vivo substrate for protein tyrosine phosphatase sigma (PTPσ) and participates in PTPσ-mediated inhibition of axon growth. Mol Cell Biol. 2007;27:208–19. doi: 10.1128/MCB.00707-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–64. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- [119].Chagnon MJ, Wu C-L, Nakazawa T, Yamamoto T, Noda M, Blanchetot C, et al. Receptor tyrosine phosphatase sigma (RPTPσ) regulates, p250GAP, a novel substrate that attenuates Rac signaling. Cell Signal. 2010;22:1626–33. doi: 10.1016/j.cellsig.2010.06.001. [DOI] [PubMed] [Google Scholar]
- [120].Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhes Migr. 2011;5:170–80. doi: 10.4161/cam.5.2.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Debant A, Serra-Pagès C, Seipel K, O’Brien S, Tang M, Park SH, et al. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rhospecific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–71. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Bateman J, Shu H, Van Vactor D. The guanine nucleotide exchange factor Trio mediates axonal development in the Drosophila embryo. Neuron. 2000;26:93–106. doi: 10.1016/s0896-6273(00)81141-1. [DOI] [PubMed] [Google Scholar]
- [123].Choquet D, Triller A. The dynamic synapse. Neuron. 2013;80:691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- [124].Ziv NE, Fisher-Lavie A. Presynaptic and postsynaptic scaffolds: dynamics fast and slow. Neuroscientist. 2014 doi: 10.1177/1073858414523321. http://dx.doi.org/10.1177/1073858414523321. [DOI] [PubMed] [Google Scholar]
- [125].Müller CS, Haupt A, Bildl W, Schindler J, Knaus H-G, Meissner M, et al. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci U S A. 2010;107:14950–7. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SGN. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–9. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- [127].Chen Y, Sabatini BL. Signaling in dendritic spines and spine microdomains. Curr Opin Neurobiol. 2012;22:389–96. doi: 10.1016/j.conb.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, et al. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci. 2013;33:13204–24. doi: 10.1523/JNEUROSCI.2381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].MacGillavry Harold D, Song Y, Raghavachari S, Blanpied Thomas A. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–22. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pagès C, et al. Interaction between GRIP and liprin-α/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- [131].Gebbink MF, Zondag GC, Koningstein GM, Feiken E, Wubbolts RW, Moolenaar WH. Cell surface expression of receptor protein tyrosine phosphatase RPTPmu is regulated by cell–cell contact. J Cell Biol. 1995;131:251–60. doi: 10.1083/jcb.131.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Campan M, Yoshizumi M, Seidah NG, Lee M-E, Bianchi C, Haber E. Increased proteolytic processing of protein tyrosine phosphatase μ in confluent vascular endothelial cells: the role of PC5, a member of the subtilisin family. Biochemistry. 1996;35:3797–802. doi: 10.1021/bi952552d. [DOI] [PubMed] [Google Scholar]
- [133].Anders L, Mertins P, Lammich S, Murgia M, Hartmann D, Saftig P, et al. Furin-, ADAM 10-, and γ-secretase-mediated cleavage of a receptor tyrosine phosphatase and regulation of β-catenin’s transcriptional activity. Mol Cell Biol. 2006;26:3917–34. doi: 10.1128/MCB.26.10.3917-3934.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Aicher B, Lerch MM, Muller T, Schilling J, Ullrich A. Cellular redistribution of protein tyrosine phosphatases LAR and PTPsigma by inducible proteolytic processing. J Cell Biol. 1997;138:681–96. doi: 10.1083/jcb.138.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Maurel P, Rauch U, Flad M, Margolis RK, Margolis RU. Phosphacan, a chondroitin sulfate proteoglycan of brain that interacts with neurons and neural cell-adhesion molecules, is an extracellular variant of a receptor-type protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1994;91:2512–6. doi: 10.1073/pnas.91.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J Immunol. 2002;169:5036–42. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- [137].Brady-Kalnay SM, Flint AJ, Tonks NK. Homophilic binding of PTPmu, a receptor-type protein tyrosine phosphatase, can mediate cell–cell aggregation. J Cell Biol. 1993;122:961–72. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Persson C, Sjöblom T, Groen A, Kappert K, Engström U, Hellman U, et al. Preferential oxidation of the second phosphatase domain of receptor-like PTP-α revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2004;101:1886–91. doi: 10.1073/pnas.0304403101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].den Hertog J, Tracy S, Hunter T. Phosphorylation of receptor protein-tyrosine phosphatase alpha on Tyr789, a binding site for the SH3–SH2–SH3 adaptor protein GRB-2 in vivo. EMBO J. 1994;13:3020–32. doi: 10.1002/j.1460-2075.1994.tb06601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].den Hertog J, Sap J, Pals C, Schlessinger J, Kruijer W. Stimulation of receptor protein-tyrosine phosphatase alpha activity and phosphorylation by phorbol ester. Cell Growth Differ. 1995;6:303–7. [PubMed] [Google Scholar]
- [141].Wasmeier C, Burgos PV, Trudeau T, Davidson HW, Hutton JC. An extended tyrosine-targeting motif for endocytosis and recycling of the dense-core vesicle membrane protein phogrin. Traffic. 2005;6:474–87. doi: 10.1111/j.1600-0854.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- [142].Martin KR, Xu Y, Looyenga BD, Davis RJ, Wu C-L, Tremblay ML, et al. Identification of PTPσ as an autophagic phosphatase. J Cell Sci. 2011;124:812–9. doi: 10.1242/jcs.080341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Haapasalo A, Kim DY, Carey BW, Turunen MK, Pettingell WH, Kovacs DM. Presenilin/γ-secretase-mediated cleavage regulates association of leukocyte-common antigen-related (LAR) receptor tyrosine phosphatase with β-catenin. J Biol Chem. 2007;282:9063–72. doi: 10.1074/jbc.M611324200. [DOI] [PubMed] [Google Scholar]
- [144].Zhang X, Guo A, Yu J, Possemato A, Chen Y, Zheng W, et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci U S A. 2007;104:4060–4. doi: 10.1073/pnas.0611665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Mulloy B, Forster MJ, Jones C, Davies DB. N.m.r. and molecular-modelling studies of the solution conformation of heparin. Biochem J. 1993;293:849–58. doi: 10.1042/bj2930849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Winter WT, Arnott S, Isaac DH, Atkins EDT. Chondroitin 4-sulfate: the structure of a sulfated glycosaminoglycan. J Mol Biol. 1978;125:1–19. doi: 10.1016/0022-2836(78)90251-6. [DOI] [PubMed] [Google Scholar]
- [147].Seiradake E, Coles CH, Perestenko PV, Harlos K, McIlhinney RAJ, Aricescu AR, et al. Structural basis for cell surface patterning through NetrinG–NGL interactions. EMBO J. 2011;30:4479–88. doi: 10.1038/emboj.2011.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- [149].Sugi T, Oyama T, Muto T, Nakanishi S, Morikawa K, Jingami H. Crystal structures of autoinhibitory PDZ domain of tamalin: implications for metabotropic glutamate receptor trafficking regulation. EMBO J. 2007;26:2192–205. doi: 10.1038/sj.emboj.7601651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Svensson G, Awad W, HÅkansson M, Mani K, Logan DT. Crystal structure of N-glycosylated human glypican-1 core protein: structure of two loops evolutionarily conserved in vertebrate glypican-1. J Biol Chem. 2012;287:14040–51. doi: 10.1074/jbc.M111.322487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Rangarajan ES, Izard T. Dimer asymmetry defines α-catenin interactions. Nat Struct Mol Biol. 2013;20:188–93. doi: 10.1038/nsmb.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Xing Y, Takemaru K-I, Liu J, Berndt JD, Zheng JJ, Moon RT, et al. Crystal structure of a full-length β-catenin. Structure. 2008;16:478–87. doi: 10.1016/j.str.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Pokutta S, Choi H-J, Ahlsen G, Hansen SD, Weis WI. Structural and thermodynamic characterization of cadherin·β-catenin·α-catenin complex formation. J Biol Chem. 2014;289:13589–601. doi: 10.1074/jbc.M114.554709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Huber AH, Weis WI. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- [155].Fujii T, Iwane AH, Yanagida T, Namba K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature. 2010;467:724–8. doi: 10.1038/nature09372. [DOI] [PubMed] [Google Scholar]
- [156].Sainlos M, Tigaret C, Poujol C, Olivier NB, Bard L, Breillat C, et al. Biomimetic divalent ligands for the acute disruption of synaptic AMPAR stabilization. Nat Chem Biol. 2011;7:81–91. doi: 10.1038/nchembio.498. [DOI] [PubMed] [Google Scholar]
- [157].Wei Z, Zheng S, Spangler Samantha A, Yu C, Hoogenraad Casper C, Zhang M. Liprin-mediated large signaling complex organization revealed by the liprin-α/CASK and liprin-α/liprin-β complex structures. Mol Cell. 2011;43:586–98. doi: 10.1016/j.molcel.2011.07.021. [DOI] [PubMed] [Google Scholar]