Abstract
Purpose
Children with specific language impairment (SLI) appear to demonstrate deficits in attention and its control. Selective attention involves the cognitive control of attention directed toward a relevant stimulus and simultaneous inhibition of attention toward irrelevant stimuli. The current study examined attention control during a cross-modal word recognition task.
Method
Twenty participants with SLI (ages 9–12 years) and 20 age-matched peers with typical language development (TLD) listened to words through headphones and were instructed to attend to the words in 1 ear while ignoring the words in the other ear. They were simultaneously presented with pictures and asked to make a lexical decision about whether the pictures and auditory words were the same or different. Accuracy and reaction time were measured in 5 conditions, in which the stimulus in the unattended channel was manipulated.
Results
The groups performed with similar accuracy. Compared with their peers with TLD, children with SLI had slower reaction times overall and different within-group patterns of performance by condition.
Conclusions
Children with TLD showed efficient inhibitory control in conditions that required active suppression of competing stimuli. Participants with SLI had difficulty exerting control over their auditory attention in all conditions, with particular difficulty inhibiting distractors of all types.
Children with specific language impairment (SLI) have deficits in cognitive control processes often included in the general category of executive functions (Gillam, Montgomery, & Gillam, 2009; Hick, Botting, & Conti-Ramsden, 2005; Im-Bolter, Johnson, & Pascual-Leone, 2006; Marton, 2008; Marton, Campanelli, Eichorn, Scheuer, & Yoon, 2014; Spaulding, 2010; van Daal, Verhoeven, & van Balkom, 2009). Executive functions include many interrelated processes, including organization and planning, maintaining and shifting set, inhibitory control, and working memory. Attention control is a critical component of many information and language processing models (Baddeley, 1996; Cowan et al., 2005). Comorbid deficits in attention are often observed in children with SLI (Cantwell & Baker, 1991; Cohen et al., 2000; Snowling, Bishop, Stothard, Chipchase, & Kaplan, 2006), and limitations in sustained and selective attention have been identified in children with SLI (Finneran, Francis, & Leonard, 2009; Hanson & Montgomery, 2002; Spaulding, 2010; Spaulding, Plante, & Vance, 2008). However, more research is needed to determine the nature of the attention difficulties and how they might affect language processing throughout childhood.
The unity and diversity of a range of executive functions in human cognition have long been argued in the literature (Duncan, Johnson, Swales, & Freer, 1997; Teuber, 1972), and as yet a universally accepted theory of executive functioning has remained elusive. Miyake et al. (2000) identified moderate correlations among three critical executive functions—shifting, updating, and inhibition—yet their analyses also suggested that the functions were clearly separable in terms of contributions to performance on several common executive function tasks. None of the three target functions were clearly related to dual task performance, indicating that yet another cognitive function might be implicated. Given that our overall conceptualization of executive functions is yet undefined, it is even more difficult to discern the relationship between executive functions and language processing in children with SLI.
Attention Control, Working Memory, and Language
Attention control is a cognitive process that maintains relevant information in an active state while preventing both internal and external distraction (Unsworth & Spillers, 2010). This is similar to what is often termed selective attention, whereby an individual chooses a stimulus to be processed more fully while suppressing all other irrelevant stimuli (Gomes, Motholm, Christodoulou, Ritter, & Cowan, 2000). Models of working memory typically involve some aspect of attention control, although the architecture of the relationship differs by model. Aspects of working memory have also been identified as critical mediators in language abilities (Baddeley, 1986, 1996; Caplan & Waters, 2013). For example, Baddeley (1986) suggested that information stored in working memory is regulated by the central executive. In contrast, Kane and Engle (Engle, 2002; Kane & Engle, 2003) proposed a model whereby working memory is mediated by executive attention, or attention control. They suggested that working memory is made up of two components—short-term memory and controlled attention—and that the attention component is the primary predictor of working memory capacity. Engle (2002) showed that working memory capacity predicted performance on tasks that measure executive control of attention, including Stroop, antisaccade, and dichotic listening tasks. Engle (2010) more recently conceptualized working memory as having both domain-specific aspects (e.g., the phonological loop and auditory, visual, or spatial stores) and the domain-general aspect of attention control, which allows an individual to keep relevant stimuli in active memory and to suppress interference from competing stimuli.
In children with SLI, limitations in working memory have been well established (e.g., Ellis Weismer, Evans, & Hesketh, 1999; Gathercole & Baddeley, 1990; Hesketh & Conti-Ramsden, 2013; Marton, Kelmenson, & Pinkhasova, 2007; Marton & Schwartz, 2003; Montgomery, 2002; Schuchardt, Bockmann, Bornemann, & Maehler, 2013). Deficits in attention control and working memory may be closely related and appear to be implicated in the language limitations in this population.
Processing Limitations and Patterns of Impairment in Children With SLI
Both language-specific deficits and hypothesized underlying processing deficits have been investigated in children with SLI. Although impairments in morphosyntactic, phonological, or auditory processing might be explained by domain-specific theories, limited processing capacity and generally slower processing speed also contribute to the heterogeneous nature and range of impairments in children with SLI. Kail and colleagues (Kail, 1994; Leonard et al., 2007; Miller, Kail, Leonard, & Tomblin, 2001; Miller et al., 2006) showed that children with SLI typically demonstrate slower reaction times (RTs) on motor, cognitive, and linguistic tasks. Slow RTs were also shown to be stable over time in children with SLI (Miller et al., 2006). RTs are thought to provide a measure of global processing speed; slower RTs in children with SLI appear to be indicative of a general processing limitation that affects all levels of language function. However, by utilizing different statistical methods of analysis, other studies identified limitations to this hypothesis (Windsor & Hwang, 1999; Windsor, Milbrath, Carney, & Rakowski, 2001): Not all children with SLI demonstrate slowing, and the slowing appears to be process specific rather than generalized.
Limited attention in children with SLI is a possible cause of performance deficits on linguistic tasks (e.g., Bishop, Carlyon, Deeks, & Bishop, 1999; Helzer, Champlin, & Gillam, 1996). There is a high comorbidity of SLI and attention-deficit/hyperactivity disorder (ADHD) diagnoses (Cantwell & Baker, 1991; Snowling et al., 2006), and differentiating between effects of the two disorders can be a challenge for clinicians and researchers alike. Redmond, Thompson, and Goldstein (2011) recently discussed the importance of identifying measures that would accurately allow for differential diagnosis between children with SLI and children with ADHD. They developed psycholinguistic profiles for participants with SLI, typical language development (TLD), and ADHD and concluded that the strongest predictors of language status were a tense marking measure (Test of Early Grammatical Impairment; Rice & Wexler, 2001) and a sentence recall task (Redmond, 2005). These measures clearly differentiated the SLI group from their peers with TLD and differentiated between the SLI and ADHD groups. According to the authors, these results call into question models that propose cognitive or attention deficits as a direct cause of language impairment. Redmond et al. (2011) did suggest, however, that attention and information processing could serve as more complex mediators or contributors to language impairment.
Using Engle's model of working memory and attention control as a guide (Engle, 2002, 2010), we assumed that children with SLI, who have known impairments in working memory and information processing, would have associated deficits in attention control. Whether these deficits are reflective of problems selecting the relevant stimulus for processing or inhibiting the irrelevant stimuli is undetermined. Stevens and colleagues (Stevens, Fanning, Coch, Sanders, & Neville, 2008; Stevens, Sanders, & Neville, 2006) presented neurophysiological evidence that attentional enhancement is limited in children with SLI and is amenable to treatment. In contrast, Spaulding (2010) suggested that suppression of both irrelevant and competing information is impaired in children with SLI. Deficits on tasks that require higher level processing involving working memory or lexical access could also be attributed to an impaired inhibitory mechanism in children with SLI (Ellis Weismer et al., 1999; Marton et al., 2007; Seiger-Gardner & Schwartz, 2008).
Research Questions
Using a paradigm that manipulated interference within a dichotic listening task, the current study systematically investigated the role of attention control in word recognition. Children with SLI and age-matched peers with TLD were presented with cross-modal stimuli: auditory words and visual images presented simultaneously. In experimental conditions, they were instructed to pay attention to one ear and ignore the other. They heard different words in each of their ears and were presented with a picture. Their task was to determine whether the word in the attended ear was the same as or different from the picture. Interference was presented in the form of competing stimuli in the channel to be ignored. Accuracy and RT were the dependent variables; performance in three experimental conditions was compared with two baselines. Given that children with SLI have historically shown slower RTs that appear to be indicative of processing limitations, it was deemed critical to measure RT in addition to behavioral accuracy. Increased RT on experimental trials compared with baseline would be reflective of active attention control. Patterns of RT and accuracy in the SLI group were expected to differ from those in peers with TLD. The primary comparisons of interest were between pairs of conditions within each group. With this in mind, the following research questions were addressed:
Do children with SLI exhibit deficits in attention control that can be related directly to their language deficits?
How does auditory interference affect word recognition abilities in children with SLI compared with their peers with TLD?
Method
Participants
Forty participants between the ages of 9 and 12 years took part in the study. Half the participants (n = 20) were language impaired (SLI), and the other half (n = 20) had TLD and served as age-matched controls. According to the statistical power calculator G*Power (Faul, Erdfelder, Lang, & Buchner, 2007), a total sample size (both groups) of 36 is needed to detect within-group differences with an alpha level of .05 and a moderate effect size of .25. Children between the ages of 9 and 12 years were targeted because evidence has shown that the ability to control auditory attention develops primarily between the ages of 5 and 7 years, with some continuing development through age 9 years (Bartgis, Lilly, & Thomas, 2003). The mean age in the TLD group was 10;9 (years;months; range = 9;1–12;1, SD = 9.5 months), and the mean age in the SLI group was 10;10 (range = 9;1–12;8, SD = 12 months). There was no significant difference between groups, F(1, 38) = 0.265, p = .610. The TLD group included 11 boys and nine girls, and the SLI group included 13 boys and seven girls. All participants were monolingual speakers of English. Participant characteristics are listed in Table 1.
Table 1.
Participant characteristics and results of standardized testing.
| Characteristic | SLI |
TLD |
||||
|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | |
| Age (months) | 131.10 | 11.60 | 109–152 | 130.10 | 9.40 | 109–145 |
| TONI | 101.10 | 11.48 | 91–135 | 112.40 | 12.60 | 109–145 |
| CELF-Core | 80.60 | 11.74 | 62–98 | 112.30 | 8.71 | 99–130 |
| CELF-Rec | 84.00 | 9.45 | 61–99 | 110.45 | 10.46 | 96–137 |
| CELF-Exp | 81.15 | 14.11 | 59–106 | 114.70 | 10.94 | 99–134 |
| CRS-R | 62.70 | 16.41 | 39–87 | 47.25 | 7.07 | 40–59 |
Note. SLI = specific language impairment; TLD = typical language development; TONI = Test of Nonverbal Intelligence–III or IV; CELF = Clinical Evaluation of Language Fundamentals–III or IV; Core = core language score/composite; Rec = receptive language score; Exp = expressive language score; CRS-R = Conners' Rating Scale–Revised. TONI and CELF scores are reported as standard scores with a mean of 100. CRS-R score is reported as a T score with a mean of 50.
All participants completed the Test of Nonverbal Intelligence (TONI) Third Edition (Brown, Sherbenou, & Johnsen, 1997) or Fourth Edition (Brown, Sherbenou, & Johnsen, 2010) and either the Clinical Evaluation of Language Fundamentals–Third Edition (CELF-3; Semel, Wiig, & Secord, 1996) or Fourth Edition (CELF-4; Semel, Wiig, & Secord, 2004), depending on the date of initial intake. Parents filled out a case history form, a socioeconomic status questionnaire (adapted from Hollingshead, 1975), and the Conners' Rating Scales–Revised (CRS-R; Conners, 1997), a screening measure for ADHD. All participants passed a binaural hearing screening (500, 1000, 2000, and 4000 Hz at 20 dB). Handedness was determined using the Edinburgh handedness survey (Oldfield, 1971).
The children with SLI scored within 1 SD of the mean on the TONI (M = 101.1, SD = 11.5) and received a total language score of more than 1.25 SD below the mean on the CELF-3 or CELF-4 (M = 80.6, SD = 11.8). Four of the 20 participants failed to meet the total language score criterion but either (a) had receptive or expressive index scores that fell more than 1.25 SD below the mean or (b) had a diagnosis by a certified speech-language pathologist and current enrollment in speech-language therapy. Children were excluded from the study if they presented with or had a history of frank motor, neurological, or social–emotional impairment. Children with a documented diagnosis of ADHD were also excluded. Parents of 18 of the 20 children in the SLI group filled out the CRS-R. Nine of these 18 children received scores higher than 65 (M = 62.7, SD = 16.4). Given this high percentage of participants and the common co-occurrence of attention and language impairments in school-aged children (Cantwell & Baker, 1991; Snowling et al., 2006), these participants were not excluded; rather, these scores were considered in later statistical analyses. It is important to note that parent report does not constitute a diagnosis. Hale, How, Dewitt, and Coury (2001), in their investigation of the discriminant validity of the Conners' scales for identifying ADHD subtypes, suggested that although the CRS-R parent and teacher ratings may aid in classification of ADHD subtypes, accurate diagnosis requires systematic observations across a number of contexts. In addition, research has shown that the use of rating scales, including the CRS-R, with children who have language impairments tends to overidentify children with language impairment as having behavioral or socioemotional disorders (Redmond, 2002).
Children with TLD also scored within 1 SD of the mean on the TONI (M = 112.4, SD = 12.6). Although both groups scored within the average range according to the norms of the TONI, there was a statistically significant group difference, F(1, 38) = 8.80, p = .005, ηp 2 = .188. This is not an unusual finding: A recent meta-analysis showed that across 131 studies published between 1995 and 2012, children with SLI scored, on average, 0.69 SD below their peers with TLD on measures of nonverbal cognition (Gallinat & Spaulding, 2014).
Children with TLD achieved total language scores on the CELF-3 or CELF-4 within the average range (M = 112.3, SD = 8.7). These scores also differed significantly from those of the SLI group, F(1, 38) = 93.98, p < .001, ηp 2 = .712. TLD participants had no history of speech, language, learning, motor, neurological, or social–emotional impairments per parent report. Parents of 12 of the 20 children in the TLD group filled out the CRS-R. None of the children received a score higher than 65 (M = 47.25, SD = 7.1), and none of the 20 participants had a documented diagnosis of ADHD.
Materials and Procedure
All auditory stimuli were monosyllabic words of high familiarity for first-grade children on the basis of Cycowicz, Friedman, Rothstein, and Snodgrass (1997). Although lexical and semantic deficits are common in children with SLI, the ages of children in this study ranged from 3 to 6 years beyond a typical first grader. Thus, all lexical items should have been familiar to all participants. A pretest naming task was administered to ensure that all children were familiar with all picture stimuli. Children in both groups labeled all items correctly on this task. Auditory stimuli were digitally recorded by a female speaker in a soundproof booth and were processed using Cool Edit Pro (1998) software. All tokens were normalized for peak root-mean-square amplitude. The audio files were cut and pasted into two-channel audio files, with one word in the right channel and another word in the left. Word pairs were constructed such that the words were not phonologically or semantically related. Phonological relatedness was controlled for by ensuring that word pairs did not share the same initial or final phonemes. Word-association norms were consulted to ensure that there was no semantic relationship between the words (Nelson, McEvoy, & Schreiber, 1998). Word pairs were matched and edited such that the onset and offset of the words occurred simultaneously. Word pairs ranged in duration from 410 to 650 ms. Example word pairs included nose–car, hat–dog, clock–brush, and cup–bed. Thus, although duration of individual words differed, the duration within each pair was matched. Auditory stimuli were presented to the participants through headphones at 70 dB SPL.
Visual stimuli were black-and-white line drawings from Snodgrass and Vanderwart (1980) representing the same highly familiar words as above (Cycowicz et al., 1997). A total of 56 different images were used. They were presented on a 17-in. personal computer screen at a distance of approximately 45 cm from the subject. Visual stimuli appeared for 1000 ms, followed by a 2000-ms interstimulus interval.
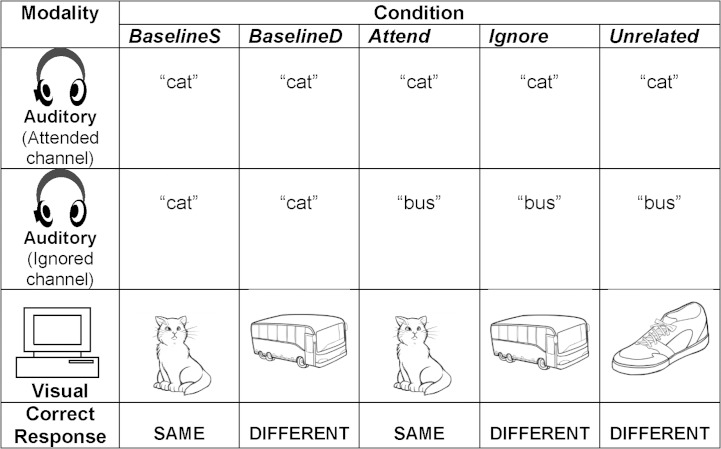
Trial lists were constructed such that five conditions occurred within each of two main trial blocks (right ear attend, left ear attend). Figure 1 depicts the five conditions. Two baseline conditions were used. In the first condition (baseline same), the auditory words presented in each ear were the same, and the auditory word matched the picture (e.g., the child heard cat binaurally and the picture was of a cat). In the second baseline condition (baseline different), the auditory words presented in each ear were again the same, but the picture did not match the words (e.g., cat was heard in both ears, but the picture was of an unrelated item such as a bus).
Figure 1.
Presentation of sample stimuli in five conditions. Auditory and visual stimuli were presented simultaneously. Visuals adapted from Snodgrass and Vanderwart (1980).
During the experimental conditions, participants were instructed to direct their attention to a particular channel (right or left ear). For example, for the right ear block, participants were told, “Pay attention only to the words in your right ear. Ignore the words in your left ear.” In the “attend” experimental condition, the visual stimulus matched the attended auditory stimulus. For example, if the word cat was presented in the attended ear and the word bus was presented in the ear to be ignored, the picture was of a cat. The correct response in this condition would be same. In the “ignore” condition, the picture matched the stimulus to be ignored (in this example, a bus). Here, the correct response is different. In the “unrelated” condition, the visual stimulus was different from both auditory stimuli (e.g., a shoe). The correct response in this condition was again different.
Thirty stimuli (word–picture pairs) were created for each condition, resulting in 150 experimental trials per ear. Thirty additional filler stimuli were presented in each trial block to balance the number of same and different responses for a total of 180 trials per ear. For each ear, participants were presented first with the baseline conditions (60 trials) followed by the experimental conditions (three blocks of 40 trials each). Within each of the experimental blocks, stimuli in the three experimental conditions and the filler trials were presented randomly. The order of presentation of trial blocks (right ear attend followed by left, or vice versa) was counterbalanced across participants.
Children were seated in front of a personal computer screen while wearing TDH-50 headphones (Telephonics, Farmingdale, NY). The participant was instructed to press a button marked same if the picture matched the word he or she heard in the attended ear and to press a button marked different if the picture did not match. These buttons were aligned vertically to avoid confusion with left and right ears and to avoid any advantage due to handedness. Children were instructed to push the buttons with their preferred or dominant hand and to press the button as quickly and accurately as possible. RT was measured from the onset of the auditory stimulus to the participant's response (button press) for each target.
Results
Children's responses were analyzed for accuracy and RT. An initial item analysis led to the removal of four items due to miscoding that had rendered the children's responses invalid. These items accounted for 1.3% of all trials. The data were then sorted by condition and examined for accuracy. Accuracy was determined by calculating the proportion of correct responses to the total number of trials. Both incorrect and missed responses (those not recorded within the 3000-ms trial window) were counted as errors. Given that the task was essentially a two-alternative forced-choice design (same or different), we considered using signal detection theory as the basis for our statistical analysis of the accuracy data. However, because of the way the trials were constructed, we were not able to clearly identify hits, misses, and false alarms. Because we were interested only in comparing conditions in which the responses were congruent, some conditions might have hits and misses, whereas other conditions would have only false alarms and correct rejections. Thus, the conditions could not be compared directly via signal detection theory. Furthermore, according to Stanislaw and Todorov (1999) and Abbey and Eckstein (2002), the correct measure in a two-alternative forced-choice design is proportion of correct responses. For the RT analyses, all errors were removed from the data set, accounting for 12% of responses overall. RTs considered outliers (±3 SD from each participant's grand mean RT) were also removed from the RT analysis. Outliers accounted for 1% of correct responses overall.
Statistical analyses were conducted by way of planned comparisons. For the reasons that follow, the a priori comparisons of interest were between a limited number of condition pairs. In the current design, note that the response in some conditions is same and in others is different. There may be an inherent difference in RT between same and different responses. Some have suggested that participants always respond more quickly when the response is same than when it is different (e.g., Goldfarb & Henik, 2006), whereas others have found no difference between the two response types (Posner & Mitchell, 1967). It is partially for this reason that the primary comparisons of interest for the study were made across conditions in which the expected responses were congruent. Planned comparisons were also used because the design of the study yielded a great number of possible comparisons, not all of which were theoretically important. Comparisons were made both within and between groups. Given the manipulation of attention within the paradigm and the theoretical basis for the comparisons (i.e., that RTs would differ between particular pairs of conditions depending on the level of auditory distraction), it was deemed appropriate to make these selected a priori comparisons (per Howell, 2010). As only correct responses were included in the RT analyses, accuracy rates are considered first.
Accuracy
Accuracy rates approached ceiling levels (> 90%) in some conditions, thereby limiting the variance and potentially yielding artifacts. Comparisons in those conditions should be interpreted cautiously. It should also be noted that accuracy in the baseline conditions, though above 90% for both groups, was not at 100%. Within each condition there were 30 trials per ear, or 60 with the right and left blocks collapsed. Thus, 90% accuracy would indicate that the children responded correctly to about 54 of 60 trials. Despite the fact that children knew the words and were able to name the pictures, some errors may simply have been response (i.e., button-press) errors. This was a speeded decision task, and children may have pressed the wrong button inadvertently, given their intent to be as fast as possible. In addition, it was a cross-modal task. All children may have had some minor difficulties integrating the auditory and visual information, even in baseline conditions. We did not assume that baseline accuracy would be at 100%; rather, the baseline values were used as comparisons for the experimental trials when distraction was introduced.
Between-groups differences were examined using a series of one-way analyses of variance by condition. Because there were five comparisons, the likelihood of Type I error was controlled by applying a Bonferroni correction. Thus, significance was set at p ≤ .01 (.05/5). Group means and standard deviations for each condition are listed in Table 2.
Table 2.
Mean accuracy rates (percentage correct) by condition for specific language impairment (SLI) and typical language impairment (TLD) groups.
| Condition | SLI |
TLD |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Baseline same | 91.64 | 6.59 | 91.12 | 6.18 |
| Baseline different | 91.41 | 4.85 | 94.83 | 3.19 |
| Attend | 83.24 | 12.34 | 85.25 | 8.28 |
| Ignore | 77.67 | 15.20 | 85.32 | 7.96 |
| Unrelated | 89.63 | 8.74 | 94.21 | 4.30 |
For the baseline-same condition, the TLD and SLI groups performed with similar accuracy, F(1, 38) = 0.067, p = .797, ηp 2 = .002. Accuracy for the baseline-different condition was significantly different, F(1, 38) = 6.936, p = .01, ηp 2 = .15. Although this result was statistically significant, it is important to note the potential ceiling effect: The number of items correct would have differed only by about two items, on average. In the attend condition, the two groups demonstrated similar accuracy rates, F(1, 38) = 0.365, p = .549. ηp 2 = .01. In the ignore condition, where differences were anticipated, the SLI group showed a slight decrease in accuracy; however, it did not reach statistical significance when compared with their peers with TLD, F(1, 38) = 3.981, p = .053, ηp 2 = .095. Despite the nearly 8-point difference in accuracy scores (equivalent to five additional incorrect items on average), the increased variability observed in the SLI group may account for the nonsignificant finding. Last, in the unrelated condition, the group differences were not statistically significant, F(1, 38) = 4.430, p = .042, ηp 2 = .10.
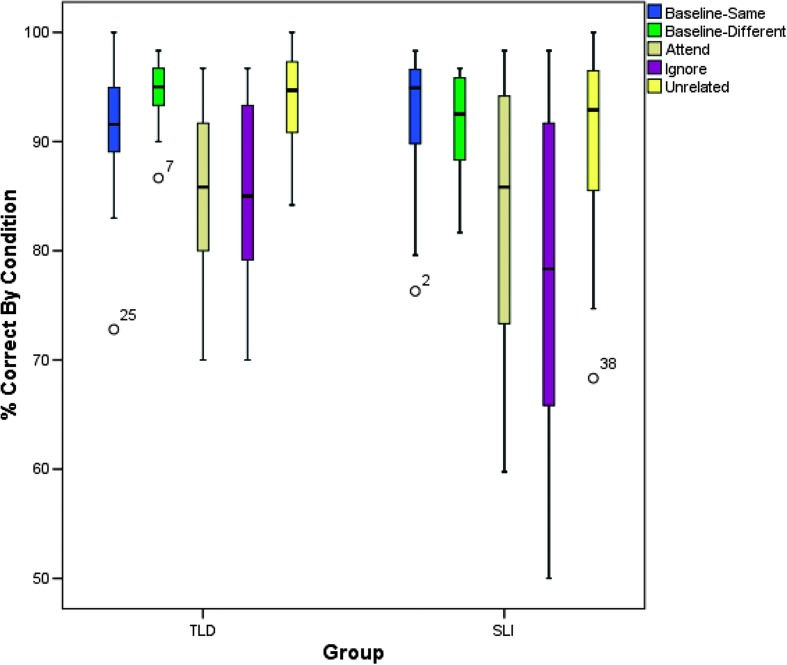
Although it was important to explore between-groups differences, we were primarily interested in within-group patterns of performance between sets of conditions. Figure 2 depicts the patterns of performance by condition for both groups. The following a priori comparisons were analyzed via repeated measures analyses of variance within each group. Given the number of comparisons (five), Type I error was again controlled for by applying a Bonferroni correction, with significance set at p ≤ .01 (.05/5). Means and standard deviations for each group were noted above. For both groups, the sphericity assumption was violated; thus, the Huynh–Feldt adjustment was used for the degrees of freedom in the analyses. In the TLD group, a main effect of condition was identified, F(3.224, 61.261) = 19.219, p < .001, ηp 2 = .503. A similar effect was observed in the SLI group, F(2.853, 54.208) = 13.638, p < .001, ηp 2 = .418. Pairwise comparisons between selected conditions were then analyzed for each group.
Figure 2.
Box plots for accuracy rates (percentage correct responses) by condition for participants with typical language development (TLD) and specific language impairment (SLI). Box plots display group medians, first and third quartiles, 10th and 90th percentiles, and outliers. Numbers associated with outliers refer to individual case numbers.
The first comparison considered was between accuracy in the baseline-same condition and in the baseline-different condition. Difference in performance on these two conditions might indicate greater difficulty on the basic lexical decision task when the visual stimulus and the auditory word were incongruous. However, there was no statistically significant difference in performance between these two conditions for either group. Thus, both groups were equally accurate in making same–different judgments in the baseline conditions. In the TLD group, the mean accuracy was significantly better in the baseline-same condition than in the attend condition (p = .003). In the SLI group, the mean accuracy in the baseline-same and attend conditions were also significantly different (p = .01). In these conditions, the participants confirmed a relationship between the attended auditory word and the picture target. The introduction of an interfering yet noncompeting stimulus in the unattended channel resulted in reduced accuracy compared with baseline for both groups.
There was no difference in accuracy in the unrelated condition compared with the baseline-different condition for either the TLD group (p = .995) or the SLI group (p = 1.00). In the unrelated condition, the participants disconfirmed a relationship between the auditory word and the picture target in the presence of an unrelated distractor. Thus, the introduction of an interfering (but unrelated) stimulus in the unattended channel when the response was different did not have an effect on accuracy in either group. The fourth comparison was between the baseline-different and ignore conditions. In both groups, the mean accuracy in the ignore condition was significantly reduced compared with the mean in the baseline-different condition (TLD: p < .001; SLI: p = .004). Here the participants were required to disconfirm the relationship between the attended word and the picture; however, the competing auditory stimulus matched the visual stimulus, requiring increased cognitive control in order to make the correct decision. Therefore, in both groups, the presence of a competing stimulus in the unattended channel had a negative effect on accuracy rates. Last, accuracy rates in the unrelated condition were compared with those in the ignore condition. Up to this point, we have compared accuracy rates in the experimental conditions with the baseline(s). Here, we compared accuracy in two experimental conditions to determine the unique effect of the competing versus noncompeting auditory stimulus in the unattended channel on accuracy rates. Both groups performed more accurately on the trials with a noncompeting stimulus (in the unrelated condition) than on those with a competing stimulus (in the ignore condition; TLD: p < .001; SLI: p = .001).
These data show that overall the groups performed with similar accuracy. Ceiling effects and variability make between-groups comparisons difficult to interpret. Within-subject analyses revealed a significant overall effect of condition for both groups, indicating that the varying degrees of auditory interference affected accuracy rates. Several a priori comparisons were considered to examine within-group patterns of performance. Results showed similar patterns in both groups, though variability was much higher in the SLI group.
RT
The RT data were first analyzed with ear of presentation as a primary factor so that we could determine whether participants with TLD or SLI showed lateralized dominance (as demonstrated by faster RTs). No significant effect of ear was detected either within or between groups; thus, the following analyses were performed with right and left ear responses collapsed.
The RT data were analyzed similarly to the accuracy data. Recall that the RT data examined correct responses only; thus, incorrect, missed, and outlier responses were removed for the following analyses. A series of comparisons addressing a priori hypotheses was completed to compare RTs (in milliseconds) between groups in each condition and then between pairs of conditions within each group. Given the number of comparisons (five), the Bonferroni correction was again applied, with significance set at p ≤ .01. Group means and standard deviations are shown in Table 3. It should be noted that although the SLI group appeared to have responded more slowly overall, none of the comparisons were statistically significant at the .01 level. For the baseline conditions, the participants with SLI performed similarly to their peers with TLD: same trials, F(1, 38) = 3.33, p = .076, ηp 2 = .081; different trials, F(1, 38) = 4.527, p = .04, ηp 2 = .106. In all experimental conditions, group differences did not reach statistical significance at the .01 level: attend, F(1, 38) = 5.695, p = .022, ηp 2 = .13; ignore, F(1, 38) = 4.190, p = .048, ηp 2 = .099; and unrelated, F(1, 38) = 5.514, p = .024, ηp 2 = .127.
Table 3.
Mean reaction times (in milliseconds) by condition for the specific language impairment (SLI) and typical language impairment (TLD) groups.
| Condition | SLI |
TLD |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Baseline-same | 1045.52 | 193.34 | 921.17 | 235.43 |
| Baseline-different | 1106.62 | 193.06 | 966.36 | 222.78 |
| Attend | 1086.02 | 219.25 | 913.93 | 236.52 |
| Ignore | 1089.54 | 253.95 | 1035.37 | 221.30 |
| Unrelated | 1163.72 | 267.86 | 980.56 | 223.77 |
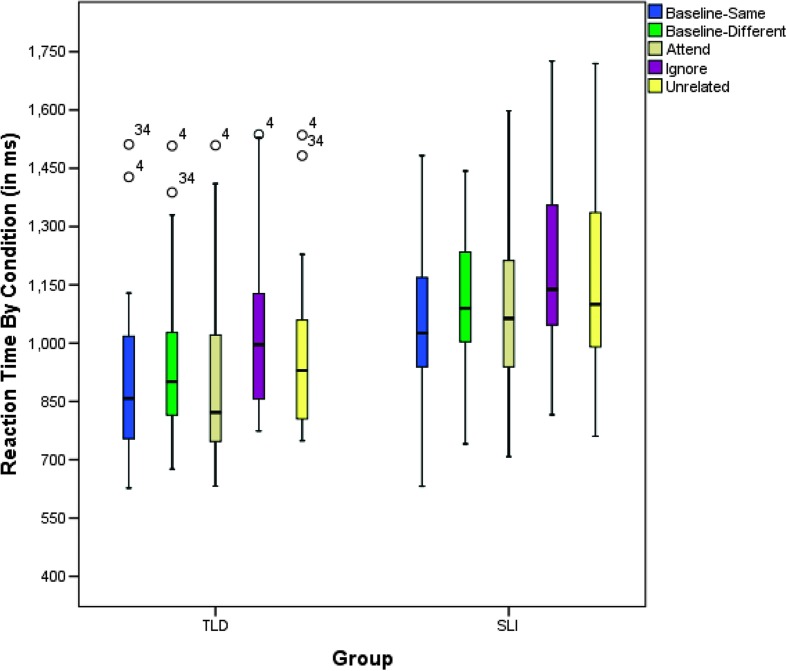
However, the primary question of interest was not whether the SLI group responded more slowly than the TLD group but rather how their RT was affected in experimental conditions compared with their own baselines. Figure 3 depicts patterns of performance across conditions for each group. Thus, the first comparison was between RTs in the baseline-same condition and RTs in the baseline-different condition. This comparison quantifies the difference in RT between same and different responses. Recall that neither group's accuracy was affected by this contrast. The difference between mean RTs in the baseline-same condition and in the baseline-different condition was not statistically significant for TLD participants (p = .095). However, participants with SLI responded somewhat faster when the response mode was same versus when it was different (p = .02). It took longer for participants with SLI to correctly disconfirm a relationship between a word and picture (i.e., they were different) than to confirm the relationship (i.e., they were the same). Next, RTs in the attend condition were compared with RTs in the baseline-same condition. The mean RTs in these conditions were similar for both groups (TLD: p = 1.0; SLI: p = 1.0). This indicates that neither group was slower to react in the attend condition, compared with baseline, even though there was a distractor present in the unattended ear.
Figure 3.
Box plots for reaction time (in milliseconds) by condition for participants with typical language development (TLD) and specific language impairment (SLI). Box plots display group medians, first and third quartiles, 10th and 90th percentiles, and outliers. Numbers associated with outliers refer to individual case numbers.
RTs in the baseline-different condition were then compared with those in the unrelated and ignore conditions. The difference between mean RTs in unrelated and baseline-different conditions was not statistically significant for either group (TLD: p = 1.0; SLI: p = .761). The comparison between ignore and baseline-different conditions, on the other hand, was significant for the TLD group (p = .001) but not for the SLI group (p = .086). This comparison should reveal the degree to which a competing lexical stimulus in the channel to be ignored affected RT. Last, RTs in the ignore condition were compared with those in the unrelated condition. The difference in RTs between these two conditions should reflect the unique effects of the competing lexical stimulus as opposed to an unrelated distractor. In the TLD group, the mean difference in RTs was statistically significant (p < .001). However, the pattern differed here for the SLI group. The difference between RTs in the ignore condition and the unrelated condition was not significant (p = .724). It appears that participants with SLI responded similarly to auditory interference in these conditions regardless of distractor type.
The RT data show that despite similar behavioral accuracy rates, the participants with SLI responded more slowly and that the TLD and SLI groups showed similar patterns of performance in only two of the five comparisons of interest. RTs in the SLI group were particularly affected in conditions for which the expected response was different.
Discussion
Children with SLI demonstrate limitations in working memory capacity and function (Ellis Weismer et al., 1999; Gathercole & Baddeley, 1990; Marton & Schwartz, 2003; Marton et al., 2007; Montgomery, 2000, 2002). Engle and colleagues (Engle, 2002, 2010; Kane & Engle, 2003) suggested that in typical individuals, working memory capacity is mediated by the domain-general aspect of attention control, or the ability to keep relevant stimuli in mind while inhibiting distractors. Using this theory as a framework, the current study focused on control of auditory attention in children with SLI. This skill in particular was of interest because it is considered an active process that requires cognitive control. Children with SLI appear to have subtle impairments in nonlinguistic cognitive skills, particularly in aspects of executive function (Marton, 2008; Noterdaeme, Amorosa, Mildenberger, Sitter, & Minow, 2001). Some researchers have postulated that deficits in attention and executive function may play a causal role in language impairment (e.g., Montgomery, Evans, & Gillam, 2009). However, most studies have focused on sustained attention or on a combined (sustained–selective) construct (Finneran et al., 2009; Spaulding et al., 2008). Given that attention is a multifaceted process and that it is closely related to other executive function skills, the present study controlled for confounding factors in the experimental task. By presenting stimuli simultaneously and requiring an immediate response, demands on working memory and sustained attention were limited.
Comparisons of accuracy and RT on pairs of conditions were examined. These comparisons were made within and between groups. Between-groups comparisons on accuracy measures were nonsignificant or were problematic to interpret due to ceiling effects, with accuracy rates that were greater than 90% in several conditions. Both groups showed statistically significant decreases in performance between baseline and experimental conditions, indicating that the presence of a distractor in the unattended channel led to decreases in accuracy.
RTs were slightly increased for participants with SLI across the board, including on baseline measures. It is possible that the basic nature of the task required increased processing for the children with SLI. Because even the baseline task was a language processing task, it may have taken children with SLI longer to recognize the auditory word, call up its representation in memory, and compare it with the visual stimulus. Although the stimuli were chosen for their phonological simplicity and high familiarity, it is possible that the task of comparing and making a lexical decision required more processing time for the participants with SLI . Slower word recognition has also been attributed to the generalized slowing hypothesis in children with SLI (Miller et al., 2001; Velez & Schwartz, 2010). As an alternative, it is possible that the dual-modality (auditory and visual) aspect of the task posed a challenge for the children with SLI. Thus, although the participants with SLI appeared to be slower overall on the experimental task, the explanation may not be as simple as a generalized slowing account would suggest. The absence of a baseline, nonlinguistic RT task in this study limits our interpretation of the slower SLI performance even in baseline conditions. However, differences on nonlinguistic RT tasks (e.g., basic motor or auditory detection) are not always significant (e.g., Kohnert & Windsor, 2004; Miller et al., 2001). Therefore, the cross-modal, linguistic nature of the experimental task likely contributed to slowed responses in the SLI group. Future investigations might explore performance on various measures that require processing in dual modalities or dual task processing.
The analyses also compared within-group patterns of performance by comparing RTs between pairs of conditions. These comparisons revealed differences between groups on three of the five comparisons. Slower RTs in the ignore condition reflect active inhibitory processing in the unattended channel, which is required to suppress the activation of the matching visual stimulus and unattended auditory stimulus. In the unrelated condition, conversely, this cross-modal activation would not be taking place because the unattended auditory word bore no relationship to the visual stimulus. In fact, children with TLD demonstrated this pattern, with significantly different RTs in the ignore condition compared with both the baseline and unrelated conditions. Children with SLI, however, did not show a difference in RT on the two conditions. It took them just as long to process the unrelated or noncompeting stimuli as the competing stimuli.
This comparison between the unrelated and ignore conditions is a critical one. The SLI group had apparent difficulty inhibiting both unrelated and competing stimuli in the unattended channel. One explanation is that children in this group were generally less efficient in processing and comparing the auditory stimuli with the visual stimulus. In the attend condition, where the visual stimulus matched the attended word, this cross-modal activation may have facilitated a rapid and correct decision even though an unrelated distractor stimulus was present in the unattended channel. However, in the absence of this facilitation, the children with SLI took longer to process the relevant auditory stimulus and make the comparison with the visual stimulus regardless of the type of distraction from the unattended channel. This suggests a more widespread problem with distractor processing rather than a problem specific to inhibitory control at the lexical level. Slower RTs could not be attributed uniquely to the active inhibitory processing required in the ignore condition; rather, children with SLI had difficulty inhibiting any distracting stimulus in this paradigm.
Measuring Attention Control in Children With SLI
Although other standardized and experimental tasks measure various aspects of attention, this study utilized a novel task to separate the effects of selective attention on language processing from other constructs such as sustained attention and working memory function. As previously noted, the accuracy data revealed no significant differences between groups on the ability to match auditory words and visual representations in the presence of auditory distractors. Previous studies (e.g., Hanson & Montgomery, 2002) used accuracy measures alone to determine that children with SLI did not differ from children with TLD on measures of auditory attention. A behavioral task without RT measurements might have led to the same incorrect conclusion in this case. The fact that both groups were relatively accurate indicated that the task was manageable and appropriate for this age group (9- to 12-year-olds). Had the SLI group been significantly less accurate on all conditions, this would have suggested that other factors, such as linguistic or auditory processing demands, may have been confounding the results. Thus, we propose that the experimental task targeted differences in attention control rather than other processing or linguistic skills.
RT data were used to measure differences in processing time between groups on the experimental task. As noted previously, results indicated a pattern of generalized slowing for the SLI group relative to the TLD group. There is some disagreement in the field about whether children with SLI are generally slower across modalities (Kail, 1994; Miller et al., 2001; Windsor & Hwang, 1999; Windsor et al., 2001) or slower only on cognitive–linguistic tasks. An additional factor should be taken into consideration in light of these results. Authors recently have attempted to differentiate between language and information processing profiles in children with SLI and ADHD (Cardy, Tannock, Johnson, & Johnson, 2010; Redmond et al., 2011). Cardy et al. (2010) compared SLI, ADHD, and TLD groups on measures of nonlinguistic RT as well as an auditory rapid temporal processing task. They found that children with ADHD were slower than children in both the TLD and SLI groups on both tasks. They suggest that given the high comorbidity of ADHD and SLI diagnoses, it is possible that previous findings suggesting generally slower processing in children with SLI could be attributed to limitations in attention. Given that nine of the 20 children in our SLI group received ADHD index scores above the screening cutoff on the CRS-R, we might also conclude that limitations in attention in general could be the cause of the slower RTs in the SLI group. To test this, performance on the experimental task for the nine participants who had elevated CRS-R scores was compared with that of the nine who scored below the cutoff (data were missing for two participants). There was no difference in performance between these two groups in terms of accuracy, F(1, 16) = 1.11, p = .308, or RT, F(1, 16) = 0.640, p = .436. Increased CRS-R scores did not relate to differences in performance within the SLI group. Therefore, general inattentiveness, as measured by the CRS-R, could not account for differences in performance on the experimental task. Granted, this comparison was made between two small samples. We might have chosen to exclude those participants, but it seemed imprudent to do so. The reality of SLI is that it is not really specific in clinical terms. Children with SLI are a notoriously heterogeneous group, and speech-language clinicians must evaluate and treat children with a variety of needs that differ somewhat in terms of language modality (receptive vs. expressive), language domain (phonology, morphology, syntax, semantics, pragmatics), and related cognitive skills (nonverbal cognition, executive function, attention). From a researcher's perspective, we need to find balance between controlling for confounding factors (e.g., cognitive skills below the average range, frank neurological or social–emotional dysfunction) and not limiting our sample such that it becomes an unrealistic comparison to clinical samples. Therefore, the children with elevated CRS-R scores were included and their performances were examined. Future studies should attempt to account for ADHD status more systematically in order to address these questions.
Clinical Implications and Directions for Future Research
The current study was also motivated by clinical concerns. Older elementary-age children with a history of language delay are at risk for a variety of continued language and learning difficulties (Rescorla, 2005). Difficulties with classification and diagnosis in this age group are also apparent, with overlapping diagnoses often including SLI, ADHD, dyslexia, and language-learning disability (Snowling et al., 2006). Better profiling of language and executive function skills in older children with SLI is of vital importance. To do this, however, we must better operationalize our definitions of executive functions in general and attention skills in particular. If we are to better discriminate between children with SLI and those with ADHD, we must be cognizant of which aspects of attention we are measuring in research and how they affect language processing. Although it may not be possible to isolate sublevels of attention, a better understanding of the relationship between attention and language processing would be helpful. The results of the current study seem to indicate that the attention problems measured on the CRS-R are not the same as those implicated in language processing tasks. An intriguing option to consider is that the difference is one of vigilance and self-regulation (in ADHD) versus cognitive control of attention (in SLI). However, this hypothesis should be tested systematically by controlling variables in the clinical versus control groups or by improved experimental design. Thus, converging evidence in the literature points to limitations in attention in children with SLI. Primary aims of research going forward should be to better define the aspects of attention that are impaired, examine how those aspects of attention affect language processing, and examine whether and how they develop over time in both SLI and TLD populations.
Apparent difficulties in dual-task performance also suggest that future investigations should aim to more accurately and definitively identify areas of weakness in children with SLI. Previous studies have also identified difficulties with dual-task performance in this population (Marton & Schwartz, 2003; Seiger-Gardner & Schwartz, 2008). The current task could be modified to control for effects of modality (auditory, visual, and cross-modal stimuli), domain (e.g., linguistic vs. nonlinguistic stimuli), or complexity (e.g., high vs. low processing load). Additional experiments might assess performance in a variety of dual tasks.
Summary and Conclusions
The current study utilized a novel task to examine control of auditory attention and its relationship to language processing in children with and without SLI. It contributes to a growing body of literature identifying impairments in attention and executive function in children with SLI. By utilizing a task that specifically measured attention control, the experiment avoided confounds with related constructs such as sustained attention and working memory.
Overall, children with TLD were efficient in processing linguistic stimuli, even in the presence of auditory distractors. Children with SLI were less efficient at processing the linguistic stimuli in the presence of distraction. They were slower to process lexical items when the items were presented in a cross-modal format regardless of interference. Overall, the evidence points to deficits in cognitive control of attention in children with SLI that relate directly to the children's language processing limitations.
Acknowledgments
This research was conducted as Kristen R. Victorino's dissertation under the direction of Richard G. Schwartz. Preparation of this article was supported by National Institute on Deafness and Other Communication Disorders Grant 5RO1 DC5RO1DC01141 to Richard G. Schwartz.
Funding Statement
This research was conducted as Kristen R. Victorino's dissertation under the direction of Richard G. Schwartz. Preparation of this article was supported by National Institute on Deafness and Other Communication Disorders Grant 5RO1 DC5RO1DC01141 to Richard G. Schwartz.
References
- Abbey C. K., & Eckstein M. P. (2002). Classification image analysis: Estimation and statistical inference for two-alternative forced-choice experiments. Journal of Vision, 2(1), 66–78. [DOI] [PubMed] [Google Scholar]
- Baddeley A. D. (1986). Working memory. Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- Baddeley A. D. (1996). Exploring the central executive. Quarterly Journal of Experimental Psychology: Section A, 49(1), 5–28. [Google Scholar]
- Bartgis J., Lilly A. R., & Thomas D. G. (2003). Event-related potential and behavioral measures of attention in 5-, 7-, and 9-year-olds. Journal of General Psychology, 130, 311–335. [DOI] [PubMed] [Google Scholar]
- Bishop D. V. M., Carlyon R. P., Deeks J. M., & Bishop S. J. (1999). Auditory temporal processing impairment: Neither necessary nor sufficient for causing language impairment in children. Journal of Speech, Language, and Hearing Research, 42, 1295–1310. [DOI] [PubMed] [Google Scholar]
- Brown L., Sherbenou R. J., & Johnsen S. K. (1997). The Test of Nonverbal Intelligence–Third Edition. Austin, TX: Pro-Ed. [Google Scholar]
- Brown L., Sherbenou R. J., & Johnsen S. K. (2010). The Test of Nonverbal Intelligence–Fourth Edition. Austin, TX: Pro-Ed. [Google Scholar]
- Cantwell D. P., & Baker L. (1991). Association between attention deficit-hyperactivity disorder and learning disorders. Journal of Learning Disabilities, 24, 88–95. [DOI] [PubMed] [Google Scholar]
- Caplan D., & Waters G. (2013). Memory mechanisms supporting syntactic comprehension. Psychonomic Bulletin & Review, 20, 243–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardy J., Tannock R., Johnson A., & Johnson C. (2010). The contribution of processing impairments to SLI: Insights from attention-deficit/hyperactivity disorder. Journal of Communication Disorders, 43, 77–91. [DOI] [PubMed] [Google Scholar]
- Cohen N. J., Vallance D. D., Barwick M., Im N., Menna R., Horodezky N. B., & Isaacson L. (2000). The interface between ADHD and language impairment: An examination of language, achievement, and cognitive processing. Journal of Child Psychology and Psychiatry, 41, 353–362. [PubMed] [Google Scholar]
- Conners K. C. (1997). Conners' Rating Scales: Revised. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Cool Edit Pro. (1998). [Computer software]. San Jose, CA: Syntrillium Software. [Google Scholar]
- Cowan N., Elliott E. M., Scott Saults J., Morey C. C., Mattox S., Hismjatullina A., & Conway A. R. (2005). On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology, 51, 42–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz Y., Friedman D., Rothstein M., & Snodgrass J. (1997). Picture naming by young children: Norms for name agreement, familiarity, and visual complexity. Journal of Experimental Child Psychology, 65, 171–237. [DOI] [PubMed] [Google Scholar]
- Duncan J., Johnson R., Swales M., & Freer C. (1997). Frontal lobe deficits after head injury: Unity and diversity of function. Cognitive Neuropsychology, 14, 713–741. [Google Scholar]
- Ellis Weismer S., Evans J., & Hesketh L. J. (1999). An examination of verbal working memory capacity in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 42, 1249–1260. [DOI] [PubMed] [Google Scholar]
- Engle R. (2002). Working memory capacity as executive attention. Current Directions in Psychological Science, 11, 19–23. [Google Scholar]
- Engle R. W. (2010). Role of working memory capacity in cognitive control. Current Anthropology, 51, S17–S26. [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., & Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Finneran D., Francis A., & Leonard L. (2009). Sustained attention in children with specific language impairment (SLI). Journal of Speech, Language, and Hearing Research, 52, 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat E., & Spaulding T. J. (2014). Differences in the performance of children with specific language impairment and their typically developing peers on nonverbal cognitive tests: A meta-analysis. Journal of Speech, Language, and Hearing Research, 57, 1363–1382. [DOI] [PubMed] [Google Scholar]
- Gathercole S. E., & Baddeley A. D. (1990). Phonological memory deficits in language disordered children: Is there a causal connection? Journal of Memory and Language, 29, 336–360. [Google Scholar]
- Gillam R., Montgomery J., & Gillam S. (2009). Memory and attention in children with language impairments. In Schwartz R. G. (Ed.), Handbook of child language disorders (pp. 201–225). New York, NY: Psychology Press. [Google Scholar]
- Goldfarb L., & Henik A. (2006). New data analysis of the Stroop matching task calls for a reevaluation of theory. Psychological Science, 17, 96–100. [DOI] [PubMed] [Google Scholar]
- Gomes H., Motholm S., Christodoulou C., Ritter W., & Cowan N. (2000). The development of auditory attention in children. Frontiers in Bioscience, 5, 108–120. [DOI] [PubMed] [Google Scholar]
- Hale J. B., How S. K., Dewitt M. B., & Coury D. L. (2001). Discriminant validity of the Conners' scales for ADHD subtypes. Current Psychology, 20, 231–249. [Google Scholar]
- Hanson R. A., & Montgomery J. W. (2002). Effects of general processing capacity and sustained selective attention on temporal processing performance of children with specific language impairment. Applied Psycholinguistics, 23, 75–93. [Google Scholar]
- Helzer J. R., Champlin C. A., & Gillam R. B. (1996). Auditory temporal resolution in specifically language-impaired and age-matched children. Perceptual and Motor Skills, 83, 1171–1181. [DOI] [PubMed] [Google Scholar]
- Hesketh A., & Conti-Ramsden G. (2013). Memory and language in middle childhood in individuals with a history of specific language impairment. PLoS One, 8(2), e56314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hick R., Botting N., & Conti-Ramsden G. (2005). Cognitive abilities in children with specific language impairment: Consideration of visuo-spatial skills. International Journal of Language & Communication Disorders, 40, 137–149. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. (1975). Four-factor index of social status. Unpublished manuscript, Yale University, New Haven, CT.
- Howell D. (2010). Fundamental statistics for the behavioral sciences (7th ed.). Belmont, CA: Wadsworth Cengage Learning. [Google Scholar]
- Im-Bolter N., Johnson J., & Pascual-Leone J. (2006). Processing limitations in children with specific language impairment: The role of executive function. Child Development, 77, 1822–1841. [DOI] [PubMed] [Google Scholar]
- Kail R. (1994). A method for studying the generalized slowing hypothesis in children with specific language impairment. Journal of Speech and Hearing Research, 37, 418–421. [DOI] [PubMed] [Google Scholar]
- Kane M. J., & Engle R. W. (2003). Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General, 132, 47–70. [DOI] [PubMed] [Google Scholar]
- Kohnert K., & Windsor J. (2004). The search for common ground, part II: Nonlinguistic performance by linguistically diverse learners. Journal of Speech, Language, and Hearing Research, 47, 891–903. [DOI] [PubMed] [Google Scholar]
- Leonard L., Weismer S., Miller C., Francis D., Tomblin J., & Kail R. (2007). Speed of processing, working memory, and language impairment in children. Journal of Speech, Language, and Hearing Research, 50, 408–428. [DOI] [PubMed] [Google Scholar]
- Marton K. (2008). Visuo-spatial processing and executive functions in children with specific language impairment. International Journal of Language & Communication Disorders, 43, 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton K., Campanelli L., Eichorn N., Scheuer J., & Yoon J. (2014). Information processing and proactive interference in children with and without specific language impairment. Journal of Speech, Language, and Hearing Research, 57, 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton K., Kelmenson L., & Pinkhasova M. (2007). Inhibition control and working memory capacity in children with SLI. Psychologia, 50, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton K., & Schwartz R. G. (2003). Working memory capacity and language processes in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 46, 1138–1153. [DOI] [PubMed] [Google Scholar]
- Miller C., Kail R., Leonard L., & Tomblin J. (2001). Speed of processing in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 44, 416–433. [DOI] [PubMed] [Google Scholar]
- Miller C., Leonard L., Kail R., Zhang X., Tomblin J., & Francis D. (2006). Response time in 14-year-olds with language impairment. Journal of Speech, Language, and Hearing Research, 49, 712–728. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., & Wager T. D. (2000). The unity and diversity of executive functions and their contributions to “complex” frontal lobe tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Montgomery J. (2000). Verbal working memory and sentence comprehension in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 43, 293–308. [DOI] [PubMed] [Google Scholar]
- Montgomery J. (2002). Understanding the language difficulties of children with specific language impairments: Does verbal working memory matter? American Journal of Speech-Language Pathology, 11, 77–91. [Google Scholar]
- Montgomery J., Evans J. L., & Gillam R. B. (2009). Relation of auditory attention and complex sentence comprehension in children with specific language impairment: A preliminary study. Applied Psycholinguistics, 30, 123–151. [Google Scholar]
- Nelson D. L., McEvoy C. L., & Schreiber T. A. (1998). The University of South Florida word association, rhyme, and word fragment norms. Retrieved from http://www.usf.edu/FreeAssociation/ [DOI] [PubMed] [Google Scholar]
- Noterdaeme M., Amorosa H., Mildenberger K., Sitter S., & Minow F. (2001). Evaluation of attention problems in children with autism and children with a specific language disorder. European Child and Adolescent Psychiatry, 10, 58–66. [DOI] [PubMed] [Google Scholar]
- Oldfield R. (1971). The assessment and analysis of handedness: The Edinburg Inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Posner M. I., & Mitchell R. F. (1967). Chronometric analysis of classification. Psychological Review, 74, 392–409. [DOI] [PubMed] [Google Scholar]
- Redmond S. (2002). The use of rating scales with children who have language impairments. American Journal of Speech-Language Pathology, 11, 124–138. [Google Scholar]
- Redmond S. (2005). Differentiating SLI from ADHD using children's sentence recall and production of past tense morphology. Clinical Linguistics & Phonetics, 19, 109–127. [DOI] [PubMed] [Google Scholar]
- Redmond S., Thompson H., & Goldstein S. (2011). Psycholinguistic profiling differentiates specific language impairment from typical development and from attention-deficit/hyperactivity disorder. Journal of Speech, Language, and Hearing Research, 54, 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla L. (2005). Age 13 language and reading outcomes in late-talking toddlers. Journal of Speech, Language, and Hearing Research, 48, 459–472. [DOI] [PubMed] [Google Scholar]
- Rice M. L., & Wexler K. (2001). Test of Early Grammatical Impairment. New York, NY: The Psychological Corporation. [Google Scholar]
- Schuchardt K., Bockmann A.-K., Bornemann G., & Maehler C. (2013). Working memory functioning in children with learning disorders and specific language impairment. Topics in Language Disorders, 33, 298–312. [Google Scholar]
- Seiger-Gardner L., & Schwartz R. (2008). Lexical access in children with and without specific language impairment: A cross-modal picture-word interference study. International Journal of Language & Communication Disorders, 43, 528–551. [DOI] [PubMed] [Google Scholar]
- Semel E., Wiig E. H., & Secord W. A. (1996). Clinical Evaluation of Language Fundamentals–Third Edition. San Antonio, TX: Psychological Corp. [Google Scholar]
- Semel E., Wiig E. H., & Secord W. A. (2004). Clinical Evaluation of Language Fundamentals–Fourth Edition. San Antonio, TX: Psychological Corp. [Google Scholar]
- Snodgrass J. G., & Vanderwart M. (1980). A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Learning, Memory, and Cognition, 12, 147–154. [DOI] [PubMed] [Google Scholar]
- Snowling M. J., Bishop D. V. M., Stothard S. E., Chipchase B., & Kaplan C. (2006). Psychosocial outcomes at 15 years of children with a preschool history of speech-language impairment. Journal of Child Psychology and Psychiatry, 47, 759–765. [DOI] [PubMed] [Google Scholar]
- Spaulding T. (2010). Investigating mechanisms of suppression in preschool children with specific language impairment. Journal of Speech, Language, and Hearing Research, 53, 725–738. [DOI] [PubMed] [Google Scholar]
- Spaulding T., Plante E., & Vance R. (2008). Sustained selective attention skills of preschool children with specific language impairment: Evidence for separate attentional capacities. Journal of Speech, Language, and Hearing Research, 51, 16–34. [DOI] [PubMed] [Google Scholar]
- Stanislaw H., & Todorov N. (1999). Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers, 31, 137–149. [DOI] [PubMed] [Google Scholar]
- Stevens C., Fanning J., Coch D., Sanders L., & Neville H. (2008). Neural mechanisms of selective auditory attention are enhanced by computerized training: Electrophysiological evidence from language-impaired and typically developing children. Brain Research, 1205, 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Sanders L., & Neville H. (2006). Neurophysiological evidence for selective auditory attention deficits in children with specific language impairment. Brain Research, 1111, 143–152. [DOI] [PubMed] [Google Scholar]
- Teuber H. L. (1972). Unity and diversity of frontal lobe functions. Acta Neurobiologia Experimentalis, 32, 617–656. [PubMed] [Google Scholar]
- Unsworth N., & Spillers G. (2010). Working memory capacity: Attention control, secondary memory, or both? A direct test of the dual-component model. Journal of Memory and Language, 62, 392–406. [Google Scholar]
- van Daal J., Verhoeven L., & van Balkom H. (2009). Cognitive predictors of language development in children with specific language impairment (SLI). International Journal of Language & Communication Disorders, 44, 639–655. [DOI] [PubMed] [Google Scholar]
- Velez M., & Schwartz R. G. (2010). Spoken word recognition in school-age children with SLI: Semantic, phonological, and repetition priming. Journal of Speech, Language, and Hearing Research, 53, 1616–1628. [DOI] [PubMed] [Google Scholar]
- Windsor J., & Hwang M. (1999). Testing the generalized slowing hypothesis in specific language impairment. Journal of Speech, Language, and Hearing Research, 42, 1205–1218. [DOI] [PubMed] [Google Scholar]
- Windsor J., Milbrath R., Carney E., & Rakowski S. (2001). General slowing in language impairment: Methodological considerations in testing the hypothesis. Journal of Speech, Language, and Hearing Research, 44, 446–461. [DOI] [PubMed] [Google Scholar]