Abstract
Background
Chicken as a delicious food for a long history, and it is well known that excess fat deposition in broiler chickens will not only induced metabolic diseases, but also lead to adverse effect in the consumer’s health. (−)-Hydroxycitric acid (HCA), a major active ingredient of Garcinia Cambogia extracts, had shown to suppress fat accumulation in animals and humans. While, the precise physiological mechanism of HCA has not yet been full clarified, especially its action in broiler chickens. Thus, this study aimed to assess the effect of (−)-HCA on lipid metabolism in broiler chickens.
Methods
A total of 120 1-day-old broiler chickens were randomly allocated to four groups, with each group was repeated three times with 10 birds. Birds received a commercial diet supplemented with (−)-HCA at 0, 1000, 2000 or 3000 mg/kg, respectively, for a period of 4 weeks ad libitum.
Results
Body weight (BW) in the 2000 and 3000 mg/kg (−)-HCA groups was significantly decreased (P < 0.05) than that in control group. A significantly decreased of serum triglyceride (TG) and density lipoprotein-cholesterol (LDL-C) content were observed in 3000 mg/kg (−)-HCA group (P < 0.05). Broiler chickens supplmented with 2000 and 3000 mg/kg (−)-HCA had pronouncedly higher hepatic lipase (HL) activity, hepatic glycogen and non-esterified fatty acid (NEFA) contents in liver (P < 0.05). Serum free triiodothyronine (FT3) and thyroxin (T4) contents were significantly higher in 3000 mg/kg (−)-HCA group (P < 0.05) compared with the control group. Supplemental (−)-HCA markedly decreased fatty acid synthase (FAS) and sterol regulatory element binding protein-1c (SREBP-1c) (P < 0.05) mRNA levels, while the mRNA abundance of adenosine 5′-monophosphate-activated protein kinaseβ2 (AMPKβ2) (P < 0.05) was significantly increased. In addition, ATP-citrate lyase (ACLY) mRNA level (P < 0.05) was significantly decreased in broiler chickens supplemented with 3000 mg/kg (−)-HCA. No differences was observed on carnitine palmitoyl transferase-I(CPT-I), while peroxisome proliferators-activated receptor α (PPARα) mRNA level (P < 0.05) was significantly increased in broiler chickens supplemented with 2000 and 3000 mg/kg (−)-HCA.
Conclusions
Supplemental (−)-HCA inhibited lipogenesis by inhibiting ACLY, SREBP-1c and FAS expression, and accelerated lipolysis through enhancing HL activity and PPARα expression, which eventually led to the reduced abdominal fat deposition in broiler chickens.
Graphical abstract.
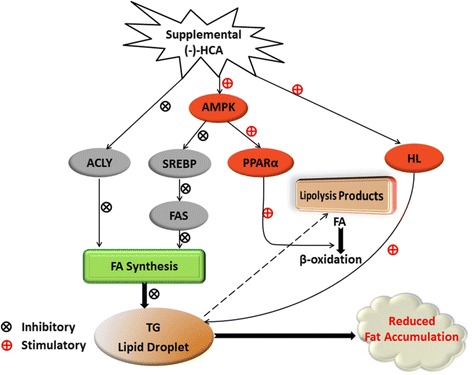
Mechanism of (−)-HCA effect on hepatic lipids metabolism.
Keywords: (−)-Hydroxycitric acid, Lipid metabolism, Broiler chickens
Background
In few decades, the principle aim of poultry production in many coutries has been to increase growth rate. Although intensive genetic selection in broilers has contributed to improve growth rates, modern bird strains often exhibit excessive body fat deposition which is not wanted by most of consumers [1], since coronary heart disease and arteriosclerosis are srongly related to the dietary intake of cholesterol. The production of brolier chickens with excess body fat also has proven to be one of the main problems encoutered within the broiler industry today, as evidenced by significant increased feed costs, decreased final product quality, and a significant economic loss to poultry processing plants [2]. The economic concern and the recognition of consumer aversion to excess fatty tissue deposition have led many poultry scientists to study the factors that are associated with fat deposition and the methods needed to reduce it [2].
(−)-Hydroxycitric acid [(−)-HCA] is the principal acid of fruit rinds of Garcinia cambogia [3]. (−)-HCA being a potent inhibitor of ATP-citrate lyase which catalyzes the extramitochondrial cleavage of citrate to oxaloacetate and acetyl-CoA, limits the availability of acetyl-CoA units required for fatty acid synthesis [4, 5]. Many studies demonstrate both in vitro and in vivo that (−)-HCA suppresses the de novo fatty acid synthesis [4, 6]. Animal studies have suggested that (−)-HCA causes weight loss [7, 8]. Moreover, it increases rates of hepatic glycogen synthesis, and decreases body weight gain [9–11]. Even though some studies have been conducted on the regulation of (−)-HCA on lipid metabolism in rats and mice, the underlying mechanisms of this physiological role of (−)-HCA are not fully understood. Importantly, no detailed information is available on the effect of (−)-HCA on lipid metabolism in broiler chickens.
Unliken mammalian species, the liver is the main site of de novo fatty acid synthesis in birds, accounting for 95 % of all lipid production in young chicks, with the adipose tissue is served only as a lipid storage site [12, 13]. In consequence, most of the endogenous body lipids are of hepatic origin and the development of adipose tissue depends on the availability of plasma triglycerides that are hydrolyzed prior to their utilization by adipocytes. Numerous studies have indicated that, among the different lipid metabolic pathways that can be altered with fat deposition, liver fatty acid metabolism is the most important [14, 15]. These results prompted researches on gene expression in the liver, especially those gene involved in fatty acid synthesis and secretion [16, 17], including peroxisome proliferators-activated receptor a (PPARα), AMP-activated protein kinase (AMPK), fatty acid synthase (FAS), sterol regulator element binding protein-1c (SREBP-1c), acetyl CoA carboxylase (ACC), carnitine palmitoyl transferase-I (CPT-I), and ATP-citrate lyase (ACLY). Despite the wealth of information available indicating that (−)-HCA alters lipid metabolism in mice, rats and humuans, little is known about the effect of (−)-HCA on lipid metabolism and hepatic gene expression in poultry, and especially in broiler chickens.
Chicken as a delicious food for a long history, and it is well known that too much abdominal fat deposition in broiler chickens will not only induced broiler ascites syndrome, sudden death and other metabolic diseases, but also lead to adverse effect in the consumer’s health. Despite the wealth of information available indicating that (−)-HCA alters lipid metabolism in mice, rats and humans, little is known about the effect of (−)-HCA on lipid metabolism and hepatic gene expression in poultry, and especially in broiler chickens. Therefore, the aim of present study was to explore the effect of (−)-HCA on lipid metabolism and hepatic lipid metabolism-related genes expression in broiler chickens which might help to identify the possible mechanism of (−)-HCA in decreasing fat deposition in adipose tissue.
Results
Growth performance
As shown in Table 1, no statistically significant differences among average daily feed intake (ADFI) and feed conversation effeciency were observed in brolier chickens, whereas body weight (BW) and average daily gain (ADG) in the 2000 mg/kg and 3000 mg/kg (−)-HCA groups were significantly lower than in the control group (P < 0.05).
Table 1.
Effect of (−)-HCA on growth performance of broiler chickens from 22 to 49d of agea
| Itemb | Supplemental (−)-HCA (mg/kg) | |||
|---|---|---|---|---|
| 0 | 1000 | 2000 | 3000 | |
| BW (g per birds) 49 d |
3460.23 ± 49.22 | 3360.03 ± 52.04 | 3337.23 ± 42.53* | 3312.83 ± 28.09* |
| ADG (g per birds per d) 22 to 49 d |
86.51 ± 1.36 | 83.51 ± 1.44 | 82.83 ± 1.16* | 82.10 ± 0.73* |
| ADFI (g per birds per d) 22 to 49 d |
177.95 ± 6.92 | 174.48 ± 7.28 | 164.22 ± 6.58 | 171.57 ± 7.19 |
| Feed conversion ratio (g :g) 22 to 49 d |
2.06 ± 0.08 | 2.09 ± 0.09 | 1.98 ± 0.08 | 2.09 ± 0.09 |
a BW = body weight; ADG = average daily gain; ADFI = average daily feed intake
b Data in each group represent mean values of three replicates, one replicate indicating the average of 25 chickens in one cage. Values are means ± SE. * P < 0.05, compared with the control group
Carcass composition
The effect of supplemental (−)-HCA on the relative weight of abdominal fat, liver, leg muscle and pectoral muscle in brolier chickens were shown in Table 2. Althought there were no statistically significant differences (P > 0.05), the aboslute and relative abdominal fat weight among all experimental groups tended to be lower than in the control group. No significant difference was observed in the relative weight of leg muscle and pectoral muscle (P > 0.05), a marked decreased in the aboslute and relative liver weight were noted in the 3000 mg/kg (−)-HCA group (P < 0.05).
Table 2.
Effect of (−)-HCA on carcass composition in broiler chickens
| Item | Supplemental (−)-HCA (mg/kg) | |||
|---|---|---|---|---|
| 0 | 1000 | 2000 | 3000 | |
| Absolute abdominal fat (g) | 103.39 ± 4.62 | 95.66 ± 6.60 | 94.55 ± 5.12 | 92.33 ± 4.41 |
| Relative abdominal fat (%) | 2.97 ± 0.12 | 2.80 ± 0.20 | 2.90 ± 0.15 | 2.90 ± 0.14 |
| Absolute liver weight (g) | 57.57 ± 3.03 | 55.52 ± 1.55 | 50.01 ± 1.2* | 48.94 ± 2.21* |
| Relative liver weight (%) | 1.65 ± 0.07 | 1.65 ± 0.04 | 1.57 ± 0.04 | 1.47 ± 0.04* |
| Relative pectoral weigh (%) | 13.88 ± 0.19 | 14.09 ± 0.24 | 13.93 ± 0.11 | 13.49 ± 0.18 |
| Relative leg weight (%) | 8.50 ± 0.19 | 8.39 ± 0.07 | 8.57 ± 0.10 | 8.63 ± 0.12 |
Values are means ± SE. Each (−)-HCA treated group represent 12 chickens at the age of 49 days, * P < 0.05, compared with the control group
Lipid metabolic parameters
The serum and hepatic TG contents were decreased, and a significant decrease was observed in the 3000 mg/kg (−)-HCA treatment group (P < 0.05) (Tables 3 and 4). Although no noticeable change was observed in the serum NEFA concentration (Table 3), while the NEFA concentration in liver was significantly increased (P < 0.05) in the 2000 mg/kg and 3000 mg/kg (−)-HCA groups than in the control group (Table 4). Serum LDL-C content was significantly lower in broiler chickens fed with 3000 mg/kg (−)-HCA than in the control group (P < 0.01), while no noticeable change was observed in the serum TC and HDL-C content following treatment with (−)-HCA compared to the control group (Table 3). Broiler chickens fed with 2000 mg/kg and 3000 mg/kg (−)-HCA resulted in a pronounced higher level of HL activity as compared to the control group (P < 0.01) (Table 4). Compared with the control group, the hepatic glycogen content also was significantly increased (P < 0.01) in broiler chickens fed 2000 mg/kg and 3000 mg/kg (−)-HCA (Table 4).
Table 3.
Effect of (−)-HCA on serum lipid parameters in broiler chickens
| Item | Supplemental (−)-HCA (mg/kg) | |||
|---|---|---|---|---|
| 0 | 1000 | 2000 | 3000 | |
| TC (mmol/L) | 3.66 ± 0.08 | 3.83 ± 0.22 | 3.42 ± 0.22 | 3.22 ± 0.14 |
| TG (mmol/L) | 0.56 ± 0.04 | 0.51 ± 0.04 | 0.48 ± 0.03 | 0.19 ± 0.01** |
| LDL-C (mmol/L) | 0.87 ± 0.13 | 0.8 ± 0.1 | 0.72 ± 0.15 | 0.46 ± 0.06* |
| HDL-C (mmol/L) | 1.72 ± 0.09 | 1.69 ± 0.07 | 1.68 ± 0.07 | 1.87 ± 0.10 |
| NEFA (μmol/L) | 1401.43 ± 110.94 | 1267.83 ± 77.74 | 1390.03 ± 141.97 | 1437.93 ± 56.08 |
Values are means ± SE. Each (−)-HCA treated group represent 12 chickens at the age of 49 days. * P < 0.05, ** P < 0.01 compared with the control group
Table 4.
Effect of (−)-HCA on liver homogenates concentration of triglycerides (TG), hepatic lipase (HL), non-esterified fatty acid (NEFA)
| Item | Supplemental (−)-HCA (mg/kg) | |||
|---|---|---|---|---|
| 0 | 1000 | 2000 | 3000 | |
| TG (mmol/g liver) | 3.31 ± 0.56 | 2.78 ± 0.40 | 2.75 ± 0.58 | 2.02 ± 0.34* |
| NEFA (μmol/L) | 65.83 ± 5.66 | 95.41 ± 9.52 | 109.90 ± 19.21* | 125.52 ± 12.37** |
| HL (U/mL) | 1.57 ± 0.11 | 1.59 ± 0.09 | 3.41 ± 0.27** | 2.68 ± 0.28** |
| Hepatic glycogen (mg/g liver) | 2.63 ± 0.23 | 3.63 ± 0.34 | 3.91 ± 0.41* | 3.91 ± 0.65* |
Values are means ± SE. Each (−)-HCA treated group represent 12 chickens at the age of 49 days. * P < 0.05, ** P < 0.01 compared with the control group
Serum metabolic hormones
As shown in Table 5, serum FT3 and T4 contents were significantly higher in the 3000 mg/kg (−)-HCA group than in the control group (P < 0.05). However, serum insulin and glucagon concentration were not affected by the addition of (−)-HCA (P > 0.05).
Table 5.
Effect of (−)-HCA on serum metabolic hormones in broiler chickens
| Item | Supplemental (−)-HCA (mg/kg) | |||
|---|---|---|---|---|
| 0 | 1000 | 2000 | 3000 | |
| Insulin (pg/mL) | 13.17 ± 0.87 | 12.85 ± 0.47 | 11.56 ± 1.03 | 13.05 ± 0.74 |
| Glucagon (pg/mL) | 496.56 ± 24.57 | 422.86 ± 21.10 | 484.43 ± 25.02 | 464.29 ± 47.38 |
| T3 (ng/mL) | 1.19 ± 0.05 | 1.21 ± 0.05 | 1.13 ± 0.06 | 1.37 ± 0.14 |
| T4 (ng/mL) | 42.82 ± 0.92 | 42.25 ± 1.58 | 42.54 ± 1.25 | 47.65 ± 1.53* |
| FT3 (fmol/ml) | 2.04 ± 0.15 | 1.98 ± 0.12 | 2.16 ± 0.21 | 3.33 ± 0.65* |
| FT4 (fmol/ml) | 6.41 ± 0.21 | 6.86 ± 0.27 | 6.31 ± 0.25 | 6.70 ± 0.26 |
Values are means ± SE. Each (−)-HCA treated group represent 12 chickens at the age of 49 days. * P < 0.05 compared with the control group
Hepatic lipid metabolic gene expression
Supplemental of (−)-HCA markedly decreased the FAS and SREBP-1c mRNA levels (P < 0.05) (Fig. 1b and d). The mRNA level of ACLY (P < 0.05) was significantly decreased in broiler chickens supplemented with 3000 mg/kg (−)-HCA (Fig. 1c), whereas the mRNA abundance of ACC (Fig. 1a) was unaffected by (−)-HCA (P > 0.05). Although no significant differences were observed in CPT-I (P > 0.05) gene expression (Fig. 2a), while the mRNA level of PPARα (P < 0.05) was significantly increased in broiler chickens supplemented with 2000 mg/kg and 3000 mg/kg (−)-HCA groups than that in the control group (Fig. 2b). Futhermore, no noticeable change was observed in the levels of AMPKα1, AMPKβ1 and AMPKγ1 gene expression (Fig. 3a, b and d), but the mRNA abundance of AMPKβ2 (P < 0.05) was significantly higher in broiler chickens supplemented with (−)-HCA groups than that in the control group (Fig. 3c).
Fig. 1.
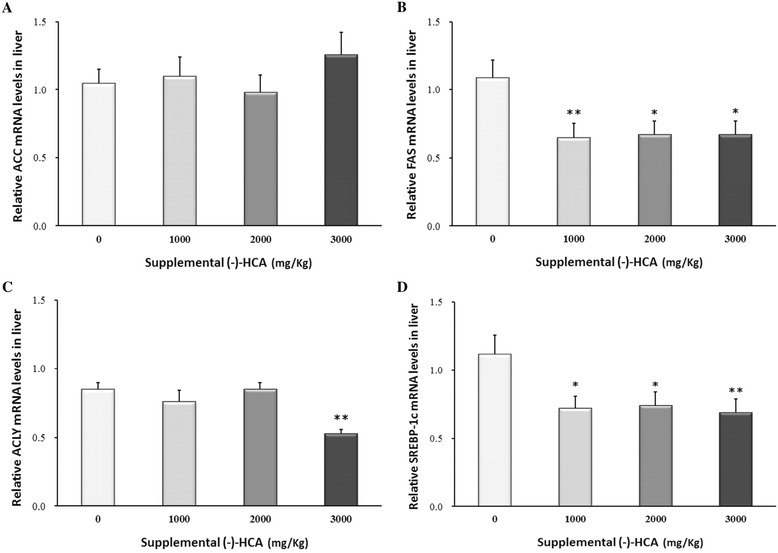
Effect of (−)-HCA on (a) ACC, (b) FAS, (c) ACLY and (d) SREBP-1c mRNA levels in the liver of broiler chickens. Values are means ± SE. (12 chickens per treatment are representative of four birds per replicate). * P < 0.05, ** P < 0.01 compared with the value of 0 mg of (−)-HCA/kg group
Fig. 2.
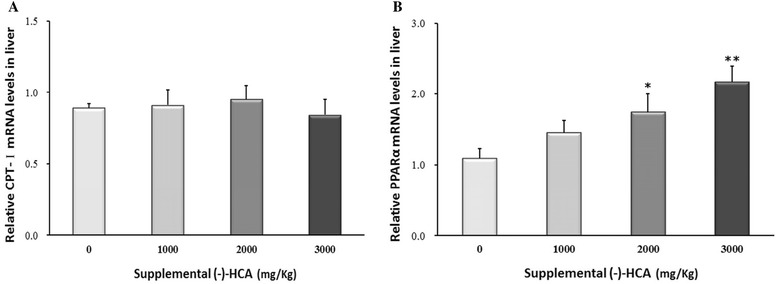
Effect of (−)-HCA on (a) CPT-I and (b) PPARα mRNA levels in the liver of broiler chickens. Values are means ± SE. (12 chickens per treatment are representative of four birds per replicate). * P < 0.05, ** P < 0.01 compared with the value of 0 mg of (−)-HCA/kg group
Fig. 3.
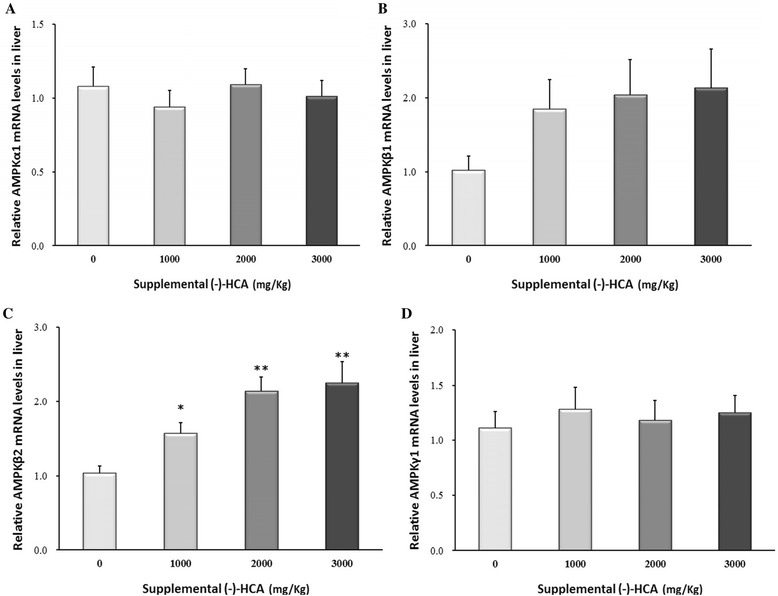
Effect of (−)-HCA on (a) AMPKα1, (b) AMPKβ1, (c) AMPKβ2 and (d) AMPKγ1 mRNA levels in the liver of broiler chickens. Values are means ± SE. (12 chickens per treatment are representative of four birds per replicate). * P < 0.05, ** P < 0.01 compared with the value of 0 mg of (−)-HCA/kg group
Discussion
Dyslipidemia is a main cardiovascular risk factor for coronary heart disease incidence and mortality [18]. Lipid metabolism disorders can accelerate the atherosclerosis process and its consequences, such as heart failure and coronary atherosclerosis [18]. Even though some drugs could be used to therapy these diseases, there would be some adverse effects at the time. It well known that “nutraceutical” products, i.e., a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease [18]. Nutraceuticals are effectively able to reduce the burden of the atherosclerosis process and coronary heart disease [19, 20]. Also, many studies have evaluated the potential role of nutraceuticals in the prevention of dyslipidaemia both in animal models [21, 22] and in humans [23, 24]. (−)-Hydroxycitric acid (HCA), an extract from the dried fruit rind of Garcinia cambogia, is widely used as an ingredient for nutritional supplements to reduce food intake, appetite and body weight. In these studies, (−)-HCA administration led to the loss of body weight and appetite reduction [25, 26], decreased energy intake [27] and increased fat oxidation [28]. Chicken as a delicious food for a long history, and it is well known that too much abdominal fat and subcutaneous fat deposition in broiler chickens will not only induced broiler ascites syndrome, sudden death and other metabolic diseases, but also lead to adverse effect in the consumer’s health. Despite the wealth of information available indicating that (−)-HCA alters lipid metabolism in mice, rats and humans, little is known about the effect of (−)-HCA on lipid metabolism and hepatic gene expression in poultry, and especially in broiler chickens.
In the present study, broiler chickens fed with (−)-HCA-containing diet for 28 days had reduced body weight and average daily gain as compared to a non-(−)-HCA control group. In addition, the absolute and relative abdominal fat weight among all experimental groups tended to be lower than in the control group, and a marked decreased in the absolute and relative liver weight were noted in the 3000 mg/kg (−)-HCA group. As a weight loss agent, many studies had report that (−)-HCA safely promotes weight loss in laboratory animals [8, 29] and humans [26, 30], and suggest food intake regulation may be a major mechanism for weight loss induced by (−)-HCA [8, 31]. However, no statistically significant differences among average daily feed intake were observed in brolier chickens supplemental with (−)-HCA. The results obtained here was similar to Sebola et al., who reports that Garcinia cambogia leaf supplementation has no effect on feed intake in the finisher stage of male Ross 308 broiler chickens [32]. This discrepancy may be due to the different kind of animal used in those studies. Present results indicated that the suppressive effect of (−)-HCA on body weight in broiler chickens is not via regulating the feed intake. Also, it is well known that the decrease in body weight gain is caused not only by a decrease in food intake but also an increase in energy expenditure (EE). Previous study shows that (−)-HCA could enhance energy expenditure in rats [33]. Although we did not measure energy expenditure, but no change in feed intake provides indirect evidence for that broiler chickens of these groups supplemental with (−)-HCA were not less efficient in using the food energy for the accretion of body mass, which suggests that energy expenditure was increased. Thyroid hormones, including T3 and T4, are recognized as key metabolic hormones, with T3 being the most functionally active form. The serum level of thyroid hormones is associated with energy production [34, 35]. In the present study, FT3 and T4 were elevated in the 3000 mg/kg (−)-HCA supplemented group. As observed previously [36], reduce of circulating thyroid hormone levels lowers the metabolic rate as a protective mechanism for the body’s energy reserves. Chen et al. also reported that broiler chickens receiving experimental diet with creatine pyruvate had greater energy expenditure, accompanying by an elevated the level of serum FT3 [37]. The suppressive effect of (−)-HCA on body weight regain is not only due to a reduction in food intake, presumably also increased thermogenesis [8]. Thus, we believed that different from mammals, the suppressive effect of (−)-HCA on body weight might be regulated not only by the feed intake, but also through increasing thyroid hormones level, which enhanced energy expenditure in broiler chickens.
Previous studies had presume that an increased fatty acid oxidation and decreased fat accumulation in liver of animal with (−)-HCA-treated contributed to decrease its weight [5, 38]. Thus, we presume that another mechanism of (−)-HCA reduced body weight could due to it inhibition the fat deposition in broiler chicken. Present study showed that the relative liver weight was significantly decreased in birds treated with (−)-HCA. This result was similar to the findings of Saito et al. who observed that Garnicia cambogia leaf supplementation reduced weight gain and liver weight of rats [39]. Kim et al. also reports that the relative liver weight was reduced in rats fed with a mixture composed of Garcinia cambogia extract, soypeptide, and L-carnitine [40]. Although previous studies [4, 5, 38] presume that fatty acid oxidation increase and fat accumulation decrease in liver of (−)-HCA-treated animal contributed to weight loss, while the physiological explanation is not clear and merits further investigation. It is reported that (−)-HCA inhibits ATP-citrate lyase and increases the cellular pool of citrate, which in turn inhibits glycolysis and thus redirects the carbon atoms for glycogen production [41]. Although serum insulin and glucagon concentration were not affected by the addition of (−)-HCA, our data showed that the hepatic glycogen content was significantly higher in the (−)-HCA groups than that of control group. In vitro and in vivo studies showed that (−)-HCA inhibits the de novo fatty acid synthesis and increases rates of glycogen synthesis [42–45]. (−)-HCA has a structure similar to citrate, which is generally known as an allosteric regulator for a number enzymes that are involved in carbohydrate and fat metabolism [46]. Therefore, administration of (−)-HCA is expected to cause metabolic ramification. Once glucose enters intracellular space across sarcolemma, it is rapidly phosphorylated to glucose 6-phosphate and converted into glycogen through the glycogen synthesis pathway and lactate through the glycolytic pathway. (−)-HCA has been found to inhibit phosphofructokinase, a key enzyme controlling glycolysis [47]. Thus, inhibition of the glycolysis pathway by (−)-HCA divert more glucose 6-phosphate into the glycogen synthesis pathway in liver. Taking account of those results, an alternative mechanism could be proposed that the (−)-HCA effect on increased rate of glycogen synthesis probably was due to its inhibitory effect on the glycolytic pathway.
Althought there were no statistically differences, the absolute and relative abdominal fat weight were tended to be lower in (−)-HCA fed broiler chickens. Similar resutls was repoted by Sebola et al., that daily supplemention with 300 mg of Garcinia cambogia leaf reduced fat pad weight by 18.75 % in male Ross 308 broiler chickens [32]. High doses of Garcinia cambogia extract are effective in suppressing fat accumulation in developing male Zucker obese rats [39]. It is well known that (−)-HCA supplementation reduces the amount of carbohydrates that are converted to body fat [44] and blocks the enzyme ATP-citrate lyase that acts as a key building block for fat synthesis. Thus, more carbohydrates will be stored as glycogen within the liver or muscle. Such transient changes in glycogen storage level do not appear to alter feed intake in broiler chickens. Therefore, it appears here that broiler chickens would likely benefit practically from (−)-HCA in terms of suppression of body fat accumulation and as such help to increase demand by consumers. Present study also found that serum TG and LDL-C content were depressed by addition of 3000 mg/kg (−)-HCA. The result was in agreement with the result of Preuss et al., who reports that after eight weeks groups treated with (−)-HCA showed significant decrease in low density lipoprotein and triglycerides levels than in the placebo group [25]. Haymamizu also reports that serum TG is lower in mice treated with 3.3 % Garcinia cambogia extract [48]. Recently, a double-blind randomized 12 week trial showed significant reductions in total cholesterol and low-density lipoprotein cholesterol in the Garcinia cambogia group, compared to the placebo group [49]. The liver is the primary site of fatty acid synthesis in poultry [50]. In the present study, supplementation of 2000 or 3000 mg/kg (−)-HCA in broiler chickens improved the liver NEFA level. In contract, the liver TG level declined in the treatment group. These results were expected, since (−)-HCA is known to inhibit ATP-citrate lyase which decreases the transformation of citrate into acetyl CoA, an essential building block for cholesterol and triglyceride biosynthesis [51]. Less acetyl CoA available for triglyceride synthesis in broiler chickens treated with (−)-HCA, may result in a decreased concentration of liver TG. It is reasonable to speculate that (−)-HCA directly stimulated the β-oxidation pathway in hepatocytes, catalyzed the conversion of TG to glycerol and fatty acid, and increased the hepatic uptake of the released NEFA [52, 53]. In addition to the above liver lipid metabolic parameters, HL is another important lipid metabolic enzyme produced primarily by the liver [54]. This study demonstrated a pronounced increase in HL activity after administration of 2000 or 3000 mg/kg (−)-HCA in broiler chickens, which result in decreased lipid synthesis [54, 55]. From the above finding, we speculated that (−)-HCA supplemental in diet not only inhibited triglyceride biosynthesis but also accelerated lipolysis, which in turn decreased the abdominal fat deposition in broiler chickens.
Many studies had certified that (−)-HCA could inhibit ATP-citrate lyase, an enzyme which plays a crucial role in energy metabolism during de novo lipogenesis [5, 26, 29, 30, 39]. In our present study, the expression of ATP-citrate lyase (ACLY) mRNA was lower in treatment group than that in control group, which indicated that (−)-HCA, similar to it action on rats and human, also regulated de novo lipogenesis in broiler chickens by inhibiting ATP-citrate lyase. Fat accumulation is complex processes that are characterized by many changes in expression of genes controlling lipogenesis and lipolysis. ATP-citrate lyase catalyzes the extramitochondrial cleavage of citrate to oxaloacetate and acetyl-CoA. Acetyl-CoA, which were catalyzed by acetyl CoA carboxylase (ACC) and fatty acid synthase(FAS), is used for the fatty acid synthesis. Although there was no observed difference in the level of ACC mRNA expression in our study, supplement of (−)-HCA markedly decreased levels of FAS. Also, we found that the level of SREBP-1c gene expression was lower than that in control group after administration of (−)-HCA. SREBP (sterol response element binding protein), the transcription factor of the leucine zipper family, have been described as regulators of biosynthesis of cholesterol and fatty acids in the liver [56]. SREBP-1c is preferentially involved in the activation of genes that control the synthesis of fatty acid [57]. As one potential regulators, SREBP-1c can directly stimulate the transcription of genes encoding FAS enzymes [58, 59]. Another interesting observation in this study was that the expression of AMPKβ2 was increased in the (−)-HCA treated groups. AMP-activated protein kinase (AMPK) has been implicated as a key regulator of physiological energy dynamics, including glucose transport and lipolysis [60], AMPKβ2 is a regulatory subunit of the AMPK [61]. The inhibition of FAS expression by AMPK activation was previously reported in primary cultured hepatocytes [62, 63]. Also, previous studies shown an inverse correlation between AMPK and SREBP-1c activities in hepatocytes and in livers of re-fed mice and ethanol-fed mice [64, 65]. Recently study shows that Ser372 phosphorylation of SREBP-1c by AMPK may contribute to the ability of polyphenols and metformin to inhibit proteolytic cleavage and nuclear translocation of SREBP-1c in hepatocytes under high glucose plus insulin conditions, thereby preventing transcription of target lipogenic genes [66]. Therefore, the significance of the expression changes of AMPKβ2, SREBP-1c, ACLY, and FAC genes indicates that these genes might be directly responsible for the effect of (−)-HCA on fatty acid synthesis and lipogenesis in broiler chickens. However, elucidation of the precise mechanism involved in the modification of these genes by (−)-HCA needs further investigation.
It is hypothesized that (−)-HCA could increase fat oxidation via inhibiting malonyl CoA formation, which in turn, would activate carnitine palmitoyl transferase-I (CPT- I) activity [67, 68]. However, no significant differences were observed in the level of CPT-Igene expression in broiler chickens supplemented with (−)-HCA. Treatment of human adipocytes with HCA-SX resulted in a significant down-regulation of 348 and induction of 366 fat- and obesity-related genes such as hormone sensitive lipase, peroxisome proliferator-activated receptors gamma (PPARγ), coactivator 1α, Leptin, and hypoxia-inducible factor-1 genes [69]. As shown in Fig. 2b, (−)-HCA treatment caused a significant up-regulation of peroxisome proliferators activated receptor α (PPARα) expression in broilers liver tissue, which was similar to a study in 3T3-L1 cells, where Hibiscus extract (HCA content in Hibiscus extract is high) significantly attenuated the expression of PPARα was observed [70]. PPARa is a member of the nuclear hormone receptor family of transcription factors. It is expressed in the liver, heart, skeletal muscle and brown adipose tissue where it regulates the expression of genes that control mitochondrial and peroxisomal fatty acid oxidation [71]. Rocchi and Auwerx has demonstrated that PPARα can increase the rate of fatty acid β-oxidation by increasing the expression of several PPARα target genes [72]. Considering the decreased TG content in serum and liver after (−)-HCA treatment, it is possible that (−)-HCA improved the expression of PPARα in liver and induced transcriptional up-regulation of enzymes involved in the β-oxidation of fatty acids, thus decreasing the TG content.
Conclusions
Present results showed that supplemental with (−)-HCA significantly decreased the body weight, TG content and increased hepatic glycogen content. The SREBP-1c, ACLY and FAS mRNA levels were significantly decreased, while AMPKβ2 and PPARα mRNA levels were significantly elevated in broiler chickens supplemental with (−)-HCA. These results indicated that supplemental (−)-HCA inhibited lipogenesis by inhibiting ACLY, SREBP-1c and FAS expression, and accelerated lipolysis through enhancing HL activity and PPARα expression, which eventually led to reduced abdominal fat deposition in broiler chickens (Fig. 4). The variance of gene expression which involved in lipid metabolism might associate with the mechanism of how (−)-HCA reduced the accumulation of fat in animal.
Fig. 4.
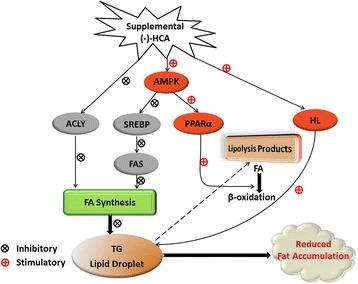
Mechanism of (−)-HCA effect on hepatic lipids metabolism. Supplemental (−)-HCA inhibited lipogenesis by inhibiting ACLY, SREBP-1c and FAS expression, and accelerated lipolysis through enhancing HL activity and PPARα expression, which eventually led to reduced abdominal fat deposition in broiler chickens. The variance of gene expression which involved in lipid metabolism might associate with the mechanism of how (−)-HCA reduced the accumulation of fat in broiler chickens
Methods
Extract of Garcinia cambogia
The Garcinia cambogia extract containing (−)-HCA was obtained from An Yun Co.Ltd (Zhengzhou, China). The carcinia cambogia contain 56-58 % (−)-hydroxycitric acid including its free and lactone form, and it also contains 12-14 % cellulose, 5.5-6 % α-D-melibiose, 2.5-3 % β-D-lactin, 1.5-2 % D-mannopyranose, 11-12 % oxophenic acid, 2-3 % octadecyl alcohol, 3.5-4 % Coenzyme A and 1.5-2 % inorganic elements. The experimental diets contained Garcinia combogia at 0, 1.7, 3.4 and 5.1 g/kg diet. These were equivalent of 0, 1000, 2000, 3000 mg/kg (−)-HCA diet.
Animal and treatment
A total of 120 1-day-old male broiler chickens (Ross 308), obtained from Jiangsu Wuxi chicken breeding company (Wuxi, China), were weighted and allocated to four treatment groups, each of which included three replicates of 10 birds. Broiler chickens were fed on the same basal diets from 1 to 49 d, including starter (1 to 21 d) and finisher (22 to 49 d) rations that were formulated according to NRC (1994) recommendations (Table 6) [73]. At the finisher phase, four groups were supplemented with 0 mg/kg, 1000 mg/kg, 2000 mg/kg or 3000 mg/kg diets (−)-HCA. The animal care and use protocol was approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University, China.
Table 6.
Composition and nutrient levels of the experimental diets
| Item | Starter | Finisher |
|---|---|---|
| Ingredient (%) | ||
| Corn | 52.6 | 57..4 |
| Wheat bran | 2.0 | 4.0 |
| Soybean meal | 31.1 | 27.0 |
| Fish meal | 6.0 | 3.0 |
| Calcium phosphate | 1.0 | 1.5 |
| Limestone | 1.2 | 1.2 |
| DL-Methionine | 0.3 | 0.1 |
| Vitamin-mineral premixa | 0.5 | 0.5 |
| Rapeseed oil | 5.0 | 5.0 |
| NaCl | 0.3 | 0.3 |
| Nutrient composition Calculated | ||
| MEb (kcal/kg) | 3.10 | 3.14 |
| CP (%) | 22.52 | 19.74 |
| Lys (%) | 1.19 | 1.08 |
| Met + cystine (%) | 0.93 | 0.71 |
| Ca (%) | 1.00 | 0.9 |
| Total P (%) | 0.80 | 0.76 |
| Available P (%) | 0.47 | 0.39 |
Nutrient level of the diets was based on National Research Council recommendations
aVitamin–mineral premix supplied the following per kilogram of diet: vitamin A, 1500 IU; vitamin D3, 200 IU; vitamin E, 10 mg; vitamin K3, 0.5 mg; thiamine, 1.8 mg; riboflavin, 3.6 mg; D-pantothenic acid, 10 mg; folic acid, 0.55 mg; pyridoxine, 3.5 mg; niacin, 35 mg; cobalamin, 0.01 mg; biotin, 0.15 mg; Fe, 80 mg; Cu, 8 mg; Mn, 60 mg; Zn, 40 mg; I, 0.35 mg; and Se, 0.15 mg
bME = metabolizable energy
Starter-phase broiler chickens were housed in lighted coops, water was provided at all times, and continuous lighting was maintained. The temperature was maintained at 32 °C for the initial 5 d and then gradually reduced according to normal management practices, until a temperature of 22 °C was achieved. Broilers were reared on the ground under natural lighting during the finisher phase. Chickens were weighed at 1, 21 and 49 d of age to determine average daily gain (ADG). Daily feed consumption per replicate was recorded, the uneaten feed was discarded, and fresh feed was replenished. Average daily feed intake (ADFI) and the feed conversion ratio were then determined. At the end of the experiment, birds were randomly selected, deprived of feed for 12 h, weighed and killed. The abdominal fat pad, liver, pectoral and leg muscles were subsequently removed and weighed.
Measurement of serum lipid parameters
Blood samples were allowed to clot at 4 °C and centrifuged at 1,500 × g for 10min before collecting the serum. Serum samples were stored at −20 °C. Concentrations of serum cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and non-esterified fatty acid (NEFA) content were measured by colorimetric enzymatic methods using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Triiodothyronine (T3), thyroxine (T4), insulin (INS), free triiodothyronine (FT3), free thyroxine (FT4) and glucagon (GLU) were measured with the RIA kits provided by Beijing North Institute of Biotechnology (Beijing, China).
Determination of hepatic lipid parameters assay
Total liver lipid content was determined on homogenized liver samples using a mixture of chloroform and methanol (2:1 v/v) according to the method of Folch [74]. The levels of hepatic TG and total TC content were determined using commercial kits (GPO-PAP and CHOD-PAP) purchased from the Nanjing Jiancheng Bioengineering Institute. The TG level in homogenates of liver was evaluated following the manufacturer’s protocols. Hepatic lipase activity and NEFA content was determined by using commercial kits from Nanjing Jianchen Biotechnology Institution. Approximately 100 mg of liver was homogenized on ice with 1 mL of physiological saline. An aliquot of these homogenates was used for protein determination by using commercial kits from Nanjing Biyuntian Biotechnology Institution and the remainder was centrifuged at 1,000 × g for 10 min at 4 °C. Clear supernatants were used for the assay. HL activity and NEFA concentrations were directly proportional to the colour intensity and were determined by measurement of absorbance at 550 and 440 nm, respectively. One unit of HL enzyme activity represents 1 mg tissue protein releasing 1 μmol of fatty acid per hour. Hepatic glycogen content was determined by using the commercial kit from Nanjing Jiancheng Biotechnology Institution.
Real-time quantitative RT-PCR
Total RNA was extracted from liver using TRIZAOL reagent (Shengxing, China), according to the manufacturer’s prtocol and quantified by measuring the optical density in a photometer (Eppendorf Biophotometer) at 260 nm. The absorption ratios (260/280 nm) for all preparations were between 1.8 and 2.0. RNA sample aliquots were subjected to electrophoresis through a 1.4 % agarose formaldehyde gel to verify their integrity. The RNA concentrations were adjusted to 1 μg/μL by measuring the optical density (OD) value, and the samples were then stored at −80 °C.
Reverse transcription was performed using the method of Zhao S [75]. An aliquot of complementary DNA sample was mixed with 20μl SYBR_Green PCR Master Mix (Roche, Switzerland) in the presence of 10 pmol of each forward and reverse primers for ACC, FAS, PPAR-α, CPT-I, SREBP-1c, ACLY, AMPKα1,AMPKβ1, AMPKβ2,AMPKγ1, and then it was subjected to PCR under standard conditions (40 cycles). As an internal control, the same RT products were also subjected to chicken β-actin RNA. All samples were analyzed in duplicate using the IQ5 Sequence Detection System and programmed to conduct one cycle (95 °C for 1min) and 40 cycles (95 °C for 20 s, 60 °C for 30 s and 72 °C for 30 s). Result (fold change) was calculated using the 2-ΔΔCt method. 2-ΔΔCt = (Ct ij – Ct β-actin j) – (Ct i1 – Ct β-actin1), where Ct ij and Ct β-actin j are the Ct for gene i and for β-actin in a pool or a sample (named j) and where Ct i1 and Ct β-actin1 are the Ct in pool 1 or sample 1, expressed as the standard. The primers were designed by premier 5.0 software (Premier Biosoft International, Palo Alto, CA, USA) and the positions are referred to chicken for SREBP-1c, ACC, PPARα, CPT-I, AMPK, FAS, ACLY and β-actins (Table 7). The primers synthesized by Invitrogen Biological Company (Shanghai, China) were designed to flank known or putative introns, preventing amplification of any contaminating genomic DNA.
Table 7.
Prime sequence of targeted genes and β-actin
| Gene | GenBank acession number |
Primer sequences (5′–3′) | Orientation | Product size (bp) |
|---|---|---|---|---|
| β-actin | L08165 | TGCGTGACATCAAGGAGAAG TGCCAGGGTACATTGTGGTA |
Forward Reverse |
300 |
| ACC | J03541 | GTTGTGGTTGGCAGAGCAAG GCACCAAACTTGAGCACCTG |
Forward Reverse |
284 |
| CPT-I | AY675193 | GGGTTGCCCTTATCGTCACA TACAACATGGGCTTCCGTCC |
Forward Reverse |
151 |
| FAS | NM205155 | TGAAGGACCTTATCGCATTGC GCATGGGAAGCATTTTGTTGT |
Forward Reverse |
96 |
| SREBP-1c | AY029224 | GTCGGCGATCCTGAGGAA CTCTTCTGCACGGCCATCTT |
Forward Reverse |
105 |
| ACLY | AJ851548 | CACCCAGAGGTGGATGTTCT GTTGCAGGCCCAATGTTAGT |
Forward Reverse |
188 |
| PPARα | AF470455 | CAAACCAACCATCCTGACGAT GGAGGTCAGCCATTTTTTGGA |
Forward reverse |
64 |
| AMPKα1 | DQ302133 | CAAGTAGTGTCTCGCACGGT GACTGATAGCTGGTCCCACG |
Forward Reverse |
133 |
| AMPKβ1 | DQ398947 | CACCAAGGATGGGGACAGAC TTCCAGATCCTGCTGCCAAG |
Forward Reverse |
127 |
| AMPKβ2 | DQ640761 | ACACTGCCTTCTTCTCCCTC GTGGGAGCTGAAAACACTGG |
Forward Reverse |
195 |
| AMPKγ1 | DQ133598 | AGGACTCCTTCAAGCCGTTG AACTCAGGCTTTGGGACCTC |
Forward Reverse |
196 |
Statistics analysis
The experimental data were expressed as the mean ± SE, and differences were considered significant when P < 0.05, as test by one-way analysis of variance (ANOVA) followed by a multiple range test, using the Statistical Packages for Social Science 16.0 and Excel 2003 in Microsoft. Replicate was considered as the experimental unit for the entire index determined.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NO. 31572483) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Abbreviations
- (−)-HCA
(−)-hydroxycitric acid
- ACC
acetyl CoA carboxylase
- ACLY
ATP-citrate lyase
- ADFI
average daily feed intake
- ADG
average daily gain
- AMPK
5′-monophosphate-activated protein kinase
- BW
body weight
- CPT- I
carnitine palmitoyl transferase- I
- FAS
fatty acid synthase
- HDL-C
density lipoprotein-cholesterol
- HL
hepatic lipase
- LDL-C
low-density lipoprotein cholesterol
- NEFA
non-esterified fatty acid
- PPARα
peroxisome proliferators-activated receptor α
- SREBP-1c
sterol regulatory element binding protein-1c
- T3
triiodothyronine
- T4
thyroxin
- TC
cholesterol
- TG
triglyceride
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HM conceived and designed the experiments. JH and LL performed the experiments. DW analyzed the data. HM and DW wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Mallard J, Douaire M. Strategies of selection for leanness in meat production. In: Leclercq B, Whitehead CC, editors. Leanness in Domestic Birds; genetic, metabolic, and hormonal aspects. Butterworths & Co Pub Ltd, INRA; 1988. p. 3–23.
- 2.Wu G, Deng X, Li J, Li N, Yang N. A potential molecular marker for selection against abdominal fatness in chickens. Poult Sci. 2006;85:1896–9. doi: 10.1093/ps/85.11.1896. [DOI] [PubMed] [Google Scholar]
- 3.Lewis Y, Neelakantan S. (−)-Hydroxycitric acid—the principal acid in the fruits of Garcinia cambogia desr. Phytochemistry. 1965;4:619–25. doi: 10.1016/S0031-9422(00)86224-X. [DOI] [Google Scholar]
- 4.Sullivan AC, Hamilton JG, Miller ON, Wheatley VR. Inhibition of lipogenesis in rat liver by (−)-hydroxycitrate. Arch Biochem Biophys. 1972;150:183–90. doi: 10.1016/0003-9861(72)90025-2. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan AC, Triscari J, Hamilton JG, Miller ON, Wheatley VR. Effect of (−)-hydroxycitrate upon the accumulation of lipid in the rat: I. Lipogenesis. Lipids. 1974;9:121–8. doi: 10.1007/BF02532136. [DOI] [PubMed] [Google Scholar]
- 6.Watson JA, Lowenstein JM. Citrate and the conversion of carbohydrate into fat fatty acid synthesis by a combination of cytoplasm and mitochondria. J Biol Chem. 1970;245:5993–6002. [PubMed] [Google Scholar]
- 7.Downs BW, Bagchi M, Subbaraju GV, Shara MA, Preuss HG, Bagchi D. Bioefficacy of a novel calcium–potassium salt of (−)-hydroxycitric acid. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2005;579:149–62. doi: 10.1016/j.mrfmmm.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Leonhardt M, Hrupka B, Langhans W. Effect of hydroxycitrate on food intake and body weight regain after a period of restrictive feeding in male rats. Physiol Behav. 2001;74:191–6. doi: 10.1016/S0031-9384(01)00547-9. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan C, Triscari J. Metabolic regulation as a control for lipid disorders. I. Influence of (−)-hydroxycitrate on experimentally induced obesity in the rodent. The American journal of clinical nutrition. 1977;30:767–76. doi: 10.1093/ajcn/30.5.767. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan AC, Triscari J, Spiegel J. Metabolic regulation as a control for lipid disorders. II. Influence of (−)-hydroxycitrate on genetically and experimentally induced hypertriglyceridemia in the rat. The American journal of clinical nutrition. 1977;30:777–84. doi: 10.1093/ajcn/30.5.777. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood M, Cleary M, Gruen R, Blase D, Stern J, Triscari J, Sullivan A. Effect of (−)-hydroxycitrate on development of obesity in the Zucker obese rat. American Journal of Physiology-Endocrinology And Metabolism. 1981;240:E72–8. [DOI] [PubMed]
- 12.Leveille GA. In vitro hepatic lipogenesis in the hen and chick. Comp Biochem Physiol. 1969;28:431–5. doi: 10.1016/0010-406X(69)91357-7. [DOI] [PubMed] [Google Scholar]
- 13.Griffin HD, Guo K, Windsor D, Butterwith SC. Adipose tissue lipogenesis and fat deposition in leaner broiler chickens. The Journal of nutrition. 1992;122:363. doi: 10.1093/jn/122.2.363. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq B, Hermier D, Guy G. Metabolism of very low density lipoproteins in genetically lean or fat lines of chicken. Reprod Nutr Dev. 1990;30:701–15. doi: 10.1051/rnd:19900607. [DOI] [PubMed] [Google Scholar]
- 15.Legrand P, Hermier D. Hepatic delta 9 desaturation and plasma VLDL level in genetically lean and fat chickens. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1992;16:289–94. [PubMed] [Google Scholar]
- 16.Douaire M, Le Fur N, el Khadir-Mounier C, Langlois P, Flamant F, Mallard J. Identifying genes involved in the variability of genetic fatness in the growing chicken. Poult Sci. 1992;71:1911–20. doi: 10.3382/ps.0711911. [DOI] [PubMed] [Google Scholar]
- 17.Daval S, Lagarrigue S, Douaire M. Messenger RN levels and transcription rates of hepatic lipogenesis genes in genetically lean and fat chickens. Genet Sel Evol. 2000;32:521–32. doi: 10.1186/1297-9686-32-5-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, Novo S, Palmiero P, Saba PS, Pedrinelli R, Ciccone MM . Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods. 2014;6:11–32.
- 19.He B. The role of omega-3 polyunsaturated fatty acids supplementation in childhood: a review. Recent Patents on Cardiovascular Drug Discovery. 2013;8:42–55. doi: 10.2174/1574890111308010006. [DOI] [PubMed] [Google Scholar]
- 20.Raatz SK, Silverstein JT, Lisa J, Picklo MJ. Issues of Fish Consumption for Cardiovascular Disease Risk Reduction. Nutrients. 2013;5:1081–97. doi: 10.3390/nu5041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alshatwi AA, Obaaid MAA, Sedairy SAA, Ramesh E, Lei KY. Black and green tea improves lipid profile and lipid peroxidation parameters in Wistar rats fed a high-cholesterol diet. Journal of Physiology & Biochemistry. 2011;67:95–104. doi: 10.1007/s13105-010-0053-3. [DOI] [PubMed] [Google Scholar]
- 22.Hsu WH, Huang YC, Lee BH, Hsu YW, Pan TM. The improvements of ankaflavin isolated from Monascus -fermented products on dyslipidemia in high-fat diet-induced hasmster. J Funct Foods. 2013;5:434–43. doi: 10.1016/j.jff.2012.11.016. [DOI] [Google Scholar]
- 23.Casas-Agustench P, Serra M, Pérez-Heras A, Cofán M, Pintó X, Trautwein EA, Ros E. Effects of plant sterol esters in skimmed milk and vegetable-fat-enriched milk on serum lipids and non-cholesterol sterols in hypercholesterolaemic subjects: a randomised, placebo-controlled, crossover study. Br J Nutr. 2012;107:1766–75. [DOI] [PubMed]
- 24.Zhao HL, Houweling AH, Vanstone CA, Jew S, Trautwein EA, Duchateau GS, Jones PJ. Action of plant sterol intervention on sterol kinetics in hypercholesterolemic men with high versus low basal circulatory plant sterol concentrations. J Am Coll Nutr. 2011;30:155–65. doi: 10.1080/07315724.2011.10719955. [DOI] [PubMed] [Google Scholar]
- 25.Preuss H, Bagchi D, Bagchi M, Rao CS, Dey D, Satyanarayana S. Effects of a natural extract of (−)-hydroxycitric acid (HCA‐SX) and a combination of HCA‐SX plus niacin‐bound chromium and Gymnema sylvestre extract on weight loss. Diabetes, Obesity and Metabolism. 2004;6:171–80. doi: 10.1111/j.1462-8902.2004.00328.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim J-E, Jeon S-M, Park KH, Lee WS, Jeong T-S, McGregor RA, Choi M-S. Does Glycine max leaves or Garcinia Cambogia promote weight-loss or lower plasma cholesterol in overweight individuals: a randomized control trial. Nutr J. 2011;10:94. [DOI] [PMC free article] [PubMed]
- 27.Westerterp-Plantenga M, Kovacs E. The effect of (−)-hydroxycitrate on energy intake and satiety in overweight humans. Int J Obes. 2002;26:870–2. doi: 10.1038/sj.ijo.0801855. [DOI] [PubMed] [Google Scholar]
- 28.Tomita K, Okuhara Y, Shigematsu N, Suh H, Lim K. (−)-Hydroxycitrate ingestion increases fat oxidation during moderate intensity exercise in untrained men. Biosci Biotechnol Biochem. 2003;67:1999–2001. doi: 10.1271/bbb.67.1999. [DOI] [PubMed] [Google Scholar]
- 29.Kang DH, Jung EY, Chang UJ, Bae S-H, Suh HJ. Psyllium husk combined with hydroxycitrate reduces body weight gain and body fat in diet-induced obese rats. Nutr Res. 2007;27:349–55. doi: 10.1016/j.nutres.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Márquez F, Babio N, Bulló M, Salas-Salvadó J. Evaluation of the safety and efficacy of hydroxycitric acid or Garcinia cambogia extracts in humans. Crit Rev Food Sci Nutr. 2012;52:585–94. doi: 10.1080/10408398.2010.500551. [DOI] [PubMed] [Google Scholar]
- 31.Louter-van de Haar J, Wielinga PY, Scheurink AJ, Nieuwenhuizen AG. Comparison of the effects of three different (−)-hydroxycitric acid preparations on food intake in rats. Nutr Metab. 2005;13:23. doi: 10.1186/1743-7075-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebola N, Ng’ambi J, Norris D, Mbajiorgu C. Effect of Garcinia cambogia leaf meal supplementation lever at finisher stage on productivity and juiciness of male ross 308 broiler chickens. Asian Journal of Animal and Veterinary Advances. 2011;6:723–30. doi: 10.3923/ajava.2011.723.730. [DOI] [Google Scholar]
- 33.Vasselli J, Shane E, Boozer C, Heymsfield S. Garcinia cambogia extract inhibits body weight gain via increased energy expenditure (EE) in rats. BETHESDA, MD 20814–3998 USA: FASEB JOURNAL. FEDERATION AMER SOC EXP BIOL 9650 ROCKVILLE PIKE; 1998. p. A505. [Google Scholar]
- 34.Hornick J-L, Van Eenaeme C, Gerard O, Dufrasne I, Istasse L. Mechanisms of reduced and compensatory growth. Domest Anim Endocrinol. 2000;19:121–32. doi: 10.1016/S0739-7240(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 35.Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002;26:45–60. doi: 10.1016/S0149-7634(01)00037-9. [DOI] [PubMed] [Google Scholar]
- 36.Buyse J, Janssens K, Van der Geyten S, Van As P, Decuypere E, Darras VM. Pre-and postprandial changes in plasma hormone and metabolite levels and hepatic deiodinase activities in meal-fed broiler chickens. Br J Nutr. 2002;88:641–53. doi: 10.1079/BJN2002741. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, MA H-T, Wang M, KONG Y-L ZOUS-X. Creatine pyruvate enhances lipolysis and protein synthesis in broiler chicken. Agric Sci China. 2011;10:1977–85. doi: 10.1016/S1671-2927(11)60199-5. [DOI] [Google Scholar]
- 38.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–6. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 39.Saito M, Ueno M, Ogino S, Kubo K, Nagata J, Takeuchi M. High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2005;43:411. doi: 10.1016/j.fct.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Kim YJ, Kim K-Y, Kim MS, Lee JH, Lee KP, Park T. A mixture of the aqueous extract of Garcinia cambogia, soy peptide and L-carnitine reduces the accumulation of visceral fat mass in rats rendered obese by a high fat diet. Genes & nutrition. 2008;2:353–8. doi: 10.1007/s12263-007-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soni M, Burdock G, Preuss H, Stohs S, Ohia S, Bagchi D. Safety assessment of (−)-hydroxycitric acid and Super CitriMax®, a novel calcium/potassium salt. Food Chem Toxicol. 2004;42:1513–29. doi: 10.1016/j.fct.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan A, Singh M, Srere PA, Glusker JP. Reactivity and inhibitor potential of hydroxycitrate isomers with citrate synthase, citrate lyase, and ATP citrate lyase. J Biol Chem. 1977;252:7583–90. [PubMed] [Google Scholar]
- 43.Berkhout TA, Havekes L, Pearce N, Groot P. The effect of (−)-hydroxycitrate on the activity of the low-density-lipoprotein receptor and 3-hydroxy-3-methylglutaryl-CoA reductase levels in the human hepatoma cell line Hep G2. Biochem J. 1990;272:181–6. doi: 10.1042/bj2720181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shara M, Ohia SE, Yasmin T, Zardetto-Smith A, Kincaid A, Bagchi M, Chatterjee A, Bagchi D, Stohs SJ. Dose-and time-dependent effects of a novel (−)-hydroxycitric acid extract on body weight, hepatic and testicular lipid peroxidation, DNA fragmentation and histopathological data over a period of 90 days. Mol Cell Biochem. 2003;254:339–46. [DOI] [PubMed]
- 45.Cheng I-S, Huang S-W, Lu H-C, Wu C-L, Chu Y-C, Lee S-D, Huang C-Y, Kuo C-H. Oral hydroxycitrate supplementation enhances glycogen synthesis in exercised human skeletal muscle. Br J Nutr. Accepted (201106 18) 2012 [DOI] [PubMed]
- 46.Munday M. Regulation of mammalian acetyl-CoA carboxylase. Biochem Soc Trans. 2002;30:1059. doi: 10.1042/bst0301059. [DOI] [PubMed] [Google Scholar]
- 47.McCune S, Foe L, Kemp RG, Jurin R. Aurintricarboxylic acid is a potent inhibitor of phosphofructokinase. Biochem J. 1989;259:925–7. doi: 10.1042/bj2590925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayamizu K, Hirakawa H, Oikawa D, Nakanishi T, Takagi T, Tachibana T, Furuse M. Effect of Garcinia cambogia extract on serum leptin and insulin in mice. Fitoterapia. 2003;74:267–73. [DOI] [PubMed]
- 49.Vasques CA, Rossetto S, Halmenschlager G, Linden R, Heckler E, Fernandez MSP, Alonso JLL. Evaluation of the pharmacotherapeutic efficacy of Garcinia cambogia plus Amorphophallus konjac for the treatment of obesity. Phytother Res. 2008;22:1135–40. [DOI] [PubMed]
- 50.Xu Z, Wang M, Mao H, Zhan X, Hu C. Effects of L-carnitine on growth performance, carcass composition, and metabolism of lipids in male broilers. Poult Sci. 2003;82:408–13. doi: 10.1093/ps/82.3.408. [DOI] [PubMed] [Google Scholar]
- 51.Jena B, Jayaprakasha G, Singh R, Sakariah K. Chemistry and biochemistry of (−)-hydroxycitric acid from Garcinia. J Agric Food Chem. 2002;50:10–22. doi: 10.1021/jf010753k. [DOI] [PubMed] [Google Scholar]
- 52.Frenkel B, Mayorek N, Hertz R, Bar-Tana J. The hypochylomicronemic effect of beta, beta’-methyl-substituted hexadecanedioic acid (MEDICA 16) is mediated by a decrease in apolipoprotein C-III. J Biol Chem. 1988;263:8491–7. [PubMed] [Google Scholar]
- 53.Frenkel B, Bishara-Shieban J, Bar-Tana J. The effect of beta, beta’-tetramethylhexadecanedioic acid (MEDICA 16) on plasma very-low-density lipoprotein metabolism in rats: role of apolipoprotein C-III. Biochem J. 1994;298:409–14. doi: 10.1042/bj2980409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuusi T, Saarinen P, Nikkila E. Evidence for the role of hepatic endothelial lipase in the metabolism of plasma high density lipoprotein2 in man. Atherosclerosis. 1980;36:589–93. doi: 10.1016/0021-9150(80)90251-8. [DOI] [PubMed] [Google Scholar]
- 55.Herbst KL, Amory JK, Brunzell JD, Chansky HA, Bremner WJ. Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wk. American Journal of Physiology-Endocrinology And Metabolism. 2003;284:E1112–8. doi: 10.1152/ajpendo.00524.2002. [DOI] [PubMed] [Google Scholar]
- 56.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–40. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 57.Bxal K, Hebbachi A, Hauton D, Brown A, Wiggins D, Patel D, Gibbons G. A role for PPARalpha in the control of SREBP activity and lipid synthesis in the liver. Biochem J. 2005;389:413–21. [DOI] [PMC free article] [PubMed]
- 58.Yin L, Zhang Y, Hillgartner FB. Sterol regulatory element-binding protein-1 interacts with the nuclear thyroid hormone receptor to enhance acetyl-CoA carboxylase-α transcription in hepatocytes. J Biol Chem. 2002;277:19554–65. doi: 10.1074/jbc.M111771200. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Yin L, Hillgartner FB. SREBP-1 integrates the actions of thyroid hormone, insulin, cAMP, and medium-chain fatty acids on ACCα transcription in hepatocytes. J Lipid Res. 2003;44:356–68. doi: 10.1194/jlr.M200283-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–20. doi: 10.1016/S0014-5793(03)00560-X. [DOI] [PubMed] [Google Scholar]
- 61.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferré P, et al. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–11. doi: 10.1128/MCB.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–7. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 64.Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–9. [DOI] [PubMed]
- 65.Yang J, Craddock L, Hong S, Liu Z. AMP-activated protein kinase suppresses LXR-dependent sterol regulatory element-binding protein-1c transcription in rat hepatoma McA-RH7777 cells. J Cell Biochem. 2009;106:414. doi: 10.1002/jcb.22024. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY-J. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. [DOI] [PMC free article] [PubMed]
- 67.Kriketos A, Thompson H, Greene H, Hill J. (−)-Hydroxycitric acid does not affect energy expenditure and substrate oxidation in adult males in a post-absorptive state. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1999;23:867–73. doi: 10.1038/sj.ijo.0800965. [DOI] [PubMed] [Google Scholar]
- 68.Ishihara K, Oyaizu S, Onuki K, Lim K, Fushiki T. Chronic (−)-hydroxycitrate administration spares carbohydrate utilization and promotes lipid oxidation during exercise in mice. The Journal of nutrition. 2000;130:2990–5. doi: 10.1093/jn/130.12.2990. [DOI] [PubMed] [Google Scholar]
- 69.Roy S, Rink C, Khanna S, Phillips C, Bagchi D, Bagchi M, Sen CK. Body weight and abdominal fat gene expression profile in response to a novel hydroxycitric acid-based dietary supplement. Gene Expr. 2003;11:5–6. [DOI] [PMC free article] [PubMed]
- 70.Kim M-S, Kim J-K, Kim H-J, Moon S-R, Shin B-C, Park K-W, et al. Hibiscus extract inhibits the lipid droplet accumulation and adipogenic transcription factors expression of 3T3-L1 preadipocytes. The Journal of Alternative & Complementary Medicine. 2003;9:499–504. doi: 10.1089/107555303322284785. [DOI] [PubMed] [Google Scholar]
- 71.Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–25. [PubMed] [Google Scholar]
- 72.Rocchi S, Auwerx J. Peroxisome proliferator-activated receptor g, the ultimate liaison between fat and transcription. Br J Nutr. 2000;84:S223. doi: 10.1079/096582197388581. [DOI] [PubMed] [Google Scholar]
- 73.Yang C, Chen A, Hong Q, Liu J, Liu J. Effects of cysteamine on growth performance, digestive enzyme activities, and metabolic hormones in broilers. Poult Sci. 2006;85:1912–6. doi: 10.1093/ps/85.11.1912. [DOI] [PubMed] [Google Scholar]
- 74.Folch J, Lees M, Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 75.Zhao S, Ma H, Zou S, Chen W. Effects of in ovo administration of DHEA on lipid metabolism and hepatic lipogenetic genes expression in broiler chickens during embryonic development. Lipids. 2007;42:749–57. doi: 10.1007/s11745-007-3068-y. [DOI] [PubMed] [Google Scholar]


