Abstract
Aims and Objectives:
Cerebral ischemic stroke is life-threatening and debilitating neurological disease, it is the third leading cause of death in the world. Studies have shown that there is a close relationship between carotid artery stenosis and ischemic cerebral vascular disease. This study is done to assess the carotid arteries with the help of color Doppler sonography and to correlate cerebrovascular accidents.
Materials and Methods:
The prospective study was carried out on 50 patients using purposive sampling technique. Risk factors such as hypertension, diabetes mellitus, smoking, and family history were documented. The data gathered from color Doppler examination consisted of peak systolic velocity of common carotid artery (CCA) and internal carotid artery (ICA), velocity ratios between CCA and ICA and plaque characteristics as seen on real-time image.
Statistical Analysis Used:
The collected data were analyzed and presented in the form of tables, figures, graphs, and diagrams wherever necessary. As this study deals with the only frequency distribution of various factors, so no tests of significance were applied.
Results:
The highest incidence of stroke was found in the male population in the age group of 60–69 years. Various risk factors included hypertension, diabetes mellitus, smoking, and family history. Of 50 patients, 12 patients showed significant stenosis (>60%). Atherosclerotic plaques were seen in 39 patients (78%).
Conclusion:
Color Doppler examination is an economic, safe, reproducible, and less time-consuming method of demonstrating the cause of cerebrovascular insufficiency in extracranial carotid artery system and will guide in instituting treatment modalities.
Keywords: Atherosclerotic plaque, color Doppler sonography, common carotid artery, internal carotid artery, peak systolic velocity, peak systolic velocity ratio, stenosis
INTRODUCTION
Cerebral ischemic stroke is a major cause of death, ranking third behind only malignancies and cardiovascular disease. Atherosclerosis of the intra and extracranial carotid vessels, leading to cerebral infarction accounts for 80% of strokes. Intracranial hemorrhage and subarachnoid hemorrhage account for the remainder. It has been conclusively proven that the risk of major stroke is higher in the first 3 months after transient ischemic attack (TIA).[1] It has been seen that 20% or more of strokes have been heralded by a TIA.[2] The highest risk of large artery stroke appears to be among patients with the highest degree of carotid stenosis, a history of diabetes, presence of asymptomatic carotid plaques, or a combination of these factors.[3]
Color Doppler sonography of carotid arteries forms an important part of the evaluation of extracranial insufficiency. Accurate diagnosis of hemodynamically significant stenosis is critical to identify patients who would benefit from surgical intervention. The value of a safe, noninvasive, and low-cost screening test is therefore of a great advantage.
Duplex sonography combining high-resolution imaging and Doppler spectrum analysis has proved to be a popular, noninvasive, accurate, and cost-effective means of detecting and assessing carotid disease. Carotid sonography has largely replaced angiography for suspected extracranial carotid atherosclerosis.[4] If timely endarterectomy of the carotid arteries is performed, many stroke cases may be prevented. This necessitates an evaluation of the extracranial carotid artery system. Carotid conventional angiography is the gold standard for detecting the severity of carotid stenosis, but it has its own disadvantages such as it is an invasive and expensive procedure. It carries a risk from contrast medium to the patients and a certain amount of morbidity. Magnetic resonance angiography is currently developing rapidly and may ultimately give similar or better results, especially for flow quantification, though at a much higher cost. Besides estimating the degree of stenosis, the biggest advantage of sonography is its ability to characterize plaque and identify plaques with higher risk of embolization. With high-resolution ultrasound, plaque can be characterized into relative risk groups containing intraplaque hemorrhage which is thought to be a precursor for plaque ulceration.[5,6] The brain is supplied by four vessels: The two internal carotid arteries (ICAs) and the two vertebral arteries [Figure 1].[7] This study was done to assess the carotid arteries with the help of color Doppler sonography and to correlate cerebrovascular accidents (CVAs) with extracranial carotid artery status.
Figure 1.
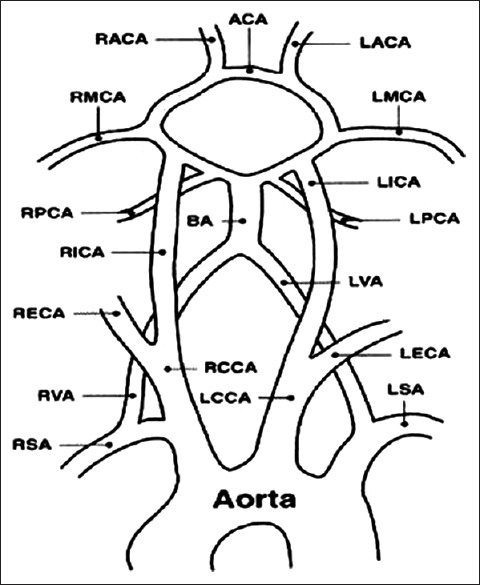
Vascular anatomy showing common carotid artery, internal carotid artery, and vertebral artery
MATERIALS AND METHODS
This descriptive study was carried out on 50 patients who had symptoms and signs of a stroke or TIAs at a tertiary care hospital in south India for a period of 2 years. Patients were selected using purposive sampling technique without any age, sex, ethnic, or socioeconomic discrimination. A detailed history and thorough physical examination were carried out on a questionnaire. Risk factors such as hypertension, diabetes mellitus, smoking, and ischemic heart disease were documented. The patients underwent computed tomography (CT) scan study prior to the color Doppler sonography of carotid arteries and findings were documented.
Cases with history, clinical and CT scan findings consistent with cerebral ischemic stroke were included in this study. Patients having symptoms suggestive of vertebrobasilar insufficiency, head injuries, and those having primary and metastatic brain tumors were excluded from the study.
Data collection techniques and tools
The data gathered from the CT scan examination consisted of: Side of the infarct – Right, left; Vascular territory – Middle cerebral artery, anterior cerebral artery, posterior cerebral artery; Cortical or subcortical infarct – Time interval between the onset of clinical signs/symptoms of ischemic stroke and CT scan performed.
The data gathered from the color Doppler examination consisted of: Peak systolic velocity (PSV) of common carotid artery (CCA) and ICA; ICA/CCA velocity ratios; Plaque characteristics; The presence of spectral broadening; and Detection and grading of carotid artery stenosis. All the examinations were performed by the same operator with a Doppler angle of 60°.
Equipment
Color Doppler sonography was done using Philips EnVisor C (Philips Medical Systems, Nederland B.V.) with a linear array transducer of 7 MHz. Prior CT scan was performed using GE Bright speed Elite, 16 slice CT scanner (GE Healthcare, Milwaukee, WI, USA). Prior ethical committee clearance was obtained to conduct this study.
Criteria used for measuring percentage of stenosis in our study
(a) ICA/CCA PSV ratios, and (b) Residual lumen diameter at most stenotic portion was compared to lumen diameter in the ICA bulb as used in Asymptomatic Carotid Atherosclerosis Study.[8] The diameter of the residual lumen and the external diameter of the artery at the same level were measured and the degree of stenosis was calculated using the relationship: Percent stenosis = (D – d) 100/D, where D is vessel wall-to-wall diameter and d, is patent vessel diameter. The gold standard has been angiography and the parameter that angiography provides is diameter stenosis and hence, in ultrasound, we also used diameter stenosis.
Analysis of data
The collected data were analyzed with the aid of a calculator and presented in the form of tables, figures, graphs, and diagrams wherever necessary. The findings are discussed in the light of findings of other similar studies. As this study deals with the only frequency distribution of various factors, so no tests of significance were applied.
RESULTS
Among the 50 patients, 36 patients (72%) were males and 14 (28%) were females. Of the 50 patients studied, 20 (40%) patients had right-sided stroke and 18 (36%) patients had left-sided stroke. Bilateral involvement was seen in 2 (4%) patients and 10 (20%) patients had TIA. Of the 12 patients with significant stenosis (>60% stenosis), the maximum patients were seen in the age group of 60–69 years that is 6 (50%) patients. Next age group was of 70–79 years with 4 (33.3%) patients. In age group 40-49 years and >80, there was only one patient in each group. None of them were in the age group of 50–59 years. Smoking and hypertension were the most prevalent risk factors for cerebral ischemic stroke [Figure 2].
Figure 2.
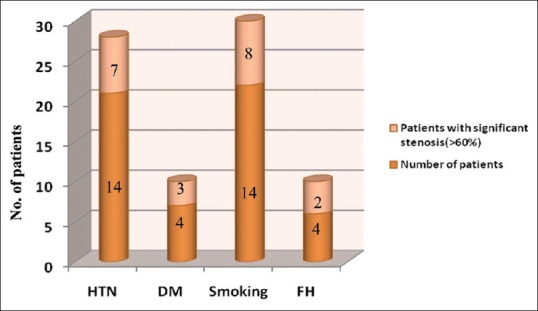
Risk Factors of cerebral ischemic stroke
CT brain findings showed normal findings in 13 cases, whereas lacunar infarcts (13 cases) were the most common pathological involvement [Table 1].
Table 1.
CT brain findings in stroke patients

Of the 12 patients with significant stenosis, none of the patients showed bilateral involvement. Eight (66.6%) patients had right-sided involvement and 4 (33.3%) patients had left-sided involvement. Seven patients have 60–79% occlusion, 3 patients have 80–89% occlusion whereas 2 patients have complete occlusion [Figure 3]. Of the total 39 patients with occlusion, 27 have occlusion <60%.
Figure 3.
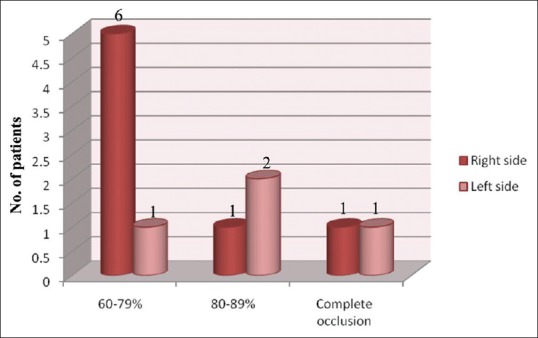
Patient with significant stenosis (>60%)
Atherosclerotic changes in the form of atheromatous plaques were found to be the main cause of obstruction. Of the 39 patients having plaque in the extracranial carotid system, 23 (59%) patients had bilateral involvement, 9 (24%) patients had plaque on right side and 7 (17%) patients had plaque on the left side. The remaining 11 (22%) patients out of 50 showed no evidence of plaque.
In our study, ICA was found to be the commonest site affected [Figure 4]. Plaques in the CCA were also seen on the right side in 1 patient and 1 on the left side. Bilateral involvement was seen in 1 patient. The bilateral homogenous plaque was the most commonly seen type of plaque [Figure 5].
Figure 4.
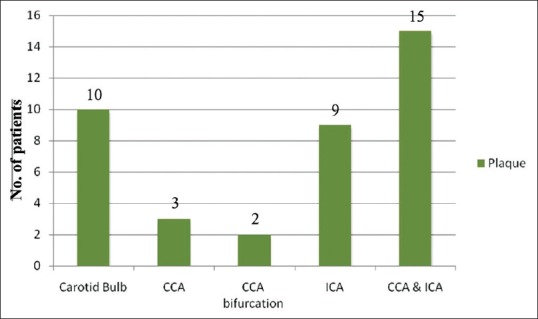
Site distribution of plaque (CCA: Common carotid artery, ICA: Internal carotid artery)
Figure 5.
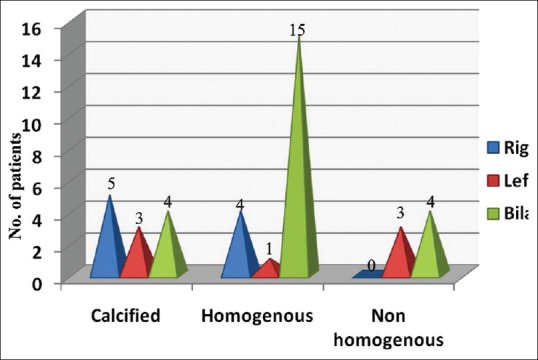
Plaque characterization
Of the 39 cases having carotid plaques, 2 cases were (5.1%) found to have a total block in which the PSV ratio was not applicable. Distribution of cases having carotid plaques, based on PSV ratio of ICA/CCA is shown in Figure 6.
Figure 6.
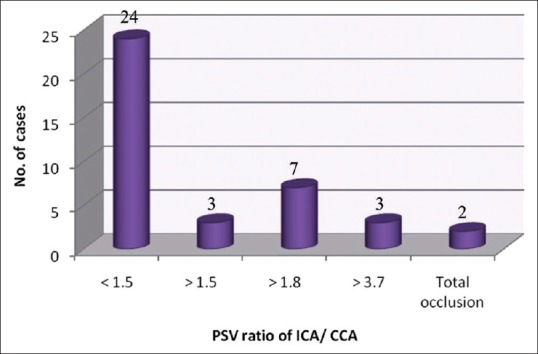
Distribution of cases having carotid plaques based on peak systolic velocity ratio of internal carotid artery/common carotid artery
DISCUSSION
About 30–60% of strokes are caused by atherosclerotic disease involving the extracranial carotid arteries usually within 2 cm of the carotid bifurcation.[9] Sonography is unique among vascular imaging procedures in that it can assess plaque composition. Sonographically detected plaque characteristics may have prognostic value and may be useful for selection of medical and surgical therapy.[10] This study was done to evaluate the extracranial carotid arterial system in the patients who presented with cerebral ischemic stroke and correlated CVA with extracranial carotid artery status.
In an earlier study, it was found that the incidence of stroke increases after 60 years of age.[11] The highest number of stroke patients in our study were found in the age group of 60–69 years which was 32% (16/50), followed by the age group between 70 and 79 years which was 26% (13/50). Iemolo et al. in his study showed that only 2.5% of stroke victims were females. In this study, 72% of the patients (36/50) were males and only 28% of the patients were females (14/50).
Lawes et al. studied 188,000 patients with hypertension out of which 6800 had stroke events.[12] In our study, of the 50 patients, 19 (38%) patients were hypertensive out of which 4 (21%) had significant stenosis. There is a positive relationship between smoking and risk of stroke. It was estimated in an earlier study that 22% of stroke was attributable to smoking.[13] Our study found 20 (40%) with a history of smoking. Of them, 6 (30%) had significant stenosis. Diabetes mellitus is another risk factor for atherosclerosis. A study conducted by Lindsberg and Roine observed that two-third of all ischemic stroke types on admission had diabetes mellitus.[14] In this study, 8 (16%) patients had diabetes mellitus of which 3 (37%) had significant stenosis.
Schulz et al. studied the family history of stroke and found that 23% of stroke patients had a positive family history.[15] In this study, family history of stroke was present in 7 (14%) patients of which 3 (42%) had significant stenosis. Cardiac diseases were ruled out in our patient since they interfere in the velocity profiles of the carotid system. A diminished cardiac output will reduce both systolic and diastolic velocities.
Normal CCA color Doppler waveform is shown in Figure 7. In the literature of ultrasound, different authors say that one of the 3 major Doppler parameters that is, PSV, EDV, or PSV ratio is the most accurate predictor of clinically significant ICA stenosis. Because a ratio compensates for the patient to patient physiological variability and also compensate for instrument variability, PSV ratio has been considered best for assessing stenosis. North American Symptomatic Carotid Endarterectomy Trial and European Carotid Endarterectomy Trial clearly demonstrated that the long-term benefits of endarterectomy were significantly greater than medical treatment in patients with 60% or 70% ICA stenosis, whether symptomatic, or asymptomatic. Second, the endarterectomy trials established 60–70% diameter reduction as clinically significant levels of ICA stenosis.[16]
Figure 7.
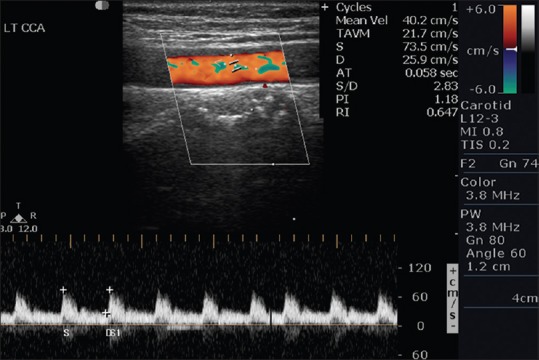
Normal Left common carotid artery color Doppler waveform in a 45-year-old male with a history of transient ischemic attack and normal brain computed tomography scan study
PSV ratio of >1.8 is an indicator of 60% or greater and a ratio of 3.7 is an indicator of more than 80% diameter stenosis. The validity of 1.5 ratio of PSV ratio of ICA/CCA is an indicator of 50% or greater stenosis. It has been found that the ratio is more accurate than PSV.[17] Using the criteria of ICA/CCA ratio, of the 10 patients with significant stenosis, 7 (70%) had significant stenosis on right side and 3 (30%) had significant stenosis on left side. On the right side, 6 patients had 60–79% stenosis and 1 patient had 80–99% stenosis. On the left side, 1 patient had 60–79% stenosis and 2 patients had 80-99% stenosis.
The duplex imaging of complete carotid occlusion was based on the absence of arterial pulsation, lumen filled with echogenic material, subnormal vessel size, and the absence of Doppler flow signals or weak Doppler signals.[18] In this study, we came across 2 patients with complete occlusion, 1 on the left and other on the right side. Severely stenosed contralateral ICA can artificially elevate ultrasound PSV since the effect was greatest when bilateral severe stenosis was present. Caution must be exercised when assessing the degree of ICA stenosis on the basis of ultrasound PSV alone.[19] We came across 1 patient who showed bilateral involvement with increased PSV > 130 on the contralateral side.
Schulte-Altedorneburg et al. found steno occlusive carotid lesion in 64% of the patients studied. He also confirmed his findings by postmortem studies.[20] In our study, 39 (78%) patients had plaques in the carotid artery of which 9 patients had plaques on the right side, 7 patients had plaques on the left side and 23 patients had bilateral involvement. Carotid bifurcation is commonly involved by the atherosclerotic plaque located distal to the origin of the carotid arteries.[21] In our study, ICA was found to be the commonest site affected by the plaque in 9 patients. In the present study, 12 patients had calcified plaques, 16 patients with strongly echogenic plaque, 7 had moderate echogenic plaque, and 4 patients showed low echogenic plaque. Soft plaques and heterogeneous plaques more positively correlate with symptoms than with any degree of stenosis and were the cause of adverse neurological events. Intraplaque hemorrhages are associated with frank ulceration and rapid progression to result in severe luminal narrowing.[22] Among the 4 low echogenic plaque lesions noted in our study, Intraplaque hemorrhage was not seen in any of the plaques. A fresh thrombus is virtually anechoic and a very old thrombus is markedly echogenic.[9] We came across 16 hyperechogenic plaques, and most of them were suggestive of very old thrombi.
Calcification occurs in plaque [Figure 8] in the areas of hemorrhage and necrosis. These calcifications generate strong reflections and distal acoustic shadows. No correlation exists between the presence of calcification and symptomatology. The risk of embolization or rapid progression would depend on plaque composition especially if it was heterogeneous, diffuse, or focal.[23] We came across 12 calcified plaques. Figure 9a shows soft plaque in left ICA 58.9% diameter stenosis and Figure 9b shows soft plaque in left ICA 31% diameter stenosis. Carotid artery stenosis of 70% or more was diagnosed reliably with the following duplex ultrasound criteria that is, a PSV of 230 cm/s or more and end diastolic velocity of 70 cm/s or more, or an ICA: CCA ratio of 3.2 or more in previous study.[24] In our study, we found 2 patients having PSV of ICA >230 cm/s. Both the patients found to have >80% stenosis. Using ICA/CCA ratio criteria, 10 patients had significant stenosis (ratio >1.8) in which 3 patients had ICA/CCA ratio >3.7. Two patients had complete occlusion on each side where no flow was detected in the vessel, hence, ICA/CCA ratio could not be assessed in them [Figure 10].
Figure 8.
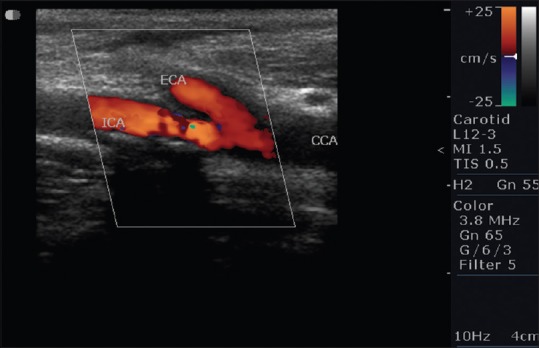
Calcific plaque at common carotid artery bifurcation in a 789-year-old female with a history of diabetes mellitus having lacunar infarcts in bilateral corona radiata
Figure 9.
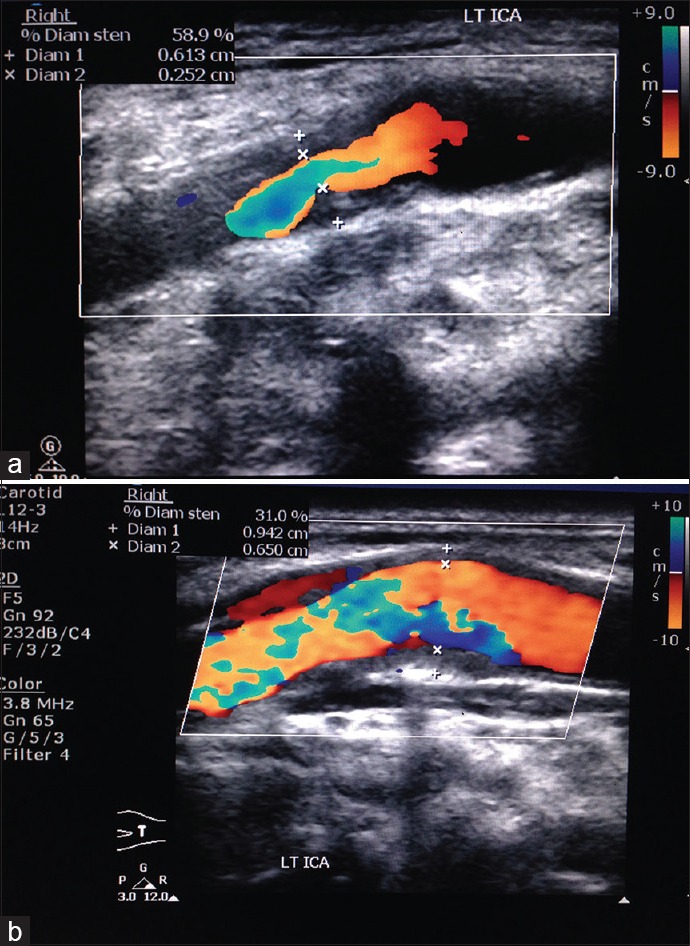
(a) Soft plaque in right carotid bulb with 58.9% stenosis in a 64-year-old male having right middle cerebral artery territory infarct. (b) Soft plaque in same patient with 31% stenosis in left proximal internal carotid artery
Figure 10.
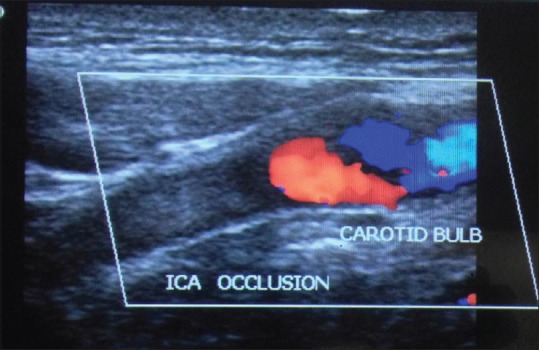
Complete occlusion of left internal carotid artery with hypoechoic plaque in a 72-year-old male with left middle cerebral artery territory infarct
A study conducted by Von Jessen and Sillesen showed that duplex scanning in 17% of patients who had TIA were found to have stenosis of 50% or greater and for the patient with persistent central neurological symptoms (stroke), stenosis was found in ≥50%.[25] We came across 12 patients with significant (>60%) stenosis of which 5 (41.6%) patients had hemiplegia, 6 (50%) patients had hemiparesis, and 1 (8.3%) patient had TIA. In our study consisting of 50 patients with cerebral ischemic stroke, carotid plaque was found in 39 (78%) patients.
According to a study conducted by Seth et al. all patients with >40% stenosis had a cortical infarct, none of the patients with >40% stenosis had a subcortical infarct. All with subcortical infarcts had either normal extracranial carotids or had <40% stenosis.[26] In our study, out of 12 patients with significant stenosis (>60%), 7 (58.3%) patients had cortical infarct, 1 (8.3%) patient had subcortical infarct, and 4 (33.34%) patients showed normal study. Of 39 patients with <60% carotid stenosis, 12 (30%) were found to have cortical infarct, 12 (30%) had subcortical infarct, and 15 (38%) showed normal findings on CT scan study. No histopathological correlation was done in our study because surgery was not done in any of the above patients. All these 50 patients were managed conservatively.
In light of the above findings, the role of carotid Doppler in detecting the site and morphology of atherosclerotic plaque and quantifying the degree of stenosis is very well justified.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bhatti TS, Harradine KL, Davies B, Earnshaw JJ, Heather BP. Urgent carotid endarterectomy can reduce the risk of stroke after a TIA. Br J Surg. 1999;86:699. doi: 10.1046/j.1365-2168.1999.0699b.x. [DOI] [PubMed] [Google Scholar]
- 2.Calanchini PR, Swanson PD, Gotshall RA, Haerer AF, Poskanzer DC, Price TR, et al. Cooperative study of hospital frequency and character of transient ischemic attacks. IV. The reliability of diagnosis. JAMA. 1977;238:2029–33. [PubMed] [Google Scholar]
- 3.Rothwell PM, Villagra R, Gibson R, Donders RC, Warlow CP. Evidence of a chronic systemic cause of instability of atherosclerotic plaques. Lancet. 2000;355:19–24. doi: 10.1016/s0140-6736(99)04470-0. [DOI] [PubMed] [Google Scholar]
- 4.Bluth EI. Evaluation and characterization of carotid plaque. Semin Ultrasound CT MR. 1997;18:57–65. doi: 10.1016/s0887-2171(97)90038-x. [DOI] [PubMed] [Google Scholar]
- 5.Fontenelle LJ, Simper SC, Hanson TL. Carotid duplex scan versus angiography in evaluation of carotid artery disease. Am Surg. 1994;60:864–8. [PubMed] [Google Scholar]
- 6.Carrol MR, Stephine RW, William JC. Diagnostic Ultrasound. 3rd ed. St. Louis, USA: Elsevier Mosby; 2005. The extracranial cerebral vessels; pp. 946–9. Ch. 27. [Google Scholar]
- 7.Williams PL, Dyson M. Gray's Anatomy. 37th ed. London: Longman Group UK Ltd; 1989. Angiology; pp. 734–43. Ch. 6. [Google Scholar]
- 8.Study design for randomized prospective trial of carotid endarterectomy for asymptomatic atherosclerosis. The Asymptomatic Carotid Atherosclerosis Study Group. Stroke. 1989;20:844–9. doi: 10.1161/01.str.20.7.844. [DOI] [PubMed] [Google Scholar]
- 9.Bluth EI, McVay LV, 3rd, Merritt CR, Sullivan MA. The identification of ulcerative plaque with high resolution duplex carotid scanning. J Ultrasound Med. 1988;7:73–6. doi: 10.7863/jum.1988.7.2.73. [DOI] [PubMed] [Google Scholar]
- 10.Iemolo F, Martiniuk A, Steinman DA, Spence JD. Sex differences in carotid plaque and stenosis. Stroke. 2004;35:477–81. doi: 10.1161/01.STR.0000110981.96204.64. [DOI] [PubMed] [Google Scholar]
- 11.Bowman TS, Sesso HD, Ma J, Kurth T, Kase CS, Stampfer MJ, et al. Cholesterol and the risk of ischemic stroke. Stroke. 2003;34:2930–4. doi: 10.1161/01.STR.0000102171.91292.DC. [DOI] [PubMed] [Google Scholar]
- 12.Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: An overview of published reviews. Stroke. 2004;35:1024. [PubMed] [Google Scholar]
- 13.Mannami T, Iso H, Baba S, Sasaki S, Okada K, Konishi M, et al. Cigarette smoking and risk of stroke and its subtypes among middle-aged Japanese men and women: The JPHC Study Cohort I. Stroke. 2004;35:1248–53. doi: 10.1161/01.STR.0000128794.30660.e8. [DOI] [PubMed] [Google Scholar]
- 14.Lindsberg PJ, Roine RO. Hyperglycemia in acute stroke. Stroke. 2004;35:363–4. doi: 10.1161/01.STR.0000115297.92132.84. [DOI] [PubMed] [Google Scholar]
- 15.Schulz UG, Flossmann E, Rothwell PM. Heritability of ischemic stroke in relation to age, vascular risk factors, and subtypes of incident stroke in population-based studies. Stroke. 2004;35:819–24. doi: 10.1161/01.STR.0000121646.23955.0f. [DOI] [PubMed] [Google Scholar]
- 16.Zweibel WJ. Introduction to Vascular Ultrasonography. 4th ed. Philadelphia: W.B. Saunders Company; 2000. Physics and instrumentation in Doppler and mode ultrasonography; pp. 17–27. Ch. 2. [Google Scholar]
- 17.Arbeille P, Desombre C, Aesh B, Philippot M, Lapierre F. Quantification and assessment of carotid artery lesions: Degree of stenosis and plaque volume. J Clin Ultrasound. 1995;23:113–24. doi: 10.1002/jcu.1870230206. [DOI] [PubMed] [Google Scholar]
- 18.Erickson SJ, Mewissen MW, Foley WD, Lawson TL, Middleton WD, Lipchik EO, et al. Color Doppler evaluation of arterial stenoses and occlusions involving the neck and thoracic inlet. Radiographics. 1989;9:389–406. doi: 10.1148/radiographics.9.3.2657897. [DOI] [PubMed] [Google Scholar]
- 19.Henderson RD, Steinman DA, Eliasziw M, Barnett HJ. Effect of contralateral carotid artery stenosis on carotid ultrasound velocity measurements. Stroke. 2000;31:2636–40. doi: 10.1161/01.str.31.11.2636. [DOI] [PubMed] [Google Scholar]
- 20.Schulte-Altedorneburg G, Droste DW, Felszeghy S, Csiba L, Popa V, Hegedüs K, et al. Detection of carotid artery stenosis by in vivo duplex ultrasound: Correlation with planimetric measurements of the corresponding postmortem specimens. Stroke. 2002;33:2402–7. doi: 10.1161/01.str.0000030111.34093.02. [DOI] [PubMed] [Google Scholar]
- 21.Zweibel WJ. Introduction to Vascular Ultrasonography. 4th ed. Philadelphia: W.B. Saunders Company; 2000. Doppler evaluation of carotid stenosis; pp. 146–51. Ch. 10. [Google Scholar]
- 22.Seeger JM, Klingman N. The relationship between carotid plaque composition and neurologic symptoms. J Surg Res. 1987;43:78–85. doi: 10.1016/0022-4804(87)90050-3. [DOI] [PubMed] [Google Scholar]
- 23.Tegos TJ, Sabetai MM, Nicolaides AN, Pare G, Elatrozy TS, Dhanjil S, et al. Comparability of the ultrasonic tissue characteristics of carotid plaques. J Ultrasound Med. 2000;19:399–407. doi: 10.7863/jum.2000.19.6.399. [DOI] [PubMed] [Google Scholar]
- 24.Carroll BA. Duplex sonography in patients with hemispheric symptoms. J Ultrasound Med. 1989;8:535–40. doi: 10.7863/jum.1989.8.10.535. [DOI] [PubMed] [Google Scholar]
- 25.von Jessen F, Sillesen HH. Occurrence of carotid stenosis in patients with cerebrovascular symptoms. Ugeskr Laeger. 1999;161:6049–52. [PubMed] [Google Scholar]
- 26.Seth SK, Solanki RS, Gupta H. Color and duplex Doppler imaging evaluation of extracranial carotid artery in patients presenting with transient ischemic attack and stroke: A clinical and radiological correlation. Indian J Radiol Imaging. 2005;15:91–8. [Google Scholar]


