Abstract
Surgical hip dislocation is the gold standard for treatment of femoroacetabular impingement (FAI). It utilizes an intermuscular and internervous approach to the hip. Concerns have been expressed that this approach causes soft tissue trauma resulting in post-operative muscle weakness of patients undergoing this procedure. We therefore asked whether surgical hip dislocation leads to (i) atrophy (decreased muscle diameter or cross-sectional area [CSA]) and (ii) degeneration (fatty infiltration) of 18 evaluated periarticular hip muscles. We retrospectively evaluated 32 patients (34 hips) following surgical hip dislocation for the treatment of FAI using pre and post-operative magnetic resonance (MR) arthrography of the hip. We evaluated muscle diameter, CSA and degree of fatty infiltration according to Goutallier for 18 periarticular hip muscles on axial and sagittal views. The mean interval between pre and post-operative MR was 1.9 ± 1.5 years (range, 0.4–6.1 years). Pre and post-operative muscle diameter and CSA of all 18 evaluated hip muscles did not differ. There was no post-operative change in the Goutallier classification for any of the evaluated 18 muscles. No muscle had post-operative degeneration higher than Grade 1 according to Goutallier. No atrophy or degeneration of periarticular hip muscles could be found following surgical hip dislocation for treatment of FAI. Any raised concerns about the invasiveness and potential muscle trauma for this type of surgery are unfounded. Level III, retrospective comparative study. See guidelines for authors for a complete description of levels of evidence.
INTRODUCTION
Post-operative alterations of the periarticular muscles have been found for basically every classic approach to the hip. Transmuscular approaches [1–3] imply the risk of substantial muscle damage resulting in gait dysfunction [4] and pain [5]. Muscle retraction and failure of repair of detached structures can also lead to functional problems. The increased risk of hip instability is an example of failure of the repair of the short external rotators in the posterior approach [6, 7]. Even intermuscular approaches such as the anterolateral approach may also result in substantial muscle alterations due to possible muscle denervation [8].
Surgical hip dislocation is an approach developed in the past decade that allows dislocating the hip without the risk of avascular necrosis [9]. It is considered the gold standard approach for management of femoroacetabular impingement (FAI) but can also be used for other indications, for example, treatment of acetabular [10, 11] and femoral fractures [12] or tumors [13]. Some authors have expressed concern that it causes more soft tissue trauma than more recently developed limited open approaches [14] or arthroscopy [15, 16]. It was even hypothesized that surgical hip dislocation is responsible for the muscle weakness in patients undergoing surgical treatment for FAI [17].
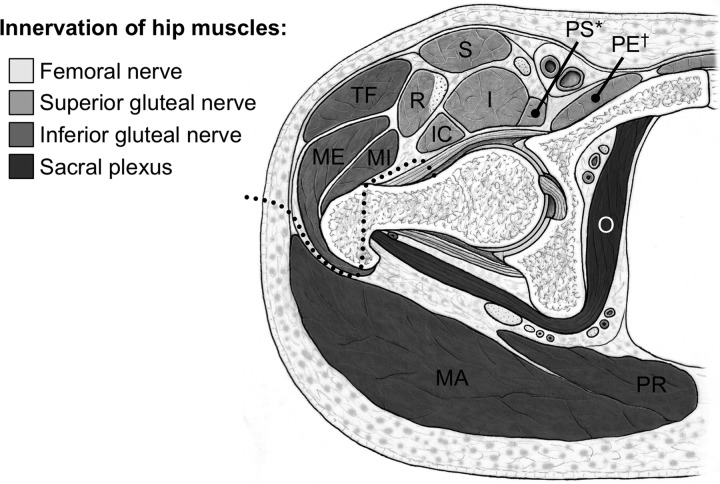
Surgical hip dislocation is essentially an intermuscular and internervous approach (Fig. 1). The majority of muscles are mobilized without extended detachment of muscle origins and insertions. This should result in minimal iatrogenic muscle trauma as shown for other intermuscular and internervous hip approaches [1]. Because surgical hip dislocation is most often used for joint preserving hip surgery in young patients, protection of the quantitative and qualitative integrity of the periarticular muscles is important. We therefore asked whether surgical hip dislocation leads to (i) atrophy (decreased muscle diameter or cross-sectional area [CSA]) or (ii) a degeneration (increased Goutallier grade [18]) of 18 evaluated periarticular hip muscles.
Fig. 1.
The surgical hip dislocation is an intermuscular and internervous approach to the hip (dotted line). The Gibson interval between the gluteus maximus (MA) and medius (ME) muscle is developed and a trochanteric osteotomy is performed. The internervous plane lies between the superior and inferior gluteal nerve. Gluteus maximus (MA), medius (ME), minimus (MI), tensor fasciae latae (TF), sartorius (S), rectus femoris (R), psoas (PS), iliacus (I), iliocapsularis (IC), pectineus (PE), obturator internus (O) and piriformis muscle (PR); *the cranial part of the psoas muscle is also supplied by direct roots of the lumbar plexus; †the caudal part of the pectineus muscle is also supplied by the obturator nerve.
PATIENTS AND METHODS
In a retrospective comparative study, we compared size and quality of hip muscles in patients before and after surgical hip dislocation for the treatment of FAI. These patients were recruited from the authors’ outpatient clinic. Inclusion criteria were pre and post-operative MR arthrographies of the hip. Sixty patients (67 hips) following surgical hip dislocation for the treatment of FAI had both pre and post-operative MR imaging (MRI). Surgeries were performed between January 2002 and December 2009. We excluded two patients (two hips) with previous surgery, one patient (one hip) with a hemiplegia and a neurologic deficit of the periarticular hip muscles, and four patients (four hips) with a known-pediatric hip disorder (two hips with slipped capital femoral epiphysis, one hip after Legg-Calvé-Perthes disease, and one hip with multiple epiphyseal dysplasia). Additionally, we excluded one patient (one hip) with a concomitant trochanteric advancement potentially affecting hip muscle quality. Twenty patients (25 hips) were excluded since they had been referred from other institutions with a preoperative MR images that had been acquired with a different MR protocol. Eventually, 32 patients (34 hips) were available for evaluation (Table I). The study was approved by the local Institutional Review Board (registration number: 16-07-13, date of issue 6 August 2013).
Table I.
Demographic data of the study group
| Parameter | Value |
|---|---|
| Number of patients (hips) | 32 (34) |
| Type of impingement (% of all hips) | |
| Cam | 12 |
| Pincer | 6 |
| Combined Cam-Pincer | 82 |
| Type of treatment (% of all hips) | |
| Offset creation only | 9 |
| Combined rim trimming and offset creation | 91 |
| Age at surgery (years) | 29 ± 8.9 (16–52) |
| Right side (% right of all hips) | 35 |
| Male hips (% male of all hips) | 38 |
| Height (m) | 1.70 ± 0.06 (1.59–1.82) |
| Weight (kg) | 70 ± 15 (53–110) |
| Body mass index (kgm2) | 24.3 ± 4.7 (19–37) |
| Interval from surgery to follow-up MRI (years) | 1.9 ± 1.5 (0.4–6.1) |
Values of continuous parameters are expressed as mean ± standard deviation with range in parentheses.
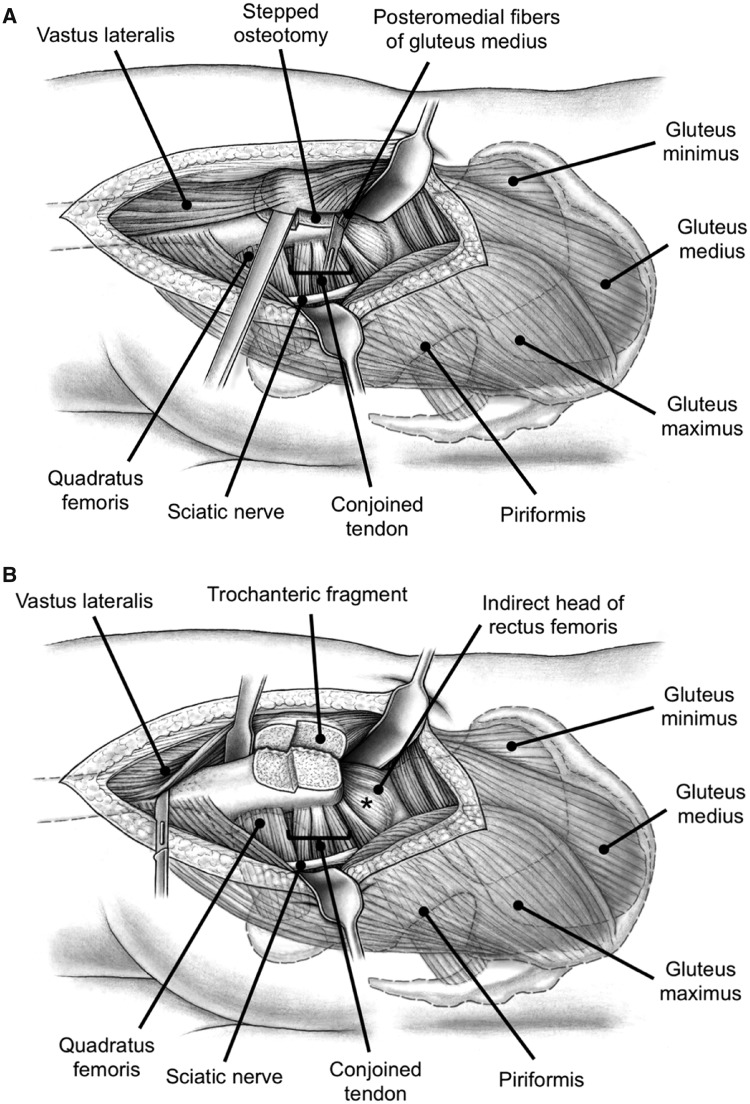
The surgical hip dislocation technique has been described in detail elsewhere [9]. Briefly, with the patient in lateral decubitus position, a straight skin incision is performed centered over the anterior third of the greater trochanter. The fascia lata is incised longitudinally and the Gibson interval between the gluteus maximus and medius muscles is developed. A trigastric osteotomy [19] of the greater trochanter is performed (Fig. 2). Starting in 2009, a stepped osteotomy has been performed (three hips [7%]) which allows advanced rehabilitation and is associated with a decreased rate of mal-/non-union compared with the flat osteotomy [19]. The trochanteric fragment includes the insertion of the gluteus minimus and medius muscles as well as the origin of the vastus lateralis muscle. The osteotomy leaves the most posteromedial portion of the gluteus medius muscle attached to the stable base of the greater trochanter (Fig. 2). This guarantees the integrity of the nutrient vessels to the femoral head (deep branch of medial circumflex femoral artery [20]). After mobilization of the greater trochanteric fragment, these fibers of the gluteus medius muscle are finally detached (Fig. 2). This is the only step of the entire approach necessitating muscle fiber detachment. The vastus lateralis muscle is mobilized from the proximal femur (Fig. 2). The insertion of the piriformis muscle at the base of the greater trochanter remains intact (Fig. 2). The capsule is developed by elevating the gluteus minimus muscle anteriorly starting at its interval to the piriformis tendon. This gives access to the posterosuperior capsular portion. The superior capsule is exposed by mobilizing the indirect head of the rectus femoris muscle (Fig. 2) and the anterior capsule by mobilizing the iliocapsularis muscle. After a z-shaped capsulotomy, the round ligament is cut and the femoral head can be dislocated for treatment of the FAI pathomorphology. Closure of the wound is initiated with a capsular suture, followed by refixation of the greater trochanter with two 3.5-mm cortical screws. The detached posteromedial fibers of the gluteus medius muscles are not reattached. The fascia of the vastus lateralis muscle can be reattached to its common site of attachment with the gluteus maximus insertion.
Fig. 2.
(A) To dislocate the hip a stepped osteotomy is performed. The trochanteric fragment includes the insertion of the gluteus minimus and medius muscles as well as the origin of the vastus lateralis muscle. The osteotomy leaves the most posteromedial portion of the gluteus medius muscle attached to the stable base of the greater trochanter. This protects the terminal branches of the medial femoral circumflex artery, which provides the blood supply to the femoral head. To mobilize the greater trochanteric fragment these fibers of the gluteus medius muscle are finally detached. (B) To further mobilize the trochanteric fragment the vastus lateralis muscle is sharply dissected from the proximal femur. Between the indirect head of the rectus femoris and piriformis muscles the postero-superior joint capsule (asterisk) can be accessed.
A total of 11 different surgeons had been involved in the 34-surgical hip dislocations. Twenty-two surgeries (65%) were performed by senior attending orthopedic surgeons (with an experience of more than 100 SHD) and eight (24%) under their direct supervision. Four surgeries (12%) were done by junior attending orthopedic surgeons (with an experience of <20 SHD).
The post-operative protocol includes immediate crutch mobilization with partial weight-bearing and restricted abduction and adduction to protect the trochanteric osteotomy. Weight bearing was restricted for 6 weeks to a maximum of 15 kg for all cases operated before 2008. After implementation of the stepped osteotomy [19], a maximum weight bearing of half of the patients’ body weight was permitted for 6 to 8 weeks. During the hospital stay, patients were kept on continuous passive motion up to 90° of flexion to prevent capsular adhesions. Stretching of the quadriceps and isometric muscle training of quadriceps and hamstrings were encouraged [19]. Uneventful healing of the greater trochanter was observed in all cases in our series. Patients were advanced to full weight bearing between 6 and 8 weeks, when the trochanteric osteotomy had healed, and abductor training was initiated for 4 to 6 more weeks. A minimum of 4 months of rehabilitation after surgery was performed in all cases.
All included patients had a pre and post-operative MR arthrogram of the hip according to a standardized technique [21]. The second MRI was performed in patients for evaluation of persistent post-operative hip pain and exclusion of potential intra-articular adhesions after a mean interval of 1.9 ± 1.5 (range, 0.4–6.1) years. The scans were performed using a Siemens Vision 1.5-T high field scanner (Erlangen, Germany) with a flexible surface coil after fluoroscopic-guided intra-articular injection of saline-diluted gadolinium-DTPA (Dotarem 1:200, Guerbert AG, Paris, France). Axial, sagittal, and coronal proton density-weighted and T1-weighted sequences were acquired. The axial slices used for measurements had a slice thickness of 3 mm and slice-to-slice distance of 3.6 mm. Commercially available software Osirix (Version 5.8, Geneva, Switzerland) was used for analysis [22].
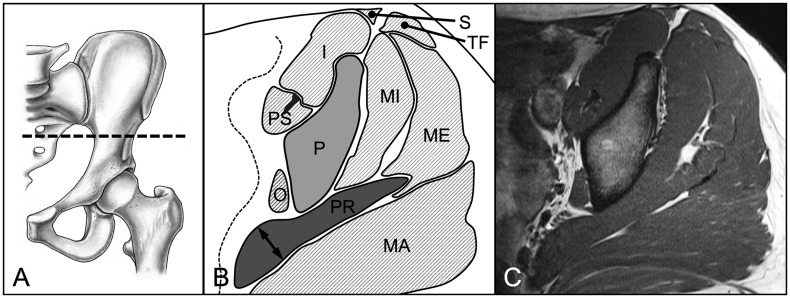
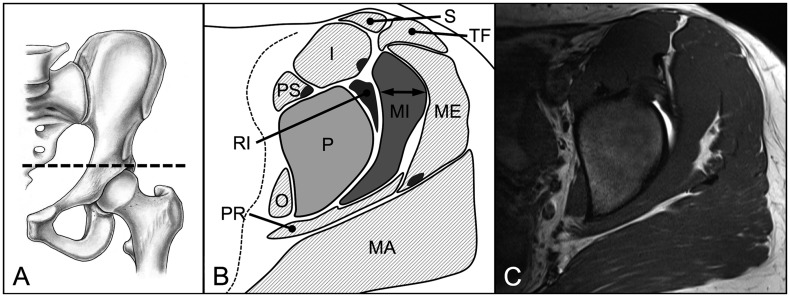
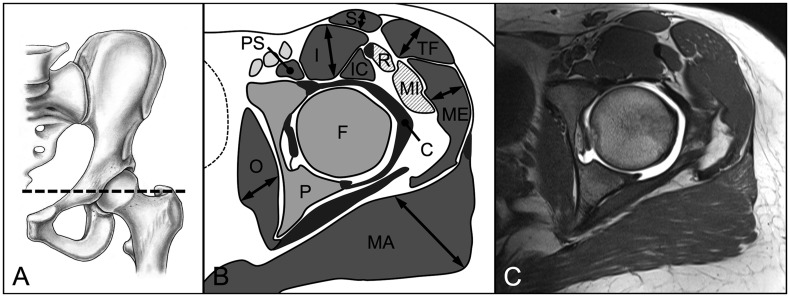
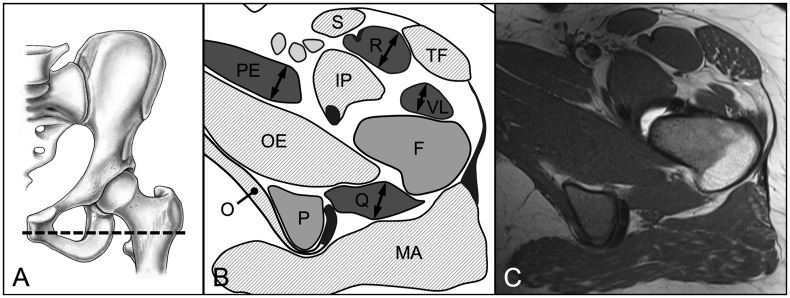
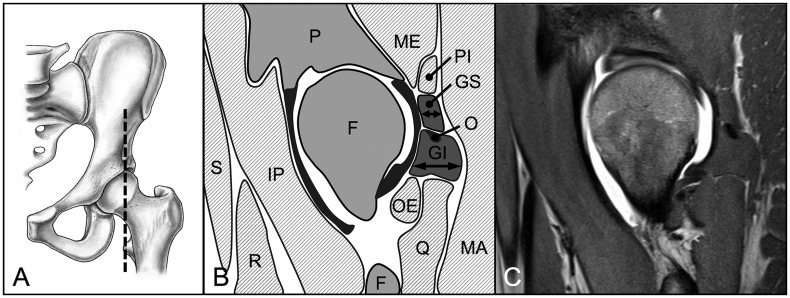
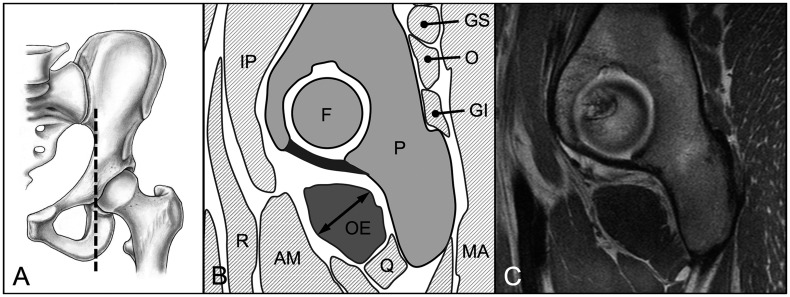
We evaluated three parameters for 18 hip muscles pre and post-operatively: muscle diameter, CSA and fatty infiltration [18]. These parameters were assessed on predefined axial and sagittal MR sections for each of the 18 evaluated muscles (Table II). We used four axial cuts at the following levels: sciatic notch (for evaluation of the piriformis muscle; Fig. 3), acetabular roof (gluteus minimus and rectus femoris muscles [indirect head]; Fig. 4), femoral head center (gluteus maximus and medius, tensor fascia latae, sartorius, iliacus, iliocapsularis, psoas and obturator internus muscles; Fig. 5) and ischial tuberosity (pectineus, rectus femoris [direct head], vastus lateralis and quadratus femoris muscles; Fig. 6). In addition, two sagittal cuts at the following positions were used: through the lateral quarter of the femoral head (gemelli superior and inferior muscles: Fig. 7) and through the transverse acetabular ligament (obturator externus muscle; Fig. 8).
Table II.
Muscle diameter, CSA, and fatty infiltration of the 18 hip muscles were assessed on axial MR views at four different levels and two different sagittal views.
| Muscle | Plane | Level/position | Description of diameter measurement | Description of CSA measurement | Figure |
|---|---|---|---|---|---|
| Gluteus maximus | Axial | Femoral head center | Maximum radial diameter | n.a. | 5 |
| Gluteus medius | Axial | Femoral head center | Maximum radial diameter | Maximum CSA | 5 |
| Gluteus minimus | Axial | Acetabular roof | Maximum diameter | Maximum CSA | 4 |
| Tensor fasciae latae | Axial | Femoral head center | Maximum radial diameter | Maximum CSA | 5 |
| Sartorius | Axial | Femoral head center | Maximum radial diameter | Maximum CSA | 5 |
| Rectus femoris, direct head | Axial | Ischial tuberosity | Maximum diameter | Maximum CSA, without tendinous portion | 6 |
| Rectus femoris, indirect head | Axial | Acetabular roof | Intactness of the tendinous insertion | n.a. | 4 |
| Vastus lateralis | Axial | Ischial tuberosity | Maximum diameter | Maximum CSA | 6 |
| Psoas | Axial | Femoral head center | Maximum radial diameter | Maximum CSA | 5 |
| Iliacus | Axial | Femoral head center | Maximum radial diameter | Maximum CSA | 5 |
| Iliocapsularis | Axial | Femoral head center | Maximum radial diameter | Maximum CSA | 5 |
| Obturator internus | Axial | Femoral head center | Maximum radial diameter | Maximum CSA, without tendinous portion | 5 |
| Obturator externus | Sagittal | Transverse ligament | Maximum diameter | Maximum CSA | 8 |
| Piriformis | Axial | Sciatic notch | Maximum diameter | Maximum CSA | 3 |
| Gemellus superior | Sagittal | Lateral ¼ of femoral head | Maximum horizontal diameter | Maximum CSA | 7 |
| Gemellus inferior | Sagittal | Lateral ¼ of femoral head | Maximum horizontal diameter | Maximum CSA | 7 |
n.a., not applicable.
Fig. 3.
(A) On the axial MR section at the level of the sciatic notch (B, C) the maximum diameter and area of the piriformis muscle (PR) were assessed. Pelvis (P), gluteus maximus (MA), gluteus medius (ME), gluteus minimus (MI), tensor fasciae latae (TF), sartorius (S), psoas (PS), iliacus (I), obturator internus (O) muscle.
Fig. 4.
(A) On the axial MR section at the level of the acetabular roof (B, C) the maximum diameter and area of the gluteus minimus muscle (MI) were assessed. Additionally, the intactness of the indirect head of the rectus femoris muscle (RI) was evaluated. Pelvis (P), gluteus maximus (MA), gluteus medius (ME), tensor fasciae latae (TF), sartorius (S), psoas (PS), iliacus (I), obturator internus (O), piriformis (PR) muscle.
Fig. 5.
(A) On the axial MR section at the level of the femoral head center (B, C) the maximum radial diameter and area of the gluteus maximus (MA) and medius (ME), tensor fasciae latae (TF), sartorius (S), iliacus (I), iliocapsularis (IC), psoas (PS), and obturator internus (O) muscles were assessed. Pelvis (P), femur (F), gluteus minimus (MI), direct head of the rectus femoris muscle (R), joint capsule (C).
Fig. 6.
(A) On the axial MR section at the level of the ischial tuberosity (B, C) the maximum diameter and area of the direct head of the rectus femoris (R), vastus lateralis (VL), quadratus femoris (Q), and pectineus (PE) muscles were assessed. Pelvis (P), femur (F), tensor fasciae latae (TF), sartorius (S), iliopsoas (IP), obturator internus (O) and externus (OE), gluteus maximus (MA) muscle.
Fig. 7.
(A) On the sagital MR section through the lateral quarter of the femoral head (B, C) the maximum horizontal diameter and area of the gemellus superior (GS) and inferior (GI) muscles were evaluated. Pelvis (P), femur (F), gluteus maximus (MA) and medius (ME), piriformis (PI), quadratus femoris (Q), obturator externus (OE), iliopsoas (IP), rectus femoris (R), sartorius (S) muscle, tendon of the obturator internus muscle (O).
Fig. 8.
(A) On the sagittal MR section through the transverse acetabular ligament (B, C) the maximum diameter and area of the obturator externus (OE) muscle were assessed. Pelvis (P), femur (F), gluteus maximus (MA), gemellus superior (GS) and inferior (GI), obturator internus (O), quadratus femoris (Q), adductor magnus (AM), rectus femoris (R), iliopsoas (IP) muscle.
The muscle diameter was measured on these MR sections for each muscle on specifically defined localizations (Table II and Figs. 3–8). As an exception, the indirect head of the rectus femoris muscle was graded as intact or not intact (Table II and Fig. 4). The CSA was measured for all periarticular hip muscles except the gluteus maximus and rectus femoris (indirect) muscles. For the gluteus maximus muscle, the CSA was not evaluated because its complete anatomical dimensions were not covered by the MR protocol. For the indirect head of the rectus femoris muscle the CSA was not determined since it comprises only the tendinous portion of this muscle. The fatty infiltration was assessed according to Goutallier [18]: Grade 0 indicates normal muscle; Grade 1 is characterized by some mild fatty streaks; Grade 2 has greater fatty streaking but more muscle than fat; Grade 3 has an equal amount of muscle and fat; and Grade 4 is defined by more fat than muscle being present. Fatty infiltration was assessed on the same MRI sections used to assess muscle diameter or CSA (Table II).
The reliability and reproducibility of muscle diameter, CSA and Goutallier classification [18] were tested using a set of 60 randomly chosen MR sections (30 pre and 30 post-operative sections). These blinded sections were analysed by two independent observers (FB and CEA) at two different occasions at least 1 month apart. Intraobserver and interobserver variations for muscle diameter and CSA were assessed using the interclass correlation coefficients (ICC) and the Goutallier classification using the kappa value (Table III).
Table III.
Results for reliability and reproducibility of muscle diameter measurement and definition of fatty infiltration
| Parameters | ICC/kappa intraobserver 1 | ICC/kappa intraobserver 2 | ICC/kappa interobserver |
|---|---|---|---|
| Diameter | 0.94 (0.92–0.96) | 0.91 (0.88–0.94) | 0.97 (0.96–0.98) |
| CSA | 0.99 (0.99–1.00) | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) |
| Fatty infiltrationa | 0.92 (0.94–0.97) | 0.86 (0.81–0.93) | 0.82 (0.73–0.88) |
Values are expressed as mean with 95% confidence interval. ICC, intraclass correlation coefficient. aAccording to Goutallier classification [18].
A normal distribution was present for all muscle diameters, which was confirmed with the Kolmorogov-Smirnov test. We used the paired t-test for comparison of muscle diameter and CSA between the pre and post-operative status. We used the McNemar test for comparison of the Goutallier classification pre and post-operatively. The integrity of the indirect head of the rectus femoris muscle was compared between pre and post-operative status using the Fisher’s exact test.
RESULTS
Pre and post-operative muscle diameter and CSA of all 18 evaluated hip muscles did not differ (Table IV).
Table IV.
Results for hip muscle diameter, CSA and fatty infiltration of the 18 evaluated muscles
| Muscle | Preoperative Diameter (cm) | Post-operative Diameter (cm) | P-value | Preoperative CSA (cm2) | Post-operative CSA (cm2) | P-value | Preoperative Goutallier Grade 1a (%) | Post-operative Goutallier Grade 1a (%) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Gluteus maximus | 3.2 ± 0.7 (2.1–4.7) | 3.1 ± 0.7 (2.2–4.7) | 0.073 | n.a. | n.a. | n.a. | 98 | 95 | 1.000 |
| Gluteus medius | 1.6 ± 0.4 (1.0–2.6) | 1.6 ± 0.5 (1.0–2.9) | 0.635 | 9.5 ± 2.5 (4.6–15.9) | 9.2 ± 2.7 (4.9–16.4) | 0.134 | 63 | 74 | 0.353 |
| Gluteus minimus | 1.6 ± 0.3 (1.1–2.0) | 1.5 ± 0.3 (0.9–2.3) | 0.209 | 11.5 ± 2.1 (8.2–15.3) | 11.6 ± 2.3 (7.8–16.0) | 0.481 | 23 | 26 | 0.808 |
| Tensor fasciae latae | 1.9 ± 0.5 (1.0–3.4) | 1.9 ± 0.5 (0.9–3.4) | 0.667 | 5.3 ± 2.0 (1.8–9.6) | 5.3 ± 2.0 (1.8–9.1) | 0.947 | 79 | 86 | 0.571 |
| Sartorius | 1.3 ± 0.3 (0.9–1.9) | 1.3 ± 0.3 (0.9–1.9) | 0.348 | 3.1 ± 0.9 (1.6–4.8) | 3.1 ± 0.9 (1.8–5.1) | 0.974 | 14 | 14 | 1.000 |
| Rectus femoris, direct head | 2.1 ± 0.4 (1.3–3.1) | 2.2 ± 0.4 (1.3–3.0) | 0.753 | 6.1 ± 1.8 (2.9–10.3) | 6.0 ± 2.0 (2.4–10.4) | 0.417 | 21 | 26 | 0.622 |
| Rectus femoris, indirect headb | 100b | 100b | 1.000 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Vastus lateralis | 1.8 ± 0.4 (1.1–2.8) | 1.7 ± 0.5 (0.9–2.6) | 0.220 | 4.1 ± 1.9 (0.8–8.8) | 3.7 ± 1.8 (0.7–8.5) | 0.131 | 58 | 72 | 0.258 |
| Psoas | 1.1 ± 0.3 (0.6–1.6) | 1.1 ± 0.2 (0.6–1.6) | 0.802 | 1.1 ± 0.4 (0.6–1.9) | 1.0 ± 0.4 (0.5–1.7) | 0.062 | 10 | 10 | 1.000 |
| Iliacus | 2.5 ± 0.4 (1.9–3.4) | 2.6 ± 0.4 (1.8–3.5) | 0.108 | 6.5 ± 1.5 (4.1–9.6) | 6.4 ± 1.5 (4.0–10.1) | 0.539 | 7 | 7 | 1.000 |
| Iliocapsularis | 0.9 ± 0.3 (0.5–1.5) | 0.9 ± 0.3 (0.4–1.5) | 0.186 | 1.6 ± 0.7 (0.8–3.0) | 1.6 ± 0.7 (0.8–3.1) | 0.403 | 7 | 9 | 0.999 |
| Obturator internus | 1.7 ± 0.4 (0.8–2.7) | 1.7 ± 0.4 (1.0–2.5) | 0.125 | 7.0 ± 2.0 (3.2–11.7) | 7.0 ± 2.0 (3.4–11.8) | 0.729 | 2 | 12 | 0.202 |
| Obturator externus | 2.6 ± 0.4 (1.9–3.4) | 2.5 ± 0.4 (1.7–3.5) | 0.108 | 7.8 ± 1.7 (4.8–11.5) | 7.8 ± 2.0 (4.5–12.9) | 0.729 | 9 | 12 | 0.999 |
| Piriformis | 1.6 ± 0.7 (0.6–3.3) | 1.6 ± 0.7 (0.7–3.0) | 0.078 | 8.9 ± 3.5 (3.9–16.5) | 8.6 ± 3.5 (3.0–16.1) | 0.161 | 12 | 19 | 0.382 |
| Gemellus superior | 0.7 ± 0.2 (0.3–1.1) | 0.7 ± 0.2 (0.3–1.2) | 0.168 | 0.7 ± 0.3 (0.3–1.5) | 0.7 ± 0.3 (0.3–1.4) | 0.068 | 2 | 14 | 0.110 |
| Gemellus inferior | 1.7 ± 0.3 (1.0–2.1) | 1.6 ± 0.3 (7–21) | 0.070 | 2.2 ± 0.6 (1.1–3.5) | 2.2 ± 0.6 (1.1–3.3) | 0.924 | 0 | 5 | 0.494 |
| Quadratus femoris | 1.6 ± 0.4 (0.8–2.8) | 1.5 ± 0.5 (0.8–2.8) | 0.222 | 4.3 ± 1.5 (2.0–8.8) | 4.2 ± 1.5 (1.8–7.5) | 0.323 | 14 | 21 | 0.571 |
| Pectineus | 1.9 ± 0.4 (1.0–2.8) | 1.9 ± 0.5 (1.1–2.8) | 0.173 | 7.9 ± 2.6 (3.7–14.7) | 7.9 ± 2.7 (3.7–16.0) | 0.688 | 26 | 35 | 0.482 |
aNone of the muscles had degeneration higher than Goutallier Grade 1. bPercentage of hips with intact indirect head of rectus femoris muscle. CSA, cross-sectional area. n.a., not applicable.
There was no post-operative change in the Goutallier classification for any of the evaluated 18 muscles (Table IV). No muscle had degeneration higher than Grade 1 according to Goutallier [18].
DISCUSSION
Surgical hip dislocation utilizes an intermuscular and internervous approach to the hip (Fig. 1). It is the gold standard approach for treatment of FAI but is also used for fracture [11, 23, 24] or tumor surgery [13]. Concerns have been expressed that the approach causes more soft tissue trauma than more recently developed limited open approaches or arthroscopy [14–16, 25]. It was even hypothesized that surgical hip dislocation causes muscle weakness in patients undergoing surgical treatment for FAI [17]. We therefore asked whether surgical hip dislocation leads to (i) atrophy and (ii) degeneration of periarticular hip muscles.
This study has several limitations. First, we included only a limited number of 34 hips for evaluation. However, this is the maximum number of hips available at our institution following surgical dislocation and with the same standardized pre and post-operative MRI protocol. Second, only symptomatic patients with persisting hip pain following surgical treatment of FAI were included in this study. These were the only patients that had a second MRI of their hip available post-operatively. Symptomatic patients after hip surgery are more likely to have pathologic MRI findings [26, 27]. Therefore, it is reasonable to assume that the symptomatic patients undergoing MR arthrography in the current study would represent those most likely to have radiological evidence of soft tissue trauma. Third, there was no consistent time interval between the surgical hip dislocation and the second MRI. The shortest time interval of 5 months in our study exceeds the required 3 months period to show post-operative fatty infiltration [3]. The grade of fatty infiltration does not change significantly from 3-month to the 1-year post-operative evaluation [3]. Based on these results from literature, one can postulate that we should not have missed any relevant qualitative and quantitative alterations of the periarticular hip muscles. Fourth, we were unable to measure the CSA of the gluteus maximus muscle which can be seen and potentially harmed during dissection in the Gibson interval. The field of view of our standard MRI did not cover the entire gluteus maximus muscle. However, since we did not find differences for its diameter and fatty infiltration, a relevant muscle damage can basically be excluded.
Soft tissue trauma has been reported for basically every surgical approach to the hip (Table V). The posterior approach has been associated with degeneration of the piriformis muscle and the conjoined tendon [28–30]. The direct lateral (transgluteal) approach may lead to atrophy and fatty degeneration of the ventral portion of the gluteus medius and minimus muscle [1, 31] and potential denervation of the tensor fascia latae muscles. The anterolateral approach may result in fatty degeneration of the anterior portion of the gluteus medius muscle with potential iatrogenic risk to the superior gluteal nerve. The anterior approach as an internervous and intermuscular approach has been associated with soft tissue damage to the tensor fascia latae and the gluteus minimus muscles [32]. Surgical detachment of muscles and/or tendons, failure of their refixation, transmuscular/tendinous approaches and iatrogenic damage of the afferent skeletal muscle innervation are the most often reported causes (Table V). In contrast to most of the studies available in literature (Table V), we could not detect any substantial changes of the quality and quantity of the periarticular hip muscles for the surgical dislocation approach. This involves in particular the gluteus minimus and medius and the vastus lateralis muscles that need to be mobilized (Fig. 2). Any raised concerns [14–17] about the invasiveness and potential muscle trauma for this type of surgery are unfounded. Surgical hip dislocation respects the blood supply to the femoral head, muscle intervals and their innervation. As with other intermuscular and internervous approaches to the hip, this implies reduced risk of surgical trauma [1, 29].
Table V.
Selected studies reporting on muscle damage following different approaches to the hip
| Author, year | Approach | Type of surgery | Modality | n (hips) | Reported muscle trauma |
|---|---|---|---|---|---|
| Lüdemann et al., [32] | Anterior | THA | MRI | 32 | Comparison of CSA and degree of fatty infiltration pre and post-operative; decreased CSA of the tensor fasciae latae muscle and increased CSA of the sartorius muscle post-operatively; increased degree of fatty infiltration of the tensor fasciae latae and gluteus minimus muscles post-operatively. |
| Bremer et al., [1] | Anterior versus transgluteal lateral | THA | MRI | 50 | Using the anterior approach, tears of the gluteus medius and minimus muscles, peritrochanteric bursa fluid, and atrophy of gluteus medius and minimus muscles were less pronounced or frequent. |
| Meneghini et al., [29] | Anterior versus posterior | THA | Cadaver | 12 | Using the anterior approach, less damage occurred to the gluteus minimus muscle or tendon; damage to the tensor fasciae latae or indirect head of the rectus femoris muscle occurred only using the anterior approach; the piriformis and conjoined tendon was detached intentionally in all hips with a posterior approach and were damaged in 50% of hips with a anterior approach; damage to the gluteus medius muscle was comparable. |
| Unis et al., [8] | Modified Watson-Jones | THA | MRI | 26 | Following a modified Watson-Jones approach, atrophy or fatty infiltration of the tensor fasciae latae was present in 62 or 42%, respectively. |
| Müller et al., [2] | Anterolateral versus direct lateral | THA | MRI | 44 | Using the direct lateral approach, the gluteus medius showed increased fatty infiltration and the tensor fasciae latae an increased CSA. |
| Vasilakis et al., [33] | Anterolateral versus modified Watson-Jones | THA | MRI | 37 | No difference in fatty infiltration for the gluteus medius and tensor fasciae latae muscles was found. |
| Pfirrmann et al., [27] | Transgluteal lateral | THA | MRI | 64 | Comparing patients with and without trochanteric pain following lateral transgluteal THA; abductor tendon defects and gluteus minimus and medius defects are more common in symptomatic patients. |
| Pellicci et al., [30] | Posterior | THA | MRI | 36 | Defects of the piriformis tendon in 43%, conjoined tendon in 57% and the quadratus femoris in 3%. |
| Khan et al., (28) | Posterior versus modified posterior | THA | MRI | 20 | Comparing fatty infiltration and volume of the piriformis and obturator internus muscles between an approach with piriformis tendon-repair and a piriformis sparing approach; for the approach with piriformis tendon-repair a decreased volume and increased fatty infiltration was found at 3-month follow-up and a comparable volume but increased fatty infiltration at 2-year follow-up for the piriformis muscle; no difference existed for the obturator internus muscle. |
| Bal et Lowe, [34] | Two-incision versus direct lateral versus posterior | THA | MRI | 32 | Minimal atrophic changes was found in hips following the two-incision approach, whereas, all hips following the direct lateral or posterior approach showed atrophic changes of gluteus minimus, medius, maximus, tensor fasciae lata, piriformis, or quadratus femoris muscle. |
| Mardones et al., [35] | Two-incision versus posterior | THA | Cadaver | 20 | Every two-incision total hip replacement caused measurable damage to the abductors, the external rotators, or both. Damage to the gluteus medius and minimus was substantially greater with the two-incision technique than with the posterior approach. |
| Van Oldenrijk et al., [36] | Direct lateral, anterior, anterolateral, posterior versus two-incision | THA | Cadaver | 25 | Four different less-invasive approaches compared with the conventional lateral approach; the less-invasive approaches did not result in less damage to the gluteus medius than the conventional approach; the less-invasive anterior approach has an increased risk of damaging the lateral femoral cutaneous nerve. |
| Dora et al., [37] | Percutaneous | Antegrade femoral nailing | Cadaver | 16 | Comparing three different nail entry points; insertion in piriformis fossa results in most damage to muscle and tendons (piriformis and obturator internus). |
| McConnell et al., [38] | Percutaneous | Antegrade femoral nailing | Cadaver | 34 | Average disruption of the tendinous portion of the gluteus medius muscle of 27% (range, 15–53%). |
THA, total hip arthroplasty; MRI, magnetic resonance imaging; CSA, cross-sectional area.
Decreased muscle strength for hip abductors and knee extension was found in patients following surgical hip dislocation for correction of FAI at a 12-month follow-up when compared with healthy volunteers [17]. The authors hypothesized that iatrogenic muscle damage during the surgical hip dislocation might be responsible for the muscle weakness in these patients [17]. The results of the current study do not support this hypothesis. The difference in muscle strength is more likely due to preexisting hip muscle weakness in patients with FAI [39]. Based on dynamometric and electromyographic measurements, it could be proven that patients with untreated FAI present with a decreased strength for adduction, abduction, flexion and external rotation in comparison to healthy volunteers [39]. In addition, a reduced preoperative ability to activate the tensor fascia latae muscle was found [39].
Studies evaluating the outcome following surgical hip dislocation in high-level athletes [40] support the statement that the surgical hip dislocation has a minimal adverse effect on the quality and quantity of the periarticular hip muscles. This is reflected by the reported athletes’ ability to resume their professional activity in literature. In a systematic review, the percentage of patients returning to the same level of sport was 95–100% for open surgical hip dislocation and 82–100% for arthroscopic treatment of FAI [40]. With the exception of a faster return to professional sports, differences in activity were not significant by 1 year when comparing surgical dislocation to arthroscopy for management of FAI prospectively [41]. Surgical hip dislocation for the treatment of FAI is an excellent muscle-preserving surgical treatment option particularly in patients with a more complex deformity that is difficult to correct by arthroscopy.
In summary, this study focused on radiological parameters to determine the effect of surgical dislocation on the periarticular muscles of the hip, providing objective evidence for the status of the periarticular musculature following the procedure. We found no significant difference in the pre and post-operative diameter, CSA or Goutallier classification of the selected periarticular muscles. We believe surgical dislocation is a safe procedure with minimal adverse effect on the periarticular musculature of the hip.
FUNDING
Funding from the Swiss National Science Foundation (SNSF) project number: PP00P3_144856 to M.T. The funding source had no role in study design, data collection, analysis, interpretation, writing or submission of the manuscript.
CONFLICT OF INTEREST
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
REFERENCES
- 1.Bremer AK, Kalberer F, Pfirrmann CW, et al. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br 2011; 93: 886–9. [DOI] [PubMed] [Google Scholar]
- 2.Müller M, Tohtz S, Dewey M, et al. Evidence of reduced muscle trauma through a minimally invasive anterolateral approach by means of MRI. Clin Orthop Relat Res 2010; 468: 3192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller M, Schwachmeyer V, Tohtz S, et al. The direct lateral approach: impact on gait patterns, foot progression angle and pain in comparison with a minimally invasive anterolateral approach. Arch Orthop Trauma Surg 2012; 132: 725–31. [DOI] [PubMed] [Google Scholar]
- 4.Svensson O, Sköld S, Blomgren G. Integrity of the gluteus medius after the transgluteal approach in total hip arthroplasty. J Arthroplasty 1990; 5: 57–60. [DOI] [PubMed] [Google Scholar]
- 5.Kohl S, Evangelopoulos DS, Siebenrock KA, et al. Hip abductor defect repair by means of a vastus lateralis muscle shift. J Arthroplasty 2012; 27: 625–9. [DOI] [PubMed] [Google Scholar]
- 6.Kwon MS, Kuskowski M, Mulhall KJ, et al. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop Relat Res 2006; 447: 34–8. [DOI] [PubMed] [Google Scholar]
- 7.Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop Relat Res 1998; 355: 224–8. [DOI] [PubMed] [Google Scholar]
- 8.Unis DB, Hawkins EJ, Alapatt MF, et al. Postoperative changes in the tensor fascia lata muscle after using the modified anterolateral approach for total hip arthroplasty. J Arthroplasty 2013; 28: 663–5. [DOI] [PubMed] [Google Scholar]
- 9.Ganz R, Gill TJ, Gautier E, et al. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br 2001; 83: 1119–24. [DOI] [PubMed] [Google Scholar]
- 10.Masse A, Aprato A, Rollero L, et al. Surgical dislocation technique for the treatment of acetabular fractures. Clin Orthop Relat Res 2013; 471: 4056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tannast M, Krüger A, Mack PW, et al. Surgical dislocation of the hip for the fixation of acetabular fractures. J Bone Joint Surg Br 2010; 92: 842–52. [DOI] [PubMed] [Google Scholar]
- 12.Henle P, Kloen P, Siebenrock KA. Femoral head injuries: Which treatment strategy can be recommended? Injury 2007; 38: 478–88. [DOI] [PubMed] [Google Scholar]
- 13.Siebenrock KA, Ganz R. Osteochondroma of the femoral neck. Clin Orthop Relat Res. 2002; 394: 211–8. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SB, Huang R, Ciccotti MG, et al. Treatment of femoroacetabular impingement in athletes using a mini-direct anterior approach. Am J Sports Med 2012; 40:1620–7. [DOI] [PubMed] [Google Scholar]
- 15.Papalia R, Del Buono A, Franceschi F, et al. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop 2012; 36:903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philippon MJ, Schenker ML. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med 2006; 25: 299–308. [DOI] [PubMed] [Google Scholar]
- 17.Luder G, Bertschy B, Rocourt MH, et al. [Functional deficits and self-perceived performance after surgical hip dislocation]. Sportverletz Sportschaden. 2011; 25: 201–7. [DOI] [PubMed] [Google Scholar]
- 18.Goutallier D, Postel JM, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994; 304: 78–83. [PubMed] [Google Scholar]
- 19.Bastian JD, Wolf AT, Wyss TF, Nötzli HP. Stepped osteotomy of the trochanter for stable, anatomic refixation. Clin Orthop Relat Res 2009; 467: 732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautier E, Ganz K, Krügel N, et al. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br 2000; 82: 679–83. [DOI] [PubMed] [Google Scholar]
- 21.Locher S, Werlen S, Leunig M, et al. [MR-Arthrography with radial sequences for visualization of early hip pathology not visible on plain radiographs]. Z Für Orthop Ihre Grenzgeb 2002; 140: 52–7. [DOI] [PubMed] [Google Scholar]
- 22.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging 2004; 17: 205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastian JD, Büchler L, Meyer DC, et al. Surgical hip dislocation for osteochondral transplantation as a salvage procedure for a femoral head impaction fracture. J Orthop Trauma 2010; 24: e113–8. [DOI] [PubMed] [Google Scholar]
- 24.Siebenrock KA, Gautier E, Woo AK, et al. Surgical dislocation of the femoral head for joint debridement and accurate reduction of fractures of the acetabulum. J Orthop Trauma 2002; 16: 543–52. [DOI] [PubMed] [Google Scholar]
- 25.Pavlou G, Salhab M, Murugesan L, et al. Risk factors for heterotopic ossification in primary total hip arthroplasty. Hip Int 2012; 22: 50–5. [DOI] [PubMed] [Google Scholar]
- 26.Blankenbaker DG, Ullrick SR, Davis KW, et al. Correlation of MRI findings with clinical findings of trochanteric pain syndrome. Skeletal Radiol 2008; 37: 903–9. [DOI] [PubMed] [Google Scholar]
- 27.Pfirrmann CW, Notzli HP, Dora C, et al. Abductor tendons and muscles assessed at MR imaging after total hip arthroplasty in asymptomatic and symptomatic patients. Radiology 2005; 235: 969–76. [DOI] [PubMed] [Google Scholar]
- 28.Khan RJ, Lam LO, Breidahl W, et al. Magnetic resonance imaging features of preserved vs divided and repaired piriformis during total hip arthroplasty: a randomized controlled trial. J Arthroplasty 2012; 27: 551–8. [DOI] [PubMed] [Google Scholar]
- 29.Meneghini RM, Pagnano MW, Trousdale RT, et al. Muscle damage during MIS total hip arthroplasty: Smith-Petersen versus posterior approach. Clin Orthop Relat Res 2006; 453: 293–8. [DOI] [PubMed] [Google Scholar]
- 30.Pellicci PM, Potter HG, Foo LF, et al. MRI shows biologic restoration of posterior soft tissue repairs after THA. Clin Orthop Relat Res 2009; 467: 940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller M, Tohtz S, Winkler T, et al. MRI findings of gluteus minimus muscle damage in primary total hip arthroplasty and the influence on clinical outcome. Arch Orthop Trauma Surg 2010; 130: 927–35. [DOI] [PubMed] [Google Scholar]
- 32.Lüdemann M, Kreutner J, Haddad D, et al. MRI-based measurement of muscle damage after minimally invasive hip arthroplasty. Orthopade 2012; 41: 346–53. [DOI] [PubMed] [Google Scholar]
- 33.Vasilakis I, Solomou E, Vitsas V, et al. Correlative analysis of MRI-evident abductor hip muscle degeneration and power after minimally invasive versus conventional unilateral cementless THA. Orthopedics 2012; 35: e1684–91. [DOI] [PubMed] [Google Scholar]
- 34.Bal BS, Lowe JA. Muscle damage in minimally invasive total hip arthroplasty: MRI evidence that it is not significant. Instr Course Lect 2008; 57: 223–9. [PubMed] [Google Scholar]
- 35.Mardones R, Pagnano MW, Nemanich JP, et al. The Frank Stinchfield Award: muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop Relat Res 2005; 441: 63–7. [DOI] [PubMed] [Google Scholar]
- 36.Van Oldenrijk J, Hoogland PV, Tuijthof GJ, et al. Soft tissue damage after minimally invasive THA. Acta Orthop 2010; 81: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dora C, Leunig M, Beck M, et al. Entry point soft tissue damage in antegrade femoral nailing: a cadaver study. J Orthop Trauma 2001; 15: 488–93. [DOI] [PubMed] [Google Scholar]
- 38.McConnell T, Tornetta P, Benson E, Manuel J. Gluteus medius tendon injury during reaming for gamma nail insertion. Clin Orthop Relat Res 2003; 407: 199–202. [DOI] [PubMed] [Google Scholar]
- 39.Casartelli NC, Maffiuletti NA, Item-Glatthorn JF, et al. Hip muscle weakness in patients with symptomatic femoroacetabular impingement. Osteoarthr Cartil 2011; 19: 816–21. [DOI] [PubMed] [Google Scholar]
- 40.Alradwan H, Philippon MJ, Farrokhyar F, et al. Return to preinjury activity levels after surgical management of femoroacetabular impingement in athletes. Arthroscopy 2012; 28: 1567–76. [DOI] [PubMed] [Google Scholar]
- 41.Zingg PO, Ulbrich EJ, Buehler TC, et al. Surgical hip dislocation versus hip arthroscopy for femoroacetabular impingement: clinical and morphological short-term results. Arch Orthop Trauma Surg 2013; 133: 69–79. [DOI] [PubMed] [Google Scholar]