Abstract
The purpose of this review is to explore the mounting evidence that primary osteoarthritis is secondary to childhood disorders such as dysplasia and/or to subtle morphologic and structural to subtle morphologic and structural abnormalities of the proximal femur and/or acetabulum that were previously unrecognized or underestimated. These structural deformities lead to early impingement through range of motion and subsequent joint degeneration. The review also presents a brief synopsis of the genetic components that influence structural morphology of the hip joint and the impact of genetic pathways on OA development. If subtle deformities can be shown to be effective predictors of OA in the general population, it may be possible to identify hips that are at risk before they progress to end-stage OA. Furthermore, if these early risk factors are modifiable, it may be possible to implement preventative measures before the requirement of total hip arthroplasty.
INTRODUCTION
Despite identification of some environmental and patient-specific risk factors, the underlying pathomechanism of osteoarthritis (OA) development of the hip remains poorly understood. As a result, with the exception of patients with obvious secondary causes of OA such as rheumatoid arthritis, avascular necrosis or severe bony dysmorphisms, the majority of patients presenting to a physician with an arthritic hip simply get grouped under the umbrella term of ‘primary’ or ‘idiopathic’ OA. In fact, on review of the data from national joint registries with regard to patients undergoing a total hip arthroplasty (THA) for arthritis, a striking figure is revealed. The most commonly reported diagnosis in over 80% of cases is primary OA [1, 2]. This finding suggests that many clinicians today continue to operate under the paradigm that degenerative changes of the hip are simply the result of a bland ‘wear and tear’ process related to axial overload of the joint, reduced contact joint area and increased pressures leading to accelerated wear. More importantly, permitting this concept that the majority of OA is idiopathic and an inevitable consequence of aging to prevail also breeds the opinion that the disease is unmodifiable at its early stages.
However, a theory that was originally proposed in 1965 by Murray [3], furthered by Harris [4] in 1986 and recently advanced by the work of Ganz [5–7] is steadily gaining support today. This hypothesis suggests that most, if not all, primary hip OA is in fact secondary to subtle morphologic and structural abnormalities of the proximal femur and/or acetabulum that were previously unrecognized or underestimated. Developmental dysplasia of the hip, Legg–Calvé–Perthes' disease (LCPD) and slipped capital femoral epiphysis (SCFE) deformities are the conspicuous example of this theory with gross distortion of joint biomechanics leading to early and severe degenerative changes of the hip joint due to excessive contact stress on the cartilage. Yet in patients without severe structural abnormalities leading to abnormal articular contact stresses, the development of OA was still being observed. This new theory proposed that subtle anatomic variations do not increase contact stresses typically, but can cause OA via an impingement mechanism. Growing evidence supports this theory that the geometry of the hip joint itself is an important risk factor for OA, with subtle architectural changes predating the radiographic appearance of primary hip OA in up to 90% of cases [3, 4, 8, 9]. This new theory focuses on motion and end-range impingement as the cause of OA as opposed to the conventional focus on axial loading of the joint. In addition, evidence suggests that several environmental and patient risk factors exist linked to the development of OA of the hip. These factors include aging, obesity, high bone density, sporting or high impact activity, occupation such as farming, genetics, childhood hip disorders or trauma [10–18]. The influence of each of these factors on the development of OA is varied. The risk of OA ranges from a 3 to 4 times greater risk with a history of hip trauma or high impact sporting activity to as high as 9.3 times greater risk of developing hip OA in farmers [12, 15, 18]. Because OA is a debilitating disease affecting approximately 15% of the world’s population [19], introduction of preventive measures through activity modification and early detection could have a significant impact on healthcare resources.
The purpose of this review is to focus on the structural morphologies of the hip that may predispose an individual to the development of OA. Additionally, a brief review of the genetic components that influence structural morphology of the hip joint and the impact of genetic pathways on OA development will be examined. If subtle deformities can be shown to be effective predictors of OA in the general population, it may be possible to identify hips that are at risk before they progress to end-stage OA necessitating a THA. Furthermore, if these early risk factors are modifiable, it may be possible to implement preventative measures before the requirement of THA.
GENETICS AND HIP DEGENERATION
When defining structural abnormalities of the hip that may predispose an individual to the development of hip OA, one cannot ignore the influence genetics. The growing importance of genes as risk factors for OA development has been seen increasingly in recent studies. For instance, in a review of patients undergoing THA for hip OA, their siblings showed a 5-fold increased risk of developing hip OA versus the general population [14]. Furthermore, it has been estimated that heritability of hip OA in women is approximately 60% [20, 21].
While genetic research on OA is in the early stages, the mounting evidence is difficult to ignore. Regions of chromosomes 2, 4, 6, 7, 11, 16, 19 and X have all been identified as home to genes involved in heritability of OA [22]. Of late, two specific genetic pathways have garnered considerable attention for their link to OA and joint shape. These pathways are the bone morphogenic protein (BMP) and wingless integration (Wnt) pathways, which yield proteins involved in bone morphogenesis. The Wnt pathway has been studied for its role in joint shape determination and development of OA. During early development, Wnt proteins are expressed in the limb bud and at sites of synovial joint formation [23]. When this gene is altered to inhibit its function in the developing animal model, the result is joint malformation with associated rapid degenerative changes [24]. In addition to determining skeletal shape during early development, the Wnt pathway is also implicated in the maintenance of bone and cartilage homeostasis in adult life [23, 25, 26]. Excessive activation of this pathway leads to cartilage breakdown and bony sclerosis as seen in OA [26]. Furthermore, variants in Wnt alleles of single nucleotide polymorphisms have been linked to increased susceptibility to OA development of the hip and knee secondary to non-optimal joint shape [27–31]. Antagonists of the Wnt pathway are currently in clinical development for treatment of sclerotic bony lesions and multiple myeloma but extension to OA treatment is unexplored [21].
The BMP family is also associated in both pathogenesis of OA and determination of joint shape. Overexpression of BMP 2 and 4 has been shown to alter the joint morphology in animal models [32]. BMPs signal through the mitogen-activated protein kinase pathway and represent proteins nested within transforming growth factor beta superfamily. The signalling initiates bone and cartilage formation during development as well as manages cell differentiation and tissue homeostasis in adult life [33]. As our understanding of the influence of genetics on the development of joint morphology grows, new treatments may be engineered that target these pathways before the onset of arthritic changes.
ACETABULAR PATHOLOGIC MORPHOLOGY AND HIP DEGENERATION
Acetabular dysplasia
While the definition of mild acetabular dysplasia is not universally accepted, the most common measurement to represent acetabular dysplasia is Wiberg’s center-edge angle (CEA) [34]. The normal range has been quoted as between 25° and 40°. Outside of these ranges are suggestive of dysplasia and <25° indicates a shallow, vertical cup orientation, which has been reported as a risk factor for OA development [35]. The prevalence of dysplasia in the population has been reported as 4.3% in men and 3.6% in women [36]. Furthermore, epidemiologic studies have retrospectively estimated that, of patients who go on to develop hip OA, 25–40% are attributable to discreet acetabular dysplasia [3, 4, 37]. When compared with a normally shaped acetabulum, a pelvis with acetabular dysplasia has defining characteristics including a shallow and more vertical socket. This morphology results in a smaller weight-bearing surface that results in increased contact stresses and focal loading of the articular cartilage and labrum beyond its physical tolerance [38, 39]. Structural instability associated with acetabular dysplasia is also proposed as a biomechanical cause of hip degeneration. The shallow, vertical cup allows the anterosuperior portion of the femoral head to migrate into areas of undercoverage (Fig. 1). This migration places increased stresses on the supporting soft tissue structures of the hip. This may result in injury to these tissues including the joint capsule, labrum and articular cartilage [3, 38–40].
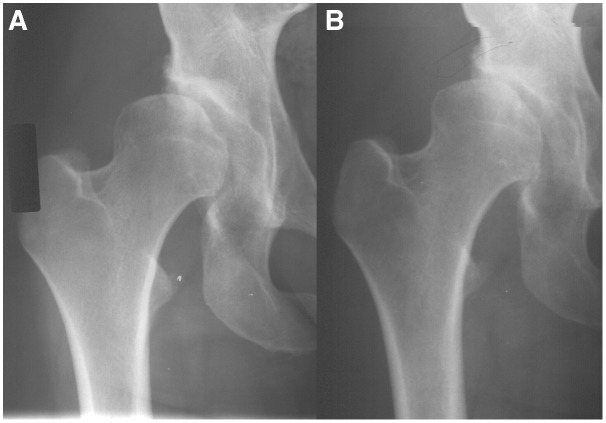
Figure 1.
A 28-year-old female with right hip dysplasia showing progression of hip arthritis over a 4-year period.
The difficulty when defining any morphologic abnormality as a risk factor for hip degeneration is proving that the abnormal morphology was present prior to the development of hip OA and not after the fact. Growing evidence, however, does seem to support this theory. A recent cohort study found that acetabular dysplasia, defined as CEA <25°, was present a mean of 6.6 years prior to radiographic appearance of OA in men and by 8.8 years prior in women [41]. The idea that dyplasia leads to hip OA was further supported by Jacobsen et al. [42, 43] who found increased risk of OA in individuals with acetabular dysplasia and by additional authors [41, 44] who identified that acetabular dyplasia at baseline was associated with increased risk of developing hip OA. Together these findings suggest that changes in acetabular shape precede the development of hip OA. In fact, the association between hip degeneration and acetabular dyplasia is further strengthened by a study in 2011 by Nicholls et al. [45] that concluded that for every loss of 1° of CEA, the risk of needing a THA for hip degeneration increased by 10.5% (range 2.0–18.2%).
Pincer morphology
While acetabular dysplasia refers to the relative undercoverage of the femoral head secondary to a shallow CEA, pincer morphology relates to relative overcoverage. With pincer morphology, an overprominent acetabular rim can result in impingement as the acetabular rim abuts against the femoral head–neck junction during motion of the hip [7]. Pincer impingement can result from either global or localized acetabular overcoverage of the femur with global overcoverage being defined as CEA >40° [46] or with protrusio acetabuli (femoral head is overlapping the ilioischial line medially) [47]. The radiological findings associated with coxa profunda are no longer appropriate for determining global coverage as they are also seen in patients with dysplasia. In localized overcoverage, there is cranial or superolateral acetabular retroversion, which is described as a posteriorly oriented acetabular opening with reference to the sagittal plane (Fig. 2) [48, 49]. In some cases, there can be a small overgrowth of the acetabulum located at the roof of the acetabulum. Pincer-type morphology is more common in middle-aged women, and the damage pattern to the hip is typically more restricted to the acetabular rim as opposed to the femoral head. Recent literature has provided increasing evidence that pincer impingement causes distinct patterns of articular cartilage and labral damage and may serve as an etiologic factor of ‘idiopathic’ OA [1, 7, 36, 50–56]. However, the process of hip degeneration does not appear to be as rapid when compared with those hips with femoral-sided morphologic pathology [6].
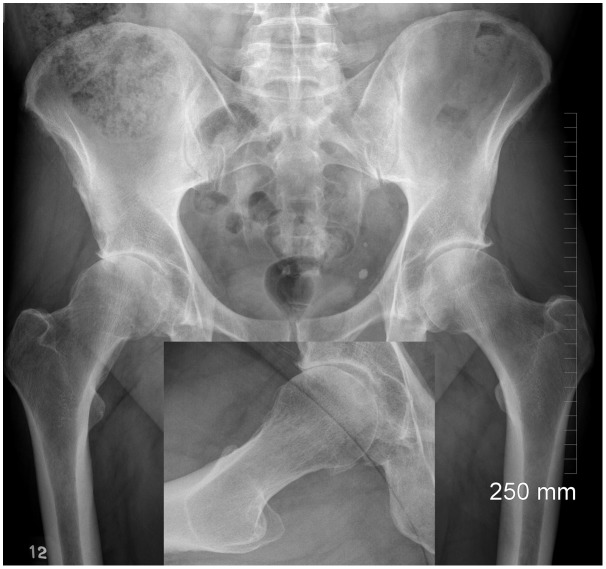
Figure 2.
Cam deformity leading to arthritis in a 36-year-old male. Inset shows lack of concavity.
As described by Ganz et al. [5, 6], in pincer-type impingement, the labrum is the first structure to fail. The early findings of labral damage are intrasubstance fissuring and ganglion formation. Eventually, bone-to-bone contact occurs on the osseous rim next to the labrum, pushing the damaged labrum forward. The labrum ultimately thins or ossifies until it is finally no longer distinguishable. This can lead to further deepening of the socket and worsening of the overcoverage. With persistent abutment, the acetabular cartilage adjacent to the labrum is the next structure to undergo degeneration and cartilage delamination can occur. With continued, unchecked abutment, the impact area on the femoral neck may develop a saddle-like callus formation with central ulcerations of the periosteum. Late in the process, secondary to chronic anterior leverage of the head in the acetabulum, there will be cartilage abrasion in the posteroinferior aspect of the acetabulum. This lesion is referred to as a contre-coup lesion [7]. Over time, this repetitive microtrauma may lead to hip OA.
Epidemiologic studies have identified the prevalence of deepened acetabular socket in 15.2% men and 19.4% women [36]. Those individuals with a deepened acetabular socket (prior to the development of hip degeneration) were 2.4 times more likely to develop hip OA versus a population without a deepened socket (Fig. 3) [36]. Similarly, Chung et al. [50] reported that people with a deepened socket and pincer deformity, defined as a CEA >45°, had a 2.3 times greater risk of developing OA in the future than their counterparts who had a CEA between 20° and 40°. Conversely, acetabular retroversion has been linked to OA in several recent studies. Labral and chondral lesions were seen in over 50% of patients with a retroverted acetabulum which may serve as precursors to the ultimate development of hip OA [57]. In fact, Kim et al. [53] identified a positive correlation between the degree of acetabular retroversion and mean joint space of the hip with the more retroverted the acetabulum, the smaller the mean joint space. Furthermore, a retrospective assessment of radiographs before development of OA identified acetabular retroversion as a risk factor for future OA development. An odds ratio (OR) of 4.7 [95% confidence interval (CI): 1.7–16.1] was reported, suggesting those with acetabular retroversion were at a considerably increased risk of developing hip degeneration versus their counterparts with an appropriately anteverted acetabulum [52].
Figure 3.
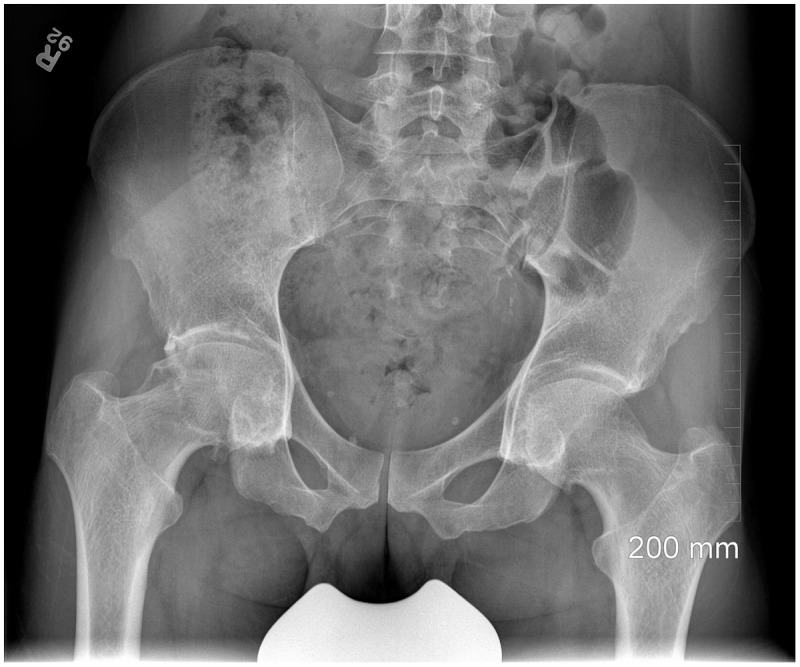
Pincer deformity leading to arthritis in a 48-year-old male with anteroposterior radiograph showing retroversion (ischial spine sign). Inset shows normal head/neck concavity.
FEMORAL PATHOLOGIC MORPHOLOGY AND HIP DEGENERATION
In addition to structural variants of the acetabulum, morphologic aspects of the femur have also been linked to the development of hip OA. Historically, the femoral morphology linked to OA has been referred to as a ‘pistol grip’ or ‘head tilt’ deformity. These terms describe a proximal femur with loss or flattening of the normal concavity of the anterosuperior region of the head–neck junction, resulting in a non-spherical femoral head with decreased femoral head–neck offset. These deformities can be seen secondary to severe SCFE or Perthes' disease. However, for deformities that lack a clear etiology but do have a clear convexity or bump at the transition zone between the femoral head and neck, the nomenclature has evolved with time and now is commonly referred to as a ‘cam’ deformity. Regardless of whether it is referred to as pistol grip or cam deformity, with these morphologic variants it is shown that the non-spherical femoral head shape leads to abnormal contact between the proximal femur and acetabular rim during movement which causes impingement of the labrum. This stress can lead to damage of the labrum and adjacent cartilage and is thought to initiate the onset of hip OA [58].
This section will review the development of hip OA secondary to femoral morphologic variants associated with pistol grip/cam deformities as well as those seen with SCFE and Perthes'. Growing evidence supports the theory that impingement secondary to a cam or pistol grip deformity with their associated loss of femoral head–neck concavity causes characteristic patterns of labral damage and subsequent articular cartilage injury and may serve as an etiologic factor of ‘idiopathic’ OA [1, 7, 36, 50–56].
Pistol grip/cam deformity
The lack of concavity associated with the pistol grip or cam deformity is typically seen on the anterosuperior aspect of the femoral head/neck junction and quantified using the alpha angle of Nötzli. In his original publication, Nötzli defined a cutoff >50.5° at the 3 o’clock position on MRI to determine the presence of cam morphology [59]. However, a recent work of Rakhra et al. [60] and Sutter et al. [61] has demonstrated the increased sensitivity and specificity of the 1:30 position compared with the 3 o’clock position in detecting the cam deformity. More specifically, using a cutoff of 60° at the 1:30 position, Sutter et al. [61] reported a sensitivity and specificity of 76% and 74.5% in diagnosing symptomatic cam-type femoroacetabular impingement (FAI). The overall prevalence in the general population is approximately 14%, but, unlike pincer deformity which is more often found in women, a cam deformity is five times more common in men [62]. In a follow-up of these asymptomatic individuals, Khanna et al. [63] found that the presence of cam deformity at the 1:30 position (anterolateral) was the most predictive of individuals becoming symptomatic.
Considerable evidence continues to mount in support of impingement secondary to a pistol grip or cam deformity as a cause of subsequent hip degeneration. Unlike pincer impingement, in the early stages of cam impingement, the labrum remains relatively uninvolved. The resultant shear forces generated by the cam impingement create forces that produce an outside-in abrasion or avulsion of the acetabular cartilage from the subchondral bone in a rather constant anterosuperior area [7]. These shear stresses cause a separation between the labrum and cartilage as the labrum is pushed outwards and the cartilage is pushed centrally. The femoral head then migrates into this defect, seen as joint space narrowing on radiograph, and at this stage, the cartilage of the femoral head becomes involved due to increased contact stress on the femoral cartilage [5, 6, 54]. Chondral avulsion in turn leads to tear or detachment of the principally uninvolved labrum. These changes eventually lead to articular degeneration and global hip OA [5, 7]. A cam lesion as a cause of hip OA is further supported by molecular studies. In 22 young adults undergoing treatment for cam-type FAI, without any radiographic evidence of OA, changes in cartilage resembling those of advanced OA were already occurring. The immunohistochemical staining of the cartilage from the pre-arthritic FAI patients showed increased staining for several OA markers including tenasin-C, cartilage oligometric protein and collagenase cleavage product. In addition, their pattern of collagen type I and II expression was similar to the findings of patients with severe OA [64–66].
A few studies have looked at the association between the presence of loss of femoral head–neck concavity and subsequent OA development (Fig. 4). A study by Gosvig et al. [36] found modest association between the presence of a pistol grip deformity and prevalent radiographic hip OA, reporting an OR of 2.2 times greater risk (95% CI 1.7–7.8). In a large retrospective series including 1007 cases with advanced hip OA, compared with 1123 controls without hip OA, an OR of 6.95 (95% CI 4.6–10.4) of developing OA was identified if a pistol grip deformity was reported [67]. Cross-sectional studies further confirm that non-spherical shape of the femoral head is associated with the development of OA as there is a mildly increased risk of requiring a THA within 19 years if non-sphericity is found versus a group with normal concavity of the femoral head [45]. Studying a large cohort of individuals (700) who underwent primary THA before the age of 50 (mean age of 40), Clohisy et al. [68] found that over 60% of the hips had plain radiographic evidence of cam-type FAI. More importantly, all contralateral hips in patients who had a total hip replacement had evidence of cam-type FAI and 73% went on to develop hip arthritis [68].
Figure 4.
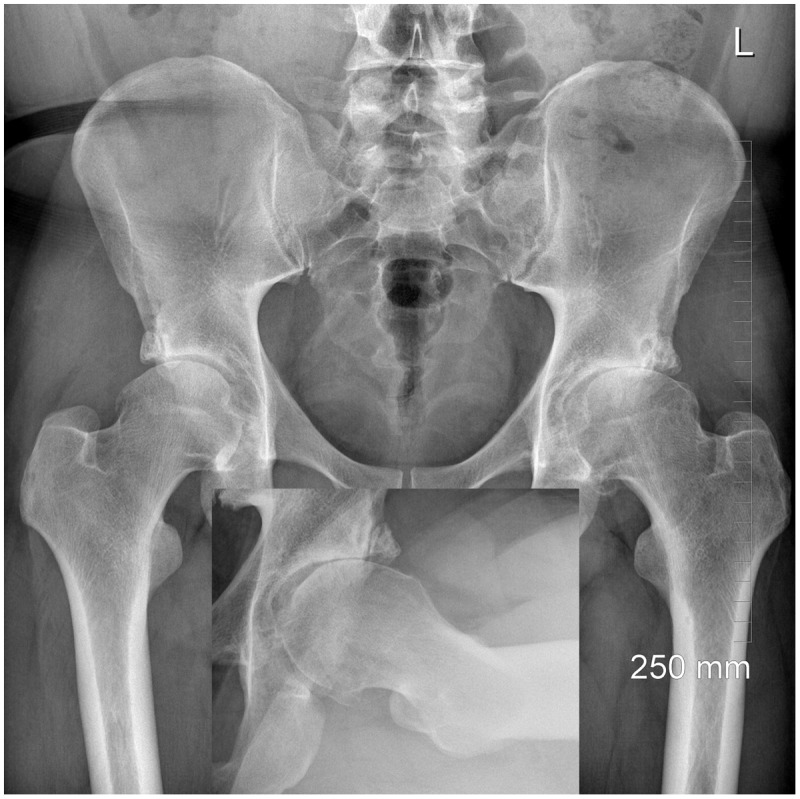
A 39-year-old male with global overcoverage and advanced arthritis of his right hip.
The severity of the cam deformity has also been correlated to OA development. An early study by Ecker et al [55] concluded that people with high alpha angles were at a slightly greater risk of developing hip OA, OR 1.09, versus those with low alpha angles. The findings from this study were furthered by the results from the CHECK study [69] which found that an alpha angle of >60° and >83° resulted in an OR of 3.67 (95% CI 1.68–8.01) and 9.66 (95% CI 4.72–19.78), respectively, for developing end-stage OA. The combination of severe cam-type deformity and decreased internal rotation (<20°) at baseline resulted in an even more pronounced adjusted OR of 25.2, and in a positive predictive value of 52.6% for developing end-stage OA [69]. In addition, the effect of worsening cam deformity was quantified by the work by Nicholls et al [45]. They identified that there was a 5.8% (2.3–9.3%) increased risk of requiring a THA for every degree of increased alpha angle. They also found that patients who underwent THA had a higher prevalence of cam deformity than did their respective controls (62.3° versus 45.8°, P < 0.001).
More refined techniques have also been used to study the total variation of the shape of the proximal femoral head/neck to investigate the influence of a cam deformity on OA development [58, 70]. One of them is statistical shape modelling (SSM), which uses an annotated outline of the objects of interest and transfers the set of annotated landmark points into a set of modes. Together, these modes describe the total variation of the shape present in the investigated population. One SSM study found that not only did proximal femoral shape change over time in subjects with hip OA, but also that even prior to radiographic findings of OA there were shape differences between OA and case controls [71]. Furthermore, Lynch et al [72] found that three distinct hip shapes or ‘modes’ were associated with incident hip OA with ORs ranging from 1.73 to 2.31. The shape of mode III, which accounted for the greatest variability in overall proximal femoral shape of the three, was characterized by a larger, aspherical femoral head size subsequently reinforcing the idea that hip shape predates the development of OA in many cases [21].
Slipped capital femoral epiphysis
SCFE is a disease common in young men of black or Polynesian descent and is often linked to obesity [73]. In the case of a severe slip, an obvious pistol grip deformity is the late result. It is believed that the resulting pistol grip deformity is caused by the healed position of the posterior and varus rotation of the femoral head in relation to an anteverted femoral neck [74, 75]. The post-severe slip morphology is further characterized by significant medial and/or posterior displacement of the femoral head, convexity at the head–neck junction, shortened femoral head, coxa vara and a short broad femoral head [76]. Similar to cam-type impingement, the abnormal post-slip morphology is thought to expose the prominent anterior metaphyseal portion of the femoral neck to the anterior surface of the acetabulum during flexion and internal rotation. The result is accelerated wear in this region ultimately leading to the development of OA [1]. In addition, the development of OA may be accelerated by the abnormal rotation of the femoral head in SCFE patients, which causes the thinner superior lateral articular cartilage on the femoral head to articulate with the acetabulum [74]. As shown by Rab [77], as the patient flexes or internally rotates their hip, there is early abutment between the femoral head and acetabular rim. This is thought to lead to increased intra-articular pressures on this already thinned articular surface. This ultimately leads to joint degeneration.
The support for a ‘severe’ slip resulting in degenerative changes of the hip is considerable, with SCFE patients undergoing THA for OA on average 11 years sooner than patients with OA without evidence of a severe slip [74]. However, support for a ‘subclinical’ SCFE resulting in OA has conflicting results. In an early study by Goodman et al. [1], they found arthritic changes in 38% of adult human skeletons with posterior slip morphology which they concluded was a risk factor for OA. The SCFE slip specimens resembled the cam-type FAI associated with decreased head–neck offset, loss of sphericity of the femoral head and the superior and anterolateral head–neck junction [74]. Further support was found in early epidemiological studies, which found that the male-to-female ratio of this deformity approximated a recent estimate of the male to female ratio of SCFE [78], which supports the hypothesis that pistol grip deformity is secondary to symptomatic or asymptomatic SCFE [36]. However, recent support for the theory that hip OA is secondary to a missed subclinical epiphyseal slip has begun to wane in favour of the theory that a cam deformity and subsequent impingement is a remodelling phenomenon. For instance, more robust epidemiological studies identify black and Polynesian people as having two to four times the incidence of SCFE; yet, they have a much lower incidence of OA (1%) versus Caucasians (3–6%) [73, 79]. If a strong link between SCFE and OA development existed, than one would expect the incidence of OA to be higher in the groups with more SCFE prevalence which is not the case. Additionally, a recent study reported cam morphotype prevalence but found no evidence of SCFE [80]. Finally, studies on morphology have shown that the direction of tilt of the capital epiphysis in SCFE (posteroinferior) differs from the anterosuperior extension of the physis seen in the cam morphotype, suggesting there is no link [80].
Legg–Calvé–Perthes' disease
LCPD is a childhood disorder characterized by a temporary loss of blood supply to the femoral head, resulting in necrosis and femoral head collapse. The disease is more commonly seen in men than women. Residual hip deformities secondary to an untreated Perthes' include growth disturbance of the proximal femoral physis, a non-spherical femoral head due to collapse, an overriding greater trochanter, coxa magna, coxa vara and secondary remodelling of the acetabulum. It is theorized that these deformities can change the mechanical function of the hip joint and contribute to impingement and subsequent hip degeneration [81]. Impingement secondary to Perthes' disease may occur as the consequence of the disease itself or as a consequence of treatment. For instance, flattening of the femoral head due to collapse results in a non-spherical femoral head, and coxa magna and coxa breva deformity leads to decreased head–neck offset. Together these produce a cam-type impingement during hip flexion and internal rotation with resultant shearing damage to the cartilage and labrum via an outside-in mechanism described in the cam FAI section.
In a recent study of 58 hips with Perthes' treated with non-operative management followed for a mean of 20 years, they found that 44% of patients had Tönnis grade 2 or above arthritis of their hip joint (Fig. 5). Additionally, clinical signs of FAI were associated with pain and with lower functional scores [82]. In a long-term study comparing patients with LCPD with controls, the study authors concluded that patients with LCPD have an increased risk of having a THA compared with a gender- and age-matched control group. They also reported that patients with LCPD have a greater risk of having radiographic OA develop compared with a gender- and age-matched control group [83, 84].
Figure 5.
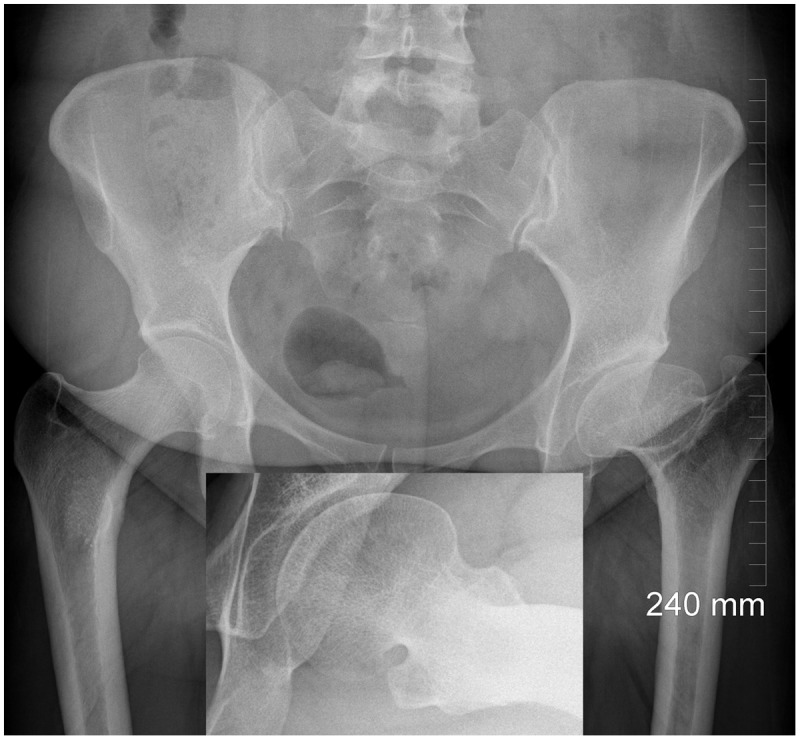
Arthritis secondary to Legg–Calve–Perthes in a 33-year-old female. Inset shows grossly aspherical femoral head.
SUMMARY
The concept that hip OA can be primary or idiopathic in nature has been increasingly challenged as our understanding of the structural morphology of the hip joint has increased. In particular, recognition of often subtle morphologic bone variants on either the acetabular or femoral side has been implicated as risk factors for subsequent hip degeneration secondary to impingement. Growing data support these structural changes as major contributors to OA development. As our understanding of abnormal hip morphology and genetics as degenerative risk factors grows, so too do our potential opportunities to intervene early in the disease process.
ACKNOWLEDGEMENTS
The authors acknowledge Peter Breithaupt for his contributions towards manuscript preparation.
FUNDING
This work was supported by the Canadian Institutes of Health Research (MOP 97778).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Goodman DA, Feighan JE, Smith AD, et al. Subclinical slipped capital femoral epiphysis. Relationship to osteoarthrosis of the hip. J Bone Joint Surg Am 1997; 79: 1489–97. [DOI] [PubMed] [Google Scholar]
- 2.Danish Hip Arthroplasty Registry. Annual Report 2004. Aarhus, Denmark: Danish Hip Arthroplasty Registry, 2004. [Google Scholar]
- 3.Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol 1965; 38: 810–82. [DOI] [PubMed] [Google Scholar]
- 4.Harris WH. Etiology of osteoarthritis of the hip. Clin Orthop Relat Res 1986; 20–33. [PubMed] [Google Scholar]
- 5.Ganz R, Parvizi J, Beck M, et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003; 112–20. [DOI] [PubMed] [Google Scholar]
- 6.Ganz R, Leunig M, Leunig-Ganz K, et al. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res 2008; 466: 264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005; 87: 1012–8. [DOI] [PubMed] [Google Scholar]
- 8.Waarsing JH, Rozendaal RM, Verhaar JA, et al. A statistical model of shape and density of the proximal femur in relation to radiological and clinical OA of the hip. Osteoarthritis Cartilage 2010; 18: 787–94. [DOI] [PubMed] [Google Scholar]
- 9.Gregory JS, Waarsing JH, Day J, et al. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis? Arthritis Rheum 2007; 56: 3634–43. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 2000; 133: 635–46. [DOI] [PubMed] [Google Scholar]
- 11.Cooper C, Inskip H, Croft P, et al. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. Am J Epidemiol 1998; 147: 516–22. [DOI] [PubMed] [Google Scholar]
- 12.Vingård E, Alfredsson L, Goldie I, Hogstedt C. Sports and osteoarthrosis of the hip. An epidemiologic study. Am J Sports Med 1993; 21: 195–200. [DOI] [PubMed] [Google Scholar]
- 13.Thelin A. Hip joint arthrosis: an occupational disorder among farmers. Am J Ind Med 1990; 18: 339–43. [DOI] [PubMed] [Google Scholar]
- 14.Lanyon P, Muir K, Doherty S, Doherty M. Assessment of a genetic contribution to osteoarthritis of the hip: sibling study. BMJ 2000; 321: 1179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croft P, Coggon D, Cruddas M, Cooper C. Osteoarthritis of the hip: an occupational disease in farmers. BMJ 1992; 304: 1269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tepper S, Hochberg MC. Factors associated with hip osteoarthritis: data from the First National Health and Nutrition Examination Survey (NHANES-I). Am J Epidemiol 1993; 137: 1081–8. [DOI] [PubMed] [Google Scholar]
- 17.Heliövaara M, Mäkelä M, Impivaara O, et al. Association of overweight, trauma and workload with coxarthrosis. A health survey of 7,217 persons. Acta Orthop Scand 1993; 64: 513–8. [DOI] [PubMed] [Google Scholar]
- 18.Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med 2000; 133: 321–8. [DOI] [PubMed] [Google Scholar]
- 19.Quintana JM, Arostequi I, Escobar A, et al. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med 2008; 168: 1576–84. [DOI] [PubMed] [Google Scholar]
- 20.Baker-LePain JC, Lane NE. Relationship between joint shape and the developmentof osteoarthritis. Curr Opin Rheumatol 2010; 22: 538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker-LePain JC, Lane NE. Role of bone architecture and anatomy in osteoarthritis. Bone 2012; 51: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdes AM, Spector TD. The contribution of genes to osteoarthritis. Med Clin North Am 2009; 93: 45–66, x. [DOI] [PubMed] [Google Scholar]
- 23.Witte F, Dokas J, Neuendorf F, et al. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns 2009; 9: 215–23. [DOI] [PubMed] [Google Scholar]
- 24.Simon-Chazottes D, Tutois S, Kuehn M, et al. Mutations in the gene encoding the low-density lipoprotein receptor LRP4 cause abnormal limb development in the mouse. Genomics 2006; 87: 673–7. [DOI] [PubMed] [Google Scholar]
- 25.Hopwood B, Tsykin A, Findlay DM, Fazzalari NL. Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor-beta/bone morphogenic protein signalling. Arthritis Res Ther 2007; 9: R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corr M. Wnt-beta-catenin signaling in the pathogenesis of osteoarthritis. Nat Clin Pract Rheumatol 2008; 4: 550–6. [DOI] [PubMed] [Google Scholar]
- 27.Lane NE, Lian K, Nevitt MC, et al. Frizzled-related protein variants are risk factors for hip osteoarthritis. Arthritis Rheum 2006; 54: 1246–54. [DOI] [PubMed] [Google Scholar]
- 28.Lories RJ, Peeters J, Bakker A, et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum 2007; 56: 4095–103. [DOI] [PubMed] [Google Scholar]
- 29.Loughlin J, Dowling B, Chapman K, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A 2004; 101: 9757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velasco J, Zarrabeitia MT, Prieto JR, et al. Wnt pathway genes in osteoporosis and osteoarthritis: differential expression and genetic association study. Osteoporos Int 2010; 21: 109–18. [DOI] [PubMed] [Google Scholar]
- 31.Waarsing JH, Kloppenburg M, Slagboom PE, et al. Osteoarthritis susceptibility genes influence the association between hip morphology and osteoarthritis. Arthritis Rheum 2011; 63: 1349–54. [DOI] [PubMed] [Google Scholar]
- 32.Duprez D, Bell EJ, Richardson MK, et al. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev 1996; 57: 145–57. [DOI] [PubMed] [Google Scholar]
- 33.Lories RJ, Luyten FP. Bone morphogenetic protein signaling in joint homeostasis and disease. Cytokine Growth Factor Rev 2005; 16: 287–98. [DOI] [PubMed] [Google Scholar]
- 34.Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint. Acta Chir Scand 1939; (Suppl. 58): 7. [Google Scholar]
- 35.Cooperman DR, Wallensten R, Stulberg SD. Acetabular dysplasia in the adult. Clin Orthop Relat Res 1983; 79–85. [PubMed] [Google Scholar]
- 36.Gosvig KK, Jacobsen S, Sonne-Holm S, et al. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am 2010; 92: 1162–9. [DOI] [PubMed] [Google Scholar]
- 37.Stulberg SD, Cordell LD, Harris WH, et al. Unrecognised childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. Hip 1975; 3: 212–30. [Google Scholar]
- 38.Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am 1995; 77: 985–9. [DOI] [PubMed] [Google Scholar]
- 39.Mavcic B, Iglic A, Kralj-Iglic V, et al. Cumulative hip contact stress predicts osteoarthritis in DDH. Clin Orthop Relat Res 2008; 466: 884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon L. Patterns of osteoarthritis of the hip . J Bone Joint Surg Br 1976; 58: 176–83. [DOI] [PubMed] [Google Scholar]
- 41.Lane NE, Lin P, Christiansen L, et al. Association of mild acetabular dysplasia with an increased risk of incident hip osteoarthritis in elderly white women: the study of osteoporotic fractures. Arthritis Rheum 2000; 43: 400–4. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsen S, Sonne-Holm S. Hip dysplasia: a significant risk factor for the development of hip osteoarthritis. A cross-sectional survey. Rheumatology 2005; 44: 211–8. [DOI] [PubMed] [Google Scholar]
- 43.Jacobsen S, Sonne-Holm S, Søballe K, et al. Hip dysplasia and osteoarthrosis: a survey of 4151 subjects from the Osteoarthrosis Substudy of the Copenhagen City Heart Study. Acta Orthop 2005; 76: 149–58. [DOI] [PubMed] [Google Scholar]
- 44.Reijman M, Hazes JM, Pols HA, et al. Acetabular dysplasia predicts incident osteoarthritis of the hip: the Rotterdam study. Arthritis Rheum 2005; 52: 787–93. [DOI] [PubMed] [Google Scholar]
- 45.Nicholls AS, Kiran A, Pollard TC, et al. The association between hip morphology parameters and nineteen-year risk of end-stage osteoarthritis of the hip: a nested case-control study. Arthritis Rheum 2011; 63: 3392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kutty S, Schneider P, Faris P, et al. Reliability and predictability of the centre-edge angle in the assessment of pincer femoroacetabular impingement. Int Orthop 2012; 36: 505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris-Hayes M, Royer NK. Relationship of acetabular dysplasia and femoroacetabular impingement to hip osteoarthritis: a focused review. PM R 2011; 3: 1055–67.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br 1999; 81: 281–8. [DOI] [PubMed] [Google Scholar]
- 49.Jamali AA, Mladenov K, Meyer DC, et al. Anteroposterior pelvic radiographs to assess acetabular retroversion: high validity of the “cross-over-sign”. J Orthop Res 2007; 25: 758–65. [DOI] [PubMed] [Google Scholar]
- 50.Chung CY, Park MS, Lee KM, et al. Hip osteoarthritis and risk factors in elderly Korean population. Osteoarthritis Cartilage 2010; 18: 312–6. [DOI] [PubMed] [Google Scholar]
- 51.Ezoe M, Naito M, Inoue T. The prevalence of acetabular retroversion among various disorders of the hip . J Bone Joint Surg Am 2006; 88: 372–9. [DOI] [PubMed] [Google Scholar]
- 52.Giori NJ, Trousdale RT. Acetabular retroversion is associated with osteoarthritis of the hip. Clin Orthop Relat Res 2003; 263–9. [DOI] [PubMed] [Google Scholar]
- 53.Kim WY, Hutchinson CE, Andrew JG, Allen PD. The relationship between acetabular retroversion and osteoarthritis of the hip. J Bone Joint Surg Br 2006; 88: 727–9. [DOI] [PubMed] [Google Scholar]
- 54.Wagner S, Hofstetter W, Chiquet M, et al. Early osteoarthritic changes of human femoral head cartilage subsequent to femoro-acetabular impingement. Osteoarthritis Cartilage 2003; 11: 508–18. [DOI] [PubMed] [Google Scholar]
- 55.Ecker TM, Tannast M, Puls M, et al. Pathomorphologic alterations predict presence or absence of hip osteoarthrosis. Clin Orthop Relat Res 2007; 465: 46–52. [DOI] [PubMed] [Google Scholar]
- 56.Tanzer M, Noiseux N. Osseous abnormalities and early osteoarthritis: the role of hip impingement. Clin Orthop Relat Res 2004; 170–7. [PubMed] [Google Scholar]
- 57.Tönnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am 1999; 81: 1747–70. [DOI] [PubMed] [Google Scholar]
- 58.Weinans H, Siebelt M, Agricola R, et al. Pathophysiology of peri-articular bone changes in osteoarthritis. Bone 2012; 51: 190–6. [DOI] [PubMed] [Google Scholar]
- 59.Nötzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br 2002; 84: 556–60. [DOI] [PubMed] [Google Scholar]
- 60.Rakhra K, Sheikh AM, Allen DJ, Beaulé PE. Comparison of MRI alpha angle measurement planes in femoroacetabular impingement. Clin Orthop Relat Res 2009; 467: 660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sutter R, Dietrich T, Zingg P, Pfirrmann CW. How useful is the alpha angle for discriminating between symptomatic patients with cam-type femoroacetabular impingement and asymptomatic volunteers? Radiology 2012; 264: 514–21. [DOI] [PubMed] [Google Scholar]
- 62.Hack K, Di Primio G, Rakhra K, Beaulé PE. Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am 2010; 92: 2436–44. [DOI] [PubMed] [Google Scholar]
- 63.Khanna V, Caragianis A, Di Primio G, et al. Incidence of hip pain in a prospective cohort of asymptomatic volunteers: is the cam deformity a risk factor for hip pain? Am J Sports Med 2014; 42: 793–7. [DOI] [PubMed] [Google Scholar]
- 64.Wilkins JM, Southam L, Mustafa Z, et al. Association of a functional microsatellite within intron 1 of the BMP5 gene with susceptibility to osteoarthritis. BMC Med Genet 2009; 10: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Lopez J, Pombo-Suarez M, Liz M, et al. Further evidence of the role of frizzled-related protein gene polymorphisms in osteoarthritis. Ann Rheum Dis 2007; 66: 1052–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev 2001; 11: 527–32. [DOI] [PubMed] [Google Scholar]
- 67.Doherty M, Courtney P, Doherty S, et al. Nonspherical femoral head shape (pistol grip deformity), neck shaft angle, and risk of hip osteoarthritis: a case-control study. Arthritis Rheum 2008; 58: 3172–82. [DOI] [PubMed] [Google Scholar]
- 68.Clohisy JC, Dobson MA, Robison JF, et al. Radiographic structural abnormalities associated with premature, natural hip-joint failure. J Bone Joint Surg 2011; 93(Suppl. 2): 3–9. [DOI] [PubMed] [Google Scholar]
- 69.Agricola R, Heijboer MP, Bierma-Zeinstra SM, et al. Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK). Ann Rheum Dis 2013; 72: 918–23. [DOI] [PubMed] [Google Scholar]
- 70.Weinans H. Periarticular bone changes in osteoarthritis. HSS J 2012; 8: 10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gregory JS, Waarsing JH, Day J, et al. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis? Arthritis Rheum 2007; 56: 3634–43. [DOI] [PubMed] [Google Scholar]
- 72.Lynch JA, Parimi N, Chaganti RK, et al. The association of proximal femoral shape and incident radiographic hip OA in elderly women . Osteoarthritis Cartilage 2009; 17: 1313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoaglund FT, Oishi CS, Gialamas GG. Extreme variations in racial rates of total hip arthroplasty for primary coxarthrosis: a population-based study in San Francisco. Ann Rheum Dis 1995; 54: 107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abraham E, Garst J, Barmada R. Treatment of moderate to severe slipped capital femoral epiphysis with extracapsular base-of-neck osteotomy. J Pediatr Orthop 1993; 13: 294–302. [DOI] [PubMed] [Google Scholar]
- 75.Aronsson DD, Loder RT, Breur GJ, Weinstein SL. Slipped capital femoral epiphysis: current concepts. J Am Acad Orthop Surg 2006; 666–79. [DOI] [PubMed] [Google Scholar]
- 76.Hoaglund FT, Steinbach LS. Primary osteoarthritis of the hip: etiology and epidemiology. J Am Acad Orthop Surg 2001; 9: 320–7. [DOI] [PubMed] [Google Scholar]
- 77.Rab GT. The geometry of slipped capital femoral epiphysis: implications for movement, impingement, and corrective osteotomy. J Pediatr Orthop 1999; 19: 419–24. [DOI] [PubMed] [Google Scholar]
- 78.Lehmann TG. Possible slipped capital femoral epiphysis in healthy 18 years old teenagers. In: 54th Nordic Orthopaedic Federation Congress. Jun 11–13 Amsterdam, The Netherlands, 2008. [Google Scholar]
- 79.Oishi CS, Hoaglund FT, Gordon L, Ross PD. Total hip replacement rates are higher among Caucasians than Asians in Hawaii. Clin Orthop Relat Res 1998; 166–74. [DOI] [PubMed] [Google Scholar]
- 80.Siebenrock KA, Ferner F, Nobel PC, et al. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res 2011; 469: 3229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim YJ, Novais EN. Diagnosis and treatment of femoroacetabular impingement in Legg–Calve–Perthes disease. J Pediatr Orthop 2011; 31(2 Suppl): S235–40. [DOI] [PubMed] [Google Scholar]
- 82.Larson AN, Sucato DJ, Herring JA, et al. A prospective multicenter study of Legg–Calve–Perthes disease: functional and radiographic outcomes of nonoperative treatment at a mean follow-up of twenty years. J Bone Joint Surg Am 2012; 94: 584–92. [DOI] [PubMed] [Google Scholar]
- 83.Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res 2011; 469: 1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Froberg L, Christensen F, Pedersen NW, Overgaard S. Long-term follow-up of a patient cohort with Legg-Calve-Perthes disease. J Pediatr Orthop B 2011; 20: 273–7. [DOI] [PubMed] [Google Scholar]