Abstract
Background
Because retroperitoneal soft tissue sarcomas (RPS) are extremely rare, there is a significant lack of clinicopathologic information to optimize the treatment strategy. The aim of this study was to evaluate the prognostic factors in RPS, with particular focus on the Ki-67 labeling index (LI).
Methods
We included the data from a total of 23 patients who received treatment for primary RPS at a single center. The variables analyzed in this study included tumor size, histological type, malignancy grade, necrosis, mitosis, and Ki-67 LI. Kaplan-Meier and Cox proportional regression analyses of overall survival (OS) were performed to identify significant prognostic variables.
Results
Of the 23 patients who underwent surgical resection, 9 (39 %) underwent simple resection of the tumor and 14 (61 %) extended resection including the adjacent organs. In the univariate analysis, a simple tumor resection and a high Ki-67 LI were associated with shorter OS. The multivariate analysis revealed that simple tumor resection and a high Ki-67 LI were independent negative prognostic factors for OS.
Conclusions
Our results suggested that combined resection of RPS and its adjacent organs improved OS. Pathologically, a high Ki-67 LI was significantly associated with negative prognosis.
Keywords: Extended resection, Ki-67, Prognostic factor, Retroperitoneal, Soft tissue sarcoma
Background
Retroperitoneal soft tissue sarcomas (RPS) are uncommon tumors, accounting for approximately 12 % of soft tissue sarcomas [1]. The mean annual incidence is approximately 2.7 per 100,000 [2], and the 5-year survival is approximately 50 % [3]. Local recurrence is common (41–68 %), and metastases occur in one third of the patients after surgical resection [3]. Local recurrence is the leading cause of death in patients with RPS. Surgery is currently the only modality that offers a chance of cure.
Extended resection including the adjacent organs is suggested regardless of the presence of neoplastic infiltration [4–7]. On the other hand, the surgery for RPS presents specific challenges due to the location in a complex space surrounded by vital structures and visceral organs, and an extended surgery is not practical in all cases [8–10]. Adjuvant therapy such as radiotherapy and/or systemic chemotherapy is usually considered because the risk of local recurrence after surgery alone is relatively high.
Prognostic factors in RPS are important for selecting appropriate therapies and follow-up after surgical resection. The currently used prognostic factors include age, tumor size, histopathological type, malignancy grade, and positive resection margins; these are combined into different prognostic systems [3, 5, 11–15]. To improve the prognostic accuracy, researchers have been trying to incorporate an assessment of the biological features, such as proliferation activity, into the prediction tools for disease prognosis. The Ki-67 labeling index (LI), assessed with immunohistochemical staining using a mouse monoclonal antibody named MIB-1 clone, and based on the proportion of Ki-67-positive tumor cells, is known to be useful for objective histopathological evaluation of the tumor proliferation activity. A high Ki-67 LI is associated with poor prognosis in soft tissue sarcomas [16, 17]. Ki-67 has also been recognized as essential for diagnosing tumor grade. The purpose of this study was to evaluate the currently used prognostic factor and to clarify the prognostic significance of Ki-67 in RPS in a Japanese single-center cohort.
Methods
Patients and data collection
The Nara Medical University institutional review board approved this study, and all participants provided informed consent. This is a retrospective study of 26 cases with RPS, treated between January 2002 and January 2014 at Nara Medical University. In three patients, only needle biopsy was carried out for the verification of pathology, because significant comorbidities and/or advanced stage made them unsuitable for a radical resection. One of these patients was treated with palliative care alone, one with radiation therapy, and one with chemotherapy. These three patients were excluded, leaving 23 cases. The type of surgery was categorized into two groups: simple resection (surgical resection of the tumor mass only) and extended resection (surgical resection of the tumor mass and the adjacent organs). All the hematoxylin and eosin (H&E)-stained specimens were re-evaluated for diagnosis by two experienced pathologists (KS and KN), following the World Health Organization (WHO) criteria for classification of soft tissue tumors [18]. The tumor variables analyzed included tumor size, histological type, malignancy grade [19], presence of necrosis, and cell mitosis.
Calculation of the Ki-67 labeling index
Immunohistochemical (IHC) staining for Ki-67 was performed with a streptavidin-biotin (SAB) complex method using the Histofine SAB-PO kit (Nichirei Co., Tokyo, Japan) according to the manufacturer’s directions. For antigen retrieval, the sections were routinely autoclaved for 10 min in 0.01 M citrate buffer (pH 6.0). The primary antibody was monoclonal mouse anti-Ki-67 antigen (clone MIB-1, Dako, Japan), ready to use at room temperature for 30 min. The sections were counterstained with Meyer’s hematoxylin and mounted with Malinol and were examined alongside H&E-stained specimens, to identify the precise locations of the lesions. Five independent areas in the sections were selected with a high-power field (×400) and saved as pictures. IHC evaluation was carried out by two investigators (YM and YT). Evaluation was performed blindly without knowledge of the patients’ outcome and other clinicopathologic characteristics. The percentages of positive cells (Ki-67 LI) were calculated. The cutoff point was determined by a receiver operating characteristics (ROC) curve for tumor recurrence.
Statistical analysis
The primary endpoint was overall survival (OS). The cutoff date of the last follow-up was December 31, 2015. The OS was computed from the date of primary tumor resection to the date of death or the last follow-up and examined using the Kaplan-Meier method. Univariate and multivariate analyses were performed for OS using the Cox proportional hazards models. All tests were two-sided. The results of Cox model analysis are reported with relative risks and 95 % confidence intervals (CIs). IBM SPSS version 21 (SPSS Inc., Chicago, IL) and PRISM software version 5.00 (GraphPad Software, Inc., San Diego, CA) were used for statistical analyses and data plotting, respectively. A P value of <0.05 was considered statistically significant.
Results
Patient characteristics and clinical and operative findings
The median patient age was 62.5 years (range 31–79 years). There were 15 male (65 %) and 8 female participants (35 %). The clinicopathologic variables of the patients and primary tumors are presented in Table 1. Nine received a simple resection of the tumor and 14 an extended resection including the adjacent organs. Type of surgery was decided by preoperative imaging or intraoperative examination. Ten patients underwent the extended resection because they were diagnosed as infiltration into the adjacent organs by preoperative imaging. In one of these ten patients, whose tumor infiltrated to the kidney and diaphragm, only the tumor and kidney were resected. Four patients underwent the extended resection because the surgeons suspected the infiltration by intraoperative examination. One patient who was diagnosed as infiltration into the adjacent organs performed the simple resection because resection of the adjacent organs was impossible for tumor adhesion. The main resected organs were the kidney (6/10, 60 %), colon (4/10, 40 %), and bladder (3/10, 30 %). In four patients, two adjacent organs were resected, and in two patients, three organs were resected en bloc. In all patients, there were no major perioperative complications more than grade 2 in Clavien classification.
Table 1.
Clinicopathologic background according to the disease status
| Variables | All (n = 23) | Simple resection (n = 9) | Extended resection (n = 14) | P value |
|---|---|---|---|---|
| Median age, years (IQR) | 62 (51.5–71) | 62 (42–70) | 62 (52.5–71) | 0.68a |
| Sex, n (%) | 0.57b | |||
| Men | 15 (65 %) | 7 (78 %) | 8 (57 %) | |
| Women | 8 (35 %) | 2 (22 %) | 6 (43 %) | |
| Follow-up, months (IQR) | 30 (18–51.5) | 19 (18–33) | 30.5 (24–87.5) | 0.12a |
| Tumor size, mm (IQR) | 77 (50–210) | 80 (50–120) | 75 (51–112) | 0.99a |
| Symptoms positive, n (%) | 0.87b | |||
| Yes | 12 (52 %) | 4 (44 %) | 8 (57 %) | |
| No | 11 (48 %) | 5 (56 %) | 6 (43 %) | |
| Infiltration into the adjacent organs, n (%) | 0.01*,b | |||
| Yes | 11 (48 %) | 1 (11 %) | 10 (71 %) | |
| No | 12 (52 %) | 8 (89 %) | 4 (29 %) | |
| Margins, n (%) | 0.94b | |||
| R0 | 4 (17 %) | 2 (22 %) | 2 (14 %) | |
| R1 | 17 (74 %) | 6 (67 %) | 11 (79 %) | |
| R2 | 2 (9 %) | 1 (11 %) | 1 (7 %) | |
| Histological type, n (%) | 0.87b | |||
| Liposarcoma | 12 (52 %) | 4 (44 %) | 8 (57 %) | |
| Leiomyosarcoma | 4 (17 %) | 2 (22 %) | 2 (14 %) | |
| MFH | 3 (14 %) | 1 (11 %) | 2 (14 %) | |
| Other | 4 (17 %) | 2 (22 %) | 2 (14 %) | |
| Tumor grade, n (%) | 0.82b | |||
| Grade 1 | 3 (14 %) | 1 (11 %) | 2 (14 %) | |
| Grade 2 | 4 (17 %) | 2 (22 %) | 2 (14 %) | |
| Grade 3 | 16 (69 %) | 6 (67 %) | 10 (71 %) | |
| Necrosis, n (%) | 0.88b | |||
| Yes | 17 (74 %) | 7 (78 %) | 10 (71 %) | |
| No | 6 (26 %) | 2 (22 %) | 4 (29 %) | |
| Mitosis, n (%) | 0.61b | |||
| 1–9/10HPF | 14 (61 %) | 5 (56 %) | 9 (64 %) | |
| 10–20/HPF | 9 (39 %) | 4 (44 %) | 5 (36 %) | |
| Ki-67 LI, n (%) | 0.42b | |||
| <25 % | 13 (56 %) | 4 (44 %) | 9 (64 %) | |
| ≥25 % | 10 (44 %) | 5 (56 %) | 5 (36 %) |
Simple resection: resection of only the tumor; extended resection: resection extended to adjacent organs; tumor grade: using the French Federation of Cancer Centers Sarcoma Group grading system
IQR interquartile range, R0 microscopically negative margin, R1 microscopically positive margin, R2 gross residual margin, MFH malignant fibrous histiocytoma, HPF high-power field, Ki-67 LI Ki-67 labeling index
aMann-Whitney test
bFisher’s exact test or chi-square with Yates’ correction
* = P <0.05
Pathological findings
The majority of the tumors were of high grade, and the most common histology was liposarcoma (52 %). Other histologies included leiomyosarcoma (17 %), malignant fibrous histiocytoma (MFH) (14 %), and others (synovial sarcoma, epithelioid sarcoma, Ewing’s sarcoma, and not otherwise-specified sarcoma). Histological type other than liposarcoma (non-liposarcoma), tumor necrosis, cell mitosis, and malignancy grade were used as prognostic factors for RPS. Cox analysis was performed to evaluate the association between these factors and OS. None of them was significant.
The Ki-67 LI was evaluated in all cases (Fig. 1). The median Ki-67 LI was 25 % (range 2–53 %). The cutoff point was determined as 25 % according to the ROC curve for tumor recurrence. To evaluate the prognostic significance of the Ki-67 LI, the Kaplan-Meier and the COX analyses were performed. In univariate analysis, tumors with a Ki-67 LI of 25 % or more showed worse prognosis than those with a Ki-67 LI of less than 25 % (P = 0.001). Non-liposarcoma histopathology was not a negative prognostic factor compared to liposarcoma histopathology in our cohort; however, there was a significant association between a high Ki-67 LI and histological type (P = 0.047, median Ki-67 LI = 28.48 and 18.90 in liposarcoma and non-liposarcoma, respectively).
Fig. 1.
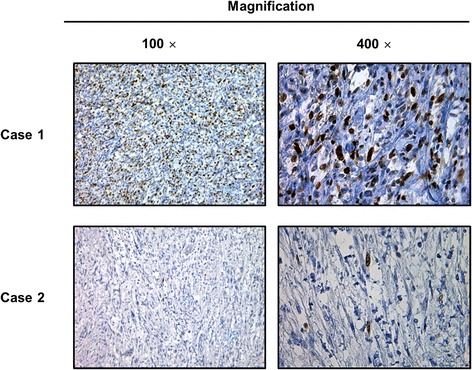
Ki-67 immunohistochemical staining of two representative cases of retroperitoneal liposarcoma. Case 1 was diagnosed with liposarcoma with a Ki-67 labeling index of 50 %. He experienced local recurrence and lung metastasis 19 months after surgical resection and died 18 months after surgical resection. Case 2 was diagnosed with liposarcoma with a Ki-67 labeling index of 2 %. He was alive without any evidence of disease recurrence at the last visit, 108 months after the surgical resection
Follow-up and overall survival
The median follow-up period was 30 months (range 6–116 months) after surgical resection. Of the 13 patients experiencing recurrence, 6 patients experienced local recurrence in the original tumor site, whereas 7 patients developed distant metastasis. The most common site of distant metastasis was lung (in four patients). Eleven patients died of tumor progression in 6 months after recurrence. Ten patients did not present evidence of disease recurrence after surgical resection, and the median OS was 52 months. In univariate Kaplan-Meier analyses, the extended resection including the adjacent organs was associated with the probability of OS (3-year OS 78 % vs. 0 %, Fig. 2b). Ki-67 LI was associated with the probability of OS (3-year OS 69 % vs. 27 %, Fig. 2c). In multivariate Cox models, age, symptoms, tumor size, resection margins, histological type, tumor grade, and necrosis were not significant prognostic factors for OS, while the type of surgery and the Ki-67 LI were significant (Table 2). In multivariate analysis, simple resection and high Ki-67 LI were independent predictive factors for decreased OS (Table 2).
Fig. 2.
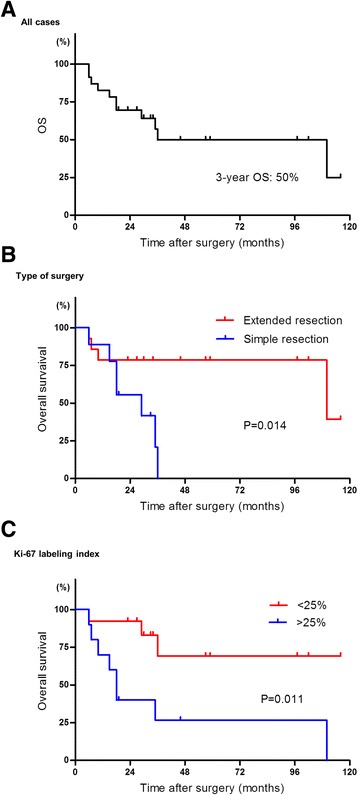
Kaplan-Meier curves of overall survival (OS) after surgical resection. a OS in all cases. All the events were due to tumor progression. b OS according to the type of surgery. The extended surgery improved OS in RPS. c OS according to the Ki-67 labeling index. P values were calculated using the log-rank test
Table 2.
Univariate and multivariate analysis of prognostic factors for overall survival
| Factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | P | HR | 95 % CI | P | |
| Age (over/under 75 years old) | 0.66 | 0.08–5.24 | 0.70 | |||
| Symptoms (symptomatic/asymptomatic) | 2.44 | 0.63–9.49 | 0.20 | |||
| Tumor size (over 10 mm/under 10 mm) | 1.49 | 0.45–4.95 | 0.52 | |||
| Type of surgery (simple/extended) | 4.88 | 1.21–19.66 | *0.026 | 3.80 | 1.25–16.59 | *0.040 |
| Surgical margins [(R1 + R2)/R0] | 3.35 | 0.42–26.90 | 0.26 | |||
| Tumor grade [grade 3/(grade 1 + 2)] | 1.49 | 0.31–7.16 | 0.71 | |||
| Necrosis (presence/absence) | 3.05 | 0.38–24.46 | 0.29 | |||
| Histologic type (non-liposarcoma/liposarcoma) | 3.82 | 0.98–14.89 | 0.054 | |||
| Ki-67 LI (≥25 %/<25 %) | 4.79 | 1.25–18.35 | *0.022 | 3.74 | 1.32–14.94 | *0.049 |
Simple: only tumor resection; extended: resection with adjacent organs
R0 microscopically negative margin, R1 microscopically positive margin, R2 gross residual margin, HR hazard ratio, CI confidence interval, Ki-67 LI Ki-67 labeling index, * = P <0.05
Discussion
RPS is a rare neoplasm and is usually locally advanced at the time of diagnosis [3]. The mainstay of treatment is surgery. The RPS borders with the surrounding organs, including the kidney, adrenal, colon, ureter, bladder, spleen, pancreas, and psoas, are usually difficult to identify. Essentially all the macroscopic total resections consist of marginal excisions through the reactive zone surrounding the tumor; therefore, the margins are microscopically positive in the majority of the patients, and the probability of local recurrence after surgery alone is high [1, 2, 7]. In this study, the resection margin had no relevance to OS. The rate of positive resection margin (R1 or R2) was very high (83 %,19/23); nevertheless, the extended resection of adjacent organs, regardless of resection margin positivity, was a significant predictor of OS.
In contrast to the more common epithelial tumors, which develop within a single organ, RPS can infiltrate multiple surrounding organs [20]. The oncologic benefit of extended surgery is associated with a lower rate of local recurrence [6, 7]. However, the feasibility of such resection is variable, depending on the exact relationships of the tumor with other structures within the anatomical complexity of the retroperitoneum. Resection of more than three adjacent organs has been shown to be associated with a significantly higher complication rate than the resection of fewer organs [10]. In this study, resections of three adjacent organs were performed in two patients and of one or two organs in 12 patients; in all these cases, there were no major perioperative complications. In one case of tumor infiltration into multiple adjacent organs, a complete resection was not attempted. Although the extended resection of adjacent organs regardless of tumor infiltration is the gold standard [6, 7, 21], significant controversy persists regarding the benefit and associated morbidity of multi-organ resection. Some authors have proposed to perform surgery with the goal of complete macroscopic resection and to resect contiguous organs only when directly infiltrated [8–10]. Four patients who subjected to extended resection did not show tumor infiltration to the adjacent organs by diagnostic imaging, but the surgeons suspected the direct infiltration to contiguous organs by intraoperative examination. All of them did not experience recurrence.
Tumor size, malignancy grade, positive margins, and histological type are currently used as prognostic factors for RPS [3, 5, 11–15, 19]. In this study, high expression of Ki-67 was a significant predictor of OS. The Ki-67 antigen is a cell cycle-associated nuclear protein and is present in all stages of the cell cycle (G1, S, G2, and M phases) in proliferating cells but is absent in G0 and early G1 phases of cells re-entering the cell cycle [22]. The antibody against Ki-67 has been documented to be useful for the diagnosis and prognosis of some neoplasms. The Ki-67 LI is correlated with shorter disease-specific survival in soft tissue sarcomas [16, 17] and is expected to be one of the adverse prognostic factors in RPS. Because the risk of local recurrence after surgery alone is very high, adjuvant therapy should be considered [3]. Adjuvant chemotherapy or radiotherapy improved local tumor control in high-risk patients [23–25]. Therefore, the high-Ki-67 LI group represents a good candidate for adjuvant therapy.
This investigation confirmed the finding that, in patients with primary RPS, extended resection of the adjacent organs was an independent prognostic factor. However, in patients whose tumors do not infiltrate the adjacent organs according to clinical imaging, it is problematic to decide if the adjacent organs should be resected en bloc. In nine patients who subjected to simple tumor resection, eight did not show tumor infiltration to the adjacent organs by diagnostic imaging or intraoperative examination. But because seven of these eight patients experienced evidence of disease recurrence, extended resection including the adjacent organs might have been required to remove the whole tumor burden. Preoperative core needle biopsy in RPS has been reported to be useful to obtain a histological diagnosis [26]. The assessment of the Ki-67 LI by preoperative biopsy would allow selection of the appropriate operation method.
The limitations of the present study include a small sample size and the retrospective analysis. As only one patient received adjuvant chemotherapy, it was difficult to assess the importance of adjuvant therapy. Eight patients received chemotherapy or radiotherapy (chemotherapy only, 5 patients; chemotherapy + radiotherapy, 3 patients) after evidence of disease recurrence.
Conclusions
Our results suggested that combined resection of RPS and its adjacent organs improved OS. Pathologically, a high Ki-67 LI was a significant predictor of OS.
Acknowledgements
Dr Shinji Tsukamoto and Dr Honoki Kanya (Department of Orthopedic Surgery, Nara Medical University, Nara, Japan) provided support for sample collection.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YM participated in the conception and design. MM, NK, and KF helped to draft the manuscript. KS participated in the analysis and interpretation of the data. SH participated in the clinical revision of the manuscript for important intellectual content. YT carried out immunoassays and performed the statistical analysis. YN, SA, and NT participated in the technical or material support. All authors read and approved the final manuscript.
Contributor Information
Yosuke Morizawa, Email: tigers.yosuke@gmail.com.
Makito Miyake, Email: makitomiyake@yahoo.co.jp.
Keiji Shimada, Email: keijishi@naramed-u.ac.jp.
Shunta Hori, Email: horimaus@gmail.com.
Yoshihiro Tatsumi, Email: takuro.birds.nest@gmail.com.
Yasushi Nakai, Email: unnkoderu@hotmail.com.
Satoshi Anai, Email: sanai@naramed-u.ac.jp.
Nobumichi Tanaka, Email: sendo@nmu-gw.naramed-u.ac.jp.
Noboru Konishi, Email: nkonishi@naramed-u.ac.jp.
Kiyohide Fujimoto, Phone: +81-744-22-3051, Email: kiyokun@naramed-u.ac.jp.
References
- 1.Gutierrez JC, Perez EA, Franceschi D, Moffat FL, Jr, Livingstone AS, Koniaris LG. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J Surg Res. 2007;141:105–14. doi: 10.1016/j.jss.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Porter GA, Baxter NN, Pisters PW. Retroperitoneal sarcoma: a population-based analysis of epidemiology, surgery, and radiotherapy. Cancer. 2006;106:1610–6. doi: 10.1002/cncr.21761. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Zlotecki RA, Hochwald SN, Hemming AW, Grobmyer SR, Cance WG. Retroperitoneal soft tissue sarcoma. Cancer. 2005;104:669–75. doi: 10.1002/cncr.21264. [DOI] [PubMed] [Google Scholar]
- 4.Stoeckle E, Coindre JM, Bonvalot S, et al. French Federation of Cancer Centers Sarcoma Group: prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–68. doi: 10.1002/1097-0142(20010715)92:2<359::AID-CNCR1331>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson IC, Whitwel DJ, Battilstuta D, et al. Surgical margin and its influence on survival in soft tissue sarcoma. ANZ J Surg. 2006;76:104–9. doi: 10.1111/j.1445-2197.2006.03615.x. [DOI] [PubMed] [Google Scholar]
- 6.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:31–7. doi: 10.1200/JCO.2008.21.5467. [DOI] [PubMed] [Google Scholar]
- 7.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–7. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 8.Hueman MT, Herman JM, Ahuja N. Management of retroperitoneal sarcomas. Surg Clin North Am. 2008;88:583–97. doi: 10.1016/j.suc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Swallow CJ, Catton CN. Improving outcome for retroperitoneal sarcomas: a work in progress. Surg Oncol Clin N Am. 2012;21:317–31. doi: 10.1016/j.soc.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Gronchi A, Pollock RE. Quality of local treatment or biopsy of tumor: which are the trump cards for loco-regional control of retroperitoneal sarcoma? Ann Surg Oncol. 2013;20:2111–3. doi: 10.1245/s10434-013-2971-0. [DOI] [PubMed] [Google Scholar]
- 11.Kiatisevi P, Asavamongkolkul A, Phimolsarnti R, Waikakul S, Benjarassamerote S. The outcomes and prognostic factors of patients with soft-tissue sarcoma. J Med Assoc Thai. 2006;89:334–42. [PubMed] [Google Scholar]
- 12.Koea JB, Leung D, Lewis JJ, Brennan MF. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol. 2003;10:432–40. doi: 10.1245/ASO.2003.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Raney RB, Jr, Crist WM, Maurer HM, Foulkes MA. Prognosis of children with soft tissue sarcoma who relapse after achieving a complete response. A report from the Intergroup Rhabdomyosarcoma study I. Cancer. 1983;52:44–50. doi: 10.1002/1097-0142(19830701)52:1<44::AID-CNCR2820520110>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 14.Yang RS, Lane JM, Eilber FR, et al. High grade soft tissue sarcoma of the flexor fossae. Size rather than compartmental status determine prognosis. Cancer. 1995;76:1398–405. doi: 10.1002/1097-0142(19951015)76:8<1398::AID-CNCR2820760815>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:739–47. doi: 10.1016/S0360-3016(03)00714-4. [DOI] [PubMed] [Google Scholar]
- 16.Sorbye SW, Kilvaer TK, Valkov A, et al. Prognostic impact of Jab1, p16, p21, p62, Ki67 and Skp2 in soft tissue sarcomas. PLoS One. 2012;7:e47068. doi: 10.1371/journal.pone.0047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa T. Histological grading and MIB-1 labeling index of soft-tissue sarcomas. Pathol Int. 2007;57:121–5. doi: 10.1111/j.1440-1827.2006.02068.x. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. World Health Organization classification of tumours of soft tissue and bone. 4. Lyon: IARC; 2013. [Google Scholar]
- 19.Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15:350–62. doi: 10.1200/JCO.1997.15.1.350. [DOI] [PubMed] [Google Scholar]
- 20.Mussi C, Colombo P, Bertuzzi A, et al. Retroperitoneal sarcoma: is it time to change the surgical policy? Ann Surg Oncol. 2011;18:2136–42. doi: 10.1245/s10434-011-1742-z. [DOI] [PubMed] [Google Scholar]
- 21.Piters PW. Resection of some—but not all—clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. J Clin Oncol. 2009;27:6–8. doi: 10.1200/JCO.2008.18.7138. [DOI] [PubMed] [Google Scholar]
- 22.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 23.Trovik LH, Ovrebo K, Almquist M, et al. Adjuvant radiotherapy in retroperitoneal sarcomas. A Scandinavian Sarcoma Group study of 97 patients. Acta Oncol. 2014;53:1165–72. doi: 10.3109/0284186X.2014.921723. [DOI] [PubMed] [Google Scholar]
- 24.Tuan J, Vitolo V, Vischioni B, et al. Radiation therapy for retroperitoneal sarcoma. Radiol. 2014;119:790–802. doi: 10.1007/s11547-013-0350-3. [DOI] [PubMed] [Google Scholar]
- 25.Angele MK, Albertsmeier M, Prix NJ, et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: a subgroup analysis of a randomized phase-III multicenter study. Ann Surg. 2014;260:749–54. doi: 10.1097/SLA.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson MJ, Martin JL, Khan AA, Hayes AJ, Thomas JM, Strauss DC. Percutaneous core needle biopsy in retroperitoneal sarcomas does not influence local recurrence or overall survival. Ann Surg Oncol. 2015;22:853–8. doi: 10.1245/s10434-014-4059-x. [DOI] [PubMed] [Google Scholar]


