Abstract
Retinal ischemia and hemorrhage are hallmarks of worsening diabetic retinopathy, which can lead to neovascularization, macular edema, and severe vision loss. Although diabetes alters expression of clotting factors and their activities, and increases retinal microthromboses, the effects of thrombotic processes on the pathogenesis of diabetic retinopathy are not fully understood. In addition to the roles of coagulation and fibrinolytic cascades in thrombosis and hemostasis, components in these systems also mediate multiple effects on the vasculature that promote inflammation. Plasma kallikrein, thrombin, and urokinase are increased in diabetic retinopathy and exert proinflammatory effects that contribute to retinal vascular dysfunction. The accumulation and activation of these and additional coagulation factors in the vitreous due to hemorrhage and chronic retinal injury in the diabetic retina may contribute to worsening of retinal inflammation and capillary dysfunction, which lead to retinal ischemia and edema. Further understanding of the role for specific coagulation factors in diabetic retinopathy may suggest new therapeutic opportunities for this vision-threatening disease.
Diabetic retinopathy is a leading cause of vision loss in working-age adults [1]. Nearly all people with 20 or more years of diabetes develop at least early stages of nonproliferative diabetic retinopathy (NPDR), which are characterized by microvascular abnormalities, including microaneurysms, retinal vascular hyperpermeability, exudates, and intraretinal “dot” hemorrhages. Worsening of these microvascular lesions associated with severe NPDR can lead to retinal ischemia and neovascularization, termed proliferative diabetic retinopathy (PDR). In addition, diabetic retinopathy can also cause diabetic macular edema (DME), which is characterized by an accumulation of fluid in the macular region resulting in impaired central vision. Although early stages of diabetic retinopathy have been primarily attributed to the effects of hyperglycemia, hypertension, dyslipidemia [2], secondary intraocular responses play a critical role in disease progression to PDR and DME. Advanced stages of diabetic retinopathy are frequently associated with regions of reduced capillary perfusion, evident by fluorescein angiography, which result in hypoxia-induced expression of vascular endothelial growth factor (VEGF). Increased intraocular concentrations of VEGF in ischemic retinopathies play important roles in both retinal neovascularization and macular edema [3]. Therefore, a key stage in the pathogenesis of diabetic retinopathy to its sight-threatening stages involves the progression from microvascular abnormalities in NPDR to a critical threshold of reduced retinal perfusion leading to retinal ischemia. The appearance of retinal ischemia and hemorrhages has implicated roles for coagulation and fibrinolytic cascades in the pathogenesis of diabetic retinopathy. However, while the effects of diabetes and hyperglycemia on coagulation and fibrinolytic systems, and their respective roles in thrombosis, have been well documented [4;5], relatively little is known about the impact of these systems on diabetic retinopathy. In addition, components of these systems also exert potent effects on inflammation, which may also contribute to the progression of diabetic retinopathy to DME independently of retinal ischemia [6]. This article examines the potential interactions between intraocular thrombotic and inflammatory systems in the pathogenesis of diabetic retinopathy.
Inflammation, thrombosis, and retinal ischemia
At the cellular level, chronic diabetes induces retinal pericyte loss and capillary endothelial cell apoptosis, which impairs blood retinal barrier function and blood flow [7]. These changes have been associated with the formation of acellular capillaries, which have been observed in trypsin digests of retinal microvascular tissue obtained from diabetic experimental animal models [8]. A number of mechanisms, including up-regulation of the angiopoietin-2/Tie2 ligand/receptor system, have been implicated in contributing to pericyte loss and increased acellular capillary segments in retina from diabetic rodents [9]. However, less is known about the processes that may contribute to degenerate capillaries in diabetic human retina and their significance in the transition from NPDR to ischemia retinopathy.
Diabetic retinopathy in animal models is associated with inflammation, including, for example, increased retinal expression of intercellular adhesion molecule-1 (ICAM-1) and leukocyte adhesion in capillaries and venules [10]. Leukocyte α4 integrin/vascular cell adhesion molecule (VCAM-1) signaling is also implicated in leukocyte adhesion and vascular leakage in diabetic retinopathy. Blockade of α4 integrin reduces retinal levels of VEGF, TNFα and NF-κB activity, as well as retinal vascular hyperpermeability and leukocyte adhesion in diabetic rats [11]. Although hyperglycemia-induced leukostasis has been implicated in retinal capillary apoptosis and occlusion in animal models of diabetic retinopathy [10], the significance of this mechanism to ischemic diabetic retinopathy, PDR, and DME is not yet available. Insulin resistance and hypertension also increase leukocyte binding to the retinal endothelium [12;13], without evidence of ischemic retinal disease, suggesting that chronic effects on leukostasis are not sufficient to cause significant retinal ischemia. A recent report has shown that retinal ICAM-1 expression and leukostasis are not altered in moderately controlled hyperglycemia (HbA1c 6–10%) and leukostasis in retinal arteries is decreased in a rodent model of type 2 diabetes with severe hyperglycemia (HbA1c >10%) [14]. Moreover, diabetes in humans can occur for decades before or without onset of ischemic retinopathy. Thus, it is unclear how the chronic effects of diabetes on leukostasis explain the onset of retinal ischemia in the progression from NPDR to PDR for a subset of affected patients. As mentioned above, the transition of diabetic retinopathy to PDR and DME is also associated with increased intraocular concentrations of VEGF. Intravitreal injection of VEGF induces retinal vascular leakage, hemorrhage and leukostasis [15;16]. Repeated intravitreal injections of VEGF in primates cause retinal capillary nonperfusion and neovascularization [17]. A recent study has reported that intravitreal administration of ranibizumab, an anti-VEGF monoclonal antibody, reduced, but did not eliminate, capillary nonperfusion in DME patients [18]. These findings suggest that VEGF, as well as potentially other cytokines and metabolic factors, induce thrombo-inflammatory processes in the retina that contribute to capillary nonperfusion and ischemia.
Diabetes is a procoagulate state and retinal microthromboses occur in diabetic retinopathy. Increased prevalence of platelet-fibrin microthrombi positive for factor XIII immunostaining have been observed in the retinal microvasculature in both donor human eyes from diabetic subjects and in experimental rodent models of diabetes, compared with their respective controls [19;20]. Although these microthrombi are associated with capillary and venule occlusion, their significance to retinal ischemia, PDR, and DME is not yet available. It is well established that diabetes induces endothelial dysfunction and injury, which can lead to increased expression of proinflammatory adhesion molecules, apoptosis, and increased leukocyte and platelet adhesion in the retinal microvasculature [10]. Immunohistochemical analyses show that microthrombi are colocalized with apoptotic retinal endothelial cells [19;20], suggesting that microthromboses are concomitant with or secondary to capillary damage. Blood platelets are more likely to adhere to the diabetic endothelium that healthy vessels [21], which may contribute to the occlusion of injured retinal capillaries. In a cross-sectional study of 227 patients with type II diabetes mellitus (119 with versus 108 without diabetic retinopathy) and 169 nondiabetic subjects, it was demonstrated that polymorphism in platelet α2β1, a receptor for collagen important for platelet adhesion and thrombus formation, is an independent risk factor for retinopathy [22]. Taken together, platelet adhesion to the injured diabetic endothelium may contribute to both ischemia and inflammation in diabetic retinopathy.
Retinal hemorrhage and inflammation
Diabetic retinopathy is also characterized by the presence of small “dot” intraretinal hemorrhages. The number and severity of these hemorrhages are frequently increased during progression from mild to moderate to severe stages of NPDR. The occurrence of these hemorrhages has been attributed, in part, to vascular hyperpermeability and microaneurysms. Although diabetes increases retinal vascular permeability via the disruption of tight junction integrity in the retinal endothelium, the loss of tight junction function alone may not be sufficient to fully explain these dot hemorrhages. Intraretinal hemorrhages require disruption of adherens junctions and breakdown of the basement membrane for erythrocytes to escape from the vasculature. In addition to these small intraretinal hemorrhages, larger flame-type hemorrhages can occur in diabetic retinopathy, especially in the presence of hypertension, which is an important risk factor for retinopathy progression. Moreover, nascent vessels that are generated during PDR are fragile, usually grow anteriorly into the vitreous, and are vulnerable to rupture and hemorrhage. Indeed, PDR can cause vitreous hemorrhage, leading to vitreoretinal disorders and tractional retinal detachment. Although intraocular hemorrhages occur at multiple levels in diabetic retinopathy and are commonly associated with advance stages of the disease, the effects of hemorrhage on retinopathy progression are not fully understood.
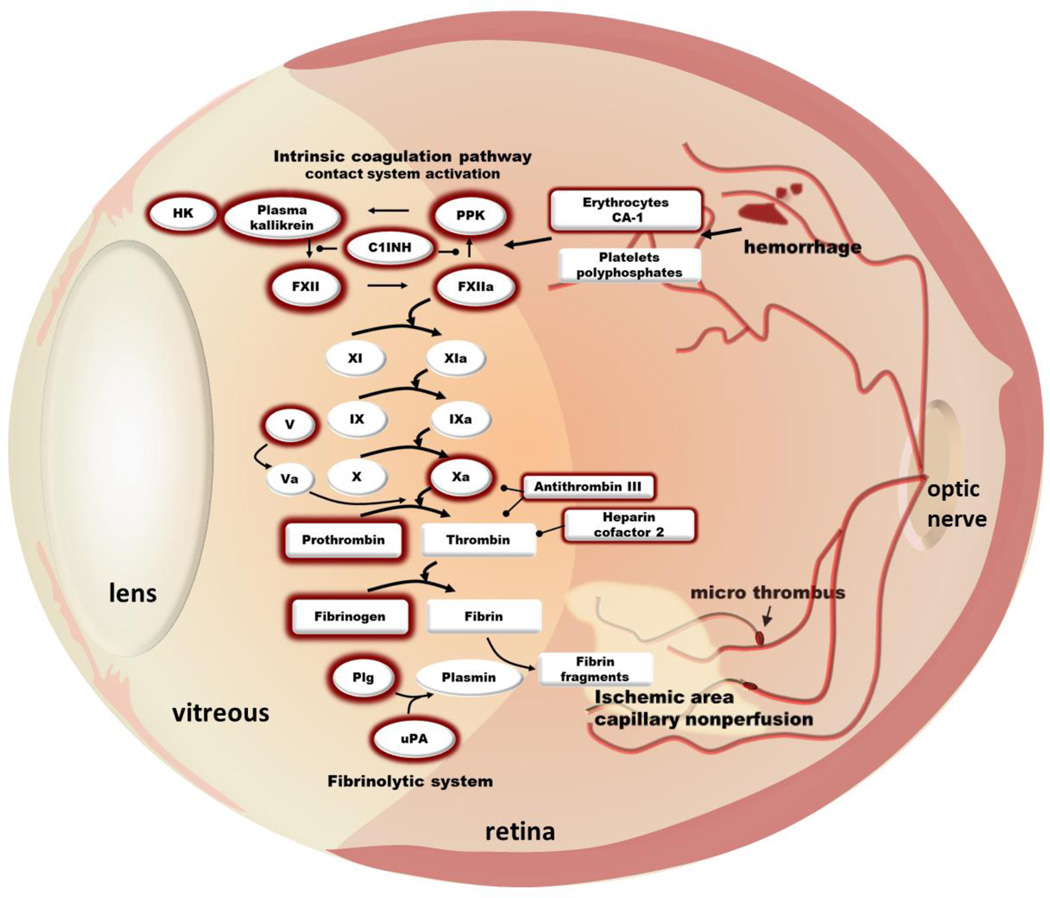
Proteomic analyses of vitreous fluid obtained from patients with advanced diabetic retinopathy have revealed that intraocular hemorrhage and plasma extravasation markedly alters the protein composition of the vitreous humor [23;24]. These studies have identified components of both coagulation and fibrinolytic cascades in the vitreous in diabetic retinopathy including prothrombin, factor V, factor XII, fibrinogen, antithrombin III, α1-anti-trypsin and heparin cofactor 2 (Figure 1). In addition to their roles in thrombosis and hemostasis, these coagulation and fibrinolytic factors increase inflammatory responses that have been implicated in diabetic retinopathy. We identified increased levels of the contact activation system components; including plasma kallikrein, factor XII (FXII), and high molecular weight kininogen (HK) in the vitreous from patients with advanced diabetic retinopathy [23]. We have also reported that intravitreal injection of plasma kallikrein exerts potent effects on retinal vascular hyperpermeabilty, leukostasis, and edema [23;25;26]. HK and FXII are plasma kallikrein’s primary substrates. Cleavage of HK by plasma kallikrein results in the production of bradykinin and thereby activation of B1 and B2 receptors, which are G-protein coupled receptors that contribute to retinal vascular inflammation and dysfunction in diabetes [6]. In addition, a number of additional substrates for this serine protease have been identified in the secretome from astrocytes and pericytes, including extracellular matrix proteins [26;27]. Liu et al. have demonstrated that plasma kallikrein binds extracellular matrix, which interferes with collagen induced platelet activation and impairs cerebral hemostasis in diabetes [28]. Abdallah et al. [29] have shown that plasma kallikrein also activates protease activated receptor (PAR)-1 and -2 on vascular smooth muscle cells, resulting in increased TNFα release. Intravitreal injections of plasma kallikrein in rats induce retinal hemorrhages, which appears similar to intraretinal dot hemorrhages observed in NPDR [26]. Plasma kallikrein also causes proteolysis of extracellular matrix components including collagen IV, a major constituent of the basement membrane [26;27]. The collagenase-like activity of plasma kallikrein may contribute to degradation of extracellular matrix and weakening of the basement membrane, thereby increasing susceptibility to vascular rupture and hemorrhage. Thus, the inflammatory effects of the contact activation system appear to be mediated by both bradykinin-dependent and -independent mechanisms. The vitreous in PDR also contains remnants of lysed erythrocytes, including carbonic anhydrase 1 and platelet and endothelial microparticles [23;30], which have been implicated in activating the contact system and thrombin [31]. Gao et al. have shown that intraocular hemorrhage results in the release of carbonic anhydrase 1 from erythrocytes, leading to increased plasma kallikrein catalytic activity [23]. This response was inhibited by either C1-Inhibitor, the primary endogenous serpin serine protease inhibitor of the kallikrein-kinin system or bradykinin BK1 and BK2 receptor antagonists. These findings indicate that the presence of lysed erythrocyte components within local ocular hemorrhage can trigger subsequent contact system components mediating retinal vascular damage in diabetic retinopathy. As mentioned above, diabetic retinopathy is also associated with platelet microparticles and adherent platelets, which may release polyphosphates and trigger FXII-mediated contact system activation [32]. These pathways could contribute, in part, to the effects of microthrombosis and hemorrhage on retinal inflammation.
Figure 1. Intraocular coagulation and fibrinolytic cascades in diabetic retinopathy.
Advanced diabetic retinopathy is characterized by the presence of intraretinal hemorrhage and capillary nonperfusion, mediated in part by leukostasis and microthrombi. Intraocular hemorrhage and plasma extravasation markedly alters vitreous protein composition in patients with advanced diabetic retinopathy. Increased levels of coagulation and fibrinolytic factors has been identified in the vitreous of patients with advanced diabetic retinopathy including plasma kallikrein (PK), factor XII (FXII), prothrombin, and urokinase plasminogen activator (uPA) (outlined in dark red). Other components detected also include high molecular weight kininogen (HK), plasma prekallikrein (PPK), C1 inhibitor (C1INH), carbonic anhydrase 1 (CA-1), and plasminogen (Plg).
In addition to the contact system, a number of other coagulation factors have been implicated in contributing to retinal inflammation in diabetic retinopathy. Proteomic data revealed that prothrombin is increased approximately 5-fold and fibrinogen (alpha, beta and gamma chain) levels were elevated more than 11-fold in the vitreous obtained from patients with PDR compared with nondiabetic subjects [24]. A recent report has implicated increased thrombin activity within the human vitreous of proliferative vitreoretinopathy patients on retinal inflammation [33]. Specifically, retinal pigmented epithelial cells exposed to vitreous samples containing high thrombin activity resulted in increased production of CCL2, CXCL8, and IL-6. Further studies demonstrated that thrombin and factor Xa stimulates IL-6, IL-8, MCP-3, and GM-CSF production by retinal pigmented epithelial cells via a PAR-1 and NF-κB pathway [34]. Moreover, thrombin-mediated activation of PAR-1 and NF-κB has also been shown to increase VCAM-1 and ICAM-1 expression - two key adhesion molecules implicated in leukocyte adhesion in diabetic retinopathy [10;35].
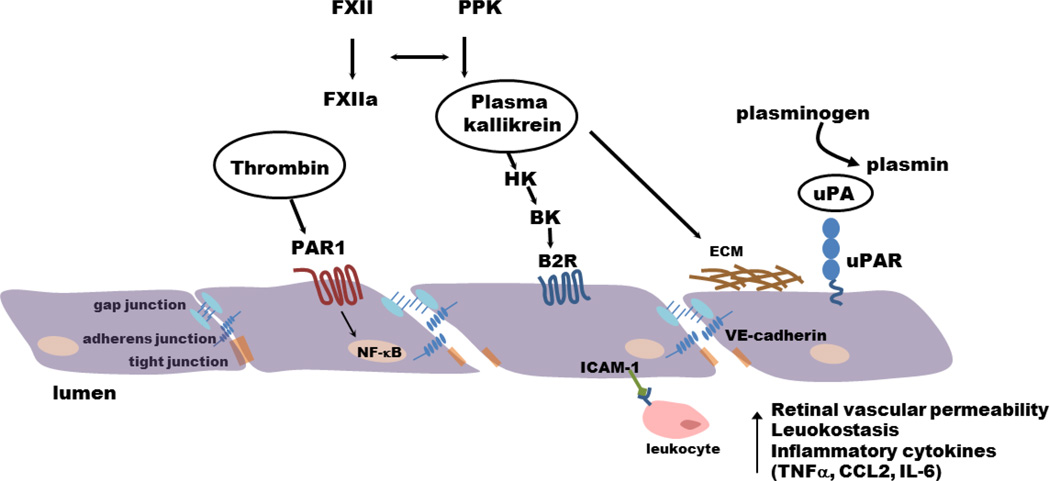
Urokinase plasminogen activator (uPA), a component of the fibrinolytic system, is increased in epiretinal membranes obtain from patients with PDR [36] and retinal uPA receptor (uPAR) expression is increased in diabetic rats [37]. Upon activation, uPA converts plasminogen to plasmin, which mediates fibrinolysis and can activate matrix metalloproteases. In addition, activation of the uPA/uPAR pathway also contributes to increased retinal vascular permeability in diabetic rats through proteolytic disruption of VE-Cadherin [38]. El-Remessy et al. [37], have also reported increased matrix metalloprotease MMP-9 activity downstream of uPA/uPAR signalling in diabetic mice retina which leads to extracellular matrix disruption in retinal vessels. Absence of uPAR in uPAR−/− mice results in blockade of diabetes induced increase in retinal vascular permeability and MMP-9 activity. Interestingly, VEGF can also directly influence levels of uPAR receptor and increase the permeability of retinal endothelial cells through the uPAR-GSK3β/β-catenin signaling pathway [37]. Taken together, these reports suggest that retinal inflammation induces endothelial dysfunction and apoptosis, which leads to microthrombosis and hemorrhage. In addition, the extravasation of activated coagulation factors can further promote retinal inflammation and endothelial dysfunction (Figure 2).
Figure 2. Activated coagulation factors promote retinal vascular permeability, leukostasis, and inflammatory cytokines.
Extravasation increases intraocular levels of thrombin, plasma kallikrein and uPA, which trigger inflammatory processes in the retina. Thrombin signals via protease-activated receptor 1 (PAR1) and NF-κB to induce inflammatory cytokines. Plasma kallikrein generates bradykinin, which can signal through B2R to increase vascular permeability, leukostasis, and edema. Plasma kallikrein also has collagenase-like activity that mediates extracellular matrix (ECM) degradation. Active uPA/uPAR signaling mediates disruption of the blood retinal barrier through proteolytic cleavage of VE-cadherin.
Multiple studies have reported that anti-inflammatory interventions, including salicylates, decreased nuclear translocation of NF-κB, expression of proinflammatory proteins, apoptosis, and capillary loss in diabetic rat retina [8]. The majority of clinical studies have not detected a significant effect of aspirin on diabetic retinopathy, reviewed by Bergerhoff et al. [39]. However, recent findings from the MADIABETES Study reported an association of aspirin use in patients with type 2 diabetes with an increased incidence of diabetic retinopathy [40]. While salicylates and other anti-inflammatory molecules have been shown to exert protective effects on the retina in diabetic animal models, the specific clinical effects of this therapeutic approach on retinal inflammation, thrombosis, and hemostasis are not yet available.
Diabetes and retinal vein occlusion
Retinal vein occlusion is an important cause of retinal vascular disease and vision loss, with an overall incidence in of about 1 to 2 percent in people over 40 years of age [41]. Santiago et al. [42] have examined the potential effects of diabetes on the prevalence of central retinal vein occlusion (CRVO) and its outcomes. This retrospective chart review of 19,648 subjects over a 4 year period showed that CRVO was observed at a similar prevalence in diabetic and nondiabetic subjects, however diabetes was associated with worse anatomical outcomes, including disc neovascularization and panretinal photocoagulation. CRVO patients with diabetes in this study included both type 1 and type 2 diabetics with 27.1±10 and 12.1±6.7 years of diabetes duration, respectively. Thus, the effects of diabetes on CRVO in outcome may be mediated, in part, by retinal vascular abnormalities associated with diabetic retinopathy. While the prevalence of CRVO is increased in a number of hypercoagulable conditions, reviewed in [41], diabetes appears to worsen CRVO outcomes rather than increase its incidence.
Conclusion
Capillary nonperfusion and intraretinal hemorrhage are associated with advanced stages of diabetic retinopathy. Although increased prevalence of microthrombi have been detected in the diabetic retina, their significance to retinal ischemia is unknown. In contrast, marked increases in coagulation and fibrinolytic factors, including plasma kallikrein, thrombin, and urokinase, have been detected in ocular samples from patients with advanced diabetic retinopathy. In addition to their roles in thrombosis and hemostasis, these factors promote retinal inflammation, vascular dysfunction, and proteolytic disruption of ECM and intercellular junctional complexes. Intraocular hemorrhage, endothelial dysfunction, and breakdown of the blood retinal barrier appears to contribute to the extravasation and activation of coagulation components in the retina and vitreous in diabetes. Inhibition of these thrombo-inflammatory pathways may provide therapeutic opportunities for diabetic retinopathy, especially for advanced stages of this disease.
Acknowledgments
The project described was supported by NIH Award Numbers EY019029, DK36836, and T32EY007145. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Literature Cited
- 1.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012 Mar;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012 Mar 29;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec 1;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 4.Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007 Aug;262(2):157–172. doi: 10.1111/j.1365-2796.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 5.Grant PJ, Kruithof EKO, Felley CP, Felber JP, Bachmann F. Short-term infusion of insulin, triacylglycerol and glucose do not cause acute increases in plasminogen activator inhibitor-1 concentrations in man. Clinical Science. 1990;79:513–516. doi: 10.1042/cs0790513. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Feener EP. Plasma kallikrein-kinin system and diabetic retinopathy. Biol Chem. 2013 Mar 1;394(3):319–328. doi: 10.1515/hsz-2012-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002 Oct;51(10):3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 8.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007 Feb;56(2):337–345. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]
- 9.Hammes HP, Lin J, Wagner P, Feng Y, vom HF, Krzizok T, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004 Apr;53(4):1104–1110. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999 Sep 14;96(19):10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iliaki E, Poulaki V, Mitsiades N, Mitsiades CS, Miller JW, Gragoudas ES. Role of alpha 4 integrin (CD49d) in the pathogenesis of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009 Oct;50(10):4898–4904. doi: 10.1167/iovs.08-2013. [DOI] [PubMed] [Google Scholar]
- 12.Phipps JA, Clermont AC, Sinha S, Chilcote TJ, Bursell SE, Feener EP. Plasma kallikrein mediates angiotensin II type 1 receptor-stimulated retinal vascular permeability. Hypertension. 2009 Feb;53(2):175–181. doi: 10.1161/HYPERTENSIONAHA.108.117663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abiko T, Abiko A, Clermont AC, Shoelson B, Horio N, Takahashi J, et al. Characterization of Retinal Leukostasis and Hemodynamics in Insulin Resistance and Diabetes: Role of Oxidants and Protein Kinase-C Activation. Diabetes. 2003 Mar;52(3):829–837. doi: 10.2337/diabetes.52.3.829. [DOI] [PubMed] [Google Scholar]
- 14.Noda K, Nakao S, Zandi S, Sun D, Hayes KC, Hafezi-Moghadam A. Retinopathy in a novel model of metabolic syndrome and type 2 diabetes: new insight on the inflammatory paradigm. FASEB J. 2014 May;28(5):2038–2046. doi: 10.1096/fj.12-215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, et al. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1) Am J Pathol. 2000 May;156(5):1733–1739. doi: 10.1016/S0002-9440(10)65044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez M, Wu Z, Moreno-Carranza B, Jeziorski MC, Arnold E, Diaz-Lezama N, et al. Vasoinhibin gene transfer by adenoassociated virus type 2 protects against. Invest Ophthalmol Vis Sci. 2011 Nov;52(12):8944–8950. doi: 10.1167/iovs.11-8190. [DOI] [PubMed] [Google Scholar]
- 17.Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996 Nov;103(11):1820–1828. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- 18.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014 Sep;121(9):1783–1789. doi: 10.1016/j.ophtha.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Yamashiro K, Tsujikawa A, Ishida S, Usui T, Kaji Y, Honda Y, et al. Platelets accumulate in the diabetic retinal vasculature following endothelial death and suppress blood-retinal barrier breakdown. Am J Pathol. 2003 Jul;163(1):253–259. doi: 10.1016/S0002-9440(10)63648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boeri D, Maiello M, Lorenzi M. Increased prevalence of microthromboses in retinal capillaries of diabetic individuals. Diabetes. 2001 Jun;50(6):1432–1439. doi: 10.2337/diabetes.50.6.1432. [DOI] [PubMed] [Google Scholar]
- 21.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001 Aug;24(8):1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 22.Matsubara Y, Murata M, Maruyama T, Handa M, Yamagata N, Watanabe G, et al. Association between diabetic retinopathy and genetic variations in alpha2beta1 integrin, a platelet receptor for collagen. Blood. 2000 Mar 1;95(5):1560–1564. [PubMed] [Google Scholar]
- 23.Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007 Feb;13(2):181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- 24.Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008 Jun;7(6):2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 25.Clermont A, Chilcote TJ, Kita T, liu J, Riva P, Sinha S, et al. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes. 2011 May;60(5):1590–1598. doi: 10.2337/db10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Clermont AC, Gao BB, Feener EP. Intraocular Hemorrhage Causes Retinal Vascular Dysfunction via Plasma Kallikrein. Invest Ophthalmol Vis Sci. 2013;54(2):1086–1094. doi: 10.1167/iovs.12-10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Gao BB, Feener EP. Proteomic identification of novel plasma kallikrein substrates in the astrocyte secretome. Translational Stroke Research. 2010;(1):276–286. doi: 10.1007/s12975-010-0039-z. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Gao BB, Clermont AC, Blair P, Chilcote TJ, Sinha S, et al. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat Med. 2011 Jan 23;(17):206–210. doi: 10.1038/nm.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdallah RT, Keum JS, El Shewy HM, Lee MH, Wang B, Gooz M, et al. Plasma kallikrein promotes epidermal growth factor receptor transactivation and signaling in vascular smooth muscle through direct activation of protease-activated receptors. J Biol Chem. 2010 Nov 5;285(45):35206–35215. doi: 10.1074/jbc.M110.171769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chahed S, Leroyer AS, Benzerroug M, Gaucher D, Georgescu A, Picaud S, et al. Increased vitreous shedding of microparticles in proliferative diabetic retinopathy stimulates endothelial proliferation. Diabetes. 2010 Mar;59(3):694–701. doi: 10.2337/db08-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Der Meijden PE, Van Schilfgaarde M, van Oerle R, Renne T, ten Cate H, Spronk HM. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012 Jul;10(7):1355–1362. doi: 10.1111/j.1538-7836.2012.04758.x. [DOI] [PubMed] [Google Scholar]
- 32.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009 Dec 11;139(6):1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastiaans J, van Meurs JC, Mulder VC, Nagtzaam NM, Smits-te NM, Dufour-van den Goorbergh DC, et al. The role of thrombin in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2014 Jul;55(7):4659–4666. doi: 10.1167/iovs.14-14818. [DOI] [PubMed] [Google Scholar]
- 34.Bastiaans J, van Meurs JC, van Holten-Neelen C, Nijenhuis MS, Kolijn-Couwenberg MJ, van Hagen PM, et al. Factor Xa and thrombin stimulate proinflammatory and profibrotic mediator production by retinal pigment epithelial cells: a role in vitreoretinal disorders? Graefes Arch Clin Exp Ophthalmol. 2013 Jul;251(7):1723–1733. doi: 10.1007/s00417-013-2335-2. [DOI] [PubMed] [Google Scholar]
- 35.Cowan C, Muraleedharan CK, O'Donnell JJ, III, Singh PK, Lum H, Kumar A, et al. MicroRNA-146 inhibits thrombin-induced NF-kappaB activation and subsequent inflammatory responses in human retinal endothelial cells. Invest Ophthalmol Vis Sci. 2014 Aug;55(8):4944–4951. doi: 10.1167/iovs.13-13631. [DOI] [PubMed] [Google Scholar]
- 36.Das A, McGuire PG, Eriqat C, Ober RR, DeJuan E, Jr, Williams GA, et al. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci. 1999 Mar;40(3):809–813. [PubMed] [Google Scholar]
- 37.El-Remessy AB, Franklin T, Ghaley N, Yang J, Brands MW, Caldwell RB, et al. Diabetes-induced superoxide anion and breakdown of the blood-retinal barrier: role of the VEGF/uPAR pathway. PLoS One. 2013;8(8):e71868. doi: 10.1371/journal.pone.0071868. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 38.Navaratna D, Menicucci G, Maestas J, Srinivasan R, McGuire P, Das A. A peptide inhibitor of the urokinase/urokinase receptor system inhibits alteration of the blood-retinal barrier in diabetes. FASEB J. 2008 Sep;22(9):3310–3317. doi: 10.1096/fj.08-110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergerhoff K, Clar C, Richter B. Aspirin in diabetic retinopathy. A systematic review. Endocrinol Metab Clin North Am. 2002 Sep;31(3):779–793. doi: 10.1016/s0889-8529(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 40.Salinero-Fort MA, San Andres-Rebollo FJ, de Burgos-Lunar C, Arrieta-Blanco FJ, Gomez-Campelo P. Four-year incidence of diabetic retinopathy in a Spanish cohort: the MADIABETES study. PLoS One. 2013;8(10):e76417. doi: 10.1371/journal.pone.0076417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macdonald D. The ABCs of RVO: a review of retinal venous occlusion. Clin Exp Optom. 2014 Jul;97(4):311–323. doi: 10.1111/cxo.12120. [DOI] [PubMed] [Google Scholar]
- 42.Santiago JG, Walia S, Sun JK, Cavallerano JD, Haddad ZA, Aiello LP, et al. Influence of diabetes and diabetes type on anatomic and visual outcomes following central rein vein occlusion. Eye (Lond) 2014 Mar;28(3):259–268. doi: 10.1038/eye.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]