Abstract
Type 1 Diabetes (T1D) is an autoimmune disease characterized by the pancreatic infiltration of immune cells resulting in T cell-mediated destruction of the insulin-producing beta cells. The successes of the Non Obese Diabetic (NOD) mouse model have come in multiple forms including identifying key genetic and environmental risk factors e.g. Idd loci and effects of microorganisms including the gut microbiota, respectively, and how they may contribute to disease susceptibility and pathogenesis. Furthermore, the NOD model also provides insights into the roles of the innate immune cells as well as the B cells in contributing to the T cell-mediated disease. Unlike many autoimmune disease models, the NOD mouse develops spontaneous disease and has many similarities to human T1D. Through exploiting these similarities many targets have been identified for immune-intervention strategies. Although many of these immunotherapies did not have a significant impact on human T1D, they have been shown to be effective in the NOD mouse in early stage disease, which is not equivalent to trials in newly-diagnosed patients with diabetes. However, the continued development of humanized NOD mice would enable further clinical developments, bringing T1D research to a new translational level. Therefore, it is the aim of this review to discuss the importance of the NOD model in identifying the roles of the innate immune system and the interaction with the gut microbiota in modifying diabetes susceptibility. In addition, the role of the B cells will also be discussed with new insights gained through B cell depletion experiments and the impact on translational developments. Finally, this review will also discuss the future of the NOD mice and the development of humanized NOD mice, providing novel insights into human T1D.
Keywords: NOD, type 1 diabetes, gut microbiota, B cells, Humanized mice
1. Introduction
Type 1 Diabetes (T1D) is an autoimmune disease characterized by the activation of autoreactive T cells and the subsequent destruction of the insulin-producing beta cells of the pancreatic Islets of Langerhans. Like many autoimmune diseases, T1D is increasing worldwide, predominantly in children [1–5], with enormous health and financial implications associated with managing T1D over a lifetime. It is therefore vital to gain as much knowledge as possible to help develop novel therapeutics to reduce this burden, with the overall aim to cure T1D. While human investigation is a major goal, particularly for the development of treatment, certain questions cannot be addressed. Many studies rely on animal models, especially those requiring initial in vivo information. One of the most used models in T1D is the Non-Obese Diabetic (NOD) mouse, which, unlike many other models studied in autoimmunity, develops spontaneous disease similar to humans. Use of this model has led to many advances, including the identification of multiple autoantigens and biomarkers that are shared by humans and which has enabled the development of therapeutic targets. While there are many important discoveries initially identified in the NOD mouse, this review focuses on 3 key areas: 1. The role of the innate immune system and gut microbiota, 2. The role of the B cells in autoimmune diabetes, 3. Humanizing the NOD mouse.
2. Animal models of Type 1 Diabetes (T1D)
There are 2 main animal models used in T1D research - the Bio-breeding (BB) rat and the NOD mouse. The BB rat model was developed in the 1970s from outbred Wistar rats. This was then followed by the NOD mouse, which originated in the inbreeding of the Cataract Shionogi (CTS) strain in the 1980s. Both the BB rat and NOD mouse exhibited polyuria, glycosuria, weight loss and lymphocytic infiltration of the islets of Langerhans within the pancreas [6, 7]. Both models have similar features to human disease; however, there are also differences, as outlined in Table 1. As T1D is T cell-mediated, the NOD model has become favored for studying the natural development of diabetogenic T cells, compared to the lymphopenic BB rat [8–11], in which lymphopenia is prominent [12] and fewer reagents are available to facilitate study. This difference provides an important research niche for the NOD model to be exploited.
Table 1.
Comparison between Human, BB rat and NOD mouse autoimmune diabetes
| Human | BB Rat | NOD | |
|---|---|---|---|
| Age at onset: | >6 months up to late adolescence |
7–14 weeks | >10 weeks |
| Ketoacidosis: | Severe | Severe | Mild |
| Lymphopenia: Insulitis: |
None DCs, Macrophages, B cells, NK cells, CD4 & CD8 T cells |
Severe DCs, Macrophages, B cells, NK cells, CD4 & CD8 T cells |
None DCs, Macrophages, B cells, NK cells, CD4 & CD8 T cells |
| Autoantigens: | Insulin, GAD, IA-2, IA-2β, ZnT8, IGRP, IAPP, HSP60, Carboxypeptidase H |
Insulin, GAD | Insulin, GAD, IA-2, IA-2β, ZnT8, IGRP, Chromogranin A |
| TCR repertoire bias: |
None | Anti-TCRVβ13 prevents diabetes |
None |
| Genetic Susceptibility: |
MHC most important, >40 non-MHC genes |
MHC most important, >10 non-MHC genes |
MHC most important, >40 non-MHC genes |
| Gender Effect: | Males and Females equally affected before puberty, small male preponderance after puberty |
Males and Females equally affected |
Females predominantly affected |
2.1. Immunopathology of the NOD mouse 2.1.1 Natural history
Innate immune cells infiltrate the pancreas of NOD mice from as early as 3 weeks of age, including dendritic cells (DCs) [13–15], macrophages [13] and neutrophils [15], prior to the infiltration of the lymphocytes. Similarly these cells are also found in the human islet infiltrate [16] and in the BB rat islets [17].
To assess the role of these subsets in the development of diabetes, individual cell populations were specifically depleted. When DCs, monocytes and macrophages were depleted, there was no lymphocytic infiltration in the pancreas at the predicted insulitis stage and diabetes was delayed [18, 19]. However, the time point of the depletion is also important [20]. Furthermore, these cells also have developmental differences [21–24] and changes to their phenotype and function [25–27] compared to diabetes resistant strains.
Infiltration of innate immune cells into the islets attracts adaptive CD4 and CD8 T cell subsets into the islets from approximately 4–6 weeks of age [28]. Both CD4 and CD8 T cells are required for diabetes development. This has been shown through genetically mutated NOD mice [29, 30], in vivo transfers into nude athymic mice [31, 32] or immunocompromised NOD.scid mice [33], and antibody immunotherapy targeting CD3 [34, 35], CD4 [36–40] and CD8 [38, 40, 41] cells, as well as Major histocompatibility complex (MHC) – MHC class I (MHC-I) [42] and MHC class II (MHC-II) [43]. The frequency of autoreactive T cells is low; however, the T cells that are autoreactive have been shown to recognize specific diabetes-related autoantigens such as those listed in Table 2. These T cells develop within the thymus and through defects in negative selection, escape into the periphery, where they fail to be controlled, and become activated leading to the specific destruction of the insulin-producing beta cells.
Table 2.
Autoantigens recognized in NOD mice by autoreactive T cells
| AUTOANTIGEN | AA* Position | MHC RESTRICTION | CLONE | CAPABLE OF INDUCING DIABETES? | REFERENCES |
|---|---|---|---|---|---|
| Insulin | A14–20 | H-2Db | AI4 | YES | [47] |
| B9–23 | I-Ag7 | 2H6 | NO (protective) | [48] | |
| B9–23 | I-Ag7 | BDC12-4.1 | YES | [49] | |
| B9–23 | I-Ag7 | BDC12-4.4 | YES | [49, 50] | |
| B12–25 | I-Ag7 | 2H6 | NO (protective) | [48] | |
| B15–23 | H-2Kd | G9C8 | YES (if activated or with CD4 T cell help) | [51, 52] | |
| B15–23 | H-2Kd | A1** | YES | [53] | |
| IGRP | 206–214 | H-2Kd | NY8.3 | YES (if activated) | [38, 54] |
| CHROMOGRANIN A | 359–372 | I-Ag7 | BDC2.5 | YES | [55] |
| 359–372 | I-Ag7 | BDC10.1 | YES | [55, 56] | |
| GAD65 | 286–300 | I-Ag7 | B16.3 | NO (protective) | [57] |
| 515–524 | H-2Kd | R1** | NO (delayed diabetes when transferred intra-peritoneally into neonatal NOD mice) |
[58] | |
| GAD67 | 515–524 | H-2Kd | C2** | UNKNOWN | [59] |
| Hsp60 | 437–460 | I-Ag7 | C9 | YES (if activated) | [60, 61] |
| ZnT8 | 345–359 | I-Ag7 | 10.8** | YES (if recipient lightly irradiated) | [62] |
Amino acid (AA)
Peptide induced responses
In addition, B cells are also involved in the development of diabetes, both as antigen presenters [15] and autoantibody secretors [44–46] and can be found within the islets of NOD mice. However, the role B cells play in diabetes pathogenesis is not fully understood and will be discussed in more detail later.
2.1.2 Genetic factors
In both NOD mice and humans, the most important genetic factor contributing to T1D susceptibility is the MHC, known as idd1 in mice (IDDM1 in humans) [63, 64]. NOD mice express MHC-II molecules I-Ag7 (ortholog of HLA-DQ), no I-E (ortholog of HLA-DR) and MHC-I H-2KdDb (MHCI), of which the I-Ag7 contributes significant susceptibility to developing diabetes. I-Ag7 has a polymorphism [65, 66], where the charged aspartate residue at position 57 of the beta chain is substituted with serine, a change which is also found in human HLA-DQA10201/B10302 (DQ8) [67] (alanine substitution for the aspartate). Substitution of the serine at amino acid position 57 in the beta chain of I-Ag7 to aspartate, the amino acid found in most other mouse strains, protected NOD mice from disease [68]. Similarly expression of I-E prevented diabetes development in NOD mice [68, 69]. In humans, the DR3/4-DQ2/8 haplotypes provide the greatest susceptibility to T1D [70], with other DR and DQ alleles providing protection [71]. To assess the roles of human HLA DR4 and DQ8 in promoting autoimmunity, we generated MHC-II deficient C57BL/6 mice (non-autoimmune) that expressed either HLA-DR4 or HLA-DQ8 [72]. Neither of these mice developed spontaneous diabetes, however, when these mice were bred onto C57BL/6 mice over-expressing B7.1 (CD80) under the rat insulin promoter (RIP), most of the HLA-DQ8 transgenic mice developed diabetes, while approximately 25% of HLA-DR4RIP-B7.1 mice developed diabetes. Upon generating HLA-DQ8DR4RIP-B7.1 transgenic mice, the diabetes incidence was similar to the HLA-DR4RIP-B7.1 mice suggesting HLA-DQ8 confers the greatest susceptibility to T1D, while HLA-DR4 limits the promotion of DQ8-mediated disease by enhancing Th2 T cell responses.
The second most important genetic susceptibility in humans relates to the level of expression of proinsulin within the thymus. Upstream of the proinsulin gene are a variable number of tandem repeats (VNTRs), which, depending on the number present, determine if the individual is protected from (140–200+ repeats, associated with increased thymic proinsulin) or susceptible to (26–63 repeats, associated with reduced thymic expression) diabetes [73]. It was hypothesized that the greater the thymic proinsulin expression, the more autoreactive T cells would be deleted by negative selection as they develop [74]. Confirmation of this hypothesis was achieved through generation of NOD mice with graded insulin levels to mimic human thymic proinsulin expression [75].
Genetically engineered NOD mice have provided a model in which genes can be deleted and/or inserted to identify their effect in mediating protection from, or susceptibility to, T1D, something we cannot do in humans. In both NOD mice and humans there are now over 40 genetic loci shown to be important in mediating T1D susceptibility, including genes relating to immune system function and regulation as well as pancreatic beta cell function (reviewed in [76]). While genome-wide association studies (GWAS) have identified susceptibility loci in humans, they do not always identify a candidate gene or its role in contributing to diabetes risk and therefore it is very important for the data to be compiled, together with functional significance [77].
Whereas in the past, a high proportion of people who developed T1D had a high genetic susceptibility profile, in recent years, individuals with lower genetic risk are also developing the disease [1]. This change in diabetes usceptibility is unlikely to have occurred by genetic shift and therefore the environment is now being interrogated in its role in promoting autoimmune diabetes.
2.1.3 Environmental factors
Environmental factors in T1D development are as important as genetic risk. In humans, this has been shown by a lack of concordance in monozygotic twin studies [78–80], geographical variation in diabetes incidence [81, 82], changes to diabetes susceptibility in migration studies [83] and, as mentioned, the accelerated rise in T1D incidence [1–5]. Therefore the environment, in association with genetic risk factors, is important for shaping the risk of developing T1D. Currently, a large international trial (TEDDY – The Environmental Determinants of Diabetes in the Young study) is underway, following children over time, to evaluate the role of specific environmental risk factors in predisposing individuals to T1D [84]. However, studying how a specific environmental factor influences human T1D is difficult due to the wide range of environmental stimuli we all encounter. Therefore, the NOD mouse model, once again, provides an invaluable source of insight into how these environmental factors may promote the development of autoimmune diabetes.
Many environmental factors in NOD mice have been implicated in altering diabetes susceptibility including exposure to dietary factors such as wheat [85, 86] and gluten [87], exposure to infectious organisms [88–90] and changes to the gut microbiota [91–95]. While the exact trigger(s) for autoimmune diabetes is unknown, the role of the gut microbiota has recently been of considerable interest.
3. The role of the gut microbiota in modifying diabetes susceptibility
Gut microbiota have co-evolved with the host over millions of years to establish a symbiotic relationship with the host, ensuring the survival of both host and microbe. However, many factors can influence changes to the composition of gut microbiota including the host genetics and immune response [91, 96, 97], usage of antibiotics or probiotics [95, 98] or inter-microbial competition [94]. The interaction between all these factors and the gut microbiota is summarized in Figure 1.
FIGURE 1. FACTORS INFLUENCING THE HOST MICROBIOTA AND DIABETES.
The gut microbiota has been shown to have a significant effect on the development of the immune system and vice versa in modifying risk of developing autoimmunity. While the bacteria can be directly influenced through the use of antibiotics and probiotics, the environmental niche in which they reside is also important. Environmental factors including sex hormones and those involved in metabolism can contribute to changes in the bacterial composition. Furthermore, the genetics of the host and the ability of the immune system to respond to the bacteria are also influential factors in shaping the gut microbiome. By altering the gut microbiota, it is possible to alter the antigens presented to the immune system and the context in which those antigens are presented i.e. in proinflammatory vs normal homeostatic turnover, the immune system can respond differently either protecting from or promoting autoimmunity. Therefore targeting different parts of this interactive network may enable tolerance and protection from disease.
3.1. The interactions between the innate immune system and the gut microbiota
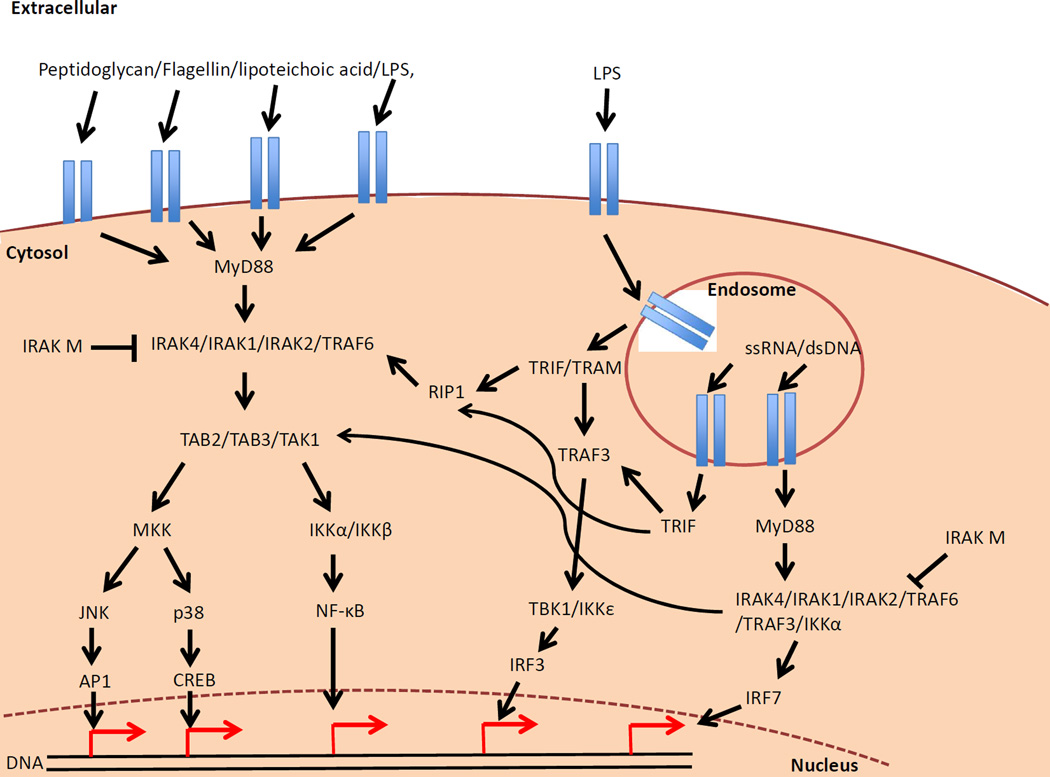
Investigations into the role innate immune cells play in the pathogenesis of T1D have focused predominantly on their interaction with the adaptive immune system via antigen presenting cells (APCs) i.e. their ability to stimulate antigen release [99], to present antigen to the diabetogenic T cells [100], their interactions with B cells [15] and their ability to promote the generation and sustained response of regulatory T cells [101]. However, the innate immune cells are also shaped by the environment, through interactions with Toll-like receptors (TLRs) and Nod-like receptors (NLRs) that recognize components of the gut microbiota (Figure 2).
FIGURE 2. TLR AND NLR SIGNALING PATHWAYS.
Pathogen-associated molecular patterns (e.g. LPS, Flagellin, bacterial peptidoglycan) bind to their specific TLR and mediate downstream effects, signaling through MyD88 and subsequently inducing proinflammatory cytokines. TLR3 and sometime TLR4 can also signal through TRIF upon binding to their ligand within the endosome, inducing proinflammatory cytokines and type I interferons to protect further cells from infection. In addition TLR7, 8 and 9 can also bind their ligand in the endosome and induce type I interferons. To help regulate these signaling pathways, molecules such as IRAK-M are present to inhibit MyD88 signaling and prevent or down-regulate the level of inflammation.
NLR signaling is believed to induce an additive response to the TLR signaling pathway. Similarly to the TLR signaling pathway, bacterial pathogen-associated molecular patterns can enter the cell and activate the cytosolic NLR signaling molecules such as NOD1/2, NLRP3 and NLRC4 (IPAF). These NLR molecules require the formation of inflammasomes (multi-subunit proteins interacting) in order to mediate their effects. While these pathways promote proinflammatory cytokines mediated through NF-KB, they also induce the activation of caspase 1, which in turn cleaves pro-IL1beta and pro-IL18 into their activated forms.
3.1.1. TLR Signaling
There are currently 12 TLRs, of which TLRs 3, 7, 8 and 9 are expressed intracellularly and TLRs 1, 2, 4, 5 and 6 are expressed extracellularly (reviewed in [102]). All but one of these TLRs utilize the downstream adaptor protein MyD88, stimulating the production of NF-κB-dependent proinflammatory cytokines and chemokines, type 1 interferon (if signaling through the endosomal TLRs) and activation of other transcription factors including cyclic AMP-responsive element-binding protein (CREB) and activator protein 1 (AP1). Therefore, signaling through these receptors is important for the TLR-expressing cells e.g. monocytes, DCs, macrophages and B cells to differentiate and become activated ([103] and reviewed in [104]). More recently, TLR signaling in T cells has also been shown to be important by providing costimulatory signals to promote survival, proliferation, cytokine secretion and antigen-specific clonal expansion (reviewed in [105]). Furthermore, these signaling events enable the gut epithelial cells to secrete antimicrobial peptides (reviewed in [106]), to undergo cell proliferation and to maintain tight junctions [107, 108], while B cells are induced to secrete IgA antibodies [109]. All of these responses collectively protect the host from infection.
Disruptions to TLR signaling, through specific genetic targeting, can increase bacterial load and pathology in response to bacterial infection [110]. However, specific TLR deficiencies can also influence the onset of autoimmunity. For example, TLR4−/− mice on the C57BL/6 background were protected from experimental autoimmune myocarditis due to a peripheral deletion of myosin-specific CD4 T cells, mediated by their increased apoptotic susceptibility when compared to wild type mice [111]. However, TLR4−/− NOD mice had accelerated autoimmune diabetes ([112] and our unpublished data). Similarly, the TLR9−/−MRL/lpr mouse developed accelerated systemic lupus erythematosus (SLE) [113], whereas TLR9−/− NOD mice are protected from autoimmune diabetes development ([97] and our unpublished data). Therefore, the role of the TLRs is different, dependent on the autoimmune disorder and the host genetic background. Further work revealed that NOD mice lacking TLR9 had decreased IFNα levels, a reduction in the number of plasmacytoid DCs and NY8.3 CD8 T cells when compared to wild type NOD mice [114]. Diabetes protection in the TLR9−/− NOD mouse also contributed to increased CD73 expression on CD4 T cells, which mediated TGF-beta dependent, MyD88-independent, immunosuppression and reduced production of proinflammatory cytokines [115].
However, some TLRs express a common phenotype regardless of the genetic background. TLR2 deficient mice are resistant to spontaneous diabetes development on the NOD background and to streptozotocin-induced diabetes in C57BL/6 mice, respectively [96]. Therefore, it was suggested that TLR2 was important for sensing beta cell death. Using Pam3Csk4 (TLR2 agonist) and dipeptidyl peptidase 4 inhibitors together (which prevent the destruction of incretin and therefore enable the hormones to reduce blood glucose), recent-onset diabetes was reversed in NOD mice [116]. This treatment was shown to increase beta cell mass and proliferation while reducing antigen-specific BDC2.5 CD4 T cell proliferation in the pancreatic lymph nodes (PLN). TLR2 has also been shown to be important in interacting with the gut microbiota and modulating insulin resistance in a T2D model [117].
It is interesting that TLR3−/− had no influence on spontaneous diabetes development in NOD mice [97] whereas TLR3 was indispensable for virus-induced diabetes in NOD [118, 119] or BALB/c mice [120] and in BB rats [121, 122]. Therefore, changing how these innate immune receptors interact with the environment is important for altering susceptibility to or protection from diabetes.
Signaling through the TLRs predominantly relies on the MyD88 adaptor protein to mediate their effects. Therefore, when MyD88 was deleted in the NOD model, it resulted in complete protection from diabetes [91]. However, when the mice were maintained in germ free (GF) conditions, they were no longer protected from diabetes development. This identified a key role for the commensal bacteria in modulating diabetes susceptibility. This was confirmed by colonizing GF MyD88−/− NOD mice with a mixture of gut microbiota, resulting in a reduction of autoimmune diabetes. Transferring microbiota from protected MyD88−/− NOD mice in non-GF conditions to conventional wild type NOD mice led to reduction of insulitis and delayed diabetes onset [94]. Stable transfer of the MyD88−/− microbiota was achieved with an increase in Lachnospiraceae and Clostridiaceae while decreasing Lactobacillacaeae compared to the control mice. The protection was also associated with higher concentrations of luminal TGF-beta and IgA and increased numbers of CD8+CD103+ T cells in the lamina propria of the large intestine.
Downstream signaling of MyD88 utilizes many different molecules to mediate its effect (Figure 2). Tan et al showed how blocking IL-1R-associated kinase M (IRAK-M, also IRAK-3), an inhibitor of MyD88 signaling, resulted in the acceleration of T1D, as early as 6–7 weeks, in IRAK-M−/− mice [123]. APCs in IRAK-M−/− NOD mice were more activated and highly capable of presenting antigen to diabetogenic T cells. This illustrates not only the importance of MyD88 signaling and its regulation in mediating autoimmunity but also that specific cellular subsets can be predominantly affected.
3.1.2. NLR Signaling
Similarly to TLR signaling, molecules known as Nucleotide oligomerization domain (NOD)-like receptors (NLRs) are intracellular proteins that detect microbial products in the cytosol, leading to activation of proinflammatory caspases and IL1-beta, with the ability to alter susceptibility to developing disease (reviewed in [124, 125]). NLR family members include NOD1 and NOD2, NLRP3, NLRC4, NLRP1 and NLRP6; however there are at least 34 NLR genes in mice. In addition to the proinflammatory role of these NLRs, NOD1 and NOD2 signaling has also been shown to stimulate antimicrobial peptide release in human epithelial cells [126, 127]. However, the role of NLRs, in the development of autoimmune diabetes is not well understood.
Interestingly, NOD2 mutations have been found in patients with Crohn’s disease, where the variants associate with a reduction in proinflammatory cytokines [128]. Therefore knowing this connection with NOD2 and Crohn’s disease, we asked whether NOD2 would be involved in mediating T1D susceptibility. To examine the role of NOD2, we recently developed NOD2 deficient NOD mice (manuscript in preparation). We found that the incidence of diabetes was very similar between the wild type and NOD2−/− NOD mice when they were co-housed. However, when they were housed separately, the NOD2−/−NOD mice were protected from developing diabetes, suggesting the microbiota play an important role in shaping this difference. However, mechanistic studies are currently under investigation. To investigate the role of the inflammasome in the immuno-pathogenesis of T1D development, we developed NLRP3-deficient NOD mice [129]. In the absence of NLRP3, NOD mice developed a reduced incidence of T1D. This protection resulted in reduced T cell activation and IFNγ secretion. Interestingly, NLRP3 could also modulate CD4 T cell chemotaxis into the islets, whereby NLRP3-deficient CD4 T cells were unable to express CCR5 and CXCL10 and therefore, the diabetogenic T cells were not able to home to the islets. This work suggests the NLRP3 inflammasome may provide a therapeutic target for the treatment of T1D.
Thus, TLR and NLR interactions with the microbiota are important in modifying diabetes susceptibility. This data, in combination with gut inflammation preceding diabetes onset [130] and diabetogenic T cells becoming activated in the gut and pancreatic lymph node (PLN) [131] aids in understanding the importance of the microbiota-gut-PLN axis in shaping diabetogenic potential and susceptibility to diabetes in NOD mice.
3.1.3. Probiotics and antibiotics modify diabetes susceptibility
Many researchers are now pursuing treatments aimed at modifying the microbiota through antibiotic or probiotic treatments. One study showed that young NOD mice that were treated with vancomycin for several months showed a reduced incidence of diabetes (~80% vs 95% in controls), accompanied by an increase of a specific species of gut bacteria (Akkermansia mucinphila) and an increase of proinflammatory CD4 T cells in the small intestine [92]. In contrast, short-term vancomycin treatment of our NOD mouse colony led to a highly accelerated incidence of diabetes, especially when the treatment started in the prenatal period (Hu, et al., manuscript in preparation). Tormo-Badia and colleagues showed recently that pregnant female NOD mice treated with a cocktail of antibiotics, not only passed on altered gut microbiota to their litters but the treatment also altered the development and function of the gut immunological system [95]. In this regard, giving probiotics, e.g. oral administration of lactobacillus casei to NOD mice, reduced the incidence of diabetes [98, 132]. Additionally, combinatorial treatment of the genetically-modified probiotic Lactococcus lactis, to secrete human proinsulin/GAD65370-575 and IL-10, with low dose anti-CD3, reversed diabetes development in NOD mice [133, 134].
Polysaccharide A (PSA), a component in the bacterial capsule of Bacteroides fragilis, was shown to induce CD4 Treg cell expansion and activation. This protected the mice from developing experimental autoimmune encephalomyelitis and intestinal inflammation, while deletion of bacterial PSA induced disease [135–137]. However, Bacteroides fragilis (PSA+) has no effect on T1D development in NOD mice (our unpublished data). No single bacterial molecule has, as yet, been shown to induce protection from T1D. On the other hand, autoreactive T cells that recognize bacterial antigens induce autoimmunity such as in uveoretinitis [138]. This supports molecular mimicry (whereby a bacterial antigen is similar to a host molecule) as a mechanism by which autoreactive T cells become activated and induce autoimmunity. However, this process has not yet been shown to have direct effect in inducing T1D.
It is clear that the microbiota as a whole is as important as the individual TLR and NLR signaling pathways and therefore multiple targets are now being pursued in developing novel therapeutics to prevent diabetes. However, an approach that targets multiple factors i.e. not just the signaling pathway but the stimulants (i.e. microbiota) too, may be more beneficial. In addition, identifying the cells that interact with the microbiota would also enable the development of more effective immune-intervention strategies.
4. The role of B cells in the pathogenesis of autoimmune diabetes
B cells are important immune cells that secrete antibodies, provide help to CD4 T cells through antigen presentation, costimulation, and cytokine production, as well as aid in the regulation of the immune response through B cell regulatory activity.
B cells also play an important role in T1D development, demonstrated by early experiments using B cell-deficient NOD mice (µMT−/−; targeted IgM gene deletion resulting in B cell developmental arrest), which rarely developed diabetes [139, 140] except for a small number of mice [141]. Furthermore, when B cells were isolated from NOD mice and transferred into µMT−/− NOD mice, they were unable to induce diabetes, whereas when the mice were repopulated with syngeneic NOD mouse bone marrow, the development of diabetes was restored [142]. This suggested that while the B cells do not directly cause T1D, they are involved in the development. Studies showed that the islet-infiltrating B cells were antigen-experienced and able to act as APCs to the islet-infiltrating T cells [143]. A specific subset of B cells known as B1a cells (CD5+CD19+CD1dmed) play an important role in the pathogenesis of T1D through activation of DCs as depleting the peritoneal B1a cells delayed diabetes onset [15, 144]. This data suggests B cells are important in mediating both the initiation and progression of T1D in NOD mice.
B cells in NOD mice have important antigen-presenting and costimulatory properties, as B cells deficient in I-Ag7 reduced the incidence of diabetes [145]. However, if costimulatory molecules including B7-1 were over-expressed locally, i.e., on the surface of the islet beta cells (under the rat insulin promoter, RIP), the µMT−/− NOD mice developed accelerated diabetes [140]. This suggests that under normal conditions, B cells are important for both antigen presentation and costimulation in the immunopathogenesis of T1D development, but are dispensable when costimulatory molecules are up-regulated locally.
B cells, like T cells, are capable of recognizing multiple antigens, dependent on their antigen-specific surface receptor, known as the B cell receptor (BCR). Through the BCR, B cells in the NOD mouse can recognize multiple diabetes-related autoantigens including GAD [46] and insulin [44, 45] resulting in autoantibody production. These autoantibodies can be detected in NOD mice as early as approximately 3 weeks of age and can help identify diabetes risk and islet inflammation. However, unlike similar autoantibodies in humans, they are not associated with the progression of islet immuno-pathology and/or diabetes onset [146–148]. In addition to islet autoantigen-reactive autoantibodies the B cells also secrete autoantibodies against systemic autoantigens including anti-nuclear antibodies in response to fragmented DNA, which may activate plasmacytoid DCs (pDCs) during diabetes pathogenesis [15, 149]. We have recently found an unexpected link between TLR4 and the antibody-producing function of B cells in NOD mice. As discussed earlier, TLR4−/− NOD mice develop accelerated diabetes, and interestingly, these mice showed reduced gut mucosal IgA secretion in vivo and in vitro (Gulden et al, unpublished data). Furthermore, it appears that more mucosal IgAs were bound to gut microbiota in TLR4−/− NOD mice compared to wild type NOD mice (Gulden et al, unpublished data). This suggests that B cells may also be important in altering the gut microbiota, which may have impact on diabetes susceptibility. Clearly, this requires further investigation.
Insulin is an important antigen that can be recognized by both T and B cells. Proinsulin is expressed in the thymus of NOD mice and humans, aiding in the deletion of autoreactive T cells, however this process is leaky and insulin-reactive T cells escape into the periphery to cause disease. Insulin-reactive B cells, on the other hand, develop within the bone marrow but they too are not fully controlled during their development and escape into the periphery to enhance diabetes pathogenesis [150, 151]. To understand how the insulin-reactive B cells develop, Thomas’ group generated a transgenic NOD mouse using the heavy chain of an anti-insulin monoclonal antibody (VH125), in combination with endogenous light chains, to form the BCR [152]. This revealed a BCR repertoire where approximately 1–3% of B cells were insulin-reactive, leading to accelerated diabetes. Interestingly, replacing 2 amino acid residues in the complementary determining region (CDR) H2 of this BCR prevented insulin recognition and subsequently the mice were protected from developing diabetes [152, 153]. Furthermore, targeting molecules downstream of BCR signaling such as BTK, through gene deletion, also protected NOD mice from developing diabetes [154]. However, expressing the insulin-specific BCR heavy chain (VH125) in the BTK-deficient NOD mice reversed this protective effect even though fewer B cells could recognize insulin. In addition, specific depletion of the insulin-reactive B cells through administration of anti-insulin monoclonal antibody prevented diabetes in NOD mice [155]. This demonstrates that autoantigen specific B cells promote autoimmunity.
4.1 B cell depletion modifies diabetes susceptibility
As B cells are important in both NOD and human T1D, we were interested in understanding how specific depletion of these cells may affect the homeostasis of immune tolerance in T1D. We generated a NOD mouse that expresses human CD20 on their B cells (hCD20 NOD). In this mouse the B cells can be depleted using 2H7, an anti-human CD20 antibody, which recognizes the same epitope as Rituximab, an antibody used in humans, at a desired time [156, 157]. Rituximab treatment has been shown to ameliorate disease in rheumatoid arthritis (RA) and SLE, among others, through apoptosis-stimulating mechanisms including complement and Fc-mediated cytotoxicity (reviewed in [158]). Temporarily depleting B cells in the hCD20 NOD mice can prevent diabetes development and reverse hyperglycemia in one third of diabetic mice [156]. Further study demonstrated that the B cell depletion promoted regulatory T cell expansion as well as altering the antigen-presenting capability of the APCs. Moreover, combined treatment with oral anti-CD3, which does not deplete T cells, significantly enhanced the immune tolerance induced by B cell depletion, through enhanced Treg expansion and conversion mediated by TGF-beta [157]. Furthermore, the combined treatment induced IL-10 and IL-27 producing dendritic cells that in turn promoted IL-10-producing CD4 T cells.
In addition to the effect on Tregs, we found that B cell depletion induced the expansion of an immunoregulatory antigen-presenting population, Gr1+CD11b+ cells, known as myeloid-derived suppressor cells [159]. This population was capable of inducing Tregs through TGF-beta and was able to suppress diabetogenic CD4 and CD8 T cells in a cell-contact dependent manner. While these cells are immunosuppressive in autoimmune diseases (reviewed in [160]), and in cancer [161], our study provides pre-clinical data for a possible target of immunotherapy in controlling diabetes development. However, these myeloid-derived suppressor cells could also induce differentiation of Th17 cells, which are important pathogenic cells in experimental autoimmune encephalomyelitis (EAE), an experimental model of human multiple sclerosis (MS) [162]. Thus, more research is needed to better understand the role of these cells in autoimmunity and in T1D.
Our studies using hCD20 NOD mice provided important pre-clinical data for the bench-to-bed side translation. The ancillary study for the clinical trial of Rituximab in patients with new-onset T1D identified that some patients had increased T cell responses to islet autoantigens [163]. To investigate the effect of B cell depletion on islet autoantigen-specific T cells, we took a bed side-to-bench “reverse translation” approach. We bred diabetogenic BDC2.5 TCR transgenic mice onto the hCD20 NOD background, generating BDC2.5hCD20 NOD mice [164]. Interestingly, anti-CD20 depletion, prior to B cell reconstitution, resulted in enhanced antigen-specific proliferation of BDC 2.5 CD4 T cells both in vitro and in vivo and accelerated diabetes upon adoptive transfer into NOD.scid mice. Unlike B cell depletion in the hCD20 NOD mice, the depletion in BDC2.5hCD20 NOD mice resulted in decreased antigen-specific Tregs [157, 159, 164]. However, upon B cell reconstitution, Tregs were significantly increased and the BDC2.5 CD4 T cells exhibited fewer proinflammatory responses and delayed transfer of diabetes into NOD.scid mice [164]. Investigation into which B cell subsets were important in regulation revealed that CD1d+CD5+ B cells showed strong antigen-specific suppression that was cell contact-dependent but independent of IL10. While B cell depletions have aided further discoveries into the complex dynamics of the immune system, it is clear that these discoveries provide important knowledge that immunotherapy can re-set the balance towards immune tolerance.
While the role of B cells in diabetes has been shown to be important for antigen presentation, autoantibody production, antibody secretion in modifying the microbiota, regulating immune responses and enhancing Treg development, there are still many unanswered questions. Thus, more research needs to be carried out including the development of humanized NOD mice.
5. Humanized NOD mice
There are different types of humanized mouse models which can be categorized into three groups – 1) introducing human gene(s) of interest into a mouse strain with or without depleting the mouse homolog gene(s) to study the function of the introduced human gene(s); 2) transplanting human cells, hematopoietic stem cells (HSC) or mature immune cells, i.e., peripheral blood mononuclear cells (PBMC) into severely immunodeficient mice to study the role of transplanted immune cells in vivo and 3) transplanting human gut microbiota into GF mice to study the effect of human gut microbiota on the body’s immunity and/or metabolism. Unlike humans, these mouse models enable us to investigate a biomedical or a disease process from the initiation stage through to fulminant disease onset in vivo. These model systems also allow researchers to investigate the affected organ/tissues such as the pancreas, which are inaccessible in live patients. In addition, humanized mice permit the identification and testing of novel therapeutics in vivo pre-clinically prior to further translation to human-based clinical trials. In this section, we will focus on the first group of humanized mice in T1D studies.
5.1. NOD mice expressing HLA molecules
The MHC is the most important genetic factor in human and mouse T1D development. Therefore researchers sought to understand how these MHC and antigen-specificities interact to initiate, or trigger, disease. Human histocompatibility leukocyte antigen DQA1*0301/DQB1*0302 (HLA-DQ8) is the HLA most commonly associated with T1D [165] and is known to bind insulin or GAD65 peptides in vitro. However, it was unknown how autoreactive T cells that targeted these antigenic specificities interacted with peptide:MHC complexes and what the functional consequence of that was. We generated one of the first generations of humanized C57BL/6 mice, in which, endogenous MHC-II (I-Ab) was deleted and replaced with HLA-DQ8 under the MHC class II promoter [166]. It is not surprising that these mice did not develop spontaneous diabetes, as they were on a T1D resistant genetic background, C57BL/6. The T1D protected phenotype demonstrated that the MHC is necessary, but not sufficient, for disease development. These mice mounted strong HLA-DQ8-restricted immune responses when immunized with GAD65 peptides (247–266 and 509–528). The immunization induced B cells to produce GAD autoantibodies in vivo and CD4 T cell proliferation in vitro through recognition of the peptides via TCR Vbeta7 and Vbeta12. Several GAD-reactive cell lines were generated from these GAD-reactive CD4 T cells and they were able to induce insulitis upon adoptive transfer in the presence of a mild insult to islet beta cells, which otherwise had no noticeable effect on insulitis induction [166]. This study also indicated that, in addition to the presence of the T1D susceptibility gene, the microenvironment of islet beta cells is also important in contributing to T1D development. To further address whether GAD-reactive CD4 T cells could cause diabetes without prior chemical insult to islets, HLA-DQ8 mice that express the co-stimulatory molecule B7-1 on their islet beta cells were generated [167]. It is intriguing that the double transgenic HLA-DQ8 B7.1 mice developed a high incidence of spontaneous diabetes in mice on the otherwise T1D resistant C57BL/6 genetic background. T cells in these mice were capable of recognizing the islet autoantigens, insulin and GAD, accompanied by increased IFN-gamma secretion. In addition, these T cells could induce diabetes upon transfer into irradiated, non-diabetic HLA-DQ8 B7.1 mice. However, this was only seen in mice expressing DQ8 as, when DQ6 was expressed with B7.1, none developed diabetes. The study confirmed the importance of HLA-DQ8 in T1D development. Interestingly, NOD mice expressing HLA-DQ8 did not develop spontaneous diabetes unless I-Ag7 was expressed or develop diabetes after cyclophosphamide treatment [168, 169]. It is noteworthy that an important factor that is often overlooked is that the removal of I-Ag7 also changes the MHC-I of NOD - KdDb to KbDb. It is known that most of diabetogenic CD8 T cells found in NOD mice are restricted to Kd [51, 170].
In NOD mice expressing human CD4 in conjunction with HLA-DQ8, GAD immunization identified T and B cell epitopes [169]. These models provided an invaluable tool to identify human GAD autoantigenic epitopes that may not have been discovered due to the low expression of GAD65 in humans and an inability to identify the antigen specificities within the islets. Similar work using these HLA-DQ8 mice also identified other antigen specificities including IA-2 [171] and peptide-binding sequence motifs shared between diabetogenic MHC-II molecules [172].
In addition to human MHC class II transgenic mice, the same approach was later used to generate mice expressing human MHC class I genes such as HLA-A2*01, HLA-A1*11 and HLA-B07*02, all of which are associated with altered genetic susceptibility to T1D [173]. However, unlike the generation of human MHC-II transgenic mice, the expression of a human HLA class I transgene requires the presence of part of the human beta 2 microglobulin, namely, a chimeric monochain construct (named HHD) encoding human beta 2 microglobulin covalently linked to human HLA-A2*01 alpha 1 and alpha 2 domains with murine alpha 3 transmembrane and cytosolic domains (from H-2Db) [174]. This HHD construct enables a greater interaction between murine CD8 and the MHC, which previously, without this construct, resulted in a low frequency of CD8 T cells [175]. HHD mice develop accelerated diabetes and express CD8 T cells recognizing murine IGRP epitopes 228–236 and 265–273, which are identical or differ by only 2 amino acids to the human IGRP sequence, respectively [174]. This demonstrates that similar autoreactive CD8 T cells can cause diabetes in humans. Furthermore, multiple proinsulin peptides were identified in these mice, as was also found in the HLA-A1*11 and HLA-B07*02 humanized NOD mice [176–178]. All of these developments, using different humanized HLA transgenic mouse strains, have enabled immunological strategies for diabetes prevention to be developed, including multi-peptide approaches or soluble peptide:HLA chimeras [179–181].
5.1.2. Developing new humanized NOD mice
Humanized NOD mice, and NOD mice in general, have provided many significant discoveries into T1D pathogenesis in humans. However, there still remain differences between the humanized mice and human patients, including a predominant female bias and a rapid progression of T1D in the mouse model, compared with a small male predominance after puberty (reviewed in [182]). In addition, while some autoantigens are shared, the sequences of these are not identical between humans and mice and thus, the antigens may encompass different epitopes. Furthermore, other factors such as the route of administration, dose and timing are also unknown when transitioning from NOD mice to humans. Thus using these mice as a preclinical model for human T1D has led to some difficulty in translating the work into humans (reviewed in [183]). Therefore, further improving humanized NOD mice is important.
Previously, NOD.scid mice, which lack T and B cells, were shown to enable more successful engraftment of human cells upon transfer, with reduced graft versus host disease severity, when compared to other commonly used mouse strains [184]. In addition, treating NOD.scid mice with anti-asialo GM1 antibody, to deplete NK cells, and breeding them to a beta 2 microglobulin deficient background prevented MHC-I antigen presentation and further enhanced human cell engraftment in these mice [185, 186]. Furthermore, T cell clones isolated from human T1D patients, when transferred into NOD.scid mice, could successfully home to the pancreas upon co-transfer of MHC-matched donor APCs, providing the human MHC for antigen presentation [187]. However, these cells failed to infiltrate the islets, which suggests other cells and/or factors may be required in order to initiate diabetes in these mice.
To address these issues, engraftment of human HSCs may provide the humanized NOD mice with a human immune system. Studies have shown that preventing the common cytokine receptor gamma chain (IL-2Rgamma chain) from functioning through truncation or genetic deletion can prevent numerous lymphoid cytokine receptors from signaling and mediating functional responses [188]. This genetic modification further enhanced human cell engraftment and enabled the development of human T and B cells when human cord blood or bone marrow was infused [189–191]. These mice have been recently used to establish optimal conditions and donors to enable successful human islet transplantation [192, 193].
NOD.scid mice lacking the IL-2Rgamma chain are also known as NSG mice and more recently have been bred to human HLA transgenic mice to provide new models to study autoimmune diabetes [194, 195]. Using this model system, Unger and colleagues showed that the adoptive transfer of an IGRP-specific T cell clone from a patient with T1D into NSG HLA-A2 transgenic mice induced islet destruction in vivo [194]. Similarly, the transfer of autoantigen-pulsed human CD4 T cells into NSG HLA-DR4 transgenic mice also led to the transferred human CD4 T cells infiltrating islets [195]. Interestingly, the islet infiltration was not restricted to autoreactive T cells from T1D patients, as CD4 T cells from healthy control subjects when pulsed with autoantigen also homed to islets. However, the severity of insulitis was greater when transferring the autoreactive CD4 T cells from patients with T1D. Therefore, these models provide key insights into the initiation and destruction of these cells and may enable cellular differences between healthy and people with T1D to be exploited for novel immunotherapeutic strategies.
One of these immunotherapeutic strategies that have been used in humanized NSG mice was anti-CD3 (Teplizumab) treatment, which did not bind to Fc receptors to deplete T cells. Preclinical studies of this treatment in NOD mice revealed sustained tolerance to pancreatic beta cells through initial reduction of T cells followed by the induction of Tregs [34, 196–198]. Interestingly, a clinical trial of Teplizumab treatment showed greater endogenous insulin preservation when used at a high dose [199]; however, there was no induction of Tregs, as was seen in mice. To identify the mechanism of action of Teplizumab in humans, Waldron-Lynch and colleagues used humanized NSG mice. The authors showed that CCR6+ T cells migrate to the small intestine from circulation upon Teplizumab treatment [200]. These CCR6+ T cells are also positive for IL-10 and can be detected in the peripheral blood. Using this knowledge, the authors studied the peripheral blood of Teplizumab-treated T1D patients and found increased expression of IL-10 within the CCR6+ Tregs in the patients. Thus, humanized mouse models have allow researchers to discover novel mechanisms and cell populations that can, in turn, provide novel ideas in developing more effective immunotherapies.
More recently, new humanized models have been established named MITRG and MISTRG [201]. These mice are deficient in mouse IL-2 receptor gamma chain and Rag genes but carry several human genes including colony-stimulating factor (GSF), granulocyte-macrophage colony stimulating factor (GMCS-F) and thyroid peroxidase (TPO) to facilitate human HSC engraftment. In addition to these human cytokines, MISTRG mice also express human signal regulatory protein alpha (SIRPalpha), which interacts with CD47 on human cells preventing phagocytes from engulfing the cells. The combination of mouse gene knock-out and human gene knock-in facilitates the development of a more expansive natural human immune system within a mouse model. While this model is currently on the BALB/c background, it is likely that this will be developed on a NOD.scid background for T1D studies. SIRPalpha has already been shown to be important for diabetes susceptibility, both in terms of polymorphic variation and the level of protein expression [202]. Therefore developing these MISTRG mice on a NOD background will provide further discoveries into the pathogenesis of autoimmune diabetes.
6. Discussion
For over 30 years, the NOD mice have been an incredibly valuable research tool, not only providing insights into genetic susceptibility and mechanisms of disease pathogenesis but also in the translational development of novel immunotherapies. In addition, the NOD model of spontaneous autoimmune diabetes is heavily used and has enabled the development of more “humanized” mice for even greater translational outcomes, across all areas of immunology. It is safe to say the NOD mouse model has been an immunological goldmine and will no doubt continue to provide insights important for translation to humans for many more years to come.
7. Personal Note
This review was written to honor Professors Diego Vergani and Giorgina-Mieli-Vergani for their seminal contribution to the field of autoimmunity, in particular the immunology of liver disease, over 4 decades. They were the pioneers of human translational immunology in autoimmune liver diseases, type 1 diabetes, lupus and more. Li Wen was privileged to be their PhD student at King’s College, where she generated hundreds of human T cell lines and clones from the peripheral blood and liver biopsies of patients with autoimmune liver diseases under their supervision. During her PhD studies, she also found high numbers of the liver resident gamma/delta T cells and alpha/beta T cells with two T cell receptors. As the Head of the Pediatric Hepatology Division, Giorgina, personally, made sure of the integrity and availability of the samples from each of the enrolled research subjects; as the lead of Autoimmune Research Group in the laboratory, Diego made sure, personally, that each of the precious samples from these pediatric patients was properly handled immediately. It was truly a dream team for human translational studies.
Susan Wong was also privileged to collaborate with Diego for human type 1 diabetes studies while working at King’s College. We greatly enjoyed the time at King’s and are honored to contribute this review article to this issue in recognition of the significant contributions of Professors Diego Vergani and Giorgina-Mieli-Vergani to the field of human autoimmunity. Previous recipients of the honor of a dedicated issue for the Journal of Autoimmunity have included Michael Sela, Ruth Arnon, Abul Abbas, Ian Mackay and Noel Rose [203–204].
Highlights.
The Non-obese diabetic mouse provides an important model for human T1D studies
Genetic and environmental factors influence T1D susceptibility
Gut microbiota modulate immune cell function and T1D development
Innate and adaptive immune cells are important in the pathogenesis of T1D
Developing humanized mice provides greater insight into human T1D
Acknowledgments
This work was supported by a Fulbright-Diabetes UK Postdoctoral Research Scholarship to JAP, grant support to FSW (MRC Grants G0901155 and MR/K021141/1, DUK grants 08/3719 and 11/4319, EFSD/NN 2014) and to LW (JDRF 5-2010-664, NIH RC1DK-087699, DK088181, DK092882) and the Yale Diabetes Research Center (P30-DK-45735).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 2.Bessaoud K, Boudraa G, Molinero de Ropolo M, de Sereday M, Marti ML, Moser M, et al. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabetic Medicine. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 3.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 4.Evertsen J, Alemzadeh R, Wang X. Increasing Incidence of Pediatric Type 1 Diabetes Mellitus in Southeastern Wisconsin: Relationship with Body Weight at Diagnosis. Plos One. 2009:4. doi: 10.1371/journal.pone.0006873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehehalt S, Dietz K, Willasch AM, Neu A, Baden-Wuerttemberg Daibet I. Epidemiological Perspectives on Type 1 Diabetes in Childhood and Adolescence in Germany - 20 years of the Baden-Wurttemberg Diabetes Incidence Registry (DIARY) Diabetes Care. 2010;33:338–340. doi: 10.2337/dc09-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakhooda AF, Like AA, Chappel CI, Murray FT, Marliss EB. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes. 1977;26:100–112. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- 7.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 8.Jackson R, Rassi N, Crump T, Haynes B, Eisenbarth GS. The BB diabetic rat. Profound T-cell lymphocytopenia. Diabetes. 1981;30:887–889. doi: 10.2337/diab.30.10.887. [DOI] [PubMed] [Google Scholar]
- 9.Elder ME, Maclaren NK. Identification of profound peripheral T lymphocyte immunodeficiencies in the spontaneously diabetic BB rat. J Immunol. 1983;130:1723–1731. [PubMed] [Google Scholar]
- 10.Jackson R, Kadison P, Buse J, Rassi N, Jegasothy B, Eisenbarth GS. Lymphocyte abnormalities in the BB rat. Metabolism. 1983;32:83–86. doi: 10.1016/s0026-0495(83)80017-1. [DOI] [PubMed] [Google Scholar]
- 11.Yale JF, Grose M, Marliss EB. Time course of the lymphopenia in BB rats. Relation to the onset of diabetes. Diabetes. 1985;34:955–959. doi: 10.2337/diab.34.10.955. [DOI] [PubMed] [Google Scholar]
- 12.Awata T, Guberski DL, Like AA. Genetics of the BB rat: association of autoimmune disorders (diabetes, insulitis, and thyroiditis) with lymphopenia and major histocompatibility complex class II. Endocrinology. 1995;136:5731–5735. doi: 10.1210/endo.136.12.7588330. [DOI] [PubMed] [Google Scholar]
- 13.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes. 1994;43:667–675. doi: 10.2337/diab.43.5.667. [DOI] [PubMed] [Google Scholar]
- 14.Bouma G, Coppens JM, Mourits S, Nikolic T, Sozzani S, Drexhage HA, et al. Evidence for an enhanced adhesion of DC to fibronectin and a role of CCL19 and CCL21 in the accumulation of DC around the pre-diabetic islets in NOD mice. Eur J Immunol. 2005;35:2386–2396. doi: 10.1002/eji.200526251. [DOI] [PubMed] [Google Scholar]
- 15.Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 16.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voorbij HA, Jeucken PH, Kabel PJ, De Haan M, Drexhage HA. Dendritic cells and scavenger macrophages in pancreatic islets of prediabetic BB rats. Diabetes. 1989;38:1623–1629. doi: 10.2337/diab.38.12.1623. [DOI] [PubMed] [Google Scholar]
- 18.Lee KU, Amano K, Yoon JW. Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes. 1988;37:989–991. doi: 10.2337/diab.37.7.989. [DOI] [PubMed] [Google Scholar]
- 19.Nikolic T, Geutskens SB, van Rooijen N, Drexhage HA, Leenen PJ. Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: a phagocyte depletion study. Lab Invest. 2005;85:487–501. doi: 10.1038/labinvest.3700238. [DOI] [PubMed] [Google Scholar]
- 20.Van Belle TL, Juntti T, Liao J, von Herrath MG. Pre-existing autoimmunity determines type 1 diabetes outcome after Flt3-ligand treatment. J Autoimmun. 2010;34:445–452. doi: 10.1016/j.jaut.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serreze DV, Gaedeke JW, Leiter EH. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: defective regulation of cytokine receptors and protein kinase C. Proc Natl Acad Sci U S A. 1993;90:9625–9629. doi: 10.1073/pnas.90.20.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serreze DV, Gaskins HR, Leiter EH. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993;150:2534–2543. [PubMed] [Google Scholar]
- 23.Prasad SJ, Goodnow CC. Intrinsic in vitro abnormalities in dendritic cell generation caused by non-MHC non-obese diabetic genes. Immunol Cell Biol. 2002;80:198–206. doi: 10.1046/j.1440-1711.2002.01074.x. [DOI] [PubMed] [Google Scholar]
- 24.Steptoe RJ, Ritchie JM, Harrison LC. Increased generation of dendritic cells from myeloid progenitors in autoimmune-prone nonobese diabetic mice. J Immunol. 2002;168:5032–5041. doi: 10.4049/jimmunol.168.10.5032. [DOI] [PubMed] [Google Scholar]
- 25.Feili-Hariri M, Morel PA. Phenotypic and functional characteristics of BM-derived DC from NOD and non-diabetes-prone strains. Clin Immunol. 2001;98:133–142. doi: 10.1006/clim.2000.4959. [DOI] [PubMed] [Google Scholar]
- 26.Welzen-Coppens JM, van Helden-Meeuwsen CG, Leenen PJ, Drexhage HA, Versnel MA. Reduced numbers of dendritic cells with a tolerogenic phenotype in the prediabetic pancreas of NOD mice. J Leukoc Biol. 2012;92:1207–1213. doi: 10.1189/jlb.0312168. [DOI] [PubMed] [Google Scholar]
- 27.Beumer W, Welzen-Coppens JM, van Helden-Meeuwsen CG, Gibney SM, Drexhage HA, Versnel MA. The gene expression profile of CD11c+ CD8α-dendritic cells in the pre-diabetic pancreas of the NOD mouse. PLoS One. 2014;9:e103404. doi: 10.1371/journal.pone.0103404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki A, Hanafusa T, Yamada K, Miyagawa J, Fujino-Kurihara H, Nakajima H, et al. Predominance of T lymphocytes in pancreatic islets and spleen of pre-diabetic non-obese diabetic (NOD) mice: a longitudinal study. Clin Exp Immunol. 1985;60:622–630. [PMC free article] [PubMed] [Google Scholar]
- 29.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 30.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, et al. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 31.Yagi H, Matsumoto M, Kunimoto K, Kawaguchi J, Makino S, Harada M. Analysis of the roles of CD4+ and CD8+ T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur J Immunol. 1992;22:2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto M, Yagi H, Kunimoto K, Kawaguchi J, Makino S, Harada M. Transfer of autoimmune diabetes from diabetic NOD mice to NOD athymic nude mice: the roles of T cell subsets in the pathogenesis. Cell Immunol. 1993;148:189–197. doi: 10.1006/cimm.1993.1101. [DOI] [PubMed] [Google Scholar]
- 33.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 34.Chatenoud L, Thervet E, Primo J, Bach JF. [Remission of established disease in diabetic NOD mice induced by anti-CD3 monoclonal antibody] C R Acad Sci III. 1992;315:225–228. [PubMed] [Google Scholar]
- 35.Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol. 2011;187:2015–2022. doi: 10.4049/jimmunol.1100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlton B, Mandel TE. Recurrence of insulitis in the NOD mouse after early prolonged anti-CD4 monoclonal antibody treatment. Autoimmunity. 1989;4:1–7. doi: 10.3109/08916938909034354. [DOI] [PubMed] [Google Scholar]
- 37.Hutchings P, O’Reilly L, Parish NM, Waldmann H, Cooke A. The use of a non-depleting anti-CD4 monoclonal antibody to re-establish tolerance to beta cells in NOD mice. Eur J Immunol. 1992;22:1913–1918. doi: 10.1002/eji.1830220735. [DOI] [PubMed] [Google Scholar]
- 38.Nagata M, Santamaria P, Kawamura T, Utsugi T, Yoon JW. Evidence for the role of CD8+ cytotoxic T cells in the destruction of pancreatic beta-cells in nonobese diabetic mice. J Immunol. 1994;152:2042–2050. [PubMed] [Google Scholar]
- 39.Makhlouf L, Grey ST, Dong V, Csizmadia E, Arvelo MB, Auchincloss H, et al. Depleting anti-CD4 monoclonal antibody cures new-onset diabetes, prevents recurrent autoimmune diabetes, and delays allograft rejection in nonobese diabetic mice. Transplantation. 2004;77:990–997. doi: 10.1097/01.tp.0000118410.61419.59. [DOI] [PubMed] [Google Scholar]
- 40.Yi Z, Diz R, Martin AJ, Morillon YM, Kline DE, Li L, et al. Long-term remission of diabetes in NOD mice is induced by nondepleting anti-CD4 and anti-CD8 antibodies. Diabetes. 2012;61:2871–2880. doi: 10.2337/db12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 42.Taki T, Nagata M, Ogawa W, Hatamori N, Hayakawa M, Hari J, et al. Prevention of cyclophosphamide-induced and spontaneous diabetes in NOD/Shi/Kbe mice by anti-MHC class I Kd monoclonal antibody. Diabetes. 1991;40:1203–1209. doi: 10.2337/diab.40.9.1203. [DOI] [PubMed] [Google Scholar]
- 43.Boitard C, Bendelac A, Richard MF, Carnaud C, Bach JF. Prevention of diabetes in nonobese diabetic mice by anti-I-A monoclonal antibodies: transfer of protection by splenic T cells. Proc Natl Acad Sci U S A. 1988;85:9719–9723. doi: 10.1073/pnas.85.24.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pontesilli O, Carotenuto P, Gazda LS, Pratt PF, Prowse SJ. Circulating lymphocyte populations and autoantibodies in non-obese diabetic (NOD) mice: a longitudinal study. Clin Exp Immunol. 1987;70:84–93. [PMC free article] [PubMed] [Google Scholar]
- 45.Michel C, Boitard C, Bach JF. Insulin autoantibodies in non-obese diabetic (NOD) mice. Clin Exp Immunol. 1989;75:457–460. [PMC free article] [PubMed] [Google Scholar]
- 46.De Aizpurua HJ, French MB, Chosich N, Harrison LC. Natural history of humoral immunity to glutamic acid decarboxylase in non-obese diabetic (NOD) mice. J Autoimmun. 1994;7:643–653. doi: 10.1006/jaut.1994.1049. [DOI] [PubMed] [Google Scholar]
- 47.Lamont D, Mukherjee G, Kumar PR, Samanta D, McPhee CG, Kay TW, et al. Compensatory mechanisms allow undersized anchor-deficient class I MHC ligands to mediate pathogenic autoreactive T cell responses. J Immunol. 2014;193:2135–2146. doi: 10.4049/jimmunol.1400997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zekzer D, Wong FS, Wen L, Altieri M, Gurlo T, von Grafenstein H, et al. Inhibition of diabetes by an insulin-reactive CD4 T-cell clone in the nonobese diabetic mouse. Diabetes. 1997;46:1124–1132. doi: 10.2337/diab.46.7.1124. [DOI] [PubMed] [Google Scholar]
- 49.Simone E, Daniel D, Schloot N, Gottlieb P, Babu S, Kawasaki E, et al. T cell receptor restriction of diabetogenic autoimmune NOD T cells. Proc Natl Acad Sci U S A. 1997;94:2518–2521. doi: 10.1073/pnas.94.6.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abiru N, Wegmann D, Kawasaki E, Gottlieb P, Simone E, Eisenbarth GS. Dual overlapping peptides recognized by insulin peptide B:9–23 T cell receptor AV13S3 T cell clones of the NOD mouse. J Autoimmun. 2000;14:231–237. doi: 10.1006/jaut.2000.0369. [DOI] [PubMed] [Google Scholar]
- 51.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA. CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 53.Ejrnaes M, Videbaek N, Christen U, Cooke A, Michelsen BK, von Herrath M. Different diabetogenic potential of autoaggressive CD8+ clones associated with IFN-gamma-inducible protein 10 (CXC chemokine ligand 10) production but not cytokine expression, cytolytic activity, or homing characteristics. J Immunol. 2005;174:2746–2755. doi: 10.4049/jimmunol.174.5.2746. [DOI] [PubMed] [Google Scholar]
- 54.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burton AR, Vincent E, Arnold PY, Lennon GP, Smeltzer M, Li CS, et al. On the pathogenicity of autoantigen-specific T-cell receptors. Diabetes. 2008;57:1321–1230. doi: 10.2337/db07-1129. [DOI] [PubMed] [Google Scholar]
- 57.Chao CC, Sytwu HK, Chen EL, Toma J, McDevitt HO. The role of MHC class II molecules in susceptibility to type I diabetes: identification of peptide epitopes and characterization of the T cell repertoire. Proc Natl Acad Sci U S A. 1999;96:9299–9304. doi: 10.1073/pnas.96.16.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Videbaek N, Harach S, Phillips J, Hutchings P, Ozegbe P, Michelsen BK, et al. An islet-homing NOD CD8+ cytotoxic T cell clone recognizes GAD65 and causes insulitis. J Autoimmun. 2003;20:97–109. doi: 10.1016/s0896-8411(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 59.Bowie L, Tite J, Cooke A. Generation and maintenance of autoantigen-specific CD8(+) T cell clones isolated from NOD mice. J Immunol Methods. 1999;228:87–95. doi: 10.1016/s0022-1759(99)00106-4. [DOI] [PubMed] [Google Scholar]
- 60.Elias D, Reshef T, Birk OS, van der Zee R, Walker MD, Cohen IR. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991;88:3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Birk OS, Elias D, Weiss AS, Rosen A, van-der Zee R, Walker MD, et al. NOD mouse diabetes: the ubiquitous mouse hsp60 is a beta-cell target antigen of autoimmune T cells. J Autoimmun. 1996;9:159–166. doi: 10.1006/jaut.1996.0019. [DOI] [PubMed] [Google Scholar]
- 62.Nayak DK, Calderon B, Vomund AN, Unanue ER. In NOD mice ZnT8 reactive T cells are weakly pathogenic but can participate in diabetes under inflammatory conditions. Diabetes. 2014;63:3438–3448. doi: 10.2337/db13-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nerup J, Platz P, Andersen OO, Christy M, Lyngsoe J, Poulsen JE, et al. HL-A antigens and diabetes mellitus. Lancet. 1974;2:864–866. doi: 10.1016/s0140-6736(74)91201-x. [DOI] [PubMed] [Google Scholar]
- 64.Singal DP, Blajchman MA. Histocompatibility (HL-A) antigens, lymphocytotoxic antibodies and tissue antibodies in patients with diabetes mellitus. Diabetes. 1973;22:429–432. doi: 10.2337/diab.22.6.429. [DOI] [PubMed] [Google Scholar]
- 65.Hattori M, Buse JB, Jackson RA, Glimcher L, Dorf ME, Minami M, et al. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986;231:733–735. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- 66.Acha-Orbea H, McDevitt HO. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci U S A. 1987;84:2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato AK, Sturniolo T, Sinigaglia F, Stern LJ. Substitution of aspartic acid at beta57 with alanine alters MHC class II peptide binding activity but not protein stability: HLA-DQ (alpha1*0201, beta1*0302) and (alpha1*0201, beta1*0303) Hum Immunol. 1999;60:1227–1236. doi: 10.1016/s0198-8859(99)00120-2. [DOI] [PubMed] [Google Scholar]
- 68.Lund T, O’Reilly L, Hutchings P, Kanagawa O, Simpson E, Gravely R, et al. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990;345:727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- 69.Nishimoto H, Kikutani H, Yamamura K, Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. Nature. 1987;328:432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- 70.Aly TA, Ide A, Jahromi MM, Barker JM, Fernando MS, Babu SR, et al. Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci U S A. 2006;103:14074–14079. doi: 10.1073/pnas.0606349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wen L, Chen NY, Tang J, Sherwin R, Wong FS. The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. J Clin Invest. 2001;107:871–880. doi: 10.1172/JCI11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 74.Pugliese A, Zeller M, Fernandez A, Zalcberg LJ, Bartlett RJ, Ricordi C, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 75.Chentoufi AA, Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes. 2002;51:1383–1390. doi: 10.2337/diabetes.51.5.1383. [DOI] [PubMed] [Google Scholar]
- 76.Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007732. doi: 10.1101/cshperspect.a007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergholdt R, Brorsson C, Palleja A, Berchtold LA, Fløyel T, Bang-Berthelsen CH, et al. Identification of novel type 1 diabetes candidate genes by integrating genome-wide association data, protein-protein interactions, and human pancreatic islet gene expression. Diabetes. 2012;61:954–962. doi: 10.2337/db11-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barnett AH, Eff C, Leslie RD, Pyke DA. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981;20:87–93. doi: 10.1007/BF00262007. [DOI] [PubMed] [Google Scholar]
- 79.Redondo MJ, Yu L, Hawa M, Mackenzie T, Pyke DA, Eisenbarth GS, et al. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44:354–362. doi: 10.1007/s001250051626. [DOI] [PubMed] [Google Scholar]
- 80.Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes. 2003;52:1052–1055. doi: 10.2337/diabetes.52.4.1052. [DOI] [PubMed] [Google Scholar]
- 81.Variation, trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet. 2000;355:873–876. [PubMed] [Google Scholar]
- 82.Bodington MJ, Muzulu SI, Burden AC. Spatial clustering in childhood diabetes: evidence of an environmental cause. Diabet Med. 1995;12:865–867. doi: 10.1111/j.1464-5491.1995.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 83.Hjern A, Söderström U. Parental country of birth is a major determinant of childhood type 1 diabetes in Sweden. Pediatr Diabetes. 2008;9:35–39. doi: 10.1111/j.1399-5448.2007.00267.x. [DOI] [PubMed] [Google Scholar]
- 84.Group TS. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci. 2008;1150:1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmid S, Koczwara K, Schwinghammer S, Lampasona V, Ziegler AG, Bonifacio E. Delayed exposure to wheat and barley proteins reduces diabetes incidence in non-obese diabetic mice. Clin Immunol. 2004;111:108–118. doi: 10.1016/j.clim.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 86.Maurano F, Mazzarella G, Luongo D, Stefanile R, D’Arienzo R, Rossi M, et al. Small intestinal enteropathy in non-obese diabetic mice fed a diet containing wheat. Diabetologia. 2005;48:931–937. doi: 10.1007/s00125-005-1718-2. [DOI] [PubMed] [Google Scholar]
- 87.Marietta EV, Gomez AM, Yeoman C, Tilahun AY, Clark CR, Luckey DH, et al. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS One. 2013;8:e78687. doi: 10.1371/journal.pone.0078687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 89.Zaccone P, Raine T, Sidobre S, Kronenberg M, Mastroeni P, Cooke A. Salmonella typhimurium infection halts development of type 1 diabetes in NOD mice. Eur J Immunol. 2004;34:3246–3256. doi: 10.1002/eji.200425285. [DOI] [PubMed] [Google Scholar]
- 90.Drescher KM, Kono K, Bopegamage S, Carson SD, Tracy S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology. 2004;329:381–394. doi: 10.1016/j.virol.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 91.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 93.Mor F, Cohen IR. Beta-lactam antibiotics modulate T-cell functions and gene expression via covalent binding to cellular albumin. Proc Natl Acad Sci U S A. 2013;110:2981–2986. doi: 10.1073/pnas.1215722110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng J, Narasimhan S, Marchesi JR, Benson A, Wong FS, Wen L. Long term effect of gut microbiota transfer on diabetes development. J Autoimmun. 2014;53:85–94. doi: 10.1016/j.jaut.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tormo-Badia N, Håkansson A, Vasudevan K, Molin G, Ahrné S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic (NOD) mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol. 2014;80:250–260. doi: 10.1111/sji.12205. [DOI] [PubMed] [Google Scholar]
- 96.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 97.Wong FS, Hu C, Zhang L, Du W, Alexopoulou L, Flavell RA, et al. The role of Toll-like receptors 3 and 9 in the development of autoimmune diabetes in NOD mice. Ann N Y Acad Sci. 2008;1150:146–148. doi: 10.1196/annals.1447.039. [DOI] [PubMed] [Google Scholar]
- 98.Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–1575. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 99.Durant S, Alves V, Coulaud J, Homo-Delarche F. Nonobese diabetic (NOD) mouse dendritic cells stimulate insulin secretion by prediabetic islets. Autoimmunity. 2002;35:449–455. doi: 10.1080/0891693021000040575. [DOI] [PubMed] [Google Scholar]
- 100.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 101.Gaudreau S, Guindi C, Ménard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 102.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 103.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol Immunol. 2013;10:103–106. doi: 10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rahman AH, Taylor DK, Turka LA. The contribution of direct TLR signaling to T cell responses. Immunol Res. 2009;45:25–36. doi: 10.1007/s12026-009-8113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ganz T. The role of antimicrobial peptides in innate immunity. Integr Comp Biol. 2003;43:300–304. doi: 10.1093/icb/43.2.300. [DOI] [PubMed] [Google Scholar]
- 107.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 109.Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 111.Gonnella PA, Waldner H, Del Nido PJ, McGowan FX. Inhibition of experimental autoimmune myocarditis: peripheral deletion of TcR Vbeta 8.1, 8.2+ CD4+ T cells in TLR-4 deficient mice. J Autoimmun. 2008;31:180–187. doi: 10.1016/j.jaut.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Gülden E, Ihira M, Ohashi A, Reinbeck AL, Freudenberg MA, Kolb H, et al. Toll-like receptor 4 deficiency accelerates the development of insulin-deficient diabetes in non-obese diabetic mice. PLoS One. 2013;8:e75385. doi: 10.1371/journal.pone.0075385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y, Lee AS, Shameli A, Geng X, Finegood D, Santamaria P, et al. TLR9 blockade inhibits activation of diabetogenic CD8+ T cells and delays autoimmune diabetes. J Immunol. 2010;184:5645–5653. doi: 10.4049/jimmunol.0901814. [DOI] [PubMed] [Google Scholar]
- 115.Tai N, Wong FS, Wen L. TLR9 deficiency promotes CD73 expression in T cells and diabetes protection in nonobese diabetic mice. J Immunol. 2013;191:2926–2937. doi: 10.4049/jimmunol.1300547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim DH, Lee JC, Lee MK, Kim KW, Lee MS. Treatment of autoimmune diabetes in NOD mice by Toll-like receptor 2 tolerance in conjunction with dipeptidyl peptidase 4 inhibition. Diabetologia. 2012;55:3308–3317. doi: 10.1007/s00125-012-2723-x. [DOI] [PubMed] [Google Scholar]
- 117.Caricilli AM, Picardi PK, de Abreu LL, Ueno M, Prada PO, Ropelle ER, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212. doi: 10.1371/journal.pbio.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Richer MJ, Lavallée DJ, Shanina I, Horwitz MS. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS One. 2009;4:e4127. doi: 10.1371/journal.pone.0004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McCall KD, Thuma JR, Courreges MC, Benencia F, James CB, Malgor R, et al. Toll-like receptor 3 is critical for coxsackievirus B4-induced type 1 diabetes in female NOD mice. Endocrinology. 2015;156:453–461. doi: 10.1210/en.2013-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, et al. Tolllike receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 121.Zipris D, Lien E, Xie JX, Greiner DL, Mordes JP, Rossini AA. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J Immunol. 2005;174:131–142. doi: 10.4049/jimmunol.174.1.131. [DOI] [PubMed] [Google Scholar]
- 122.Alkanani AK, Hara N, Lien E, Ir D, Kotter CV, Robertson CE, et al. Induction of diabetes in the RIP-B7.1 mouse model is critically dependent on TLR3 and MyD88 pathways and is associated with alterations in the intestinal microbiome. Diabetes. 2014;63:619–631. doi: 10.2337/db13-1007. [DOI] [PubMed] [Google Scholar]
- 123.Tan Q, Majewska-Szczepanik M, Zhang X, Szczepanik M, Zhou Z, Wong FS, et al. IRAK-M deficiency promotes the development of type 1 diabetes in NOD mice. Diabetes. 2014;63:2761–2775. doi: 10.2337/db13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, et al. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 125.Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schröder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 127.Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce antimicrobial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100–3111. doi: 10.1016/j.molimm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 128.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 129.Hu C, Ding H, Li Y, Pearson JA, Zhang X, Flavell RA, et al. NLRP3 deficiency protects from type 1 diabetes through the regulation of chemotaxis into the pancreatic islets. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1513509112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jaakkola I, Jalkanen S, Hänninen A. Diabetogenic T cells are primed both in pancreatic and gut-associated lymph nodes in NOD mice. Eur J Immunol. 2003;33:3255–3264. doi: 10.1002/eji.200324405. [DOI] [PubMed] [Google Scholar]
- 132.Matsuzaki T, Nagata Y, Kado S, Uchida K, Kato I, Hashimoto S, et al. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. 1997;105:643–649. doi: 10.1111/j.1699-0463.1997.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 133.Takiishi T, Korf H, Van Belle TL, Robert S, Grieco FA, Caluwaerts S, et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest. 2012;122:1717–1725. doi: 10.1172/JCI60530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robert S, Gysemans C, Takiishi T, Korf H, Spagnuolo I, Sebastiani G, et al. Oral Delivery of Glutamic Acid Decarboxylase (GAD)-65 and IL10 by Lactococcus lactis Reverses Diabetes in Recent-Onset NOD Mice. Diabetes. 2014;63:2876–2887. doi: 10.2337/db13-1236. [DOI] [PubMed] [Google Scholar]
- 135.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 136.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 137.Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 138.Horai R, Zarante-BlaDes CR, Dillenburg-Pilla P, Silver PB, Chen J, Jittayasothorn Y, et al. Activation of an autoreactive T cell receptor by endogenous microflora provokes autoimmunity in an immunologically privileged site. Keystone, CO, USA: Keystone symposia - Gut Microbiota Modulation of Host Physiology: The Search for Mechanism; 2015. Mar, pp. 1–6. [Google Scholar]
- 139.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wong FS, Visintin I, Wen L, Granata J, Flavell R, Janeway CA. The role of lymphocyte subsets in accelerated diabetes in nonobese diabetic-rat insulin promoter-B7-1 (NOD-RIP-B7-1) mice. J Exp Med. 1998;187:1985–1993. doi: 10.1084/jem.187.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang M, Charlton B, Gautam AM. Development of insulitis and diabetes in B cell-deficient NOD mice. J Autoimmun. 1997;10:257–260. doi: 10.1006/jaut.1997.0128. [DOI] [PubMed] [Google Scholar]
- 142.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 143.Puertas MC, Carrillo J, Pastor X, Ampudia RM, Alba A, Planas R, et al. Phenotype and functional characteristics of islet-infiltrating B-cells suggest the existence of immune regulatory mechanisms in islet milieu. Diabetes. 2007;56:940–949. doi: 10.2337/db06-0428. [DOI] [PubMed] [Google Scholar]
- 144.Kendall PL, Woodward EJ, Hulbert C, Thomas JW. Peritoneal B cells govern the outcome of diabetes in non-obese diabetic mice. Eur J Immunol. 2004;34:2387–2395. doi: 10.1002/eji.200324744. [DOI] [PubMed] [Google Scholar]
- 145.Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SA, Schlachterman A, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163:743–750. [PubMed] [Google Scholar]
- 146.Ziegler AG, Vardi P, Ricker AT, Hattori M, Soeldner JS, Eisenbarth GS. Radioassay determination of insulin autoantibodies in NOD mice. Correlation with increased risk of progression to overt diabetes. Diabetes. 1989;38:358–363. doi: 10.2337/diab.38.3.358. [DOI] [PubMed] [Google Scholar]
- 147.Robles DT, Eisenbarth GS, Dailey NJ, Peterson LB, Wicker LS. Insulin autoantibodies are associated with islet inflammation but not always related to diabetes progression in NOD congenic mice. Diabetes. 2003;52:882–886. doi: 10.2337/diabetes.52.3.882. [DOI] [PubMed] [Google Scholar]
- 148.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Humphreys-Beher MG, Brinkley L, Purushotham KR, Wang PL, Nakagawa Y, Dusek D, et al. Characterization of antinuclear autoantibodies present in the serum from nonobese diabetic (NOD) mice. Clin Immunol Immunopathol. 1993;68:350–356. doi: 10.1006/clin.1993.1137. [DOI] [PubMed] [Google Scholar]
- 150.Acevedo-Suárez CA, Hulbert C, Woodward EJ, Thomas JW. Uncoupling of anergy from developmental arrest in anti-insulin B cells supports the development of autoimmune diabetes. J Immunol. 2005;174:827–833. doi: 10.4049/jimmunol.174.2.827. [DOI] [PubMed] [Google Scholar]
- 151.Henry-Bonami RA, Williams JM, Rachakonda AB, Karamali M, Kendall PL, Thomas JW. B lymphocyte “original sin” in the bone marrow enhances islet autoreactivity in type 1 diabetes-prone nonobese diabetic mice. J Immunol. 2013;190:5992–6003. doi: 10.4049/jimmunol.1201359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J Immunol. 2001;167:5535–5538. doi: 10.4049/jimmunol.167.10.5535. [DOI] [PubMed] [Google Scholar]
- 153.Thomas JW, Hulbert C. Somatically mutated B cell pool provides precursors for insulin antibodies. J Immunol. 1996;157:763–771. [PubMed] [Google Scholar]
- 154.Kendall PL, Moore DJ, Hulbert C, Hoek KL, Khan WN, Thomas JW. Reduced diabetes in btk-deficient nonobese diabetic mice and restoration of diabetes with provision of an anti-insulin IgH chain transgene. J Immunol. 2009;183:6403–6412. doi: 10.4049/jimmunol.0900367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Henry RA, Kendall PL, Thomas JW. Autoantigen-specific B-cell depletion overcomes failed immune tolerance in type 1 diabetes. Diabetes. 2012;61:2037–2044. doi: 10.2337/db11-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hu C, Ding H, Zhang X, Wong FS, Wen L. Combination treatment with anti-CD20 and oral anti-CD3 prevents and reverses autoimmune diabetes. Diabetes. 2013;62:2849–2858. doi: 10.2337/db12-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gürcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9:10–25. doi: 10.1016/j.intimp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 159.Hu C, Du W, Zhang X, Wong FS, Wen L. The role of Gr1+ cells after anti-CD20 treatment in type 1 diabetes in nonobese diabetic mice. J Immunol. 2012;188:294–301. doi: 10.4049/jimmunol.1101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang H, Bi Y, Han F, Lu Y, Wang J, Zhang Z, et al. Myeloid-derived suppressor cells in immunity and autoimmunity. Expert Rev Clin Immunol. 2015:1–9. doi: 10.1586/1744666X.2015.1052794. [DOI] [PubMed] [Google Scholar]
- 161.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. 2012;189:4295–4304. doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Herold KC, Pescovitz MD, McGee P, Krause-Steinrauf H, Spain LM, Bourcier K, et al. Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol. 2011;187:1998–2005. doi: 10.4049/jimmunol.1100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiang Y, Peng J, Tai N, Hu C, Zhou Z, Wong FS, et al. The dual effects of B cell depletion on antigen-specific T cells in BDC2.5NOD mice. J Immunol. 2012;188:4747–4758. doi: 10.4049/jimmunol.1103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Todd JA, Acha-Orbea H, Bell JI, Chao N, Fronek Z, Jacob CO, et al. A molecular basis for MHC class II--associated autoimmunity. Science. 1988;240:1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- 166.Wen L, Wong FS, Burkly L, Altieri M, Mamalaki C, Kioussis D, et al. Induction of insulitis by glutamic acid decarboxylase peptide-specific and HLA-DQ8-restricted CD4(+) T cells from human DQ transgenic mice. J Clin Invest. 1998;102:947–957. doi: 10.1172/JCI2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wen L, Wong FS, Tang J, Chen NY, Altieri M, David C, et al. In vivo evidence for the contribution of human histocompatibility leukocyte antigen (HLA)-DQ molecules to the development of diabetes. J Exp Med. 2000;191:97–104. doi: 10.1084/jem.191.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Raju R, Munn SR, Majoribanks C, David CS. Islet cell autoimmunity in NOD mice transgenic for HLA-DQ8 and lacking I-Ag7. Transplant Proc. 1998;30:561. doi: 10.1016/s0041-1345(97)01404-8. [DOI] [PubMed] [Google Scholar]
- 169.Liu J, Purdy LE, Rabinovitch S, Jevnikar AM, Elliott JF. Major DQ8-restricted T-cell epitopes for human GAD65 mapped using human CD4, DQA1*0301, DQB1*0302 transgenic IA(null) NOD mice. Diabetes. 1999;48:469–477. doi: 10.2337/diabetes.48.3.469. [DOI] [PubMed] [Google Scholar]
- 170.DiLorenzo TP, Graser RT, Ono T, Christianson GJ, Chapman HD, Roopenian DC, et al. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor alpha chain gene rearrangement. Proc Natl Acad Sci U S A. 1998;95:12538–12543. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kudva YC, Deng YJ, Govindarajan R, Abraham RS, Marietta EV, Notkins AL, et al. HLA-DQ8 transgenic and NOD mice recognize different epitopes within the cytoplasmic region of the tyrosine phosphatase-like molecule, IA-2. Hum Immunol. 2001;62:1099–1105. doi: 10.1016/s0198-8859(01)00308-1. [DOI] [PubMed] [Google Scholar]
- 172.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest. 2005;115:2268–2276. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, et al. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Takaki T, Marron MP, Mathews CE, Guttmann ST, Bottino R, Trucco M, et al. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol. 2006;176:3257–3265. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 175.Irwin MJ, Heath WR, Sherman LA. Species-restricted interactions between CD8 and the alpha 3 domain of class I influence the magnitude of the xenogeneic response. J Exp Med. 1989;170:1091–1101. doi: 10.1084/jem.170.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hassainya Y, Garcia-Pons F, Kratzer R, Lindo V, Greer F, Lemonnier FA, et al. Identification of naturally processed HLA-A2--restricted proinsulin epitopes by reverse immunology. Diabetes. 2005;54:2053–2059. doi: 10.2337/diabetes.54.7.2053. [DOI] [PubMed] [Google Scholar]
- 177.Antal Z, Baker JC, Smith C, Jarchum I, Babad J, Mukherjee G, et al. Beyond HLA-A*0201: new HLA-transgenic nonobese diabetic mouse models of type 1 diabetes identify the insulin C-peptide as a rich source of CD8+ T cell epitopes. J Immunol. 2012;188:5766–5775. doi: 10.4049/jimmunol.1102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li Y, Zhou L, Zhang J, Guo B, Meng G, Chen X, et al. Identification of autoreactive CD8(+) T cell responses targeting chromogranin A in humanized NOD mice and type 1 diabetes patients. Clin Immunol. 2015;159:63–71. doi: 10.1016/j.clim.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 179.Niens M, Grier AE, Marron M, Kay TW, Greiner DL, Serreze DV. Prevention of “Humanized” diabetogenic CD8 T-cell responses in HLA-transgenic NOD mice by a multipeptide coupled-cell approach. Diabetes. 2011;60:1229–1236. doi: 10.2337/db10-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pow Sang L, Majji S, Casares S, Brumeanu TD. Long-term silencing of autoimmune diabetes and improved life expectancy by a soluble pHLA-DR4 chimera in a newly-humanized NOD/DR4/B7 mouse. Hum Vaccin Immunother. 2014;10:693–699. doi: 10.4161/hv.27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin M, Stoica-Nazarov C, Surls J, Kehl M, Bona C, Olsen C, et al. Reversal of type 1 diabetes by a new MHC II-peptide chimera: “Single-epitope-mediated suppression” to stabilize a polyclonal autoimmune T-cell process. Eur J Immunol. 2010;40:2277–2288. doi: 10.1002/eji.200940094. [DOI] [PubMed] [Google Scholar]
- 182.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 183.Roep BO. Are insights gained from NOD mice sufficient to guide clinical translation? Another inconvenient truth. Ann N Y Acad Sci. 2007;1103:1–10. doi: 10.1196/annals.1394.018. [DOI] [PubMed] [Google Scholar]
- 184.Greiner DL, Shultz LD, Yates J, Appel MC, Perdrizet G, Hesselton RM, et al. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. Am J Pathol. 1995;146:888–902. [PMC free article] [PubMed] [Google Scholar]
- 185.Yoshino H, Ueda T, Kawahata M, Kobayashi K, Ebihara Y, Manabe A, et al. Natural killer cell depletion by anti-asialo GM1 antiserum treatment enhances human hematopoietic stem cell engraftment in NOD/Shi-scid mice. Bone Marrow Transplant. 2000;26:1211–1216. doi: 10.1038/sj.bmt.1702702. [DOI] [PubMed] [Google Scholar]
- 186.Kollet O, Peled A, Byk T, Ben-Hur H, Greiner D, Shultz L, et al. beta2 microglobulin-deficient (B2m(null)) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood. 2000;95:3102–3105. [PubMed] [Google Scholar]
- 187.van Halteren AG, Kardol MJ, Mulder A, Roep BO. Homing of human autoreactive T cells into pancreatic tissue of NOD-scid mice. Diabetologia. 2005;48:75–82. doi: 10.1007/s00125-004-1613-2. [DOI] [PubMed] [Google Scholar]
- 188.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 189.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 190.Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 191.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–15673. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 193.Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Unger WW, Pearson T, Abreu JR, Laban S, van der Slik AR, der Kracht SM, et al. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLoS One. 2012;7:e49213. doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Viehmann Milam AA, Maher SE, Gibson JA, Lebastchi J, Wen L, Ruddle NH, et al. A humanized mouse model of autoimmune insulitis. Diabetes. 2014;63:1712–1724. doi: 10.2337/db13-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 198.Chatenoud L. Immune therapy for type 1 diabetes mellitus-what is unique about anti-CD3 antibodies? Nat Rev Endocrinol. 2010;6:149–157. doi: 10.1038/nrendo.2009.275. [DOI] [PubMed] [Google Scholar]
- 199.Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Waldron-Lynch F, Henegariu O, Deng S, Preston-Hurlburt P, Tooley J, Flavell R, et al. Teplizumab induces human gut-tropic regulatory cells in humanized mice and patients. Sci Transl Med. 2012;4:118ra12. doi: 10.1126/scitranslmed.3003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wong AS, Mortin-Toth S, Sung M, Canty AJ, Gulban O, Greaves DR, et al. Polymorphism in the innate immune receptor SIRPα controls CD47 binding and autoimmunity in the nonobese diabetic mouse. J Immunol. 2014;193:4833–4844. doi: 10.4049/jimmunol.1401984. [DOI] [PubMed] [Google Scholar]
- 203.Steinman L, Shoenfeld Y. From defining antigens to new therapies in multiple sclerosis: honoring the contributions of Ruth Arnon and Michael Sela. J Autoimmun. 2014;54:1–7. doi: 10.1016/j.jaut.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 204.Gershwin ME, Shoenfeld Y. Abul Abbas: an epitome of scholarship. J Autoimmun. 2013;45:1–6. doi: 10.1016/j.jaut.2013.07.006. [DOI] [PubMed] [Google Scholar]