Abstract
Background
Afaitnib has shown anti-tumor activity against metastatic EGFR-mutated NSCLC after prior failure to first generation EGFR-TKI and chemotherapy. We prospectively evaluated the efficacy and safety of afatinib in Chinese patients who previously failed first-generation TKI and chemotherapy under a compassionate use program (CUP) and compared to the erlotinib cohort.
Methods
Patients who suffered from metastatic EGFR-mutated NSCLC previously responsive to first-generation TKI and chemotherapy received afatinib until progression, loss of clinical benefits or intolerable toxicity. Treatment response, survival and safety were evaluated and compared to the erlotinib cohort.
Results
Twenty-five and 28 patients received afatinib and erlotinib respectively. More patients in the afatinib group had worse performance status (ECOG 2) than the erlotinib group (p = 0.008). After a median follow-up of 12.1 months, afatinib demonstrated comparable objective response rate (ORR) (20.0 % vs. 7.1 %, p = 0.17) but significantly higher disease control rate (DCR) (68.0 % vs. 39.3 %, p = 0.04) compared to erlotinib. Median progression-free survival (PFS) (4.1 months [95 % CI, 2.7–5.5 months] vs. 3.3 months [95 % CI, 2.2–4.3 months], p = 0.97) and overall survival (OS) were not different between the two groups (10.3 months [95 % CI, 7.5–13.0 months] vs. 10.8 months [95 % CI, 7.4–14.2 months], p = 0.51). Multivariate analyses revealed that age ≤70 years and time to progression (TTP) ≥18 months for the 1st TKI therapy were prognostic of PFS (p = 0.006 and p = 0.008 respectively). Afatinib caused less rash (60.0 % vs. 67.9 %, p = 0.04) but more diarrhea (60.0 % vs. 10.7 %, p = 0.002) compared to erlotinib.
Conclusion
Afatinib produced encouraging clinical efficacy as 2nd TKI therapy with manageable safety profiles in our Chinese patients after failure to another TKI and systemic chemotherapy.
This study was registered at ClinicalTrials.gov (NCT02625168) on 3rd December 2015.
Keywords: Afatinib, Erlotinib, Epidermal growth factor receptor mutation, Tyrosine-kinase inhibitor, Non-small-cell lung cancer
Background
First-generation epidermal growth factor receptor tyrosine-kinase inhibitors (EGFR-TKI) including geiftinib and erlotinib have been the standard first-line treatment for metastatic non-small-cell lung cancer (NSCLC) harboring activating EGFR mutation. Global and regional phase III randomized-controlled trials demonstrated that the median progression-free survival (PFS) after gefitinib or erlotinib ranged from 9 to 13 months with the longest PFS of 13.1 months seen in OPTIMAL study using erlotinib [1–7]. Emergence of T790M mutation is the most common mechanism of acquired resistance to EGFR-TKI, accounting for about 50–60 % of patients who developed disease progression after EGFR TKI [8–10].
Afatinib, regarded as second-generation EGFR-TKI, is an irreversible ErbB family blocker. It was approved as first-line treatment for EGFR-mutated advanced NSCLC in European Union and some other countries in 2013. It exhibits an inhibitory effect on T790M-mutated NSCLC in in-vitro studies, apart from the expected inhibition on exon 19 deletion and L858R point mutation [11, 12]. The LUX-Lung1 study published in 2010 has demonstrated efficacy with improvement in progression-free survival (3.3 months) for those who had taken afatinib 50 mg daily compared to those who had placebo, after previous treatment with gefitinib or erlotinib for at least 12 weeks and at least one line of platinum-based chemotherapy [13]. More recently, Khan et al. also revealed similar efficacy of afatinib in the same clinical setting in a Named Patient Use (NPU) program conducted in the United Kingdom [14]. To the best of our knowledge, there has been so far no randomized-controlled trials comparing the efficacy of afatinib with gefitinib/erlotinib (collectively grouped as first-generation EGFR-TKI in the latter text) in those who had prior failure to first-generation EGFR-TKI for their metastatic EGFR-mutated NSCLC. For the current analysis, we prospectively evaluated the efficacy and safety profiles of afatinib as 3rd or 4th line treatment after prior failure to systemic chemotherapy and first-generation EGFR-TKI under a Boehringer Ingelheim sponsored Compassionate Use Program (CUP), with comparison of our historical cohort who received erlotinib after previous failure to systemic chemotherapy and first-generation EGFR-TKI.
Methods
Study design
This study was approved by the ethics committee of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (Reference number UW 13–396). It was commenced in January 2013 with the last patient recruited in February 2014. All patients gave their written informed consent before recruitment into this study. We prospectively evaluated the use of afatinib as 3rd or 4th line treatment after progression to one line of first-generation EGFR-TKI therapy and one to two lines of systemic chemotherapy under this CUP. All patients had documented EGFR activating mutations before the start of afatinib. Determination of EGFR mutation analysis of all patients was described previously [15]. Formalin-fixed paraffin-embedded tumor biopsies before starting 1st TKI therapy were retrieved. Briefly, tumor enrichment was performed by micro-dissection under light microscopy. Genomic DNA was extracted using QIAmp DNA FFPE Tissue kit (Qiagen, Hilden, Germany), followed by polymerase chain reaction (PCR) amplification of EGFR exons 18 to 21 using intron-based primers and sequenced in both forward and reverse directions. The last date of data capture for statistical analysis was on 31st March 2015. The trial was registered with ClinicalTrials.gov (NCT02625168).
Study population
Patients who had EGFR-mutated metastatic NSCLC with prior documented objective response to first-generation TKI (gefitinib or erlotinib) for 6 months and prior treatment of at least 1 line of systemic chemotherapy were eligible to join the CUP offered by Boehringer-Ingelheim Pharma GmbH, Ingelheim, Germany. Patients who had received anti-vascular endothelial growth factor antagonist but not anti-EGFR monoclonal antibody in their previous courses of treatment, either alone or in combination with systemic chemotherapy were allowed to join this CUP. In addition, patients who had asymptomatic brain metastases who had not been on corticosteroids for the treatment of their brain metastases for at least 14 days prior to afatinib or erlotinib treatment were also eligible for this study. All recruited patients had baseline computed tomography scan of the brain, thorax and abdomen with at least 1 evaluable target lesion defined by Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1 and adequate serum hematological, hepatic and renal function as defined by LUX-Lung1 study [16].
Treatment
The treating physicians then decided the starting dose of afatinib of either 50 mg, 40 mg or 30 mg once daily continuously. After commencement of afatinib, they had regular clinical follow up every 2 weeks for 4 weeks then every 4 weeks until permanent discontinuation of afatinib or death. They also had regular imaging with computed tomography (CT) scan every 8–10 weeks for tumor response evaluation according to RECIST version 1.1 performed by two independent board certified radiologists blinded to study treatment [16]. Any discrepancies between the two radiologists on tumor response assessment were resolved by consensus. Treatment interruption was needed for those who developed grade ≥ 3 adverse event until it was returned to grade 1 or less. Then afatinib could be resumed but at a one lower dose level. Those who received afatinib 30 mg daily as the initial starting dose would discontinue afatinib permanently if they developed grade ≥3 events.
Assessment of efficacy and safety profiles
All treatment-related toxicities were collected and graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [17]. Objective response (OR) included complete response and partial response while disease control (DC) included complete response, partial response and stable disease according to RECIST 1.1. The primary study endpoint was PFS, defined as time from the date of start of afatinib to the date of objectively determined progressive disease or death from any cause). Secondary study endpoints were overall survival (OS, time from the date of start of afatinib to date of death from any cause), time to progression (TTP) started from the date of afatinib commencement to the date of objectively determined progressive disease and safety profiles. All these parameters of all patients in the afatinib group in this study were compared to a historical cohort of all patients who received erlotinib after prior failure to gefitinib and at least one line of systemic chemotherapy in our department from January 2009 to December 2011, with the same inclusion and exclusion criteria as for the patients who received afatinib in this study. All patients in this erlotinib historical cohort received erlotinib at 150 mg once daily, and they were assessed by the same imaging modalities for treatment response evaluation, as well the same departmental protocol for safety profiles and survival outcomes as for those who received afatinib in this study.
Statistical analysis
Mann–Whitney U tests were used for comparison of non-parametric variables and chi-square tests were performed for baseline and posttreatment discrete variables. Kaplan-Meier methods with log-rank tests were employed for comparison of each prespecified survival endpoints and Cox proportional hazard models were used for prognostic factors for PFS after afatinib or erlotinib in univariate and multivariate analyses, with afatinib versus erlotinib, age, sex, performance status, smoking status, histology, TTP for 1st TKI therapy, time interval between 1st TKI and afatinib or erlotinib, TTP for all lines of prior chemotherapy, time interval between last chemotherapy and afatinib or erlotinib as covariates. All statistical analyses were performed by Statistical Package for Social Sciences (SPSS) version 20 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
The patient characteristics were shown in Table 1. The median follow-up duration was 12.1 months (range 4.1–28.7 months) for the afatinib group and 12.2 months (range 0.4–48.7 months) for the erlotinib group. Twenty-five and 28 patients received afatinib and erlotinib respectively in this study after initial failure to first-generation TKI and chemotherapy. Six (24.0 %) and 13 (46.4 %) patients in the afatinib and erlotinib group respectively had asymptomatic brain metastases at baseline. They all had either gross tumor removal or radiation therapy for their brain metastases before study commencement. Four patients in the afatinib group had tumor re-biopsy before commencing afatinib and their recurrent tumors all harbored T790M mutation in addition to exon 19 deletion. Of them, one had a further L883V mutation on exon 21 and another patient had small cell transformation. More patients in the afatinib group had worse Eastern Cooperative Oncology Group (ECOG) performance status 2 compared to the erlotinib group (p = 0.008). Also the median duration of 1st TKI therapy was longer in the afatinib group (14.5 vs. 9.2 months, p = 0.02). Two, 21 and 2 patients received afatinib 50 mg, 40 mg and 30 mg daily respectively while all patients in the erlotinib group received erlotinib at 150 mg daily as the starting dose.
Table 1.
Patient characteristics
| Afatinib (n = 25) (%) | Erlotinib (n = 28) (%) | p-value | |
|---|---|---|---|
| Age (range) | 63 (42–85) | 59 (36–80) | 0.59 |
| Sex (male/female) | 11/14 | 10/18 | 0.54 |
| ECOG | |||
| 0 | 1 (4.0) | 0 (0.0) | 0.01 |
| 1 | 12 (48.0) | 24 (85.7) | |
| 2 | 12 (48.0) | 4 (14.3) | |
| 0/1 vs. 2 | 13 (52.0) vs. 12 (48.0) | 24 (85.7) vs. 4 (14.3) | 0.008 |
| Smoking status | 0.88 | ||
| Never smokers | 22 (88.0) | 25 (89.3) | |
| Current or past smokers | 3 (12.0) | 3 (10.7) | |
| Histology | 0.31 | ||
| Adenocarcinoma | 23 (92.0) | 28 (100.0) | |
| Squamous cell carcinoma | 1 (4.0) | 0 (0.0) | |
| Bronchoalveolar carcinoma | 1 (4.0) | 0 (0.0) | |
| Initial EGFR mutation status at diagnosis | 0.79 | ||
| exon 18 mutation | 0 | 1 | |
| exon 19 deletion | 11 | 13 | |
| exon 19 substitution mutation | 1 | 1 | |
| L858R | 8 | 10 | |
| L861Q | 0 | 2 | |
| double mutations | 1 | 1 | |
| EGFR mutation status with re-biopsy before afatinib or erlotinib | |||
| T790M alone | 4 | unknown | NA |
| Brain metastasis before afatinib or erlotinib | 6 (24.0) | 13 (46.4) | 0.09 |
| 1st TKI therapy | NA | ||
| Gefitinib | 14 (56.0) | 28 (100) | |
| Erlotinib | 11 (44.0) | 0 (0) | |
| Median duration of therapy (months, range) | 14.5 (3.52–40.64) | 9.2 (2.63–24.61) | 0.02 |
| Median Time to progression (months range) | 13.9 (0.66–40.15) | 9.1 (2.52–24.57) | 0.14 |
| Best response | 0.42 | ||
| CR | 1 (4.0) | 1 (3.6) | |
| PR | 23 (92.0) | 23 (82.1) | |
| SD | 0 (0.0) | 3 (10.7) | |
| PD | 1 (4.0) | 1 (3.6) | |
| Number of lines of prior chemotherapy before afatinib or erlotinib | 0.08 | ||
| 1 | 14 (56.0) | 22 (78.6) | |
| 2 | 11 (44.0) | 6 (21.4) | |
| First-line chemotherapy before afatinib or erlotinib | 25 (100) | 28 (100) | 0.88 |
| Pemetrexed + cisplatin | 3 (12.0) | 6 (21.4) | |
| Pemetrexed + carboplatin | 9 (36.0) | 7 (25.0) | |
| Paclitaxel + carboplatin | 4 (16.0) | 4 (14.3) | |
| Gemcitabine + carboplatin | 5 (20.0) | 5 (17.9) | |
| Carboplatin | 2 (8.0) | 2 (7.1) | |
| Pemetrexed | 2 (8.0) | 4 (14.3) | |
| Median duration of therapy (months, range) | 3.50 (0.69–17.97) | 2.96 (0.66–17.02) | 0.85 |
| Median time to progression (months, range) | 3.35 (0.69–17.97) | 3.48 (0.85–16.95) | 0.76 |
| Second-line chemotherapy before afatinib or erlotinib | 11 (44.0) | 6 (21.4) | 0.08 |
| Pemetrexed + carboplatin | 1 (4.0) | 0 (0.0) | |
| Paclitaxel + carboplatin | 2 (8.0) | 2 (7.1) | |
| Gemcitabine + carboplatin | 5 (20.0) | 4 (14.3) | |
| Docetaxel | 1 (4.0) | 0 (0.0) | |
| Vinorelbine | 1 (4.0) | 0 (0.0) | |
| Pemetrexed | 1 (4.0) | 0 (0.0) | |
| Median duration of therapy (months, range) | 2.30 (0.66–9.63) | 2.92 (0.69–4.34) | 0.91 |
| Median time to progression (months, range) | 3.09 (0.66–10.28) | 3.25 (0.72–4.44) | 0.74 |
| Median time interval between 1st TKI therapy and afatinib or erlotinib (months, range) | 8.38 (2.30–54.28) | 6.39 (2.56–20.07) | 0.15 |
| Median time interval between last chemotherapy and afatinib or erlotinib (months, range) | 2.79 (0.46–34.28) | 2.58 (0.23–17.05) | 0.49 |
Abbreviations: CR complete response, EGFR epidermal growth factor receptor, NA not applicable, PD progressive disease, PR partial response, SD stable disease, TKI tyrosine-kinase inhibitor
Treatment efficacy
ORR for afatinib was 20.0 % while that for erlotinib was (7.1 %, p = 0.17) (Table 2). DCR was higher with afatinib (68.0 %) than with erlotinib (39.3 %, p = 0.04). ORR of brain metastases was similar between the afatinib group (12.0 %) and the erlotinib group (14.3 %, p = 0.81). Time to progression and the duration of treatment of two TKI groups did not differ. Median PFS for the afatinib group was 4.1 months (95 % confidence interval [CI], 2.7–5.5 months) and 3.3 months (95 % CI, 2.2–4.4 months) for the erlotinib group (p = 0.97) (Fig. 1a). Median OS was also similar, 10.3 months (95 % CI, 7.5–13.0 months) for afatinib group and 10.8 months (95 % CI, 7.4–14.2 months) for erlotinib (p = 0.51) (Fig. 1b). More patients in the afatinib group received the respective TKI beyond radiological progression until symptomatic progression (39.1 % vs. 14.8 %, p = 0.05). 2 (8.0 %) patients in the afatinib group and 1 (5.6 %) patient in the erlotinib group were still receiving their respective TKI without disease progression at the time of publication.
Table 2.
Treatment outcomes in afatinib and erlotinib arm
| Afatinib (%) | Erlotinib (%) | p-value | |
|---|---|---|---|
| Best response | 0.09 | ||
| CR | 0 (0.0) | 0 (0.0) | |
| PR | 5 (20.0) | 2 (7.1) | |
| SD | 12 (48.0) | 9 (32.1) | |
| PD | 8 (32.0) | 17 (60.7) | |
| Objective response rate | 5 (20.0) | 2 (7.1) | 0.17 |
| Disease control rate | 17 (68.0) | 11 (39.3) | 0.04 |
| Objective response of brain metastases | 3 (12.0) | 4 (14.3) | 0.81 |
| Median duration of treatment (months, range) | 4.5 (0.2–22.7) | 3.3 (0.3–48.7) | 0.52 |
| Median time to progression (months, range) | 3.3 (0.2–12.6) | 3.3 (0.3–14.4) | 0.77 |
| Median PFS (95 % CI) (months) | 4.1 (2.7–5.5) | 3.3 (2.2–4.4) | 0.97 |
| Median OS (95 % CI) (months) | 10.3 (7.5–13.0) | 10.8 (7.4–14.2) | 0.51 |
Abbreviations: CI confidence interval, CR complete response, PD progressive disease, PFS progression-free survival, PR partial response, SD stable disease, TKI tyrosine-kinase inhibitor
Fig. 1.
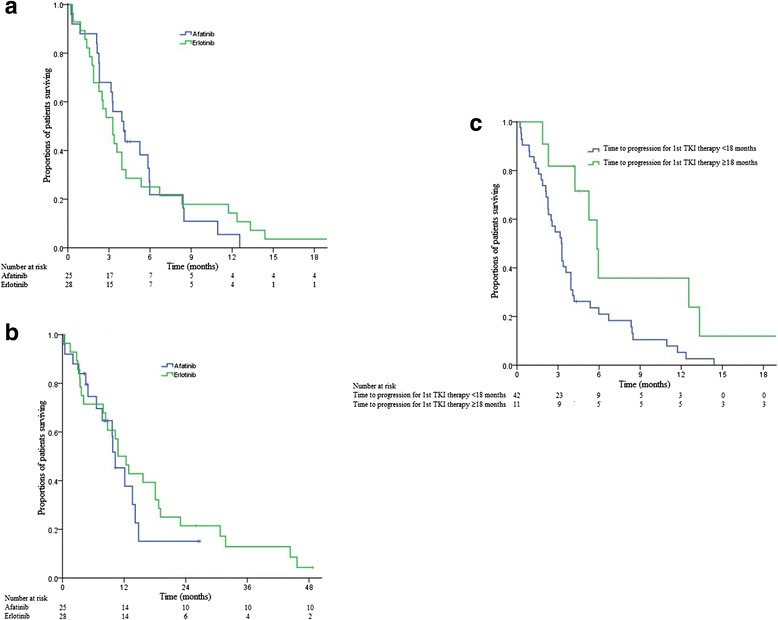
Kaplan-Meier plots illustrating survival outcomes in patients treated with afatinib or erlotinib as 2nd tyrosine-kinase inhibitor (TKI) therapy after previous failure to first-generation TKI and chemotherapy. a. Progression-free survival (PFS) in the afatinib and erlotinib group. b. Overall survival (OS) in the afatinib and erlotinib group. c. PFS comparing those whose time to progression to 1st TKI therapy was ≥18 months versus those whose time to progression to 1st TKI therapy was <18 months
In the afatinib group, median PFS was similar between those with exon 19 deletion (3.9 months [95 % CI, 2.2–5.7 months]) and L858R mutation (4.1 months [95 % CI, 1.5–6.7 months], p = 0.94). Insignificant difference in median PFS was also noted between patients with exon 19 deletion (3.6 months [93 % CI, 2.3–4.9 months]) and L858R mutation (2.5 months [95 % CI, 1.3–3.7 months], p = 0.31) in the erlotinib cohort. In addition, afatinib was not found to produce longer median PFS (4.2 months [95 % CI, 1.2–7.2 months]) than erlotinib in patients whose tumors exhibited exon 19 deletion (3.6 months [95 % CI, 2.2–4.9 months, p = 0.70). Similarly no statistical significance in median OS was noted between patients who received afatinib (14.2 months [95 % CI, 6.0–22.3 months]) and who received erlotinib (18.1 months [95 % CI, 9.7–26.4 months], p = 0.28) for their tumors which harbored exon 19 deletion. No PFS or OS advantage with afatinib was also noticed in those who had L858R mutation in their tumous compared to those who received erlotinib.
In particular, one of our study patients with previous gefitinib- and chemotherapy-responsive metastatic bronchoalveolar carcinoma which harbored exon 19 deletion had a dramatic and long-lasting response to afatinib for 12.6 months before further disease progression (Fig. 2). For the 4 patients with documented T790M mutation before starting afatinib, 1 had partial response (T790M and exon 19 deletion), 2 had stable disease (one with T790M, exon 19 deletion and small cell carcinoma and the other with T790M, exon 19 deletion and L833V mutation) and the remaining 1 patient (T790M, exon 19 deletion and L833V mutation) had his disease progressed with afatinib. Their TTP ranged from 2.3 to 6.0 months.
Fig. 2.
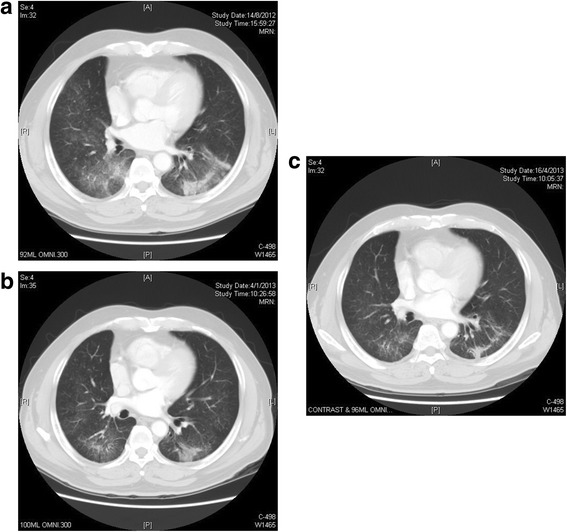
Computed tomography images of one of our study patients with metastatic bronchoalveolar carcinoma which harbored exon 19 deletion treated with afatinib as 2nd TKI therapy after failure to gefitinib and chemotherapy. a. Baseline images showing diffuse ground glass opacities representing tumor infiltrates in lower lobes of both lungs. b. CT images at 3 months after afatinib showing significant reduction of tumor infiltrates. c. CT images at 6 months after afatinib showing further response and tumor shrinkage to afatinib
Univariate and multivariate analysis of PFS and OS
Univariate analysis revealed that age ≤70 years (Hazard ratio [HR], 0.50; 95 % CI, 0.25–0.86, p = 0.008) and TTP to 1st TKI therapy for ≥18 months (HR, 0.38; 95 % CI, 0.18–0.83, p = 0.01) conferred a longer PFS for afatinib or erlotinib as 2nd TKI therapy (Table 3). They were also the only prognostic factors for PFS in multivariate analysis (HR, 0.48; 95 % CI, 0.21–0.74, p = 0.006 and HR, 0.39; 95 % CI, 0.16–0.80,; p = 0.008 respectively). The median PFS for afatinib or erlotinib in patients whose TTP to 1st TKI therapy ≥18 months was 5.8 months (95 % CI, 4.9–6.8 months) as compared to 3.3 months (95 % CI, 2.5–4.0 months) in patients whose TTP to 1st TKI therapy <18 months (Fig. 1c). No parameters were identified as significant prognostic factors for OS.
Table 3.
Univariate and multivariate analyses of prognostic markers for PFS
| Univariate analysis (p-value) | Multivariate analysis (p-value) | |
|---|---|---|
| Afatinib vs. erlotinib | 0.97 | ND |
| Age ≤70 years | 0.008 | 0.006 |
| Sex | 0.79 | ND |
| Smoking status | 0.25 | ND |
| Histology | 0.62 | ND |
| Performance status | 0.66 | ND |
| Time to progression for 1st TKI therapy | 0.09 | 0.06 |
| Time to progression ≥18 months for 1st TKI therapy | 0.01 | 0.008 |
| Time to progression for all lines of chemotherapy treatment before 2nd TKI therapy | 0.41 | ND |
| Time interval between end of 1st TKI therapy and start of afatinib or erlotinib | 0.40 | ND |
| Time interval between end of last chemotherapy treatment and start of afatinib or erlotinib | 0.88 | ND |
Note: Only covariates found significant in univariate analysis (p < 0.1) were considered in multivariate analysis
Abbreviations: ND not done, TKI tyrosine-kinase inhibitor
Post-discontinuation treatment
Seven (28.0 %) and 10 (35.7 %) patients in the afatinib and erlotinib group respectively received further systemic chemotherapy after cessation of their respective TKI therapy, without any statistical significance (p = 0.55). Similarly, 2 (8.0 %) and 2 (7.1 %) patients in the afatinib and erlotinib group respectively received another TKI therapy following discontinuation of their afatinib/erlotinib therapy (p = 0.91). All patients had only 1 line of post-discontinuation chemotherapy or TKI following cessation of afatinib/erlotinib, except that 2 patients (1 in afatinib group and 1 in erlotinib group) who received 2 lines of post-discontinuation chemotherapy. The number of lines of post-discontinuation chemotherapy and TKI did not differ between the two TKI groups (p = 0.53 and p = 0.91 respectively).
Toxicity profiles
Treatment-related toxicities differed for afatinib as compared to erlotinib group, as shown in Table 4. Acneiform rash (both all grades and grade ≥3 events) was more commonly seen with erlotinib than with afatinib. However diarrhea was the more frequent and dose-limiting complication in patients who received afatinib, leading to hypokalemia in 2 patients. Their diarrhea completely subsided after temporary afatinib suspension and the dose of afatinib was subsequently reduced from 40 mg daily to 30 mg daily. No recurrence of grade 3 diarrhea occurred following this dose reduction. In addition, more patients who received afatinib were found to have impaired liver function. However this was limited to grade 1 event only with no grade ≥2 events. Treatment interruption was similar between the afatinib and erlotinib group (28.0 % vs. 28.6 % respectively, p = 0.96). Dose reduction secondary to treatment-related complications did not differ between the two groups neither (24.0 % vs. 17.9 %, p = 0.58). No patients in either group discontinued afatinib or erlotinib respectively due to treatment-related toxicity.
Table 4.
Treatment-related toxicity profiles
| Afatinib | Erlotinib | p-value | ||||
|---|---|---|---|---|---|---|
| All grades (%) | ≥Grade 3 (%) | All grades (%) | ≥Grade 3 (%) | All grades | ≥Grade 3 | |
| Rash | 15 (60.0) | 0 (0.0) | 19 (67.9) | 5 (17.9) | 0.04 | 0.03 |
| Diarrhea | 15 (60.0) | 7 (28.0) | 3 (10.7) | 1 (3.6) | 0.002 | 0.01 |
| Mucositis | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.29 | NA |
| Paronychia | 2 (8.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.31 | NA |
| Impaired liver function | 6 (24.0) | 0 (0.0) | 1 (3.6) | 0 (0.0) | 0.02 | NA |
| Hypokalemia | 2 (8.0) | 2 (8.0) | 0 (0.0) | 0 (0.0) | 0.13 | 0.13 |
Abbreviation: NA not applicable
Discussion
Though first-generation EGFR-TKI with gefitinib or erlotinib has been the standard first-line treatment for metastatic EGFR-mutated NSCLC as demonstrated in various phase 3 randomized-controlled clinical trials [1–7], resistance against these first-generation TKI eventually develops after a median treatment duration of 9 to 13 months. It is believed to originate from the emergence of clones with the ability of generating genetic alterations which have survival advantages under the selective pressure of the current TKI treatment [18]. The most common mechanism of acquired resistance is the presence of T790M mutation on exon 20, accounting for about 50–60 % of known mutations of acquired TKI resistance [8–10]. When T790M mutation was introduced in vitro into sequences that contained exon 19 deletion and L858R mutation, the resultant proteins were found more resistant to gefitinib in the constructs which contained T790M [9]. Afatinib was found effective in reducing tumor size in transgenic mice with T790M-L858R mutation and other exon 20 insertion EGFR mutations [11]. Other mechanisms of acquired resistance to TKI include MET amplification, HER amplification, small cell transformation and rarely secondary mutations for instance BRAF mutation have been implicated [8, 19–24]. Rebiopsy of growing tumors after progression to 1st TKI therapy has caught rising attention recently and enabled us to comprehend the change in mutation patterns which may better predict the overall prognosis and guide subsequent therapy [10, 25]. In our study, 4 of our patients had documented posttreatment T790M mutation with or without extra mutations in addition to the pre-existing pretreatment EGFR mutations before commencement of afatinib. One had partial response, two had stable disease and the last patient had disease progression after afatinib. This echoed with previous findings that afatinib exhibited some antitumor activity against T790M mutation.
Strategies to treat EGFR-mutated NSCLC with acquired resistance to initial TKI therapy have been continuously evolving. Rechallenge with gefitinib or erlotinib in previously TKI-responsive NSCLC upon disease progression was able to slow down the pace of clinical deterioration and stabilization of enlargement of some lesions [26, 27]. More recently two Korean studies tested the clinical efficacy of erlotinib after initial failure to gefitinib and demonstrated the very modest and limited antitumor activity, unfortunately the median time to progression was around 2 months and more than 70 % of patients developed progressive disease [28, 29]. Another small study also echoed the short duration of treatment with the dismal median PFS of 2 months [30].
Afatinib has been studied in patients with prior failure to first-generation TKI. In the phase II/III LUX-Lung 1 study, significant improvement in median PFS from 1.1 to 3.3 months was revealed as compared to placebo despite a lack of improvement in OS [13]. It was found to be more potent against T790M compared to first-generation TKI. The treatment results of our study was also comparable with that in LUX-Lung1 study (Table 5). However its efficacy was limited by more potent inhibition against wild-type EGFR and subsequent toxicity which impairs the delivery of adequate dosing to the tumors [13]. In our study, diarrhea was the leading and dose-limiting complication which necessitated treatment interruption and dose reduction. However, acneiform rash was less common and severe with afatinib compared to erlotinib in our study, which might be a special feature in Chinese patients (Table 5). Another pan-HER inhibitor dacomitinib was also investigated in this setting after prior failure to first-generation TKI in the National Cancer Institute of Canada BR.26 trial but it failed to meet its primary survival endpoint, though the outcome in the EGFR mutant subgroup remains to be reported [31]. Third-generation TKI specially designed to block T790M including CO-1686 and AZD9291 have been evolving and tested currently in phase II/III trials [32, 33]. In 2015, the phase Ib/II studies on CO-1686 and AZD9291 demonstrated an extremely encouraging objective response rate of 29 and 21 % respectively in patients without T790M mutation and 59 and 61 % respectively in patients with T790M mutation [34, 35]. This has resulted in recent approval of AZD9291 for the treatment of patients who develop T790M mutation in their metastatic NSCLC by Food and Drug Administration (FDA) of the United States. More interestingly, they lacked the activity against wild-type EGFR leading to relatively fewer incidences of rash and diarrhea. Another approach for maximizing inhibition against acquired resistance is the combination of EGFR-TKI and anti-EGFR monoclonal antibody, leading to an ORR of 30 % and median PFS of 4.7 months revealed in a phase Ib/II trial [36, 37].
Table 5.
Comparison of baseline patient characteristics, treatment outcomes and selected toxicity profiles after afatinib as 2nd TKI therapy in LUX-Lung1 and current study
| LUX-Lung1 study | Current study | |||
|---|---|---|---|---|
| Number of patients | 390 | 25 | ||
| Age (range) | 58 (30–85) | 63 (42–85) | ||
| Male/female (%) | 159 (40.8)/231 (59.2) | 11 (44.0)/14 (56.0) | ||
| ECOG performance status (%) | ||||
| 0 | 92 (23.6) | 1 (4.0) | ||
| 1 | 268 (68.7) | 12 (48.0) | ||
| 2 | 30 (7.7) | 12 (48.0) | ||
| Prior EGFR-TKI therapy (%) | ||||
| Erlotinib | 215 (55.1) | 14 (56.0) | ||
| Gefitinib | 152 (39.0) | 11 (44.0) | ||
| Both | 23 (5.9) | 0 (0) | ||
| Number of lines of prior chemotherapy (%) | ||||
| 1 | 231 (59.2) | 14 (56) | ||
| 2 | 156 (40.0) | 11 (44) | ||
| 3 | 3 (0.8) | 0 (0) | ||
| Objective response (%) | ||||
| Partial response | 29 (7.4) | 5 (20.0) | ||
| Stable disease | 198 (50.8) | 12 (48.0) | ||
| Disease control (%) | 227 (58.2) | 17 (68.0) | ||
| Median progression-free survival in months (range) | 3.3 (2.8–4.4) | 4.1 (2.7–5.5) | ||
| Median overall survival in months (range) | 10.8 (10.0–12.0) | 10.3 (7.5–13.0) | ||
| Selected toxicity profiles (%) | All grades (%) | ≥Grade 3 (%) | All grades (%) | ≥Grade 3 (%) |
| Rash | 305 (78.2) | 56 (14.4) | 15 (60.0) | 0 (0) |
| Diarrhea | 339 (86.9) | 66 (16.9) | 15 (60.0) | 7 (28.0) |
| Mucositis/stomatitis | 237 (60.8) | 12 (3.1) | 1 (4.0) | 0 (0) |
| Paronychia/nail effect | 153 (39.2) | 20 (5.1) | 2 (8.0) | 0 (0) |
| Hypokalemia | 34 (8.7) | 11 (2.8) | 2 (8.0) | 2 (8.0) |
Abbreviations: ECOG Eastern Cooperative Oncology Group, EGFR epidermal growth factor receptor, TKI tyrosine-kinase inhibitor
Though there were no statistical significant differences in PFS and OS between afatinib and erlotinib, afatinib was found to have better disease control and borderline better objective response as compared to erlotinib. Of much interest, more patients had worse performance status (ECOG 2) and were treated with 2 previous lines of chemotherapy in the afatinib group as compared to those who received erlotinib. They inherently had very limited treatment options because of their borderline physical fitness and capabilities. In fact 20 (80 %) patients received afatinib as the last line of treatment before they succumbed to the disease and more patients received afatinib beyond disease progression as compared to those in the erlotinib group (p = 0.05). Nonetheless, they still enjoyed similar PFS and OS with afatinib as compared to those with better performance status who received erlotinib.
We found that age ≤70 years and longer TTP to 1st TKI therapy ≥18 months were prognostic factors of longer PFS to 2nd TKI therapy (irrespective of whether afatinib or erlotinib), in both univariate and multivariate analyses. Other factors especially the time interval between 1st TKI and afatinib or erlotinib were not prognostic. This might be contrary to one postulation that longer interval between 1st and 2nd TKI may promote re-growth of TKI-sensitive clones leading to continued response when TKI was rechallenged. However this postulation has been gradually superseded by the notion of tumor rebiopsy to delineate the latest mutational status before initiation of further targeted treatment. We did not perform tumor rebiopsy before commencement of afatinib or erlotinib in our study as this was not mandatory according to LUX-Lung1 study. This may be one of our study limitations. Tumor rebiopsy shall become a norm before commencement of 2nd EGFR-TKI therapy after failure to the first one especially when patients were advised to join the clinical trials using T790M-specific TKI [38]. The relatively small sample size was another limitation. In addition, comparison of afatinib with erlotinib was not performed in a randomized-controlled trial basis though data for the patients in the erlotinib cohort were prospectively collected. It is difficult to be carry out such randomized-controlled trial, however, having realized the very limited efficacy of erlotinib after prior failure to gefitinib shown in previous studies [26–30]. Notwithstanding, our study provided important clinical information on the efficacy and safety of afatinib as 2nd TKI therapy and its comparable anti-tumor activity but with a different toxicity profile compared to erlotinib in this setting.
Conclusion
Our study demonstrated the ability of afatinib to prolong disease progression with similar survival outcomes but different toxicities compared to those who received erlotinib, and a comparable efficacy at least as comparable as that shown in LUX-Lung1 study.
Acknowledgments
We thanked Boehringer-Ingelheim Pharma GmbH, Ingelheim & Co. KG, Germany to supply afatinib to our patients in this study.
Abbreviations
- EGFR
epidermal growth factor receptor
- TKI
tyrosine-kinase inhibitor: CUP, Compassionate use program
- NSCLC
non-small-cell lung cancer
- ECOG
Eastern Cooperative Oncology Group
- ORR
objective response rate
- DCR
disease control rate
- PFS
progression-free survival
- OS
overall survival
- TTP
time to progression
- NPU
named patient use
- PCR
polymerase chain reaction
- RECIST
Response Evaluation Criteria for Solid Tumors
- CT
computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- SPSS
Statistical Package for Social Sciences
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors fulfilled the authorship criteria with substantial contribution to the conception and study design. VHFL, DKCL, KOL, PML, TWL and DLWK recruited patients and collected the data. VHFL, TSC, DKLC and KOL performed statistical analysis of the data. All the authors drafted the manuscript, and all read and approved the final manuscript before submission.
Contributor Information
Victor H. F. Lee, Phone: 852-2255-4352, Email: vhflee@hku.hk
Dennis K. C. Leung, Email: leungkcd@ha.org.hk
Tim-Shing Choy, Email: ctsz01@ha.org.hk.
Ka-On Lam, Email: lamkaon@hku.hk.
Pui-Mei Lam, Email: lammei411@yahoo.com.hk.
To-Wai Leung, Email: leungtw@ha.org.hk.
Dora L. W. Kwong, Email: dlwkwong@hku.hk
References
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Eng J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small lung cancer harboring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomized phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small cell lung cancer (OPTIMAL, CTONG-0802): A multicenter, open-label, randomized, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicenter, open-label, randomized phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 6.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 7.Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: First-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–28. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 8.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 10.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616–22. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–11. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap TA, Vidal L, Adam J, Stephens P, Spicer J, Shaw H, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28:3965–72. doi: 10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- 13.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo in patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13:528–38. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 14.Khan F, Ottensmeier C, Popat S, Dua D, Dorey N, Ellis S, et al. Afatinib use in non-small cell lung cancer previously sensitive to epidermal growth factor receptor inhibitors: The United Kingdom Named Patient Programme. Eur J Cancer. 2014;50:1717–21. doi: 10.1016/j.ejca.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Tam IY, Chung LP, Suen WS, Wang E, Wong MC, Ho KK, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res. 2006;12:1647–53. doi: 10.1158/1078-0432.CCR-05-1981. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours; revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (assessed May 28, 2009)
- 18.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakowski MF, Ladanyi M, Kris MG. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med. 2006;355:213–5. doi: 10.1056/NEJMc053610. [DOI] [PubMed] [Google Scholar]
- 20.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 23.Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR mutant lung cancers that lack the second-site EGFR T790M mutation. Cancer Discov. 2012;2:922–33. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci. 2012;109:E2127–33. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hata AKN, Katakami N, Yoshioka H, Takeshita J, Tanaka K, Nanjo S, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to EGFR-TKI: Comparison between T790M mutation-positive and -negative populations. J Clin Oncol. 2012;30(15_Suppl);abstr 7528.
- 26.Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 27.Yokouchi H, Yamazaki K, Kinoshita I, Konishi J, Asahina H, Sukoh N, et al. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer. 2007;7:51. doi: 10.1186/1471-2407-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho BC, Im CK, Park MS, Kim SK, Chang J, Park JP, et al. Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol. 2007;25:2528–33. doi: 10.1200/JCO.2006.10.4166. [DOI] [PubMed] [Google Scholar]
- 29.Lee DH, Kim SW, Suh C, Yoon DH, Yi EJ, Lee JS. Phase II study of erlotinib as a salvage treatment for non-small-cell lung cancer patients after failure of gefitinib treatment. Ann Oncol. 2008;19:2039–42. doi: 10.1093/annonc/mdn423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa DB, Nguyen KS, Cho BC, Sequist LV, Jackman DM, Riely GJ, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008;14:7060–7. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfizer announces 2 top-line results from 2 phase III trials of dacomitinib in patients with refractory advanced non-small cell lung cancer [press release]. New York, NY: Pfizer Inc; January 27, 2014.
- 32.Soria J, Sequist LV, Gadgeel S, Goldman J, Wakelee H, Varga A, et al. First-in-human evaluation of CO-1686, an irreversible highly selective TKI of mutations of EGFR (activating and T790M). 15th World Conference on Lung Cancer; October 27–30 2013; Sydney, New South Wales, Australia.
- 33.Ranson M, Pao W, Kim D, Kim DW, Kim SW, Ohe Y, et al. AZD9291: an irreversible, potent and selective tyrosine kinase inhibitor of activating EGFR and resistance T790M mutations in advanced NSCLC. 15th World Conference on Lung Cancer; October 27–30 2013; Sydney, New South Wales, Australia.
- 34.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–9. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 35.Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–99. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 36.Janjigian YY, Groen HJ, Horn L, Smit EF, Fu Y, Wang F, et al. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib. J Clin Oncol. 2011;29 (suppl; abstr 7525).
- 37.Janjigian YY, Smit EF, Horn L, Groen HJ, Camidge R, Gettinger S, et al. Activity of afatinib/cetuximab in patients (pts) with EGFR mutant non-small cell lung cancer (NSCLC) and acquired resistance (AR) to EGFR inhibitors. Ann Oncol. 2012;23 (suppl 9;abstr 12270).
- 38.Yu HA, Arcila M, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR TKI therapy in 155 patients with EGFR mutant lung cancers. Clin Cancer Res. 2013;19:2240–47. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]


