Many cells in the human body are quiescent—that is, they are not actively dividing to create new cells, but can reenter the cell division cycle and proliferate at some later time. Among the cells that can survive in a quiescent state are lymphocytes that can become activated to mount an immune response, and dermal fibroblasts that can be called upon to aid in wound healing. The ability to enter and exit quiescence appropriately, and to remain viable while quiescent, is central to tissue homeostasis and the response to potentially life-threatening challenges. Yet, despite the central role of quiescence in normal physiology and pathophysiology, fundamental questions about quiescence remain unanswered but are currently being addressed.
Since the 1960s, it has been debated whether quiescence represents a distinct state outside the proliferative cell cycle (see the figure)—the so-called G0 phase. One model posits that cells progress through the cell cycle at different rates, and respond to extracellular conditions by adjusting their probability of entering S phase (when DNA is replicated), the “transition probability” (1). In this scenario, cells exhibit a continuous distribution of G1 lengths, and “quiescence” is just an extended G1 phase (the phase that precedes S phase). The opposing model posits that a distinct quiescent state exists outside the proliferative cell cycle, and that cells enter this state when conditions are unsuitable for division (2).
figure. G0 or G1?
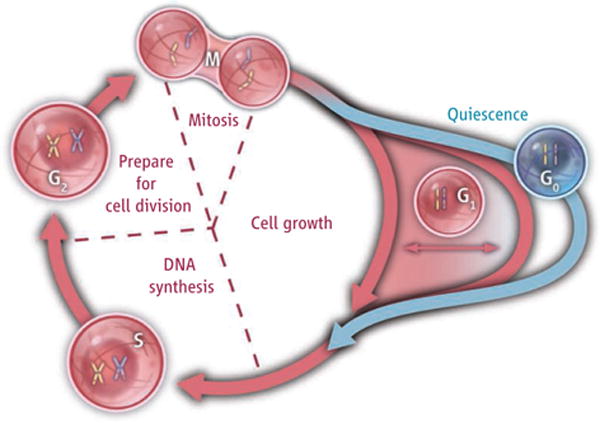
The cell division cycle is divided into a growth phase (G1), a DNA synthesis stage (S), a waiting phase (G2), and mitosis (M). Cells that exit this proliferative cycle, but can reenter later, are quiescent. Whether quiescent cells constitute a distinct state (G0) (blue) or a continuum of G1 (red) is debated.
There is no debate about whether an adipocyte is in a different state than a neuron, or even whether senescent cells that have exhausted their life span are in a distinct state (they are no longer able to divide, and unlike the quiescent cells, cannot reenter the cell cycle). So why does debate persist about quiescence? Part of the answer is that cells do not take on new visible, morphological characteristics upon quiescence. They do not accumulate lipid droplets like adipocytes, or appear flattened like some senescent cells.
There are many ways to probe cells besides simply looking at them, and multiple approaches to define what is distinct about quiescence. In yeast cells, quiescence has been associated with resistance to stresses, decreased translation rate, and a thicker cell wall (3), but these properties are also associated with slow growth. In mammalian cells, research on quiescence has focused primarily on inhibitors of cyclin-dependent kinases, the enzymes that drive cell cycle progression. The amount of the cyclin-dependent kinase inhibitor p27Kip1, for instance, increases in quiescent cells (4), and plays an important role in initiating and maintaining quiescence (5). However, increased expression of cyclin-dependent kinase inhibitors is also induced upon entry into senescence and differentiation, and may be more closely associated with permanent exit from the cell cycle than quiescence (6).
Quiescence has also been characterized by monitoring gene expression patterns, an approach that has provided insight into the distinctive properties of quiescent cells and may ultimately yield better quiescence markers. Quiescent cells show reduced transcription of some genes and increased expression of others (6–10). Some of these changes include down-regulation of genes associated with cell cycle progression and up-regulation of genes classified as tumor suppressors and genes associated with remodeling of the extracellular environment. This finding is consistent with a model in which quiescent cells are distinct from cycling cells. It also suggests that quiescent cells do not just decrease the expression of genes involved in cell proliferation, but also take on new properties, such as actively inhibiting senescence, differentiation, and programmed cell death (apoptosis) (11, 12).
However, although quiescent cells have a transcriptional profile distinct from that of proliferating cells, and even from that of the G1 subpopulation within a proliferating sample (6), they may not have a different quiescence signature from the G1 cells in a slowly growing population. In yeast, which can be grown at carefully controlled rates by providing limiting amounts of specific metabolites, expression of more than a quarter of genes correlates with growth rate (13). Further, expression of the same genes changes when yeast face nutrient limitation or starvation (and their growth rate decreases) (14). These results are consistent with a model in which there is a continuous distribution of growth rates (during the G1 phase), and changes in gene expression in different environments reflect a shift toward a slowing or hastening of cell cycle progression.
In mammalian cells, however, which are more likely to enter quiescence in response to an absence of growth factors or situational cues rather than to an absence of nutrients, there is evidence that quiescence does not reflect a longer G1 phase, but a distinct state. Cells induced into quiescence by the same method but for different lengths of time express different gene expression patterns and have different functional phenotypes (6, 15). This is true even when comparing two conditions in which the cells have essentially stopped dividing. Further, the changes that occur when mammalian cells arrest their cell cycles in G1 do not recapitulate the changes that occur in response to a quiescence signal in terms of gene expression changes or functional changes (6, 11).
Ultimately, does it matter whether quiescence is a distinct state? There are at least two arguments for the importance of distinguishing between quiescent and proliferating cells. One is that research would likely proceed more rapidly if there were a clear classification scheme for quiescence. If scientists performing an experiment on different days, in different ways, in different laboratories, or in different model systems had mechanisms to ensure that they were or were not studying the same cell state, it would be easier to translate research advances across laboratories, species, and fields. Another argument for better defining the quiescent state as distinct from the cell cycle is that there are likely many diseases that represent a manifestation of cells either dividing when they ought to be quiescent, or failing to divide when they should. If there are important characteristics of quiescent cells that distinguish them from proliferating cells, then identifying and understanding these characteristics would likely have therapeutic applications for a wide range of disorders involving the immune response, wound healing, fibrosis, and cancer.
Perhaps finding a universal molecular marker(s) specific for the quiescent state is unrealistic. Maybe the very qualities that are characteristic of quiescent cells also make it more challenging to determine their distinctiveness. Apoptotic cells can shred their DNA into bits, creating a convenient marker for researchers, because they will never replicate again. Quiescent cells, by contrast, cannot deviate too strongly from the proliferative state to which they will return upon short notice.
Is cell quiescence a distinct state or does it represent a series of growth rates that cells use to adjust to their environment?
References
- 1.Smith JA, Martin L. Proc Natl Acad Sci USA. 1973;70:1263. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardee AB. Proc Natl Acad Sci USA. 1974;71:1286. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray JV, et al. Microbiol Mol Biol Rev. 2004;68:187. doi: 10.1128/MMBR.68.2.187-206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polyak K, et al. Genes Dev. 1994;8:9. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 5.Fero ML, et al. Cell. 1996;85:733. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 6.Coller HA, et al. PLoS Biol. 2006;4:e83. doi: 10.1371/journal.pbio.0040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams JG, Penman S. Cell. 1975;6:197. doi: 10.1016/0092-8674(75)90010-0. [DOI] [PubMed] [Google Scholar]
- 8.Schneider C, King RM, Philipson L. Cell. 1988;54:787. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Adler AS, Segal E, Chang HY. PLoS Genet. 2007;3:e91. doi: 10.1371/journal.pgen.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gos M, et al. Cell Prolif. 2005;38:107. doi: 10.1111/j.1365-2184.2005.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sang L, Coller HA, Roberts JM. Science. 2008;321:1095. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohrin M, et al. Cell Stem Cell. 2010;7:174. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brauer MJ, et al. Mol Biol Cell. 2008;19:352. doi: 10.1091/mbc.E07-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klosinska MM, Crutchfield CA, Bradley PH, Rabinowitz JD, Broach JR. Genes Dev. 2011;25:336. doi: 10.1101/gad.2011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemmons JMS, et al. PLoS Biol. 2010;8:e1000514. doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]


