Abstract
Tinnitus is the perception of a “phantom sound” and has a high prevalence. Although many therapies have been investigated within the last decades, there is still no effective standard therapy. Animal studies and human functional imaging studies revealed that tinnitus perception is associated with many complex changes in multiple brain structures. There is growing evidence that brain stimulation might be able to interrupt the local altered neuronal activity and hereby inhibit tinnitus perception. In this editorial review, an update is given on the most promising targets for brain stimulation. Promising structures for stimulation are the dorsal cochlear nucleus, the inferior colliculus and the medial geniculate body of the thalamus. For cortical stimulation, the auditory cortex is considered as a target. Nevertheless, the field is waiting for evidence from well-designed clinical trials, based on supporting evidence from experimental/mechanistic research, to support or discourage the application of brain stimulation in tinnitus.
Key Words: Deep brain stimulation, electric stimulation, neuromodulation, tinnitus, treatment
Currently, up to 15% of the general population suffers chronically from the perception of a “phantom sound”, also known as tinnitus.[17,36] This is defined as the perception of a sound in the absence of an external source. Due to lack of awareness and an aging population, the prevalence is still rising. The most severe degree of tinnitus is experienced by 2.4% of the population and is associated with insomnia, depression, and even suicide.[3,27] Although many therapies are being developed in the last years, there is still no effective standard therapy.[19,25] Current therapies mostly focus on treating the distress caused by tinnitus instead of reducing the actual phantom sound. Nevertheless, many patients do not benefit from the current approaches and become severe and chronic tinnitus sufferers. In these patients, neuromodulation-based treatments can be a promising option. Several preclinical and clinical studies demonstrated beneficial effects.[50] From coincidental findings in Parkinson's disease patients who also had tinnitus and were treated with deep brain stimulation (DBS), we know that stimulation can alter or even completely diminish the perception of tinnitus.[41] Since central nervous system changes especially occur in the chronic patients, it can be expected that these refractory, chronic, and often severe sufferers are the best candidates for neuromodulation. In this editorial review, pathophysiological changes associated with tinnitus and the potential of neuromodulation to interfere with these changes are discussed. Based on the latest preclinical and clinical studies, brain stimulation of subcortical auditory targets, nonauditory structures, and relevant cortical structures are reviewed. Furthermore, cochlear stimulation, as well as the novel approach trigeminal nerve stimulation to treat tinnitus are discussed.
Animal studies and human functional imaging studies revealed that tinnitus perception is associated with many complex changes in several different brain structures. The generally accepted hypothesis is that neuronal changes occur in both auditory and nonauditory brain structures, most often as a compensating mechanism on reduced input from the auditory nerve caused by cochlear hair cell damage, which is associated with hearing loss. These central neuronal changes include an increase in spontaneous firing rate, synchronized activity, bursting activity, and tonotopic reorganization.[15,40] Tinnitus perception is the result of dysfunction of multiple involved brain structures. The exact working mechanism of DBS is unknown, but different theories describe a combined excitatory and inhibitory effect.[6,10,24,35] DBS has been shown to be able to reduce an increased spontaneous activity as this therapy inhibits the elevated bursting activity in the subthalamic nucleus in Parkinson's disease patients.[5] It can be expected that modulation of one arbitrary part in the complex tinnitus pathways can disrupt pathological neuronal activity and thereby alter tinnitus perception or distress caused by this phantom sensation.[44,50]
Complex interactions within and between auditory and nonauditory brain structures are present in tinnitus. Every change in neuronal activity causes a cascade of changes in direct and indirect connected brain areas. An important role of the limbic system has been implied, as studies have shown that attention and emotions can influence tinnitus perception.[23,27] To simplify the complex pathways, classical and nonclassical auditory pathways are distinguished, not taking descending projections into account. Following the classical pathway, the cochlear nerve fibers end in the ipsilateral cochlear nucleus (CN), further project to the central part of the mainly contralateral inferior colliculus (IC), and subsequently to the medial geniculate body (MGB) and primary auditory cortex. Brain structures in the nonclassical auditory pathway are interestingly less tonotopically organized than in the classical pathway and have additional connections with the limbic system and caudate nucleus. The central part of the IC connects to the dorsal and external nucleus of the IC, which in turn projects to the dorsal and medial part of the MGB. From these parts of the MGB, connections project to the amygdala, secondary auditory cortex, and association auditory cortex.[21,36] The loudness of tinnitus does not always correlate with the burden and impact of tinnitus on life quality, suggesting a substantial role of nonauditory brain structures in the pathophysiology of chronic tinnitus. The importance of auditory-limbic interactions has been emphasized by Rauschecker et al., who propose a failing neural “noise cancelation” mechanism of (para)limbic structures as the underlying cause of tinnitus suffering.[39]
Multiple targets can be proposed in which DBS might have an advantageous effect on tinnitus perception, as shown in Figure 1. Within the auditory pathway, three nuclei should be considered. First, multiple preclinical and clinical studies suggest that the dorsal CN (DCN) plays an important role in the development of tinnitus and could, therefore, be a target for DBS in tinnitus. An increased bursting activity is found in both the DCN and ventral CN.[20,53] Ablation of this structure in an animal study resulted in a decrease in neuronal hyperactivity in higher output structures.[33] In a human study, patients who did not have a functioning auditory nerve received an auditory brainstem implant in the DCN. The majority of successfully implanted patients (6/10) reported a reduction in tinnitus perception or even complete suppression (1/10) during stimulation.[47] Side effects of stimulation that have been described include facial pain and ocular vibration, although some studies do not mention any side effects.[34,47] Effects of stimulation on hearing in patients with intact auditory nerves are not known. Second, IC stimulation might have an effect on tinnitus perception since studies have shown an increased spontaneous activity and neuronal synchrony in the contralateral IC in tinnitus.[4,7,8,28] Almost all ascending auditory brainstem projections converge in the IC. Electrical stimulation of the IC in patients with unilateral deafness showed some side effects including the perception of unpleasant sounds, paresthesia, dizziness, facial twitches, and temperature changes.[31] Third, the MGB is a possible target in the auditory pathway. It is known that the thalamus plays an important role in tinnitus, as thalamotomies have shown attenuation of tinnitus. Integration of auditory and limbic information occurs in the thalamus and more specifically, the amygdala receives auditory input from the MGB.[23] The MGB has an important role in tinnitus, since the ventromedial prefrontal cortex and nucleus accumbens (NAc) might be able to tune out the pathophysiological tinnitus signal by projecting to the MGB.[30,39] Although the role of the MGB in tinnitus is less intensively investigated as compared to the DCN and IC, it can be expected that stimulation of this specific thalamic structure can influence tinnitus perception and distress. Side effects of electrical stimulation of the MGB are not known. Thalamic stimulation for movement disorders, however, has been proven to be safe with only a few reversible side effects.[22] The MGB is better accessible with stereotaxy than deeper auditory structures and, therefore, the risks of surgery are expected to be relatively low.
Figure 1.
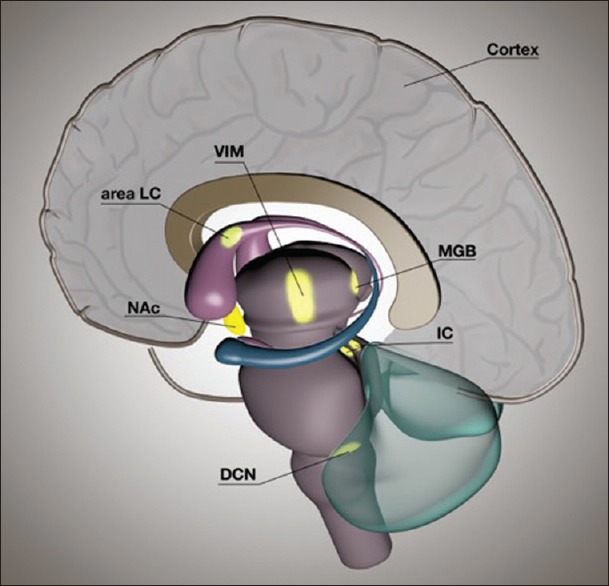
A schematic representation of a sagittal view of a human brain showing possible targets for brain stimulation to treat tinnitus. Auditory structures include the dorsal cochlear nucleus, inferior colliculus, medial geniculate body of the thalamus, and auditory cortex. Nonauditory structures are the nucleus accumbens, locus of caudate neurons (area LC), and ventral intermediate nucleus of the thalamus
Coincidental findings in patients with movement disorders who were treated with DBS, taught us that stimulation of nonauditory targets can attenuate tinnitus. Stimulation of the ventral intermediate nucleus of the thalamus (VIM) in Parkinson's disease patients who also suffered from tinnitus improved tinnitus in three out of seven patients.[41] Furthermore, two case reports described a decreased tinnitus perception after a cerebrovascular accident in the putamen and caudate nucleus and after perioperative focal vascular injury in a locus of the caudate nucleus (area LC).[29,32] A clinical study where patients with movement disorders were temporarily stimulated in area LC revealed a decrease in tinnitus loudness in all patients.[9] Although we do not know much about the role of the VIM or caudate nucleus in the pathophysiology in tinnitus, abovementioned findings are encouraging. The NAc, also known as the “reward center” of the brain, has a role in tinnitus distress according to clinical electroencephalographic findings.[51] It is hypothesized that DBS of the NAc in tinnitus would disrupt the abnormal functioning NAc in tinnitus patients in a way that tinnitus perception would be inhibited.[39] DBS of the NAc has been performed in obsessive-compulsive disorder patients and is associated with a risk of hypomania.[11] Human functional magnetic resonance imaging studies and preclinical studies have demonstrated the involvement of the amygdala and hippocampus in tinnitus, and these areas could, therefore, be considered as possible DBS targets.[43] Side effects such as negative emotions have appeared in some patients during stimulation and make these areas less suitable for the treatment of tinnitus with DBS.
Other neuromodulation-based approaches have also been suggested. In this respect, modulating the activity of relevant cortical structures has been performed. Transcranial magnetic stimulation (TMS) is a noninvasive technique in which strong magnetic field impulses can alter neuronal activity not only in cortical but also in areas connected to the cortex. Repetitive TMS can induce residual inhibition and suppress tinnitus loudness temporarily.[50] The effect of another noninvasive therapy, transcranial direct current stimulation, has been evaluated in a meta-analysis.[46] Overall, 39.5% of the patients responded with an average decrease in tinnitus intensity of 13.5%. This effect can last for an hour or longer. Another method of modulating the cortical activity is by extradural electrical stimulation. Stimulation of the primary auditory cortex and/or the secondary auditory cortex can be successful in suppressing severe, refractory tinnitus.[45] De Ridder et al.[12] implanted auditory cortex electrodes in 43 tinnitus patients who all showed benefit from two placebo controlled TMS sessions. In this technique, the electrodes are secured on the dura of the auditory cortex, which is reached via a craniotomy (2 cm × 6 cm), guided by functional magnetic resonance imaging. Despite that all patients responded to TMS, only 67% responded to cortical stimulation with an average suppressing effect of 51%. Side effects of stimulation are limited and only occurred at high frequency or high-intensity stimulation. Symptoms as a feeling of intoxication, word finding difficulties, dizziness, vertigo, hearing perception changes, feeling of “aural pressure,” and out of body, experiences were described. Complications can be severe. Epileptic seizures occurred in 3 of 43 patients and of the 4 patients, who were implanted following the intradural technique, one had an intracranial hemorrhage, and one developed an intracranial abscess. In another recent study, chronic electrical stimulation of the auditory cortex was applied in nine patients. The authors did not find a general objective efficiency.[16] Overall, cortical electrical stimulation might become a beneficial treatment option for a subgroup of severe tinnitus patients.[13,26]
Besides DBS and cortical neuromodulation approaches, some other concepts have been described. Intracochlear stimulation via cochlear implantation is a viable treatment option in patients with tinnitus and unilateral of bilateral severe or profound hearing loss.[2,49] In patients with bilateral hearing loss, a systematic review concluded a reduction of mean tinnitus score of 25–72% and a total suppression of tinnitus in 8–45% of patients.[38] Standard clinical stimulation, stimulation independent of an acoustic input, and even inaudible stimulation can be effective. This suggests an effect of central neuroplastic changes besides the effect of a shift in attention from tinnitus to environmental sounds.[1] Another technique that can indirectly influence central tinnitus-related neuronal activity is noninvasive transcutaneous electric nerve stimulation (TENS).[45] The cochlear nuclei receive somatosensory, nonauditory inputs besides auditory inputs from the vestibulocochlear nerve. Preclinical studies showed that transcutaneous electrical stimulation of the branches of the trigeminal nerve and parts of the dorsal column cause modulation of neuronal activity in the DCN.[54] TENS of the median nerve, temporomandibular joint, parts of the external ear, and upper cervical nerve C2 can be used to inhibit tinnitus perception temporarily in some patients.[18,37,48,52] Recently, electrical stimulation of branches of the trigeminal nerve or the trigeminal ganglion has been proposed as a potential treatment modality for tinnitus.[14,42,45]
In conclusion, developments in the field of neuromodulation are promising for patients with severe tinnitus. Several types of neuromodulation-based approaches are being investigated. The general mechanism of action is that neuromodulation interferes with pathological neuronal activity and thereby can attenuate distress or perception of tinnitus. In this respect, increased neuronal activity is found in the DCN, IC, MGB, and auditory cortex. These regions are, therefore, potential targets for brain stimulation. It is impossible to reach these regions selectively and precisely with noninvasive stimulation methods. When surgery is considered, then the MGB is a more accessible target. Furthermore, the MGB is an important relay station where the auditory and limbic structures interact. Tinnitus perception can be influenced by superficial stimulation techniques, which attenuate abnormal auditory cortex activity. Up to date, only a subgroup of tinnitus patients responded to auditory cortex stimulation. From the nonauditory structures, stimulation of the VIM, caudate nucleus (area LC), and NAc have potential to interfere with tinnitus. Using a bottom-up approach with cochlear stimulation or TENS of somatosensory inputs of the DCN, tinnitus percept can be modified in some cases.
Although much is happening at the moment, the field is waiting for evidence from well-designed clinical trials, based on supporting evidence from experimental/mechanistic research, to support or discourage the application of brain stimulation in tinnitus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank Geertjan van Zonneveld for the three-dimensional reconstruction of the potential brain stimulation targets for tinnitus.
Footnotes
Contributor Information
Gusta van Zwieten, Email: gusta.van.zwieten@mumc.nl.
Jasper V. Smit, Email: jasper.smit@maastrichtuniversity.nl.
Ali Jahanshahi, Email: a.jahanshahianvar@maastrichtuniversity.nl.
Yasin Temel, Email: y.temel@mumc.nl.
Robert J. Stokroos, Email: robert.stokroos@mumc.nl.
REFERENCES
- 1.Arts RA, George EL, Griessner A, Zierhofer C, Stokroos RJ. Tinnitus suppression by intracochlear electrical stimulation in single-sided deafness: A prospective clinical trial – Part I. Audiol Neurootol. 2015;20:294–313. doi: 10.1159/000381936. [DOI] [PubMed] [Google Scholar]
- 2.Arts RA, George EL, Stokroos RJ, Vermeire K. Review: Cochlear implants as a treatment of tinnitus in single-sided deafness. Curr Opin Otolaryngol Head Neck Surg. 2012;20:398–403. doi: 10.1097/MOO.0b013e3283577b66. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson A, Ringdahl A. Tinnitus – A study of its prevalence and characteristics. Br J Audiol. 1989;23:53–62. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- 4.Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease. Mov Disord. 2002;17(Suppl 3):S145–9. doi: 10.1002/mds.10156. [DOI] [PubMed] [Google Scholar]
- 6.Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55(12 Suppl 6):S13–6. [PubMed] [Google Scholar]
- 7.Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007;228:168–79. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res. 1995;82:158–78. doi: 10.1016/0378-5955(94)00174-o. [DOI] [PubMed] [Google Scholar]
- 9.Cheung SW, Larson PS. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC) Neuroscience. 2010;169:1768–78. doi: 10.1016/j.neuroscience.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Chiken S, Nambu A. Mechanism of deep brain stimulation: Inhibition, excitation, or disruption? Neuroscientist. 2015 doi: 10.1177/1073858415581986. pii: 1073858415581986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Koning PP, Figee M, van den Munckhof P, Schuurman PR, Denys D. Current status of deep brain stimulation for obsessive-compulsive disorder: A clinical review of different targets. Curr Psychiatry Rep. 2011;13:274–82. doi: 10.1007/s11920-011-0200-8. [DOI] [PubMed] [Google Scholar]
- 12.De Ridder D, Vanneste S, Kovacs S, Sunaert S, Menovsky T, van de Heyning P, et al. Transcranial magnetic stimulation and extradural electrodes implanted on secondary auditory cortex for tinnitus suppression. J Neurosurg. 2011;114:903–11. doi: 10.3171/2010.11.JNS10197. [DOI] [PubMed] [Google Scholar]
- 13.De Ridder D, Vanneste S. Auditory cortex stimulation might be efficacious in a subgroup of tinnitus patients. Brain Stimul. 2014;7:917–8. doi: 10.1016/j.brs.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Dehmel S, Cui YL, Shore SE. Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol. 2008;17:S193–209. doi: 10.1044/1059-0889(2008/07-0045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Engelhardt J, Dauman R, Arné P, Allard M, Dauman N, Branchard O, et al. Effect of chronic cortical stimulation on chronic severe tinnitus: A prospective randomized double-blind cross-over trial and long-term follow up. Brain Stimul. 2014;7:694–700. doi: 10.1016/j.brs.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am. 2003;36:239–48. doi: 10.1016/s0030-6665(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 18.Herraiz C, Toledano A, Diges I. Trans-electrical nerve stimulation (TENS) for somatic tinnitus. Prog Brain Res. 2007;166:389–94. doi: 10.1016/S0079-6123(07)66037-3. [DOI] [PubMed] [Google Scholar]
- 19.Hoare DJ, Kowalkowski VL, Kang S, Hall DA. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope. 2011;121:1555–64. doi: 10.1002/lary.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaltenbach JA. Summary of evidence pointing to a role of the dorsal cochlear nucleus in the etiology of tinnitus. Acta Otolaryngol Suppl. 2006;556:20–6. doi: 10.1080/03655230600895309. [DOI] [PubMed] [Google Scholar]
- 21.Kandel ER, Schwartz JH, Jessell TM. New York: McGraw-Hill; 2000. Principles of Neural Science. [Google Scholar]
- 22.Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001;16:464–8. doi: 10.1002/mds.1089. [DOI] [PubMed] [Google Scholar]
- 23.Kraus KS, Canlon B. Neuronal connectivity and interactions between the auditory and limbic systems.Effects of noise and tinnitus. Hear Res. 2012;288:34–46. doi: 10.1016/j.heares.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–35. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 25.Landgrebe M, Azevedo A, Baguley D, Bauer C, Cacace A, Coelho C, et al. Methodological aspects of clinical trials in tinnitus: A proposal for an international standard. J Psychosom Res. 2012;73:112–21. doi: 10.1016/j.jpsychores.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langguth B, De Ridder D. Tinnitus: Therapeutic use of superficial brain stimulation. Handb Clin Neurol. 2013;116:441–67. doi: 10.1016/B978-0-444-53497-2.00036-X. [DOI] [PubMed] [Google Scholar]
- 27.Langguth B, Landgrebe M, Kleinjung T, Sand GP, Hajak G. Tinnitus and depression. World J Biol Psychiatry. 2011;12:489–500. doi: 10.3109/15622975.2011.575178. [DOI] [PubMed] [Google Scholar]
- 28.Lanting CP, De Kleine E, Bartels H, Van Dijk P. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol. 2008;128:415–21. doi: 10.1080/00016480701793743. [DOI] [PubMed] [Google Scholar]
- 29.Larson PS, Cheung SW. A stroke of silence: Tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. J Neurosurg. 2013;118:192–4. doi: 10.3171/2012.9.JNS12594. [DOI] [PubMed] [Google Scholar]
- 30.Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron. 2011;69:33–43. doi: 10.1016/j.neuron.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim HH, Lenarz T, Anderson DJ, Lenarz M. The auditory midbrain implant: Effects of electrode location. Hear Res. 2008;242:74–85. doi: 10.1016/j.heares.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Lowry LD, Eisenman LM, Saunders JC. An absence of tinnitus. Otol Neurotol. 2004;25:474–8. doi: 10.1097/00129492-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Manzoor NF, Licari FG, Klapchar M, Elkin RL, Gao Y, Chen G, et al. Noise-induced hyperactivity in the inferior colliculus: Its relationship with hyperactivity in the dorsal cochlear nucleus. J Neurophysiol. 2012;108:976–88. doi: 10.1152/jn.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthies C, Thomas S, Moshrefi M, Lesinski-Schiedat A, Frohne C, Battmer RD, et al. Auditory brainstem implants: Current neurosurgical experiences and perspective. J Laryngol Otol Suppl. 2000;27:32–6. doi: 10.1258/0022215001904699. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism (s) of action of deep brain stimulation: Activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–48. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Møller A. Textbook of Tinnitus. New York: Springer-Verlag; 2011. Epidemiology of tinnitus in adults. [Google Scholar]
- 37.Møller AR, Møller MB, Yokota M. Some forms of tinnitus may involve the extralemniscal auditory pathway. Laryngoscope. 1992;102:1165–71. doi: 10.1288/00005537-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Ramakers GG, van Zon A, Stegeman I, Grolman W. The effect of cochlear implantation on tinnitus in patients with bilateral hearing loss: A systematic review. Laryngoscope. 2015;125:2584–92. doi: 10.1002/lary.25370. [DOI] [PubMed] [Google Scholar]
- 39.Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–26. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–74. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, Burchiel KJ, Anderson VC, Martin WH. Deep brain stimulation effects in patients with tinnitus. Otolaryngol Head Neck Surg. 2009;141:285–7. doi: 10.1016/j.otohns.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog Brain Res. 2007;166:107–23. doi: 10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shulman A, Strashun AM, Afriyie M, Aronson F, Abel W, Goldstein B. SPECT imaging of brain and tinnitus-neurotologic/neurologic implications. Int Tinnitus J. 1995;1:13–29. [PubMed] [Google Scholar]
- 44.Smit JV, Janssen ML, Schulze H, Jahanshahi A, Van Overbeeke JJ, Temel Y, et al. Deep brain stimulation in tinnitus: Current and future perspectives. Brain Res. 2015;1608:51–65. doi: 10.1016/j.brainres.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 45.Soleymani T, Pieton D, Pezeshkian P, Miller P, Gorgulho AA, Pouratian N, et al. Surgical approaches to tinnitus treatment: A review and novel approaches. Surg Neurol Int. 2011;2:154. doi: 10.4103/2152-7806.86834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song JJ, Vanneste S, Van de Heyning P, De Ridder D. Transcranial direct current stimulation in tinnitus patients: A systemic review and meta-analysis. ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/427941. 427941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soussi T, Otto SR. Effects of electrical brainstem stimulation on tinnitus. Acta Otolaryngol. 1994;114:135–40. doi: 10.3109/00016489409126031. [DOI] [PubMed] [Google Scholar]
- 48.Steenerson RL, Cronin GW. Tinnitus reduction using transcutaneous electrical stimulation. Otolaryngol Clin North Am. 2003;36:337–44. doi: 10.1016/s0030-6665(02)00164-0. [DOI] [PubMed] [Google Scholar]
- 49.Tyler RS, Rubinstein J, Pan T, Chang SA, Gogel SA, Gehringer A, et al. Electrical stimulation of the cochlea to reduce tinnitus. Semin Hear. 2008;29:326–32. doi: 10.1055/s-0028-1095892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanneste S, De Ridder D. Noninvasive and invasive neuromodulation for the treatment of tinnitus: An overview. Neuromodulation. 2012;15:350–60. doi: 10.1111/j.1525-1403.2012.00447.x. [DOI] [PubMed] [Google Scholar]
- 51.Vanneste S, Plazier M, der Loo EV, de Heyning PV, Congedo M, De Ridder D. The neural correlates of tinnitus-related distress. Neuroimage. 2010;52:470–80. doi: 10.1016/j.neuroimage.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 52.Vanneste S, Plazier M, Van de Heyning P, De Ridder D. Transcutaneous electrical nerve stimulation (TENS) of upper cervical nerve (C2) for the treatment of somatic tinnitus. Exp Brain Res. 2010;204:283–7. doi: 10.1007/s00221-010-2304-5. [DOI] [PubMed] [Google Scholar]
- 53.Vogler DP, Robertson D, Mulders WH. Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci. 2011;31:6639–45. doi: 10.1523/JNEUROSCI.6538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C, Martel DT, Shore SE. Transcutaneous induction of stimulus-timing-dependent plasticity in dorsal cochlear nucleus. Front Syst Neurosci. 2015;9:116. doi: 10.3389/fnsys.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]


