Abstract
Rationale
Prenatal exposure to nicotine has been linked to accelerated risk for different psychiatric disorders, including conduct disorder, attention deficit hyperactivity disorder (ADHD) and drug abuse. We examine a potential link between prenatal nicotine exposure, hyperactivity, anxiety, nicotine consumption, and cognitive performance in rats.
Methods
Adolescent offspring of females exposed during pregnancy to 0.06 mg/ml nicotine solution as the only source of water and of a group of pair-fed females, used as a control for anorexic effects of nicotine, were evaluated in a battery of tests, including locomotor activity, the elevated plus maze, two-bottle free-choice nicotine solution consumption, the five-choice serial reaction time test (5-CSRTT) and a delay-discounting test. All tests were conducted between postnatal day (PND) 25 and PND 50.
Results
Nicotine-exposed animals expressed hyperactivity, increased number of open arms entries in the elevated plus maze and increased numbers of anticipatory responses in the 5-CSRTT. Decreased aversion for nicotine solution in the free-choice test and decreased numbers of omission errors in the 5-CSRTT were observed both in nicotine-exposed and pair-fed offspring. Neither nicotine exposure nor pair-feeding had an effect on impulsive choice in a delay-discounting test.
Conclusions
Our study confirms deleterious effects of prenatal nicotine exposure on important aspects of behaviour and inhibitory control in adolescent rats and supports epidemiological findings that show increased levels of symptoms of ADHD and related disorders among those whose mothers smoked during their pregnancy. It also suggests a link between food restriction during pregnancy and addiction-related behaviours in offspring.
Keywords: Nicotine, Gestation, Hyperactivity, Impulsivity, Adolescence, ADHD
Introduction
Gestational tobacco exposure has numerous consequences, including placental dysfunctions, intrauterine growth retardation, reduced birth weight and an increased risk for pre-term delivery and still birth (Ernst et al. 2001; Jauniaux and Burton 2007; Winzer-Serhan 2008). It has been linked to increased risk for childhood onset psychiatric disorders including attention deficit hyperactivity disorder (ADHD), conduct disorder and substance abuse (particularly smoking) in adolescence (Milberger et al. 1998; Roberts et al. 2005; Weissman et al. 1999). Animal studies have provided compelling evidence that nicotine can be the single most important factor triggering the negative effect of smoking on neurodevelopment (Ernst et al. 2001; Slotkin 2008).
Children of mothers who smoked during pregnancy display more externalising behaviours (e.g. aggression, overactivity) (Orlebeke et al. 1997, 1999); deficits in learning, memory and sustained attention; increased impulsivity (performance on a response inhibition task and continuous performance task); and lower scores on overall cognitive function (Fried et al. 2003; Fried and Watkinson 2001; Jacobsen et al. 2007; Kristjansson et al. 1989; McCartney et al. 1994). Importantly, a higher General Cognitive Index of the McCarthy Scales of Children’s Abilities based on cognitive measures of perceptual performance as well as quantitative and verbal skills was reported in 3-year-old children (N=935) whose mothers quit smoking during pregnancy (Sexton et al. 1990), as compared with those whose mothers continued to smoke; this finding suggests the existence of a critical period for tobacco effects on foetal development.
Although there seems to be overwhelming evidence for a deleterious effect of in utero exposure to tobacco smoking on behaviour and cognition later in life and an increased risk for childhood onset psychiatric disorders, it is difficult to separate these effects from other confounding environmental and genetic factors (D’Onofrio et al. 2008; Thapar et al. 2009), e.g. it has been suggested that common genetic vulnerability factors may exist for both maternal smoking and offspring ADHD that may explain the increased rate of ADHD among children of smokers (Maher et al. 2002; Munafo and Johnstone 2008). Relative effects of environmental and genetic factors on ADHD-like phenotype can be disentangled in animal models.
Findings in rats consistently show lower birth weight in offspring exposed to nicotine in utero (Paulson et al. 1994; Schneider et al. 2010), which is similar to the results of human studies (Eskenazi et al. 1995; Salihu et al. 2005). Maternal and foetal malnutrition from anorexic effects of tobacco smoking (Jo et al. 2002) may mediate some of these effects; however, the weight of tobacco-exposed children (Day et al. 1992) and nicotine-exposed rats (Schneider et al. 2010, 2011) recovers over time.
Prenatal exposure to nicotine has also been shown to lead to hyperactivity in rats (Tizabi et al. 2000; Vaglenova et al. 2004), but other reports are in conflict with these findings (LeSage et al. 2006; Romero and Chen 2004; Schneider et al. 2011; Shacka et al. 1997). Rats exposed to nicotine in utero also show several cognitive impairments including attention and memory deficits (Levin et al. 1993, 1996; Sorenson et al. 1991) and impairments in acquisition and retention of avoidance behaviour (Genedani et al. 1983; Vaglenova et al. 2008). However, again, these findings are not entirely consistent since some studies found no decrement in avoidance behaviour and spatial learning (Bertolini et al. 1982; Paulson et al. 1994). Several factors could contribute to the disparities between studies, including the dose of nicotine, route and duration of administration, the exposure period and sex of the animals. In addition, the age of the exposed animals at testing (adolescents vs. adults) and methodological differences in the behavioural paradigms are also likely to be important.
Thus, impairments in attention, memory and learning described in rats exposed to nicotine in utero are consistent with the cognitive deficits found in psychiatric disorders such as ADHD, but it is important to use measures of cognitive dimensions that can be more directly linked to attentional problems and impulsivity observed in children and adolescents. Such comparable aspects of cognitive performance can be measured by the five-choice serial reaction time test (5-CSRTT) and delay-discounting tests in rats. Reaction time, response accuracy, omission errors and anticipatory responses, thought to reflect processes related to attention and impulsivity, can be assessed with the 5-CSRTT, and choice impulsivity can be assessed in delay-discounting paradigms (Winstanley et al. 2006).
We recently reported evidence from the 5-CSRTT for increased inattentiveness and impulsivity in adult rats prenatally exposed to nicotine (Schneider et al. 2011), but these aspects of cognitive function have yet to be reported for adolescent animals. Adolescence is a key period for increased behavioural problems and initiation of substance abuse in humans (Paus et al. 2008) and for the onset of anxiety disorders, bipolar disorder, depression, eating disorders, psychosis and substance abuse (Rutherford et al. 2010; Wermter et al. 2010). Similar behavioural and cognitive changes can be seen or induced in adolescent rodents (for reviews, see Adriani and Laviola 2004; Marco et al. 2011), exhibiting neurobehavioural characteristics typical for adolescence, such as excitatory amino acid overshoot in the prefrontal cortex, initiation of puberty, and the beginning of independent functioning (Spear 2000).
In the current study, we evaluate in an animal model whether prenatal nicotine exposure influences motor activity, anxiety and cognitive functions in adolescence. In addition, maturational and developmental data were collected and nicotine consumption measured in a two-bottle free choice test. Our previous studies reported a significant anorexic effect of nicotine in pregnant rats which might have confounded the effects of in utero nicotine exposure in offspring; an additional control group of pair-fed females was therefore used in the present study. Nicotine-exposed offspring were not crossfostered to normal dams as it has been previously shown that crossfostering, per se, has no impact on postnatal somatic growth retardation, cognitive ability and neurochemical changes in the offspring (Ribary and Lichtensteiger 1989; Schneider et al. 2011), but can increase anxiety (Vaglenova et al. 2004).
Our results confirm direct deleterious effects of prenatal nicotine exposure on important aspects of behaviour and inhibitory control in adolescent rats and suggest that the direct effects of nicotine may, at least in part, contribute to epidemiological findings that show increased levels of ADHD symptoms and related disorders among those whose mothers smoked during pregnancy.
Materials and methods
Subjects
Both male (N=30) and female (N=90) Lister hooded rats (Harlan Olac, Bicester, UK) were used. They were housed individually (except during mating) and had ad libitum access to food and drinking fluids (tap water or nicotine solutions). Females (205–269 g at the beginning of the study) were weighed three times during the week preceding the start of the experiment. The average weight was calculated for each rat. The rats were divided according to a randomised block design balanced by body weights into the following groups: nicotine exposure (Nic, n=40), control group (Con, n=25) and control pair-fed animals (Con-pf, n=25). An additional group of females (n=8) was used to assess nicotine blood levels during gestation. National and institutional guidelines for housing and treatment were followed. Animals were maintained in a temperature-controlled environment (21±1°C) at 50 % humidity and on a 12-h light/dark cycle.
Drug
Nicotine bitartrate (Sigma-Aldrich, Sigma, Dorset, UK) was dissolved in the drinking water at varying concentrations. Nicotine-containing water was adjusted to the pH of drinking water (pH 7) with 0.001 N NaOH. Doses are presented as those of nicotine base.
Pair-feeding
Two groups of females (Nic=25, Con-pf=25) were matched by weight at the beginning of the study (Con-pf=232.8±15.3, Nic=233.3±15.4 g, means ± S.D.). Pair-fed animals were provided each day with the amount of food eaten by matched nicotine-exposed animals on the previous day; their access to water was not restricted.
Nicotine consumption and nicotine blood level
As in our previous studies (Schneider et al. 2010, 2011), animals were exposed to nicotine through drinking water, which has the advantage that treatment is episodic, as it occurs only when animals drink, and thus resembles human exposure patterns (Jauniaux and Burton 2007), and is relatively stress-free. In brief, 40 females were habituated to increasing concentrations of nicotine solution (0.02, 0.04, 0.06 mg/ml) in tap water as the only source of fluid for 3 weeks before mating. The final nicotine concentration used in the present study (0.06 mg/ml) was previously shown to induce cognitive aberrations in adult rats (Schneider et al. 2011), but only mild teratogenic effects in offspring (Schneider et al. 2010). In rare cases (10 times all together in six different animals) when females were drinking less than 10 ml of nicotine solution per day, supplementary access to tap water was provided for 5 min, which was not included in the analysis of fluid intake. Nicotine treatment was terminated on the day that pups were delivered. Control and pair-fed females (N=50) continued to receive tap water. The females (n=8) used to evaluate nicotine blood levels during the second week of pregnancy were exposed to nicotine in an identical manner, and nicotine concentrations were determined using tail vein blood and gas chromatography.
Mating
All females were controlled according to their estrous cycle. Females in proestrous and estrous were mated during the dark phase of the day at the beginning of the fourth week of nicotine exposure. Nicotine solution was not withheld before mating. The day on which a vaginal plug or spermatozoa were found in the vaginal smear was defined as gestational day 0.
Pregnancy
Pregnant females from the nicotine and control groups were weighed twice weekly. A 0.06-mg/ml nicotine solution was used throughout pregnancy and its consumption was assessed daily. Food consumption was evaluated three times a week, except in nicotine-exposed animals used for pair-feeding, for which food consumption was controlled daily.
Birth measures
All dams were checked twice daily (before 8 a.m. and after 4.30 p.m.) starting a few days before delivery. Deliveries completed by 8 a.m. were assigned to postnatal day 1 (PND 1). Pups born later that day were assigned to PND 1 on the following morning. Litters were examined on PND 1 for obvious morphological anomalies (e.g. missing digits, facial malformations, etc.), sexed by relative ano-genital distance and, in the case of litters with more than eight offspring, culled randomly to eight pups with equal numbers of males and females per litter whenever possible. Offspring were evaluated throughout the lactation period in terms of reflex development and neuromuscular maturation. Tests were selected from standard neurobehavioural developmental test batteries (Adams 1986).
Developmental milestones
Fourteen control litters (58 males), 13 nicotine-exposed litters (55 males) and 16 pair-fed litters (67 males) were used to assess development and maturation in offspring. The dam was first removed from the home cage and specific tests measuring reflex development, motor coordination and muscle strength were applied to the offspring. All testing was conducted between 9.00 a.m. and 4.00 p.m.
To assess righting reflex, each pup was given two successive trials per day from PND 2 to 5 and the time from being placed in a supine position until it righted itself onto all four feet was recorded. The cut-off time was 30 s. Surface righting reflects the development of labyrinthine and body righting mechanisms as well as vestibular function and motor development.
Negative geotaxis was observed daily from PND 7 to PND 10; pups were timed for completing a 180 ° turn within 30 s when placed in a head-down position on a 25 ° inclined wooden surface. Rats were given two consecutive trials per day and the mean was calculated. Negative geotaxis reflects vestibular function, motor development and activity.
Forelimb grip strength was assessed on PNDs 14 and 17. A steel wire (20 cm long, about 0.3 cm thick) was supported between two poles of wood 25 cm above the table covered with soft towels. The latency to fall off the wire grasped by both forepaws was measured with a maximum time of 20 s and is a measure of muscle strength.
Maturational milestones
Pups from each litter were weighed on PNDs 1, 5, 10, 15, and 20. The emergence of physical maturation landmarks were noted, including pinnae detachment (PND 3), incisor eruption (PNDs 7–10), fur appearance (PND 9) and eye opening (PNDs 12–17).
Tests in adolescence
Offspring previously used to assess maturational and developmental milestones were divided according to a split-litter design. The four male siblings coming from the same litter were randomly assigned to be used in different sets of experiments: (1) locomotor activity and the elevated plus maze, (2) the 5-CSRTT, (3) a delay-discounting test and (4) a two-bottle free-choice nicotine consumption test. Animals were kept four per cage. All tests were conducted between PND 25 and PND 40, except the 5-CSRTT that continued to PND 50, on groups of 10–12 animals coming from litters with a minimum of four males (10 Con, 12 Con-pf and 11 Nic). The adolescent period in rodents was defined according to previous literature (Adriani and Laviola 2004; Spear 2000).
Spontaneous locomotor activity and locomotor habituation
The number of cage crosses was assessed in adolescent animals during 60-min test sessions in photocell activity cages measuring 30×30×30 cm (Schneider et al. 2010). The animals were exposed to the same activity cages on nine consecutive days. Data were analysed for blocks of 15 min.
Elevated plus maze
The elevated plus maze consisted of four arms (50×10 cm), with two arms enclosed by walls 40 cm high and two open arms; the maze was elevated 50 cm above the ground. Behaviour was tested in a dark room with a 40-W bulb hung 60 cm above the central part of the maze. The percentage of time spent in the open arms compared to the time spent in both open and enclosed arms and the percentage of open arm entries compared to both open and enclosed arm entries were used as anxiety measures. Prior to the single 5-min exposure to the plus maze, each rat was placed for 5 min in an open field to facilitate exploratory behaviour in the plus maze apparatus (Pellow et al. 1985).
Two-bottle free choice nicotine consumption test
The procedure for nicotine consumption was based on previously published protocols (Maehler et al. 2000), with some modifications. Twelve rats from each group were singly housed in standard cages and provided with food and two bottles of fluid. One bottle contained nicotine dissolved in 10 % sucrose solution and the other bottle contained 10 % sucrose solution only. The bottle positions were rotated every day. The first nicotine concentration tested was 0.01 mg/ml nicotine solution followed 8 days later by 0.005 mg/ml and again 2 days later by 0.0025 mg/ml nicotine solution. Bottles were filled with fresh solutions daily, weighed and placed in the home cages. Approximately 23.5 h later, the bottles were removed and weighed again and a difference score was calculated (gram). A cage with a sham 10 % sucrose solution bottle was filled and weighed using the same procedures to determine liquid loss due to evaporation and bottle handling. Animals were weighed every 2 days.
Five-choice serial reaction time test
The 5-CSRTT was conducted in standard operant conditioning chambers (Cenes Ltd., Cambridge, UK). The training phases of the experiments were based on procedures described elsewhere (Hahn et al. 2002; Schneider et al. 2011). Thirty-five adolescent rats (Nic=12, Con=12, Con-pf=11) were assessed in the 5-CSRTT. They were kept in pairs 1 week before starting the 5-CSRTT. The mean weight of each animal was calculated as the average of the three weights from that week. The start point for each individual rat on the growth curve was identified and its body weight was reduced to 85 % of its free-feeding weight by restricting the amount of food given during the following week. The experiment started on the fourth day of food restriction. Training was initiated by habituation to the chamber and magazine training, followed by attentional training beginning with all response holes illuminated for 10 s (stimulus duration, SD), followed by the introduction of progressively more demanding task parameters (Table 1). In the final stage of training, a stimulus light in a randomly chosen hole was illuminated for 1 s. If a subject nose-poked into a hole while it was illuminated or within 5 s after the light had terminated (limited hold), a 45-mg food pellet (BioServ, Frenchtown, NJ, USA) was delivered into the food tray and a correct response was registered. A response into any other hole during that time was recorded as an incorrect response and resulted in a 5-s time-out during which the house light was extinguished. A failure to respond before the end of the limited hold was registered as an omission error and resulted in a 5-s time-out. The next trial was initiated immediately after a correct response was made or at the end of the time-out that followed an incorrect response. The mean duration of the inter-trial interval (ITI) was 5 s; individual ITI varied randomly within the range 0.625–9.375 s, except in the last stage of the experiment where a 15-s variable ITI was used. Responses during inter-trial intervals were recorded as anticipatory responses and resulted in a 3-s time-out (responses during the time-outs were not counted as anticipatory responses). All training and test sessions lasted for 30 min. Rats were advanced into consecutive experimental stages when their accuracy (percent correct responses) reached 70 %, number of omissions was no higher than 30 % and latency to correct responses reached half of the stimulus duration.
Table 1.
Effects of exposure to nicotine (Nic) and pair-feeding (Con-pf) on body weight, solution consumption and food consumption in female rats used for breeding
| Con | Con-pf | Nic | p level | ||
|---|---|---|---|---|---|
| Mean for a week before mating (mean ± S.E.M.) | Body weight [g] | 250.02±3.79 | 223.73±3.23a | 213.27±4.97a | p<0.001 |
| Solution consumption [ml] | 24.74±0.76 | 20.42±0.99a | 13.98±0.68a,b | p<0.001 | |
| Food consumption [g] | 15.56±0.22 | na | 13.45±0.31a | p<0.001 | |
| Mean for the whole pregnancy (mean ± S.E.M.) | Body weight [g] | 284.47±4.23 | 259.63±2.52a | 246.64±5.76a | p<0.001 |
| Solution consumption [ml] | 43.87±1.98 | 38.44±1.37a | 22.23±1.28a,b | p<0.001 | |
| Food consumption [g] | 20.66±0.34 | na | 18.62±0.71a | p<0.001 |
na not applicable
Post hoc Bonferroni LSD test results significant at least at p<0.01 vs. Con
Post hoc Bonferroni LSD test results significant at least at p<0.05 vs. Con-pf
Several performance measures were recorded: percentage of correct responses (accuracy)=100×(correct responses / (correct+incorrect responses), as a measure of spatial attention; percentage of omission errors (omissions)=100×(omission errors / stimuli presented), reflecting attention but also influenced by the general rate of responding; latency of correct responses=the mean time between stimulus onset and a nose-poke in the correct hole; latency of incorrect responses=the mean time between stimulus onset and a nose-poke in an incorrect hole; anticipatory responses as percentage of trials=100×total number of responses in ITIs / number of trials, as a measure of impulsive responding; reinforcers earned, equal to absolute number of correct responses in a session, as a measure of overall success of task performance.
Delay-discounting paradigm
Standard experimental chambers (Campden Instruments, London, UK) were contained in sound-insulated, ventilated enclosures. The chambers were fitted with two retractable levers separated by a recess in which 45 mg pellets of food could be presented. White noise was present at all times to mask external sounds. The experiments were controlled by programmes written with the Arachnid system (Paul Fray, Cambridge, UK) running under RISC OS on Acorn computers.
Three separate groups of adolescent rats (n=10) were assessed in the delay-discounting test. They were habituated to the experimental chambers during two 30-min sessions with food pellets being delivered every 30 s. Training was conducted over four phases and was based on previously described experimental procedures (Schneider et al. 2011). In the first phase, rats were trained to press the left or right levers on alternate sessions to receive a 45-mg food pellet (BioServ). Each 30-min session consisted of 60 trials. Subjects were trained for seven sessions until all earned at least 50 rewards per session. The second phase was identical to the first one except that both levers were presented at the same time. Animals were trained for six sessions and lever preference (right vs. left) was assessed for each rat. In the third phase, each rat had one lever designated as the ‘immediate’ delivery lever (one pellet) and one lever as the ‘delay’ delivery lever (three pellets), but no delay was introduced in this phase. The ‘immediate’ delivery lever was designated as the lever preferred by the rat during phase two. This method of lever assignment was used to overcome the potential impact of lever preference on performance during the delayed-reward phase. Rats were trained in 30 min sessions consisting of 60 free-choice trials (two levers presented). The third phase lasted for 4 days until all animals had shown at least 70 % preference for the ‘delay’ lever. During the fourth phase, delay was introduced between pressing of the ‘delay’ lever and food delivery. Each session consisted of 60 trials, divided into six blocks of 10 trials, with trials spaced apart by 30 s. Each 10-trial block began with two ‘forced’ trials in which either the left or the right lever was presented in random order for every pair of trials, followed by eight ‘choice’ trials in which both levers were presented. The fourth phase lasted for 4 days with the delay to the larger reward increased daily according to the sequence 3, 6, 12, and 24 s. Choice ratios (delay-lever presses/total lever presses) were calculated for each rat at each delay using the choice trial responses (i.e. excluding single lever trials) summed across the six consecutive blocks.
Statistical analysis
Behavioural data were analysed using one- or two-factor ANOVA followed by Bonferroni modified Least Significant Difference test (LSD) for post hoc analysis. For maturational and developmental data, litter was used as the unit for statistical analysis. Thus, the data subjected to statistical analyses were means for all male animals in each litter, rather than results for individual animals within litters. Non-parametric Kruskal–Wallis ANOVA was used to assess mating efficacy and for analysis of a two-bottle free choice nicotine consumption test and was followed by Dunnett post hoc tests. The 5-CSRTT percentage data for accuracy and omissions were arc-sine-transformed, and latency data were log-transformed (Hahn et al. 2002). Spearman’s rank correlation test was used to correlate measures obtained in the 5-CSRTT and Fisher test to assess the significance of the difference between correlation coefficients. For those variables assessed multiple times, age (PND) and day of training were used as repeated measures. All tests of significance were performed at alpha=0.05 using Unistat 5.6 (Unistat Ltd, London, UK). All data are presented as means ± S.E.M. if not otherwise stated.
Results
Nicotine exposure before and during pregnancy
Three weeks of pre-exposure to increasing doses of nicotine as the only source of water and food restriction in pair-fed animals resulted in decreased body weight before mating (F(2, 40)=22.0, p<0.001) in both groups compared to Con animals (post hoc comparisons p<0.001 in each case). During the last week of habituation, when the final concentration of nicotine solution was used, solution consumption (F(2, 40)=38.2, p<0.001) was decreased in the nicotine-exposed and pair-fed groups (p<0.001 vs. Con) with the Nic group consuming even less solution than the Con-pf group (p<0.01). Food consumption was decreased in the Nic group compared to Con animals (F(1, 25)=30.71, p<0.001). Lower body weight (F(2, 40)=20.3, p<0.001), decreased solution consumption (F(2, 40)=48.4, p<0.001) and decreased food consumption (F(1, 25)=6.87, p<0.01) were also observed in pregnant pair-fed and nicotine-exposed animals compared to the Con group (at least p<0.05). The Nic group consumed even less solution than the Con-pf group (p<0.01). There was no difference in body weight between the Nic and the Con-pf group either during pre-exposure to nicotine or pregnancy (Table 2).
Table 2.
Consecutive steps during the 5-CSRTT training
| Step | Stimulus duration (s) |
Spatial (S), non-spatial (NS) |
Limited hold (s) |
Mean inter-trial interval (s) |
Incorrect time-out (s) |
Anticipatory time-out (s) |
Number of sessions |
|---|---|---|---|---|---|---|---|
| 1 | 10 | NS | 10 | 5 | 0 | 0 | 3 |
| 2 | 10 | S | 10 | 5 | 0 | 0 | 9 |
| 3 | 5 | S | 5 | 5 | 5 | 3 | 5 |
| 4 | 1 | 5 | 5 | 5 | 5 | 3 | 6 |
| 5 | 1 | S | 5 | 15 | 5 | 3 | 3 |
Nicotine blood levels
The mean plasma nicotine blood level in the second week of gestation was 81.1±23.2 ng/ml (means ± S.D.), which is at the upper end of the dose range for heavy smokers (Benowitz et al. 2009). There was no difference in mean nicotine solution consumption per kilogramme body weight per day between the groups of nicotine-exposed pregnant females used for nicotine blood tests or for offspring delivery (F(1, 19)=4.46, n.s.; 77.32±8.9 vs. 90.0±15.4 ml/kg, respectively).
Litter characteristics
There were no between-group differences in any of the litter characteristics recorded in the study: number of animals per litter (Con=9.0±0.28, Con-pf=9.13±0.34, Nic=8.54±0.69), the numbers of females and males per litter (Con=4.14±0.51, Con-pf=4.25±0.41, Nic=4.08±0.43 and Con=4.14±0.39, Con-pf=4.25±0.4, Nic=4.08±0.37, respectively) and sex ratio (Con=0.53±0.05, Con-pf=0.54±0.04, Nic=0.52±0.02); however, a significantly smaller percentage of nicotine-exposed females became pregnant compared to both Con and Con-pf groups (Con=14/25, Con-pf=16/25, Nic=13/40; χ2=18.19, p=0.0001).
Postnatal growth and maturation
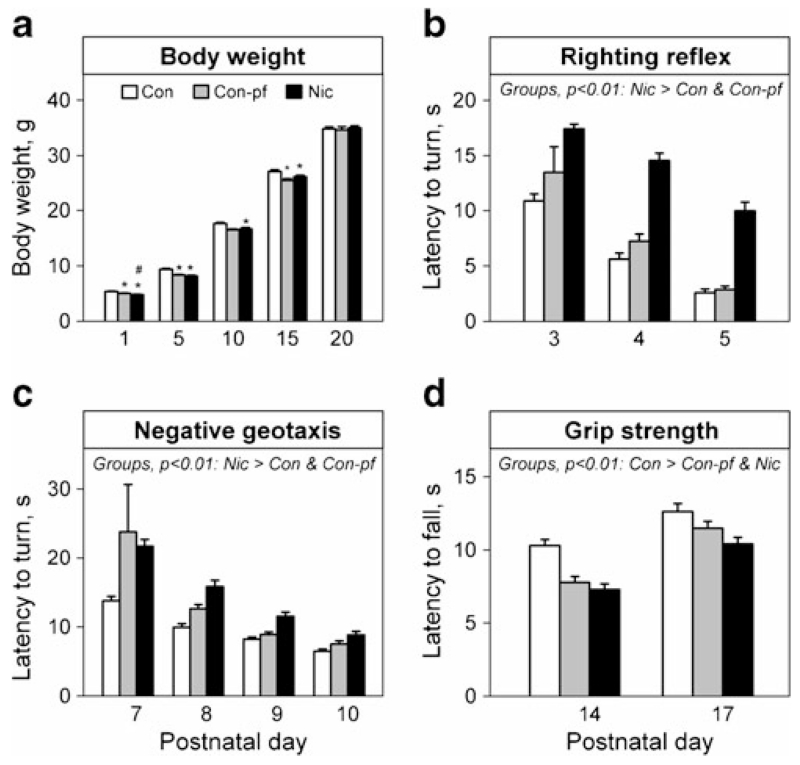
There was no between-group difference in the body weight of the offspring during the first 20 PNDs (F(2, 30)=1.31, n.s.); however, a significant difference in weight gain (group×PND) was observed (F(8, 855)=2.94, p<0.01). Post hoc analysis revealed decreased body weights in Nic and Con-pf animals compared to the Con group on PNDs 1, 5 and 10, in the Nic group vs. the Con-pf group on PND 1, and in the Con-pf group vs. the Con group on PND 15 (Fig. 1a). The other maturational measures (pinnae detachment, fur appearance, incisor eruption and eye opening) did not differ between the groups.
Fig. 1.
Birth weights (a), motor co-ordination (b, c) and muscle strength (d) in male rats prenatally exposed to nicotine (Nic) and in the offspring of pair-fed females (Con-pf). Data are shown as means ± S.E.M. Litter (Con=14, Con-pf=16, Nic=13) was used as a unit for analysis. Post hoc Bonferroni LSD test results significant at least at p<0.05 are marked as asterisk vs. the control group (Con), number sign (#) vs. the Con-pf group
Neurobehavioural development
The ontogeny of the righting reflex was delayed in animals prenatally exposed to nicotine compared to both Con and Con-pf animals (Fig. 1b; F(2, 30)=26.4, p<0.001; post hoc results p<0.01 in all cases). Rats in all groups showed decreased latencies to right themselves onto all four feet from a supine position over the consecutive sessions (F(2, 30)=60.9, p<0.001). There was no group×PND interaction.
The ontogeny of negative geotaxis was significantly delayed in rats prenatally exposed to nicotine when compared to the Con but not to the Con-pf group (Fig. 1c; (F(2, 30)=3.21, p=0.05; post hoc analysis p<0.01). All groups decreased the latencies to turn 180 ° over the consecutive sessions (F(3, 30)=17.4, p<0.001). There was no group×PND interaction.
Both the Nic and Con-pf groups had decreased grip strength compared to Con animals on PNDs 14 and 17 (Fig. 1d; F(2, 30)=8.3, p<0.01). All groups showed increased grip strength over the consecutive sessions (F(1, 30)=78.3, p<0.001). There was no group×PND interaction.
Tests in adolescent rats
Locomotor activity
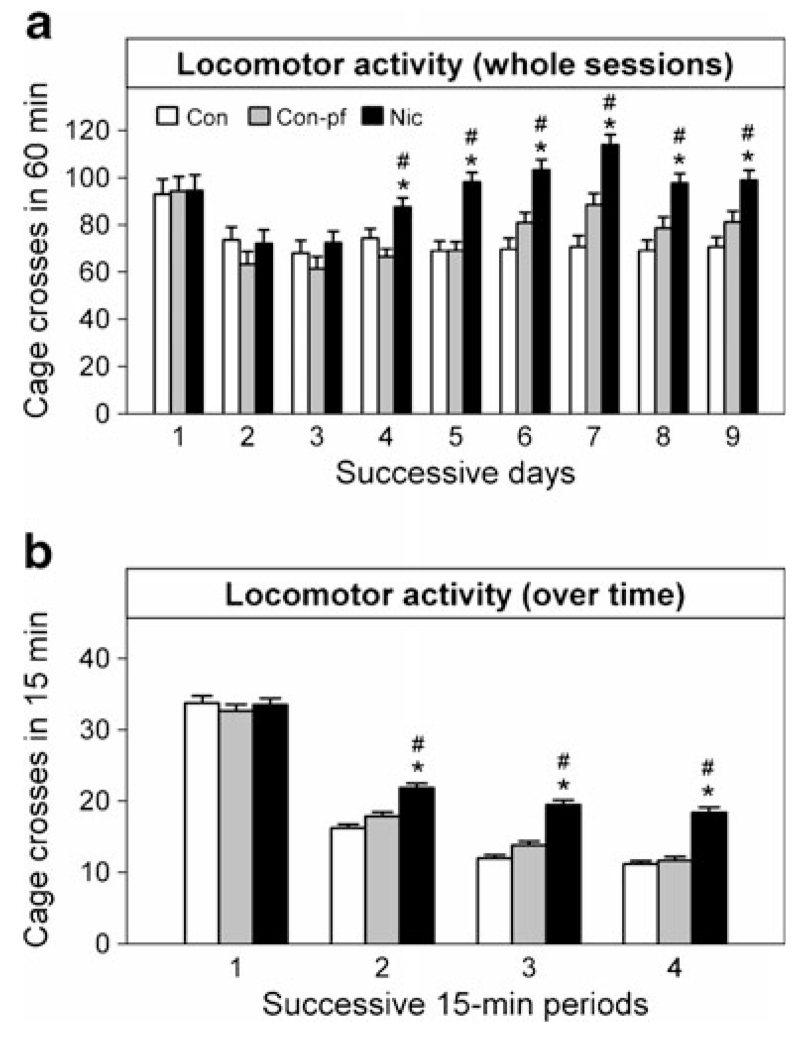
There was a significant effect of group (F(2, 33)=7.82, p=0.001), day of testing (F(8, 33)=10.8, p<0.001) and group×day of testing interaction (F(16, 3,828)=3.28, p<0.001). Post hoc analysis revealed increased activity in the Nic group compared to the Con and Con-pf groups from the fourth to the ninth day of testing (Fig. 2a, all post hoc comparisons p<0.01). When 15-min within-session periods were used in the analysis, significant group×within-session period interaction was observed (Fig. 2b; F(6, 3,843)=8.06, p<0.001) with increased activity in the Nic group compared to both Con and Con-pf animals in all within-session periods, except the first 15 min, suggesting decreased locomotor habituation in the Nic group (Fig. 2b).
Fig. 2.
Locomotor activity in adolescent rats prenatally exposed to nicotine (Nic) and in the offspring of pair-fed females (Con-pf) assessed in nine daily 60-min sessions (a) and mean within-session activity for all the sessions (b). Data are shown as means ± S.E.M., n=12 for all groups. Post hoc Bonferroni LSD test results significant at least at p<0.05 are marked as asterisk vs. the control group (Con), number sign (#) vs. the Con-pf group
The elevated plus maze
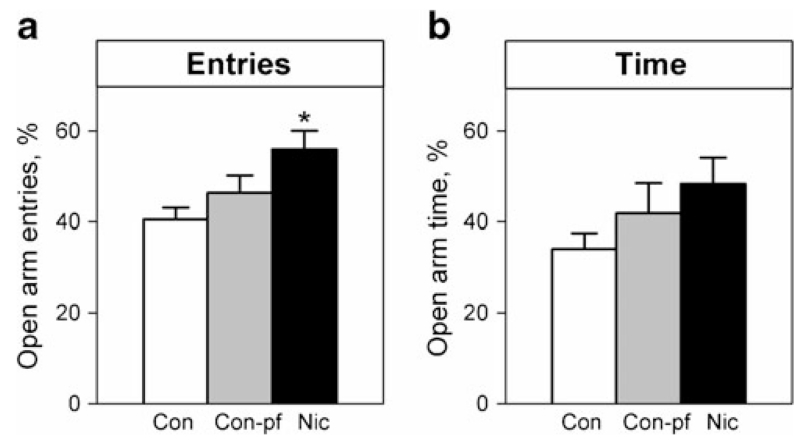
There was a significant between-group difference in the percentage of open arm entries (Fig. 3a, F(2, 33)=4.78, p<0.05), but not in the percentage of time spent in the open arms (Fig. 3b, F(2, 33)=1.71, n.s.), with an increased number of open arm entries in the Nic group compared to the Con group (post hoc comparison p<0.01). There was no between-group difference in the number of closed arm entries (Con=8.42±0.54, Con-pf=7.08±0.61, Nic=7.83±0.73).
Fig. 3.
Open arm entries (a) and time spent in open arms (b) in the elevated plus maze test in adolescent rats prenatally exposed to nicotine (Nic) and in the offspring of pair-fed females (Con-pf). Data are shown as means ± S.E.M.; n=12 for all groups. Post hoc Bonferroni LSD test results significant at least at p<0.05 are marked as asterisk vs. the control group (Con)
Two-bottle free choice nicotine consumption test
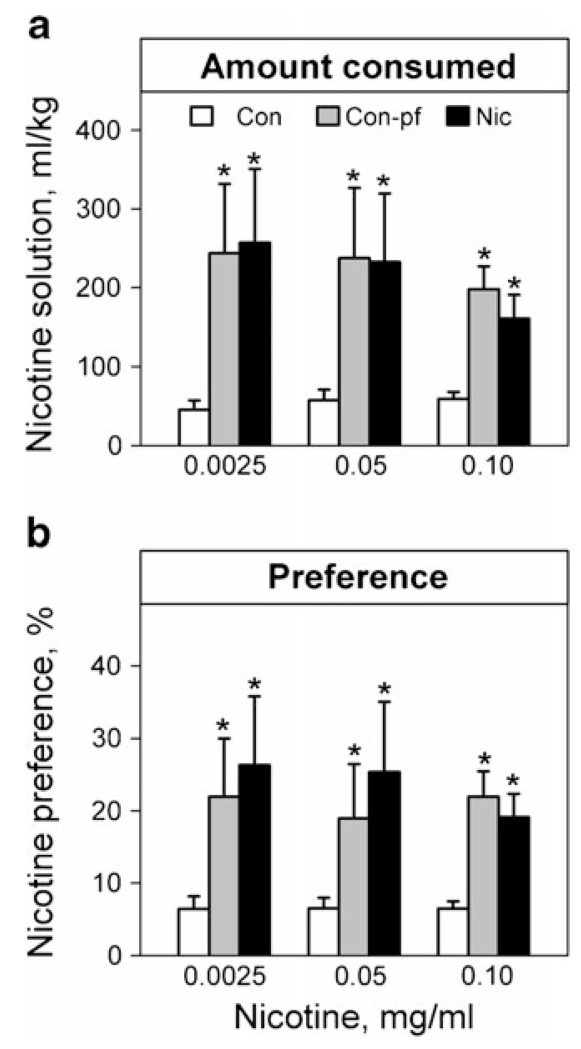
There was a significant effect of group for nicotine solution consumption per kilogramme body weight for all three nicotine solution concentrations (Fig. 4a, 0.01 mg/ml, χ2=32.0, p<0.001; 0.005 mg/kg, χ2=7.21, p=0.03; 0.0025 mg/ml, χ2=11.2, p=0.003) as well as for nicotine solution aversion (Fig. 4b, 0.01 mg/ml, χ2=30.5, p<0.001; 0.005 mg/kg, χ2=8.74, p=0.01; 0.0025 mg/ml, χ2=8.93, p=0.01). Post hoc analysis revealed increased nicotine solution consumption and reduced aversion in both the Nic and the Con-pf groups compared to Con animals (all comparisons p<0.05 at least). There was no between-group difference in sucrose solution consumption in any stage of the test.
Fig. 4.
Nicotine solution consumption (a) and nicotine solution preference (b) in adolescent rats prenatally exposed to nicotine (Nic) and in the offspring of pair-fed females (Con-pf). Data are shown as means ± S.E.M.; n=12 for all groups. Note that all data show aversions to nicotine solutions relative to water. Post hoc Bonferroni LSD test results significant at least at p<0.05 are marked as asterisk vs. the control group (Con)
Five-choice serial reaction time task
There were several between-group differences during acquisition of the task. During the phase when the duration of the visual stimuli was 10 s, there was a trend towards between-group difference in anticipatory responses (F(2, 32)=1.5, p=0.06) with both the Nic and Con-pf groups showing more anticipations than Con animals (Con=52.8±2.98, Con-pf=67.7±3.8, Nic=69.8±3.66). When the duration of visual stimuli was set to 5 s, there was a significant difference in the number of anticipatory responses (F(2, 32)=4.45, p<0.05), with the Nic group showing more anticipations than the Con group (p<0.001; Con=26.9±1.3, Con-pf=34.3±2.1, Nic=41.1±2.7). Numbers of omission errors also differed between groups (F(2, 32)=2.32, p=0.05), with the Nic group showing fewer omission errors than the Con group (p<0.001; Con=34.9 ± 1.2, Con-pf=31.5 ± 1.7, Nic=28.0±1.0).
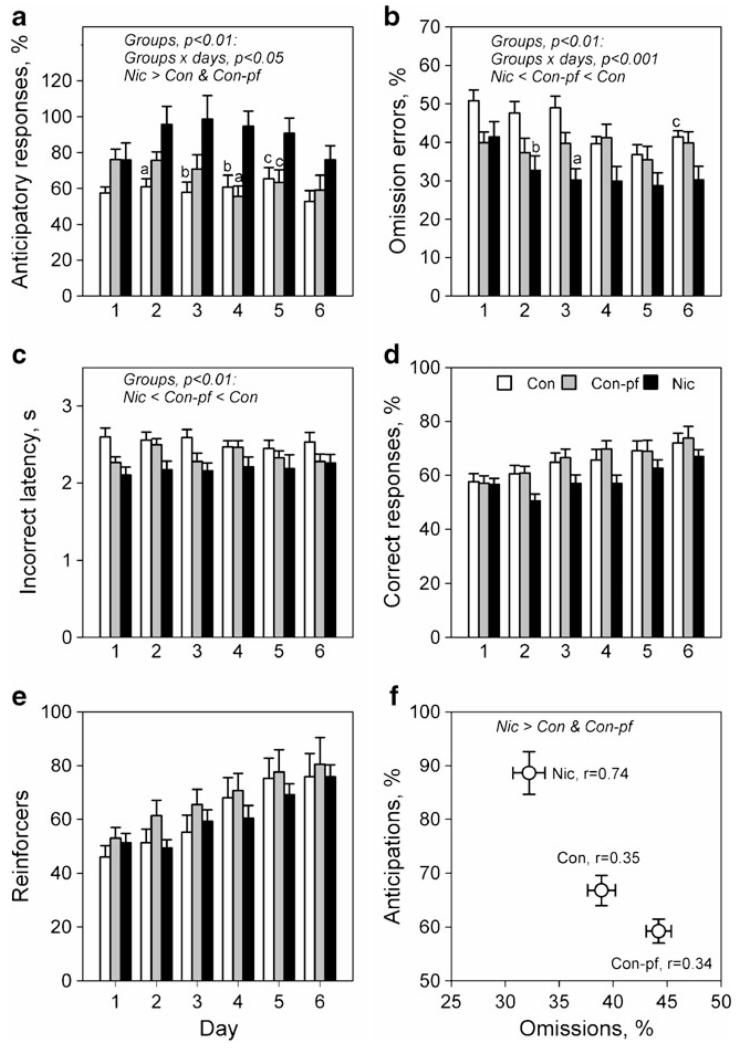
At the stage where a 1-s stimulus duration was used, the performance of the Nic and Con-pf groups differed significantly from that of the Con group (Fig. 5). Under this condition, adolescent rats prenatally exposed to nicotine exhibited an increased percentage of anticipatory responses in comparison to both the Con and Con-pf groups (Fig. 5a; F(2, 32)=6.77, p<0.01; post hoc p<0.001, either), decreased number of omission errors (Fig. 5b; F(2, 32)=5.45, p<0.01; post hoc p<0.001, either) and a shorter latency to incorrect responses (Fig. 5c; F(2, 32)=6.28, p<0.01; post hoc p<0.01 at least). The Con-pf group displayed decreased numbers of omission errors (p<0.05) and shorter latencies to incorrect responses (p<0.05) compared to the Con group. There was no between-group difference in accuracy (Fig. 5d; F(2, 32)=2.44, n.s.), reinforcers earned (Fig. 5e; F(2, 32)=0.6, n.s.) or in latency to correct responses (F(2, 32)=3.08, n.s.). Anticipatory responses were negatively correlated with omission errors for all animals (Fig. 5f; r=−0.62, p<0.001); however, the strength of this correlation was higher in the Nic group (Con r=−0.34, Con-pf r=−0.35, Nic r=−0.74) compared to both Con (z=1.97, p<0.05) and Con-pf (z=1.91, p<0.05) groups.
Fig. 5.
Performance in the 5-CSRTT in adolescent rats prenatally exposed to nicotine (Nic, n=12) and in the offspring of control (Con, n=12) and pair-fed females (Con-pf, n=11). Data are shown as means ± S.E.M. from 6 days of the final stage of the procedure when 1-s stimulus duration was used: number of anticipations (a), percentage omissions (b), latency to incorrect responses (c), percentage correct responses (d), number of correct responses (e) and correlation between anticipations and omissions (f). For post hoc test results for the Nic vs. the Con and Con-pf groups: a, p<0.001; b, p<0.01; c, p<0.05
There was a significant effect of day for all variables, except latency to incorrect responses, shown in Fig. 5 (smallest F(3, 160)=3.36, p<0.001) that was attributable to a progressive improvement of performance over the 6 days for accuracy, numbers of reinforcers earned, number of omissions and anticipations. Significant group×day interaction was found for the number of anticipations and omission errors (Fig. 5a, b; F(10, 160)=2.10, p<0.05 and F(10, 160)=3.24, p<0.001, respectively).
Introduction of 15-s ITIs led to increased number of omission errors (F(1, 32)=36.2, p<0.0001) and anticipatory responses (Fig. 5a; F(1, 32)=264.4, p<0.0001) and decreased latency to incorrect responses (Fig. 5c; F(1, 32)=45.0, p<0.0001, respectively), but had no impact on accuracy (Fig. 5d; F(1, 32)=2.44, n.s.), when the last day under the 5-s ITI was compared to the first day of 15-s ITI. There was no group×ITI interaction, suggesting a similar impact of this manipulation on all groups of animals.
Delay-discounting test
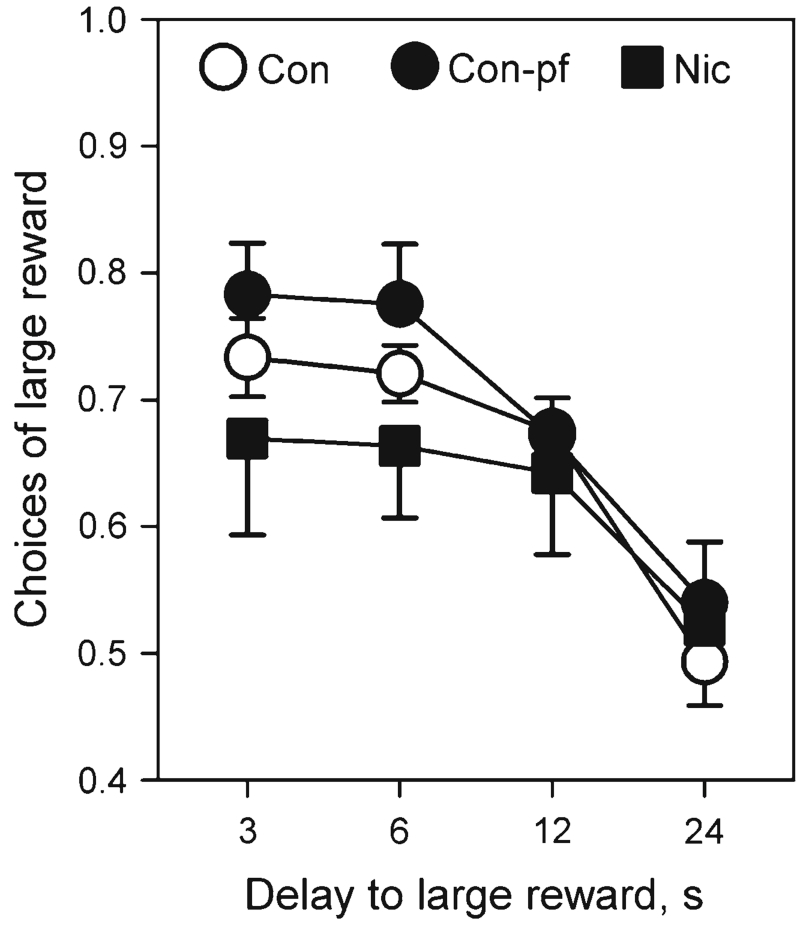
All groups chose the large reward on at least 70 % of trials when the delay to the large reward was 0 s. As the delay to the large reward increased, the preference of all groups of rats shifted towards the smaller but more immediate reward (Fig. 6; delay: F(3, 27)=18.2, p<0.0001); however, there was no significant effect of nicotine exposure or pair-fed feeding on choice behaviour at the different delays (group: F(2, 27)=1.03, n.s.; delay×group: F(6, 81)=0.55, n.s.). All groups reached random level of performance at the delay of 24 s and, therefore, the study was terminated.
Fig. 6.
Delay-discounting in adolescent rats prenatally exposed to nicotine (Nic) and in the offspring of pair-fed females (Con-pf). Data are shown as mean ± S.E.M.; n=10 for all groups
Discussion
Following gestational exposure to nicotine, the offspring were found to have lower birth weights, slower weight gain before weaning and delayed sensorimotor development, and to be hyperactive during multiple exposures to a familiar environment. During adolescence, they displayed less anxiety-related behaviour in the elevated plus maze, consumed more nicotine solution and made more anticipatory responses (impulsivity) but fewer omission errors in the 5-CSRTT. Some of these deficits might be explained by the effects of under-nutrition during pregnancy, as the offspring of pair-fed females that showed lower birth weights and slower weight gain before weaning also displayed increased nicotine consumption in adolescence and decreased numbers of omission errors in the 5-CSRTT. All the deficits found in the Con-pf group were seen in nicotine-exposed animals, but aberrations in activity, decreased anxiety and impulsivity were observed only in the Nic group. Neither nicotine exposure nor pair-feeding had an effect on impulsive choice in the delay-discounting test.
Nicotine exposure, pair-feeding and litter characteristics
In line with previous animal studies (e.g. Murrin et al. 1987; Schneider et al. 2010, 2011) pregnant females exposed to a nicotine solution as the only source of fluid showed decreased body weight gain and lower solution and food consumption compared to Con animals (Table 1). Both decreased solution consumption and decreased body weights were also observed in pair-fed animals; however, the Nic group consumed even less solution than the Con-pf group. There was no difference in body weight between these two groups. Prenatal exposure to nicotine had no effect on the litter size, numbers of males and females per litter or the sex ratio, suggesting only mild teratogenicity of both the nicotine dose regimen used and the pair-feeding procedure; however, a significantly smaller percentage of nicotine-exposed females became pregnant compared to both the Con and Con-pf groups, suggesting that nicotine exposure might have an impact on fertility-related processes, an observation that accords with human studies showing decreased fertility and increased time to conception among women who smoke cigarettes (Jensen et al. 1998; Munafo et al. 2002).
Developmental changes
Both birth weight and weight gain during the first 15 days of life were decreased by prenatal exposure to nicotine and the pair-feeding procedure (Fig. 1a). This has been shown previously in rats (Paulson et al. 1994; Schneider et al. 2011) and is similar to the results of human studies (Eskenazi et al. 1995). The long-term significance of lower birth weight is still unclear but studies in humans have found an association of low birth weight with long-term cognitive deficits (Hack et al. 1994; Taylor et al. 2004, 2011), and also with increased risk of ADHD (Hultman et al. 2007). The other maturational measures (pinnae detachment, fur appearance, incisor eruption and eye opening) did not differ between the groups. In contrast, developmental measures including righting reflex (also vs. the Con-pf group), negative geotaxis and latency to fall in the grip strength test were all compromised in nicotine-exposed animals. Offspring of pair-fed females displayed only a shorter latency to fall in the grip strength tests. Our results agree with previous studies showing developmental deficits in rodents exposed prenatally to similar doses of nicotine (Ajarem and Ahmad 1998; Peters and Ngan 1982; Schneider et al. 2010). The delay in attaining these skills might be due to damage or poor development of the motor and vestibular systems of the brain (Springate 1981).
Locomotor activity
In utero nicotine exposure has been associated with ‘hyperactivity’ in humans as measured by a combined parental rating of restlessness, being fidgety, unable to settle and easily distracted (Kotimaa et al. 2003). Interestingly, overactivity in ADHD has been shown to be more pronounced under familiar (habituated) and unstimulating conditions and to normalise in novel or stimulating environments (Antrop et al. 2000; Sagvolden et al. 1998). The latter observation prompted us to evaluate activity during multiple exposures to the same environment. We found increased activity in the Nic group compared to both the Con and Con-pf groups after three daily exposures between days 4 and 9 of testing, which might resemble the more pronounced hyperactivity under familiar (less novel) conditions in children with ADHD. Importantly, hyperactivity in the Nic group was observed after the first 15 min of exposure to the familiar environment, suggesting decreased locomotor habituation (Fig. 2b). Prenatal exposure to nicotine in rats has been previously shown to lead to hyperactivity (Tizabi et al. 2000; Vaglenova et al. 2004), hypoactivity (LeSage et al. 2006; Romero and Chen 2004) or to have no impact at all (Schneider et al. 2011; Shacka et al. 1997). Our results suggest that this discrepancy might be related not only to pharmacological factors such as different doses of nicotine and routes of administration but may also depend upon the age of testing (adulthood vs. adolescence) or the amount of exposure to the apparatus, particularly whether the environment appeared to be novel or familiar to the animal, reflecting exploratory or habituated behaviour, respectively. It might therefore be necessary to use multiple and prolonged daily exposures to observe clear effects of prenatal exposure to nicotine on activity.
Elevated plus maze
Nicotine-exposed animals made more entries into but spent similar amount of time in the open arms as compared with Con animals. In a classical interpretation of elevated plus maze results, adolescent nicotine-exposed animals seemed to display reduced basal levels of anxiety during the exploration of the apparatus, a behavioural pattern that may also suggest increased novelty-seeking behaviour (Redolat et al. 2009). This interpretation was strengthened by the lack of between-group differences in activity as measured by the number of closed arm entries. Sensation-seeking behaviour is more frequent in adolescence (Steinberg 2004) and is associated with ADHD (e.g. Barkley and Cox 2007; McNamara et al. 2008; Murphy and Barkley 1996). Novelty-seeking has also been linked to increased impulsivity both in humans and rodents (for reviews, see Evenden 1999a; Laviola et al. 2003) and identified as a significant contributor to drug use and abuse (review in Bardo and Dwoskin 2004).
Nicotine consumption
Both the Nic and Con-pf groups showed increased consumption of nicotine solution and reduced aversion for nicotine solutions at all concentration used. There was no between-group difference in the consumption of 10 % sucrose solutions. Neither group increased solution consumption with decreasing nicotine concentrations. So far, there are no data showing a relationship between gestational famine in humans or food restriction in animals and increased risk for smoking or nicotine intake; however, food or protein restriction in animals leads to increased maternal stress and dysregulation of the stress response system (Guarnieri et al. 2012) and dopamine system dysfunction (Vucetic et al. 2010) in offspring, both of which may be implicated in decreased nicotine aversion found in our study in the Con-pf group.
In contrast, there is a vast literature linking prenatal nicotine exposure to increased risk of substance abuse, particularly smoking, in humans (Cornelius et al. 2000; Roberts et al. 2005; Wakschlag et al. 1997; Weissman et al. 1999) and to increased nicotine self-administration in rats (Klein et al. 2003; Levin et al. 2006). However, an increased intake of nicotine solutions in oral choice tests does not necessarily indicate an enhanced potential for self-administering the drug or developing dependence on it; such findings need to be linked to actions on nicotinic receptors by, for example, demonstrating sensitivity to nicotine antagonists. Interestingly, recent studies suggest that ADHD symptoms, even at levels below the clinical threshold, are significantly associated with risk for smoking (Fuemmeler et al. 2007; Kollins et al. 2005; McClernon et al. 2008) and with a higher daily smoking rate among young adolescents (Kollins et al. 2005). Moreover, retrospective reports of childhood ADHD symptoms among young adults suggest that hyperactivity/impulsivity symptoms are stronger predictors of regular smoking than inattentive symptoms (Kollins et al. 2005).
Five-choice and delayed discounting tasks
Previous studies have demonstrated deficits in learning and memory in adult rats prenatally exposed to nicotine (Levin et al. 1993; Vaglenova et al. 2008) as well as impairments in measures of attention, impulsive responding and variability of reaction times using the 5-CSRTT and the lack of differences in choice impulsivity measured in the delay-discounting task (Schneider et al. 2011).
In the present study, we have observed an increase in anticipatory responses and a decrease in omission errors during the training phases of the study (5 s SD) in the Nic group compared to Con animals. During the attentional phase (1 s SD) there was no between-group difference in accuracy or in the number of reinforcers earned, but the Nic group showed more anticipatory responses, fewer omission errors and decreased latency to incorrect responses compared to both the Con and Con-pf groups, while Con-pf animals made fewer omission errors and displayed shorter latencies to incorrect responses compared to the Con group. Anticipatory responses were negatively correlated with the number of omission errors for all animals; however, this correlation was stronger in the Nic group (Fig. 5f). Due to the short-lasting adolescent period in rats, it was not possible to continue training until stable baseline performance was reached in any of the three groups; however, decreasing significance levels for differences between the Con and Nic groups for anticipatory responses (day 2, p<0.001 vs. day 6, p<0.09) as well as our results for adult rats prenatally exposed to nicotine (Schneider et al. unpublished data) suggest that those differences might disappear with prolonged training. Furthermore, strong correlations between the increased number of anticipations and the decreased number of omission errors in the Nic group accompanied by normal accuracy and numbers of reinforcers earned may suggest that behavioural disinhibition (leading to an increase in impulsive anticipatory responses) decreases the number of omissions and thus enables these animals to deal with attentional demands of the task. Such behaviour may mask attentional problems in these animals and this aspect requires further study. Decreased number of omissions in Con-pf animals cannot be explained by the same mechanism, but may be related to their increased activity (shorter latencies to incorrect responses). Importantly, adolescents whose mothers smoked during pregnancy show increased impulsivity, but not attentional problems in vigilance tests (Fried and Watkinson 2001; Kristjansson et al. 1989) compared to healthy controls, which might resemble the results we obtained in adolescent rats. Introduction of 15 s ITIs resulted in increased numbers of anticipatory responses and omission errors in all groups, but had no impact on accuracy.
In the delay-discounting task, which measures a specific aspect of choice impulsivity, there was no difference between nicotine-exposed, pair-fed offspring and control rats. There are no data on delay discounting test performance in children exposed to nicotine in utero, but not diagnosed as ADHD, and an association between ADHD and performance on delay-discounting tasks was not observed in adolescents (Scheres et al. 2006). The discrepancy in our findings between impulsive responding indexed by anticipatory responses in the 5-CSRTT and the delay-discounting test in the Nic group is not unexpected also because they measure entirely different aspects of impulsivity, consistent with the non-unitary nature of impulsive behaviour in humans (Evenden 1999b; Moeller et al. 2001) and animals (Winstanley et al. 2006).
Study limitations
The present study has two main limitations. Firstly, we used nicotine treatment only until the animals’ birth, which is an equivalent of the first two trimesters in humans (Bayer et al. 1993; Clancy et al. 2007). It has been previously shown that nicotine interferes with critical developmental events that occur during this period, such as neurogenesis and early synaptogenesis (Dwyer et al. 2008), and results in a plethora of behavioural aberrations (e.g. Schneider et al. 2011; Tizabi et al. 2000; Vaglenova et al. 2004). Potentially important effects of nicotine exposure during early postnatal life in rats, which is equivalent to the third trimester of human gestation (Clancy et al. 2007), will need to be assessed in future studies. Also, it might be suggested that termination of nicotine exposure at parturition might lead to withdrawal symptoms in the dams and changes in maternal behaviour. While the effects of withdrawal on both the dams and pups were not examined in this study, no unusual behaviours were noted when chronic administration ended. Importantly, previous studies have shown that withdrawal from similar doses of nicotine contributes little, if anything either to growth deficits or to abnormal development of brain cell (Slotkin et al. 1993) and that nicotine administration through drinking water in rodents has a very limited impact on maternal behaviour (Heath et al. 2010). Secondly, although females from the pair-fed group consumed significantly less water than control animals, nicotine-exposed females consumed even less water than the pair-fed group. In contrast to prenatal under-nutrition (Nunez et al. 2008; Zhang et al. 2010), direct tests on the behavioural effects of gestational dehydration in rats do not seem to have been published and its impact on the performance measures used in this study cannot be fully excluded. However, it seems unlikely that the differences in maternal dehydration level can target specifically some aspects of behavioural disinhibition, like increased locomotor activity, decreased anxiety and impulsivity, which were observed only in the Nic group, but not in other groups, like decreased latency to incorrect responses and changes in the number of omission errors found in both the Nic and Con-pf groups. Nicotine exposure would be a much more plausible explanation for the observed behavioural aberrations.
Summary
Prenatal nicotine exposure in adolescent rats led to hyperactivity in a familiar environment, decreased anxiety in the elevated plus maze, higher nicotine consumption and behavioural disinhibition characterised by increased anticipatory responses, decreased omission errors and shorter latency to incorrect responses in the 5-CSRTT. Some of these effects might have been mediated by gestational under-nutrition as offspring of pair-fed females also showed higher nicotine consumption, fewer omission errors and shorter latencies to incorrect responses in the 5-CSRTT; however, the latter findings were of significantly greater magnitude in nicotine-exposed animals. These findings confirm direct deleterious effects of prenatal nicotine exposure on important aspects of behaviour and inhibitory control in adolescent rodents. The observed behavioural pattern resembles components of the hyperactive/impulsive symptom domain in ADHD. Thus, our results support epidemiological studies showing increased levels of ADHD symptoms among those whose mothers smoked during pregnancy (Milberger et al. 1998; Thapar et al. 2003; Weissman et al. 1999) and challenge the conclusion that the observed association between ADHD and maternal smoking in pregnancy is mediated entirely by genetic effects (D’Onofrio et al. 2008; Thapar et al. 2009). The precise mechanisms by which such long-term impacts on behaviour arise remain unknown, but are likely to involve epigenetic changes induced by exposure to the environmental factors (Mill and Petronis 2008) and this will be elucidated by our future studies.
Acknowledgments
The research was supported by a grant from the Wellcome Trust (079314).
Footnotes
Conflict of interest Philip JE Asherson has received funding for his work on advisory boards, consultancy or industry-sponsored educational activities from Janssen-Cilag, Eli-Lilly, Shire and Flynn Pharma. Ian P Stolerman has received compensation in the past 3 years for professional services to Elsevier Science Publishers, Springer-Verlag and the US National Institute on Drug Abuse. The other authors declare no conflict of interest.
Contributor Information
T. Schneider, Section of Behavioural Pharmacology, Institute of Psychiatry P048, King’s College London, De Crespigny Park, London SE5 8AF, UK; Experimental Psychology, University of Oxford, South Parks Road, Oxford OX1 3UD, UK
L. Bizarro, Departamento de Psicologia do Desenvolvimento e da Personalidade, Instituto de Psicologia, Universidade Federal do Rio Grande do Sul, Rua Ramiro Barcellos 2600, Porto Alegre, RS 90035-003, Brazil
P. J. E. Asherson, MRC Social Genetic and Developmental Psychiatry Centre, Institute of Psychiatry P080, King’s College London, De Crespigny Park, London SE5 8AF, UK
I. P. Stolerman, Section of Behavioural Pharmacology, Institute of Psychiatry P048, King’s College London, De Crespigny Park, London SE5 8AF, UK
References
- Adams J. Methods in behavioral teratology. Plenum; York: 1986. pp. 67–97. [Google Scholar]
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998;59:313–318. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- Antrop I, Roeyers H, Van OP, Buysse A. Stimulation seeking and hyperactivity in children with ADHD. J Child Psychol Psychiatry. 2000;41:225–231. [PubMed] [Google Scholar]
- Bardo MT, Dwoskin LP. Biological connection between novelty- and drug-seeking motivational systems. Nebr Symp Motiv. 2004;50:127–158. [PubMed] [Google Scholar]
- Barkley RA, Cox D. A review of driving risks and impairments associated with attention-deficit/hyperactivity disorder and the effects of stimulant medication on driving performance. J Safety Res. 2007;38:113–128. doi: 10.1016/j.jsr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini A, Bernardi M, Genedani S. Effects of prenatal exposure to cigarette smoke and nicotine on pregnancy, offspring development and avoidance behavior in rats. Neurobehav Toxicol Teratol. 1982;4:545–548. [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nicotine Tob Res. 2000;2:45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day N, Cornelius M, Goldschmidt L, Richardson G, Robles N, Taylor P. The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicol Teratol. 1992;14:407–414. doi: 10.1016/0892-0362(92)90051-b. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Prehn AW, Christianson RE. Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight. Am J Public Health. 1995;85:395–398. doi: 10.2105/ajph.85.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden J. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999a;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999b;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;23:421–430. doi: 10.1016/s0892-0362(01)00160-x. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25:427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J Pediatr Psychol. 2007;32:1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- Genedani S, Bernardi M, Bertolini A. Sex-linked differences in avoidance learning in the offspring of rats treated with nicotine during pregnancy. Psychopharmacology (Berl) 1983;80:93–95. doi: 10.1007/BF00427504. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Brayton CE, Richards SM, Maldonado-Aviles J, Trinko JR, Nelson J, Taylor JR, Gourley SL, DiLeone RJ. Gene profiling reveals a role for stress hormones in the molecular and behavioral response to food restriction. Biol Psychiatry. 2012;71:358–365. doi: 10.1016/j.biopsych.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birth weights under 750 g. N Engl J Med. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Horst NK, Picciotto MR. Oral nicotine consumption does not affect maternal care or early development in mice but results in modest hyperactivity in adolescence. Physiol Behav. 2010;101:764–769. doi: 10.1016/j.physbeh.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Torrang A, Tuvblad C, Cnattingius S, Larsson JO, Lichtenstein P. Birth weight and attention-deficit/hyperactivity symptoms in childhood and early adolescence: a prospective Swedish twin study. J Am Acad Child Adolesc Psychiatry. 2007;46:370–377. doi: 10.1097/01.chi.0000246059.62706.22. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32:2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83:699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Adult and prenatal exposures to tobacco smoke as risk indicators of fertility among 430 Danish couples. Am J Epidemiol. 1998;148:992–997. doi: 10.1093/oxfordjournals.aje.a009576. [DOI] [PubMed] [Google Scholar]
- Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Pfaff DW, Vandenbergh DJ. Laternal nicotine exposure increases nicotine preference in periadolescent male but not female C57B1/6 J mice. Nicotine Tob Res. 2003;5:117–124. doi: 10.1080/14622200307257. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Kotimaa AJ, Moilanen I, Taanila A, Ebeling H, Smalley SL, McGough JJ, Hartikainen AL, Jarvelin MR. Maternal smoking and hyperactivity in 8-year-old children. J Am Acad Child Adolesc Psychiatry. 2003;42:826–833. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- Kristjansson EA, Fried PA, Watkinson B. Maternal smoking during pregnancy affects children’s vigilance performance. Drug Alcohol Depend. 1989;24:11–19. doi: 10.1016/0376-8716(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Gustaf E, Dufek MB, Pentel PR. Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol Biochem Behav. 2006;85:575–583. doi: 10.1016/j.pbb.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993;15:251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol Biochem Behav. 2006;85:669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Wilkerson A, Jones JP, Christopher NC, Briggs SJ. Prenatal nicotine effects on memory in rats: pharmacological and behavioral challenges. Brain Res Dev Brain Res. 1996;97:207–215. doi: 10.1016/s0165-3806(96)00144-7. [DOI] [PubMed] [Google Scholar]
- Maehler R, Dadmarz M, Vogel WH. Determinants of the voluntary consumption of nicotine by rats. Neuropsychobiology. 2000;41:200–204. doi: 10.1159/000026660. [DOI] [PubMed] [Google Scholar]
- Maher BS, Marazita ML, Ferrell RE, Vanyukov MM. Dopamine system genes and attention deficit hyperactivity disorder: a meta-analysis. Psychiatr Genet. 2002;12:207–215. doi: 10.1097/00041444-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Marco EM, Macri S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox Res. 2011;19:286–307. doi: 10.1007/s12640-010-9205-z. [DOI] [PubMed] [Google Scholar]
- McCartney JS, Fried PA, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicol Teratol. 1994;16:269–276. doi: 10.1016/0892-0362(94)90048-5. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Fuemmeler BF, Kollins SH, Kail ME, Ashley-Koch AE. Interactions between genotype and retrospective ADHD symptoms predict lifetime smoking risk in a sample of young adults. Nicotine Tob Res. 2008;10:117–127. doi: 10.1080/14622200701704913. [DOI] [PubMed] [Google Scholar]
- McNamara J, Vervaeke SL, Willoughby T. Learning disabilities and risk-taking behavior in adolescents: a comparison of those with and without comorbid attention-deficit/hyperactivity disorder. J Learn Disabil. 2008;41:561–574. doi: 10.1177/0022219408326096. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol. 1998;27:352–358. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- Mill J, Petronis A. Pre- and peri-natal environmental risks for attention-deficit hyperactivity disorder (ADHD): the potential role of epigenetic processes in mediating susceptibility. J Child Psychol Psychiatry. 2008;49:1020–1030. doi: 10.1111/j.1469-7610.2008.01909.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Munafo M, Murphy M, Whiteman D, Hey K. Does cigarette smoking increase time to conception? J Biosoc Sci. 2002;34:65–73. [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC. Genes and cigarette smoking. Addiction. 2008;103:893–904. doi: 10.1111/j.1360-0443.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- Murphy K, Barkley RA. Attention deficit hyperactivity disorder adults: comorbidities and adaptive impairments. Compr Psychiatry. 1996;37:393–401. doi: 10.1016/s0010-440x(96)90022-x. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Ferrer JR, Zeng WY, Haley NJ. Nicotine administration to rats: methodological considerations. Life Sci. 1987;40:1699–1708. doi: 10.1016/0024-3205(87)90020-8. [DOI] [PubMed] [Google Scholar]
- Nunez H, Ruiz S, Soto-Moyano R, Navarrete M, Valladares L, White A, Perez H. Fetal undernutrition induces overexpression of CRH mRNA and CRH protein in hypothalamus and increases CRH and corticosterone in plasma during postnatal life in the rat. Neurosci Lett. 2008;448:115–119. doi: 10.1016/j.neulet.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC. Increase in child behavior problems resulting from maternal smoking during pregnancy. Arch Environ Health. 1997;52:317–321. doi: 10.1080/00039899709602205. [DOI] [PubMed] [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC. Child behavior problems increased by maternal smoking during pregnancy. Arch Environ Health. 1999;54:15–19. doi: 10.1080/00039899909602231. [DOI] [PubMed] [Google Scholar]
- Paulson RB, Shanfeld J, Vorhees CV, Cole J, Sweazy A, Paulson JO. Behavioral effects of smokeless tobacco on the neonate and young Sprague Dawley rat. Teratology. 1994;49:293–305. doi: 10.1002/tera.1420490409. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Peters MA, Ngan LL. The effects of totigestational exposure to nicotine on pre- and postnatal development in the rat. Arch Int Pharmacodyn Ther. 1982;257:155–167. [PubMed] [Google Scholar]
- Redolat R, Perez-Martinez A, Carrasco MC, Mesa P. Individual differences in novelty-seeking and behavioral responses to nicotine: a review of animal studies. Curr Drug Abuse Rev. 2009;2:230–242. doi: 10.2174/1874473710902030230. [DOI] [PubMed] [Google Scholar]
- Ribary U, Lichtensteiger W. Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J Pharmacol Exp Ther. 1989;248:786–792. [PubMed] [Google Scholar]
- Roberts KH, Munafo MR, Rodriguez D, Drury M, Murphy MF, Neale RE, Nettle D. Longitudinal analysis of the effect of prenatal nicotine exposure on subsequent smoking behavior of offspring. Nicotine Tob Res. 2005;7:801–808. doi: 10.1080/14622200500262840. [DOI] [PubMed] [Google Scholar]
- Romero RD, Chen WJ. Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol Biochem Behav. 2004;78:675–681. doi: 10.1016/j.pbb.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Rutherford HJ, Mayes LC, Potenza MN. Neurobiology of adolescent substance use disorders: implications for prevention and treatment. Child Adolesc Psychiatr Clin N Am. 2010;19:479–492. doi: 10.1016/j.chc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behav Brain Res. 1998;94:61–71. [PubMed] [Google Scholar]
- Salihu HM, Aliyu MH, Kirby RS. In utero nicotine exposure and fetal growth inhibition among twins. Am J Perinatol. 2005;22:421–427. doi: 10.1055/s-2005-915219. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Schneider T, Bizarro L, Asherson PJ, Stolerman IP. Gestational exposure to nicotine in drinking water: teratogenic effects and methodological issues. Behav Pharmacol. 2010;21:206–216. doi: 10.1097/fbp.0b013e32833a5bb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Ilott N, Brolese G, Bizarro L, Asherson PJ, Stolerman IP. Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology. 2011;36:1114–1125. doi: 10.1038/npp.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Fox NL, Hebel JR. Prenatal exposure to tobacco: II. Effects on cognitive functioning at age three. Int J Epidemiol. 1990;19:72–77. doi: 10.1093/ije/19.1.72. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Fennell OB, Robinson SE. Prenatal nicotine sex-dependently alters agonist-induced locomotion and stereotypy. Neurotoxicol Teratol. 1997;19:467–476. doi: 10.1016/s0892-0362(97)00063-9. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, Seidler FJ. Impact of fetal nicotine exposure on development of rat brain regions: critical sensitive periods or effects of withdrawal? Brain Res Bull. 1993;31:319–328. doi: 10.1016/0361-9230(93)90224-y. [DOI] [PubMed] [Google Scholar]
- Sorenson CA, Raskin LA, Suh Y. The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacol Biochem Behav. 1991;40:991–993. doi: 10.1016/0091-3057(91)90117-k. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Springate JE. The neuroanatomic basis of early motor development: a review. J Dev Behav Pediatr. 1981;2:146–150. [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Filipek PA, Juranek J, Bangert B, Minich N, Hack M. Brain volumes in adolescents with very low birth weight: effects on brain structure and associations with neuropsychological outcomes. Dev Neuropsychol. 2011;36:96–117. doi: 10.1080/87565641.2011.540544. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004;10:149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Rutter M, Harold G. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66:722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Russell LT, Nespor SM, Perry DC, Grunberg NE. Prenatal nicotine exposure: effects on locomotor activity and central [125I]alpha-BT binding in rats. Pharmacol Biochem Behav. 2000;66:495–500. doi: 10.1016/s0091-3057(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150:159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, Pandiella N, Birru S. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol Learn Mem. 2008;90:527–536. doi: 10.1016/j.nlm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, Reyes TM. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168:359–370. doi: 10.1016/j.neuroscience.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Wermter AK, Laucht M, Schimmelmann BG, Banaschweski T, Sonuga-Barke EJ, Rietschel M, Becker K. From nature versus nurture, via nature and nurture, to gene × environment interaction in mental disorders. Eur Child Adolesc Psychiatry. 2010;19:199–210. doi: 10.1007/s00787-009-0082-z. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci. 2008;13:636–649. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li N, Yang J, Zhang T, Yang Z. Effects of maternal food restriction on physical growth and neurobehavior in newborn Wistar rats. Brain Res Bull. 2010;83:1–8. doi: 10.1016/j.brainresbull.2010.06.005. [DOI] [PubMed] [Google Scholar]