Abstract
Chromatin is a critical regulator of neural plasticity, but basic principles of chromatin function in neurons are unclear. In this issue of Neuron, Maze et al. (2015) establish histone H3.3 turnover as a novel mechanism contributing to CNS gene regulation, synaptic plasticity, and cognition.
Chromatin regulation is an indispensable feature of neural plasticity that mediates a wide range of behaviors, including memory formation, reward learning, drug responses, stress, and adaptations to early-life experiences (Day et al., 2013; Feng and Nestler, 2013; Miller et al., 2008; Szyf et al., 2007). The effects of chromatin regulation are exerted primarily through changes in gene expression, with chromatin acting as a gatekeeper of DNA accessibility. DNA is packaged into the nucleus by coiling around a core octamer of histone proteins, consisting of two copies each of H2A, H2B, H3, and H4 to create nucleosomes, the building blocks of chromatin. Histone proteins are modified in several ways to alter access to DNA and influence transcription, but studies in the nervous system have focused almost exclusively on posttranslational modifications of histone tails, leaving entire branches of chromatin regulation virtually unexplored. Histone variant exchange is one such branch that was suggested to be a key regulator of neural plasticity and cognitive function (Michod et al., 2012; Santoro and Dulac, 2012; Zovkic et al., 2014), but our understanding of histone variants and their diverse functions in the nervous system is still in its infancy. In this issue of Neuron, Maze and colleagues (2015) utilize powerful analytical chemistry techniques to implicate dynamic exchange of the histone variant H3.3 into the chromatin core particle as a new mechanism controlling cognitive function in the CNS.
Histone variants are nonallelic histone proteins that are found across species and replace their canonical counterparts in the nucleosome (see Table 1). Although different variants exhibit variable degrees of structural and functional diversity compared to canonical histones, a primary distinction between the two is in their capacity for synthesis outside of cell replication. In contrast to canonical histones, whose deposition in the core octamer is replication dependent, histone variants are capable of replication-independent deposition, suggesting that their functions in chromatin could be a plasticity mechanism in postmitotic neurons. Maze and colleagues address this previously unexplored question through a comprehensive characterization of H3.3 function and chromatin dynamics over development and in the mature nervous system, identifying histone turnover as a novel regulator of neural plasticity. Moreover, besides directly implicating H3.3 in neural plasticity and memory, the findings of Maze et al. overturn a fundamental assumption among many classical epigeneticists, i.e., stability of the core composition of the chromatin particle in nonreplicating cells.
Table 1.
Canonical Histones and Histone Variants
| Histone Family | Canonical Histones | Variant Histones |
|---|---|---|
| H3 | H3.1, H3.2 | H3.3, CenH3, H3.4, H3.5 (primate specific: H3.Y, H3.X) |
| H2A | H2A.1, H2A.2 | H2A.Z, H2A.X, macroH2A1, macroH2A2, H2A.Bbd |
| H2B | H2B | H2BE, H2BFWT, hTSH2B (human specific: spH2B) |
| H4 | H4 | No known variants |
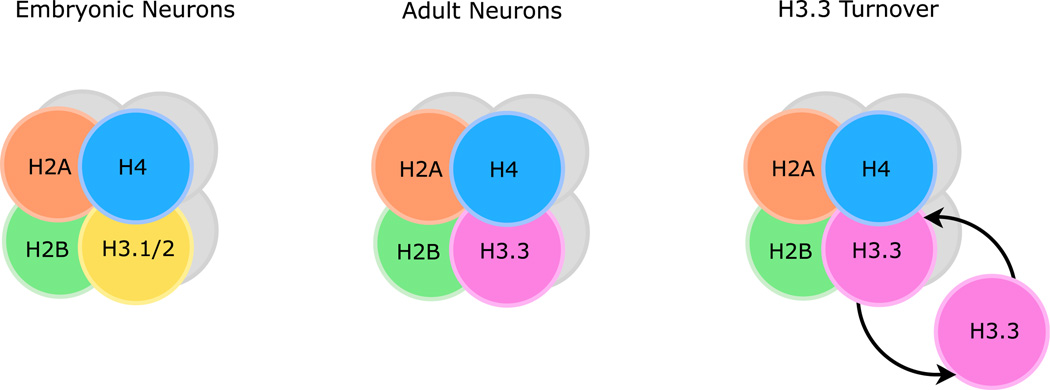
The H3 histone family consists of two canonical replication-dependent variants, H3.1 and H3.2, and a single replication-independent variant, histone H3.3. Although additional replication-independent variants of H3 do exist, CenH3 is specifically enriched in centromeres, and H3.4 and H3.5 are specific to the testes (Filipescu et al., 2014), making H3.3 the sole replication-independent H3 in the brain. In contrast to H3, the H2A histone family has several fully or partially replication-independent variants (Skene and Henikoff, 2013), suggesting a potentially unique role for H3.3 in neural function. Given its exclusive replication-independent status in the H3 family, Maze et al. (2015) hypothesized that H3.3 expression is developmentally regulated in nondividing cells of the nervous system and that its turnover is an essential regulator of neural plasticity. In an impressive series of experiments, the authors demonstrated that H3.3 accumulates in both neurons and glia during maturation, yet maintains a slow steady-state and a rapid activity-induced turnover rate, thus establishing histone turnover as a novel regulator of neural plasticity (see Figure 1).
Figure 1. Neuronal H3.3 Occupancy during Development and in Adulthood.
In embryonic neurons, canonical histones H3.1 and H3.2 make up the majority of H3, whereas H3.3 accumulates with age to become the dominant H3 in adult neurons. Despite its high expression levels, H3.3 maintains both constitutive and activity-induced turnover, implicating H3.3 dynamics as a novel regulator of neural plasticity.
In their initial experiments, the authors utilized fluorescence-activated cell sorting (FACS) to isolate both neurons and glia, allowing them to make cell-type-specific distinctions that are often overlooked in studies of neural plasticity. Using mass spectrometry, they demonstrated that H3.3 starts off as a minor variant in embryonic chromatin in both neurons and glia, then accumulates to make up more than half of all neuronal H3 by weaning (3 weeks of age in mice). Levels continue to increase throughout life until H3.3 becomes the dominant H3 in aged mice (2 years of age), making up more than 94% of all H3 in neurons and more than 80% in glia. This trajectory was remarkably similar in human brains, in which H3.3 represented the smallest fraction of H3 in the fetal brain until it increased to more than 93% of total H3 in adolescence (age 14), remaining stable thereafter (up to 72 years of age). This incredible level of H3.3 accumulation suggests that virtually the entire pool of H3 throughout the entire neuronal genome is turned over across the lifespan, irrespective of the transcriptional activity status of overlying genes.
A long-standing premise in chromatin research is that histones are highly stable proteins with long half-lives, a notion that is incompatible with evidence for H3.3 saturation in adult neurons given that histone dynamics are essential for transcription (Weber and Henikoff, 2014). To determine the extent to which H3.3 remains dynamic in postnatal neurons, Maze and colleagues utilized SILAC (stable isotope labeling of amino acids in cell culture), which involves the delivery of heavy labeled lysines to mice through the diet, which can then be measured to obtain an index of histone turnover. Using this approach, they found that approximately one-third of the total H3.3 pool was replaced over a 4 week period even though the overall levels of H3.3 did not change, suggesting that H3.3 was undergoing constitutive turnover, whereas H3.1 and H3.2 remained static. Once again, the presence of constitutive H3.3 turnover was confirmed in the human brain using the “bomb-pulse” method, which measures the decay in radioactive carbon produced by aerial nuclear weapons testing in 1955–1963. Together, these data convincingly demonstrate that histones are much less stable than previously thought, suggesting that histone turnover is an essential feature of chromatin function in the brain.
The tremendous level of H3.3 saturation observed in the adult nervous system also poses a challenge to another tenet of H3.3 function in other model systems, which find that H3.3 is a strong marker of active chromatin (Hake and Allis, 2006). To examine the association between H3.3 binding and gene expression after H3.3 accumulation, the authors compared gene expression data from RNA sequencing with H3.3 binding data from ChIP sequencing in embryonic and adult neurons, periods when H3.3 represents a minor and a major variant, respectively. Consistent with prior studies, H3.3 enrichment in gene bodies and promoters was associated with active gene expression in embryonic neurons. However, this relationship did not hold true in adult neurons, suggesting that H3.3 is not a marker of active chromatin in the adult nervous system. Another line of research that connects H3.3 to active chromatin holds that H3.3 tends to preferentially associate with active posttranslational modifications to exert positive effects on gene expression. To determine whether this holds true in neurons, the authors again used SILAC to differentiate between dynamic and static H3.3 and showed that only embryonic H3.3 is associated with activity-promoting posttranslational modifications, whereas this effect disappeared in adult neurons. These data reinforce the notion that classical models of H3.3 function do not hold true in the mature nervous system, where H3.3 becomes ubiquitously represented throughout the neuronal genome. Moreover, these data suggest that histone turnover can occur independently from active posttranslational modifications, proposing that these two processes are distinct and must be considered separately in the adult brain.
Having dissociated H3.3 turnover from active posttranslational modifications, Maze et al. (2015) next set out to investigate how H3.3 turnover is affected by neuronal activity. First, they measured H3.3 expression in response to several stimulation protocols in cultured neurons and in vivo using optogenetic stimulation of the medial prefrontal cortex. In both cases, they showed that neuronal activity increased H3.3 expression levels, which translated into potentiated H3.3 incorporation into chromatin of active genes. To determine whether H3.3 dynamics are also subject to regulation by naturally occurring environmental stimuli, mice were exposed to 4 weeks of environmental enrichment, beginning at 3 weeks of age. Compared to normally housed mice, enriched housing resulted in increased expression and turnover of H3.3 in the hippocampus, with hippocampal neurons exhibiting potentiated H3.3 incorporation compared to normally housed controls. These data show that, in contrast to canonical H3, whose expression was unchanged by environmental enrichment, H3.3 is subject to experience-mediated regulation in neurons. Moreover, the occurrence of long-term changes specifically in H3.3 suggests that replication-independent histone variants are a novel target for regulating long-term outcomes associated with distinct environmental experiences.
Given the highly dynamic and experience-sensitive nature of H3.3 turnover, Maze et al. (2015) hypothesized that this process is involved in regulating neural plasticity and cognitive function in mature rodents, a hypothesis that was well supported in an extensive series of experiments. To isolate the effect of histone dynamics, the authors utilized a novel approach to manipulate H3.3 turnover without creating functionally deleterious nucleosome-free regions, a common concern in histone depletion studies. Specifically, they employed a knockdown approach that utilizes chained microRNAs to knock down two distinct H3.3 coding genes simultaneously, allowing them to inhibit all sources of H3.3. This manipulation resulted in selective depletion of the free H3.3 replacement pool, while leaving chromatinized H3.3 intact. Beginning with cultured neurons, the authors showed that blocking H3.3 dynamics resulted in reduced spine density and the development of both excitatory and inhibitory synapses in cultured neurons, an effect that was rescued by replacing H3.3, but not the canonical H3 histones.
To demonstrate the relevance of H3.3 for neural plasticity in vivo, the role of H3.3 in regulating spine density was confirmed by delivering the microRNA constructs into areaCA1 of the hippocampus using AAV. With this approach, they showed that H3.3 knockdown reduced both spine density and the amplitude of miniature excitatory post synaptic currents (mEPSC), supporting the hypothesis that H3.3 turnover is a novel regulator of neural plasticity. Consistent with the ability of H3.3 to mediate key aspects of hippocampal plasticity, the authors showed that H3.3 turnover is also important for regulating two hippocampus-dependent cognitive tasks, novel object recognition and contextual fear conditioning, with performance on both tasks being impaired by H3.3 knockdown. These data add histone turnover to the list of chromatin modifications that regulate cognitive function.
The most direct role of chromatin on neural plasticity and behavior is linked to its effects on transcription, suggesting that the effects of H3.3 turnover on plasticity involve changes in gene expression. The authors confirmed the link between transcription and H3.3 dynamics by using the chained microRNA approach to inhibit H3.3 turnover, which impaired plasticity-related gene expression in response to depolarization. Interestingly, a comparison of H3.3 ChIP and RNA sequencing in depolarized neurons showed that H3.3 dynamics were preferentially correlated with activity of late-responding plasticity genes, indicating that H3.3 exhibits category-specific gene regulation. Moreover, the link with active genes occurred preferentially in gene bodies rather than promoters, a phenomenon that was validated in hippocampi of mice housed under environmentally enriched conditions. Compared to normally housed mice, enriched mice exhibited greater H3.3 enrichment in gene bodies of both neurons and glia, with preferential turnover of genebody H3.3 occurring on more active genes. The tremendous degree of consistency in data between neurons and glia shows that H3.3 turnover in gene bodies is a universal property of gene expression in the brain that is not specific to particular genes, since distinct sets of genes were preferentially expressed in neurons and glia.
Histone turnover involves a cycle of H3.3 removal, degradation, and incorporation of new H3.3 into chromatin, but factors that regulate these processes are not well defined, particularly in neurons. Prior studies have implicated several factors in H3.3 regulation (Skene and Henikoff, 2013), but Maze et al. showed that depolarization specifically enhances interactions between H3.3 and its chaperone Hira, which is necessary for depolarization- induced H3.3 incorporation. In contrast to known deposition candidates, regulation of H3.3 removal from chromatin is poorly understood, and Maze et al. provided the first evidence implicating proteasome-mediated H3.3 eviction and degradation in neurons. This novel role for the proteasome is particularly interesting in light of recent evidence for proteasomal regulation of learning and memory, as well as its regulation of H2A variants outside the brain (Walters and Zovkic, 2015).
Conclusions
Chromatin is subject to complex regulatory mechanisms that impact neural plasticity through effects on gene expression. Despite this crucial role, there is a tremendous gap in our understanding of basic principles of chromatin regulation, particularly in the brain. In an impressive series of studies, Maze et al. (2015) implicate histone turnover as a fundamental new mechanism of neural plasticity and cognitive function, adding to growing evidence for a vital role of histone variants in the brain (see Table 2). Histone turnover is a distinct process from histone subunit exchange, in which histones that make up the nucleosome are replaced by other subtypes. Rather than being replaced, H3.3 remains at a constant level while also maintaining its capacity for both constitutive and activity-induced turnover, which is critical for gene regulation and cognition. Although these studies focused on the H3 family, histone turnover likely has a universal role across various histone types, and future studies of this process, in conjunction with histone variant exchange, will undoubtedly elucidate fundamental mechanisms of gene regulation in the nervous system. Interestingly, recent studies have identified additional replication-independent H3 variants in primates, H3.X and H3.Y (Wiedemann et al., 2010), which along with the findings of Maze et al. introduce the intriguing possibility that additional diversification of H3 may regulate neuronal function in people.
Table 2.
Studies Implicating Histone Variants in Cognitive Function
| Variant | Region | Behavioral Effect of Target Histone Depletion | Reference |
|---|---|---|---|
| H3.3 | Hippocampus | Impaired fear conditioning and novel object recognition | Maze et al. (2015) |
| H2A.Z | Hippocampus | Enhanced fear conditioning | Zovkic et al. (2014) |
| H2BE | Main olfactory epithelium | Impaired odor discrimination | Santoro and Dulac (2012) |
The work of Maze et al. (2015) is also illustrative of an ongoing tectonic shift in the epigenetics field. A founding principle in epigenetics was the concept that epigenetic marks are stable in cells and also heritable in organisms, providing lifelong continuity of cell phenotype and cell fate determination. This founding assumption was based on the readily observable fact that cells stably maintain their phenotype over a lifespan, implying stable underlying biochemical epigenetic mechanisms. The principal molecular cornerstones of this presumed stability were DNA cytosine methylation and the long-lived histone subunits that comprise the octameric core of the chromatin particle. Thus, the basic assumption was that “irreversible” chemical reactions underlay cellular information storage. Work over the last decade has made clear that these early models of epigenetic stability are incorrect. Thus, it has been discovered that DNA cytosine methylation is chemically reversible in nondividing cells, as is histone lysine/arginine methylation, and most recently, breakthrough work that includes these new finding by Maze et al. indicates that dynamic regulation of the chromatin core particle can be added to this list (Zovkic et al., 2014).
It is clear that transient signals can trigger lifelong changes in cell function, and by definition, epigenetic mechanisms contribute to this form of cellular information storage. However, the prior model of epigenetic changes being permanent due to an underlying magic bullet of irreversible chemical reactions needs to be replaced by an understanding of cellular persistence being subserved by ongoing dynamic but bistable biochemical reactions (Nicolis and Prigogine, 1977). The costs of dynamic bistability are 2-fold—a requirement for ongoing energy input to defeat the second law of thermodynamics, and the possibility of accumulation of errors. In the case of cells, the cost of errors is phenomena such as aging, memory degradation, and oncogenesis. However, the evolutionary benefits of cellular changes being subserved by dynamic bistable reactions are manifold—allowing the acquisition of acquired change and such phenomena as stimulus-induced homeostatic regulation, cellular and neural plasticity, and organismal learning.
REFERENCES
- Day JJ, Childs D, Guzman-Karlsson MC, Kibe M, Moulden J, Song E, Tahir A, Sweatt JD. Nat. Neurosci. 2013;16:1445–1452. doi: 10.1038/nn.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Nestler EJ. Curr. Opin. Neurobiol. 2013;23:521–528. doi: 10.1016/j.conb.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipescu D, Müller S, Almouzni G. Annu. Rev. Cell Dev. Biol. 2014;30:615–646. doi: 10.1146/annurev-cellbio-100913-013311. [DOI] [PubMed] [Google Scholar]
- Hake SB, Allis CD. Proc. Natl. Acad. Sci. USA. 2006;103:6428–6435. doi: 10.1073/pnas.0600803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Wenderski W, Noh K, Bagot RC, Tzavaras N, Purushothaman I, Elsässer S, Guo Y, Ionete C, Hurd YL, et al. Neuron. 2015;87:77–94. doi: 10.1016/j.neuron.2015.06.014. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michod D, Bartesaghi S, Khelifi A, Bellodi C, Berliocchi L, Nicotera P, Salomoni P. Neuron. 2012;74:122–135. doi: 10.1016/j.neuron.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. Neurobiol. Learn. Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolis G, Prigogine I. Self-Organization in Nonequilibrium Systems: From Dissipative Structures to Order through Fluctuations (Wiley) 1977 [Google Scholar]
- Santoro SW, Dulac C. eLife. 2012;1:e00070. doi: 10.7554/eLife.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Henikoff S. Development. 2013;140:2513–2524. doi: 10.1242/dev.091439. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver I, Meaney M. Reprod. Toxicol. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Walters BJ, Zovkic IB. Neuroscience. 2015;300:39–52. doi: 10.1016/j.neuroscience.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Weber CM, Henikoff S. Genes Dev. 2014;28:672–682. doi: 10.1101/gad.238873.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann SM, Mildner SN, Bönisch C, Israel L, Maiser A, Matheisl S, Straub T, Merkl R, Leonhardt H, Kremmer E, et al. J. Cell Biol. 2010;190:777–791. doi: 10.1083/jcb.201002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovkic IB, Paulukaitis BS, Day JJ, Etikala DM, Sweatt JD. Nature. 2014;515:582–586. doi: 10.1038/nature13707. [DOI] [PMC free article] [PubMed] [Google Scholar]