Abstract
Normal energy metabolism is characterized by periodic shifts in glucose and fat oxidation, as the mitochondrial machinery responsible for carbon combustion switches freely between alternative fuels according to physiological and nutritional circumstances. These transitions in fuel choice are orchestrated by an intricate network of metabolic and cell signaling events that enable exquisite crosstalk and cooperation between competing substrates to maintain energy and glucose homeostasis. By contrast, obesity-related cardiometabolic diseases are increasingly recognized as disorders of metabolic inflexibility, in which nutrient overload and heightened substrate competition result in mitochondrial indecision, impaired fuel switching, and energy dysregulation. This Perspective offers a speculative view on the molecular origins and pathophysiological consequences of metabolic inflexibility.
Introduction
A staggering 68% of U.S. adults classify as obese or overweight. Increased adiposity is associated with insulin resistance, hypertension, hepatic steatosis, dyslipidemia, glucose intolerance, and hyperinsulinemia. Collectively known as the metabolic syndrome, this constellation of comorbidities raises the risk of developing cardiovascular disease and type 2 diabetes. In general, these are diseases of energy surplus, caused in large part by physicalinactivity and overconsumptionof calorically dense processed foods. Drug discovery efforts aimed at curtailing the epidemic spread of metabolic disease have focused heavily on mechanisms governing systemic glucose and lipid balance and the interplay between nutrient supply and insulin sensitivity. In most cases, the onsetofinsulin resistance comes early in disease development and plays a central role in the etiology of late-stage complications. Because insulin orchestrates systemic flux and disposal of glucose, fatty acids, and amino acids, resistance to the actions of the hormone gives rise to a metabolic storm of aberrant nutrient partitioning. Among the key features of this storm is an apparent stiffness in mitochondrial substrate selection, such that various organs and cell types fail to appropriately adjust fuel choice in response to nutritional circumstances. This phenomenon, dubbed “metabolic inflexibility,” has gained growing attention as a hallmark of cardiometabolic disease and a potential cause of cellular dysfunction. Thus, emerging evidence implies that metabolic health deteriorates as mitochondria lose their capacity to switch freely between alternative forms of carbon energy. This Perspective considers the physiological relevance of substrate choice and the molecular consequences of mitochondrial indecision.
Metabolic Flexibility and the Freedom of Choice
Mitochondria, the respiratory engines of the cell, consume oxygen to “burn” carbon intermediates derived from three principal nutrients: fatty acids, glucose, and amino acids. These fuels can each be catabolized to acetyl-CoA, which serves as the universal substrate that feeds the tricarboxylic acid cycle (TCAC). Each turn of the TCAC releases carbons in the form of CO2 while also generating reducing equivalents (NADH and FADH2) that drive the electron transport chain (ETC) and oxidative phosphorylation (OXPHOS), a less powerful but more efficient and higher capacity ATP regenerating system than glycolysis. The ETC/ OXPHOS system requires oxygen as the final electron acceptor, resulting in the production of water. Cellular rates of CO2 production relative to oxygen consumption, or the respiratory quotient (RQ), fluctuate between 0.7 and 1.0 and provide an approximation of mitochondrial fuel use under typical conditions in which amino acids contribute only minimally as an oxidative substrate. A high RQ is indicative of glucose oxidation, whereas a low RQ reflects predominately fat oxidation.
Normal physiology is characterized by diurnal oscillations in whole-body RQ, reflective of a metabolically flexible state in which mitochondria switch freely between substrates (fat and sugar) based on nutritional and physiological cues. For the purpose of this discussion, a metabolically sensitive and flexible system is defined as one in which nutrient and energetic signals are rapidly propagated and appropriately interpreted to elicit finely tuned adjustments in fuel partitioning. The physiological importance of metabolic plasticity cannot be understood without first considering the evolutionary pressure for mitochondria to choose fat as a fuel source when systemic glucose reserves are threatened. Whereas lipids provide an abundant, carbon-rich energy source for most tissues, the brain has limited capacity for fat catabolism and therefore relies heavily on a continuous supply of glucose. During periods of food restriction or sustained exercise, protection against hypoglycemia is accomplished by having more versatile tissues (e.g., cardiac, skeletal muscle, liver) convert to a lipid-based respiratory economy. At a systemic level, this switch in metabolic currency is mediated in large part by the counter-regulatory hormones, insulin and glucagon, both of which exert strong influence on adipose tissue lipolysis. Fasting lowers the insulin:glucagon ratio, which stimulates hydrolysis of adipose tissue triacylglycerol and thereby increases delivery of free fatty acids to the periphery. Lipolysis also occurs in tissues such as muscle and liver, further facilitating rapid provision of lipid fuel.
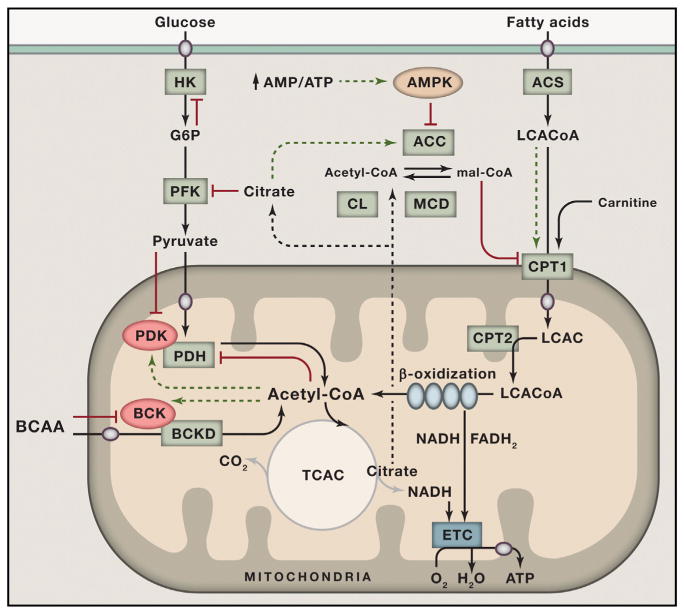
As first recognized by Randle (Randle, 1998), systemic changes in energy supply and demand are also monitored and controlled locally, such that glucose consumption is suppressed when fat oxidation increases. For example, increased supply and oxidation of fatty acids leads to cellular accumulation of acetyl-CoA, NADH, and ATP, which allosterically inhibit pyruvate dehydrogenase (PDH), the mitochondrial enzyme complex that couples glycolysis to glucose oxidation (Figure 1). This same set of allosteric effectors activates a family of PDH kinases that phosphorylate the complex, further inhibiting its catalytic activity (Sugden and Holness, 2006). Randle’s glucose-fatty acid cycle hypothesis further proposed that a rise in cellular citrate inhibits phosphofructokinase-1 and that lowering of glycolysis and pyruvate oxidation results in accumulation of glucose-6-phosphate (G6P), leading to allosteric inhibition of hexokinase 2 (HK2) in muscle and heart and diminished glucose uptake. This reciprocal regulation of substrate selection not only preserves glucose for the brain, but also relieves pyruvate from duties as an oxidative fuel while permitting its use as a gluconeogenic precursor in liver or an anaplerotic substrate that refills the TCAC in muscle and heart.
Figure 1. Nutrient Sensing and Signaling Regulate Substrate Selection during Fasting and Feeding.
Glucose and fatty acids serve as the primary catabolic substrates that provide acetyl-CoA to the tricarboxylic acid cycle. The pathways of glucose and fat oxidation are reciprocally regulated by several key metabolic intermediates and signals. During fasting, elevated acetyl-CoA derived from high rates of β-oxidation lowers glucose oxidation by allosterically inhibiting PDH and by activating its inhibitory kinase, PDK. Conversely, feeding and glucose surplus restrict fat oxidation by increasing production of malonyl-CoA, which inhibits CPT1. Citrate acts as a signal of plenty that limits glycolytic flux by inhibiting PFK and lowers β-oxidation by giving rise to cytoplasmic acetyl-CoA and malonyl-CoA via CL and ACC, respectively. During periods of energy deficit, an increase in the cellular AMP/ATP ratio activates AMPK, which phosphorylates and inhibits ACC while also activating MCD, thereby relieving malonyl-CoA-mediated inhibition of CPT-1 and promoting fat oxidation. Catabolism of branched-chain amino acids (BCAA) is regulated by BCKD, which is feedback inhibited by acyl-CoA products of the complex due to activation of its inhibitory kinase, BCK. Increased cellular concentrations of pyruvate, BCAA, and fatty acyl-CoAs promote their own catabolism by antagonizing the inhibitory actions of PDK, BCK, and malonyl-CoA, respectively.
ACC, acetyl-CoA carboxylase; ACS, acyl-CoA synthetase; AMPK, 5’ AMP-activated kinase; BCAA, branched-chain amino acids; BCKD, branched-chain ketoacid dehydrogenase; BCK, BCKD kinase; CL, citrate lyase; CPT1, carnitine palmitoyltransferase 1; ETC, electron transport chain; G6P, glucose 6 phosphate; HK, hexokinase; LCAC, long-chain acylcarnitine; LCACoA, long-chain acyl-CoA; MCD, malonyl-CoA decarboxylase; PFK, phosphofructokinase; PDH, pyruvate dehydrogenase; PDK, PDH kinase. Red indicates inhibition; green indicates activation; circles are transporters.
Another important alternative fuel source during starvation comes as a result of proteolysis and amino acid catabolism, which is regulated, in part, by the mitochondrial branched-chain ketoacid dehydrogenase (BCKD) complex. Like PDH, this complex is allosterically inhibited by NADH and the acyl-CoA esters that arise during branched-chain amino acid catabolism and is covalently inactivated by phosphorylation via BCKD kinase. Also noteworthy, the branched-chain amino-acid-derived α-ketoacid substrates of BCKD inhibit this kinase and thereby promote complex activity when amino acids are present in excess (Shimomura et al., 2001). This regulatory strategy conserves cellular proteins during short periods of fasting and promotes amino acid catabolism in response to a protein-rich diet and during prolonged starvation or exercise when muscle protein breakdown is activated.
By contrast, the nutrient and hormonal milieu elicited by the postprandial state favors glucose uptake, glycolysis, and pyruvate oxidation and a corresponding suppression of fatty acid catabolism. The mechanisms governing the meal-induced switch from fatty acid to glucose oxidation first came to light through the elegant work of McGarry and colleagues (McGarry, 2002), who discovered that feeding increases tissue levels of a precursor for de novo lipogenesis, malonyl-CoA, that also serves as a potent allosteric inhibitor of carnitine palmitoyltransferase-1 (CPT-1) (Figure 1). Positioned on the outer mitochondrial membrane, CPT-1 converts long-chain fatty acyl-CoAs to long-chain acylcarnitines that are able to traverse the inner membrane. Accordingly, CPT-1 acts as the gateway for entry of fatty acids into the mitochondrial matrix. Production of malonyl-CoA, the gatekeeper, increases when glucose is plentiful, thus restricting β-oxidation (McGarry, 2002). Additionally, the glucose-induced rise in pyruvate inhibits the PDKs and thereby favors dephosphorylation and activation of the PDH complex. This step results in refilling of the TCAC and net export of citrate. Upon delivery to the cytoplasm, citrate is cleaved by ATP-citrate lyase to oxaloacetate and acetyl-CoA, the latter of which is then carboxylated and converted to malonyl-CoA by one of two isoforms of acetyl CoA carboxylase (ACC). Citrate also acts as an allosteric activator of ACC in a feedforward manner. In sum, an influx of glucose into the TCAC results in mitochondrial export of carbon metabolites that serve as negative regulators of fat oxidation to facilitate a robust switch in fuel selection. Transitions from a fed state back to a state of energy deficit (e.g., fasting or exercise) relieve the inhibition fat oxidation through activation of the energy stress sensor, 5’ AMP-activated kinase (AMPK), which phosphorylates and inactivates ACC, thereby lowering malonyl-CoA levels and increasing CPT-1 activity.
Collectively, the mechanisms originally described by Randle and McGarry represent key components of a sophisticated metabolic network that monitors and responds to the local and systemic nutrient environments to maintain glucose homeostasis. Transitions between fasting and feeding trigger a wave of metabolite signals that guide mitochondrial fuel choice and regulate carbon trafficking (Figure 1). Metabolic intermediates arising from fat catabolism act as negative regulators of glucose oxidation and vice versa. Crosstalk and cooperation between competing substrates enable mitochondria to choose the energy source that is most appropriate for a particular physiological state. Preferential selection of glucose during the fasted-to-fed transition prevents hyperglycemia, whereas the switch to alternative fuels (lipids and amino acids) during periods of food deprivation defends against hypoglycemia and ensures organism survival.
Metabolic Congestion Results in Mitochondrial Indecision
It is important to consider that human physiology evolved to cope with dramatic fluctuations in energy supply and demand during periods of feast and famine or hunting/gathering. Thus, episodes of refueling were typically preceded by a sustained period of energy deficit. By contrast, physiology in the modern era is characterized by a steady influx of competing fuels. A large body of evidence suggests that overnutrition and unabated substrate competition lead to a state of metabolic insensitivity and inflexibility, characterized by distorted nutrient sensing, blunted substrate switching, and impaired energy homeostasis.
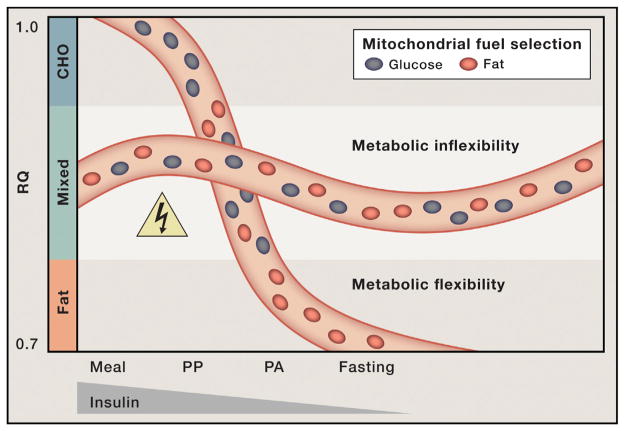
The concept of metabolic inflexibility was first introduced by Kelley and colleagues, who monitored gas exchange across the leg to examine substrate switching in healthy compared to obese and diabetic subjects (Figure 2) (Kelley et al., 1999). Lean, healthy subjects shifted from a low RQ in the fasted state to a high RQ during a hyperinsulinemiceuglycemic clamp, an infusion procedure that mimics the fed state by increasing plasma insulin concentrations while holding blood glucose constant at basal levels. When this same test was applied to obese and type 2 diabetic subjects, the transition to the “fed” state was accompanied by only a marginal change in RQ. Therefore, the insulin-resistant individuals continued to oxidize a fixed mixture of fats and carbohydrates regardless of the nutritional context. Additionally, emerging evidence suggests that increased amino acid supply and catabolism (which elicits an intermediate RQ) might also contribute to obesity-related perturbations in fuel use (Newgard, 2012). Thus, in the context of chronic over-feeding, competition between substrates escalates, cooperation is lost, and the mitochondria are left in a state of indecision characterized by persistent oxidation of all three major fuels. This phenomenon of blunted fuel switching has now been described in a variety of clinical settings, including obesity (Prior et al., 2014), diabetes (Ukropcova et al., 2007), heart disease (Turer et al., 2010), nonalcoholic steatohepatitis (Lee et al., 2014), poly-cysticovarian syndrome (Di Sarra et al., 2013), and physical inactivity (Bergouignan et al., 2013). Whereas most of these studies evaluated substrate use by skeletal muscle, heart, and/or liver, emerging evidence shows that similar perturbations in fuel switching and nutrient responsiveness manifest in other organs and cell types, including adipose tissue (Sparks et al., 2009), macrophages (Asterholm et al., 2012a), and monocytes (Liu et al., 2012).
Figure 2. Mitochondrial Indecision Results in Metabolic Inflexibility.
In healthy, metabolically flexible states, consumption of a high-carbohydrate (CHO) meal together with a small rise in blood insulin levels elicit a surge in the respiratory quotient (RQ; VCO2/VO2), indicative of a robust shift from fatty acid to glucose oxidation. During the postprandial (PP) hours following a meal, mitochondria consume a mixture of fats and carbohydrate. Progression toward the postabsorptive (PA) state and prolonged fasting are accompanied by increased fat oxidation and a corresponding decline in the RQ. Mitochondrial capacity to switch freely between oxidative fuels depending on the nutritional context is lost in obese, metabolically inflexible individuals. Persistent oxidation of a mixture of carbon fuels increases mitochondrial congestion and risk of metabolic hazard.
Metabolic inflexibility can also be induced experimentally in rodents with high-fat feeding (Koves et al., 2008; Newgard, 2012) or as a consequence of one of numerous and disparate genetic manipulations used to mimic various metabolic disease states (Asterholm et al., 2012b; Koves et al., 2008; Vadvalkar et al., 2013). In many cases, mitochondrial capacity to switch between fuels is coupled to changes in cellular and/or tissue functions, such as insulin action, glucose disposal, lipolysis, lipid storage, cardiac contractility, immune function, and inflammatory response (Asterholm et al., 2012a, 2012b; Koves et al., 2008; Liu et al., 2012; Sparks et al., 2009; Vadvalkar et al., 2013). Moreover, sluggish responses to a change in nutrient load appear to extend beyond substrate selection and are observed at the level of gene and protein expression. Thus, robust induction or suppression of a wide range of transcripts, which typically occurs during the fasting-to-fed transition, is attenuated in models of disease (Gao et al., 2014; Jans et al., 2011). This consequence is not surprising considering that many of the aforementioned signaling events and metabolic intermediates that mediate the glucose-fatty acid cycle also influence gene transcription via direct or indirect mechanisms. Although this mode of regulation is unlikely to influence immediate responses to a meal and/or insulin stimulation, nutrient-induced modulation of mRNA and protein abundance probably reflects cellular anticipation of prolonged stress and/or priming of the network for the next meal. This type of hormetic adaptation builds regulatory reserve and enhances the capacity of the network to monitor and cope with future metabolic insults. Accordingly, perturbations in meal-induced transcriptional/ translational reprogramming might compromise nutrient sensitivity in response to chronic metabolic pressures (Gao et al., 2014).
Mitochondrial Overload Leads to Metabolic Gridlock
Research to delineate the molecular origins of metabolic inflexibility has focused largely on the glucose-fatty acid cycle and/or aberrant production of malonyl-CoA. Although these mechanisms certainly weigh heavily on fuel selection, the finding that inflexibility is associated with or can be provoked by a broad range of clinical and experimental circumstances suggests that the molecular basis of this condition reaches beyond dysregulation of PDH and/or CPT-1. It is important to consider that: (1) fuel selection occurs at the level of the mitochondrion, (2) carbon substrates are the source of the electrons that feed the ETC, and (3) oxidative phosphorylation satisfies 70%–90% of ATP demand in most cells. Accordingly, this organelle is ideally positioned to monitor and transmit energy and nutrient status throughout the metabolic network (Anderson et al., 2009; Mailloux et al., 2013; Muoio and Neufer, 2012; Newman et al., 2012). This mitocentric model of nutrient sensing and partitioning suggests that cellular energy charge and shifts in flux control are integrated and executed as a function of mitochondrial carbon load.
To conceptualize a network model of metabolic inflexibility, the spatiotemporal features of carbon flux through the metabolic interstates of an organism transitioning from fasting to feeding can be viewed in a manner analogous to the onset of rush hour traffic. When volume is light, free-flowing traffic remains highly responsive to internal cues based on operator decisions and mechanical function, as well as external inputs from traffic signs and signals. Under these circumstances, distance between vehicles remains generous, buffering capacity is robust, and abrupt fluctuations in traffic density and speed can occur with minimal risk of collision. As volume expands, bottlenecks at highly traveled intersections impede flow, the buffering capacity of the network diminishes, and the probability of random collision mounts. An incident at a critical node can lead to systemic paralysis, such that traffic flow remains unresponsive even when a major signal changes from red to green. The roadways reach a state of grid-lock, and the time and energy required to restore normal flow depends on the extent of the impasse and the severity of the damage.
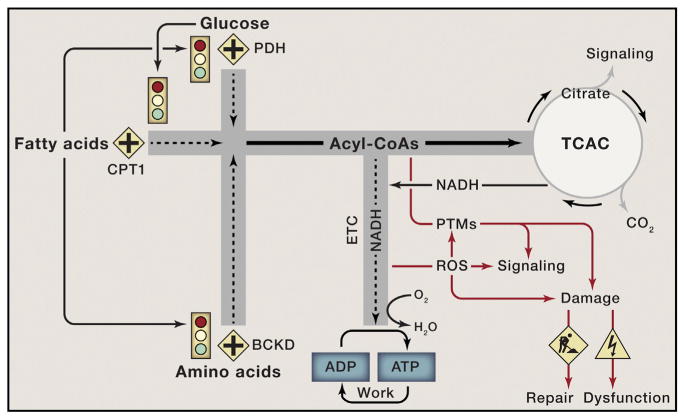
It is conceivable that a similar state of gridlock develops when organisms eat voraciously and often (Figure 3). During and immediately after each meal, carbon traffic becomes more congested and competition between substrates intensifies. Moreover, as intracellular triacylglycerol and glycogen depots reach capacity, continuous turnover of these large reservoirs imposes additional nutrient pressure on the network due to mass action. Eventually, carbon flux is perturbed, not only due to the high volume of traffic, but also as a result of distorted and conflicting signals that provoke a situation of metabolic “road rage” between the products of CPT-1, PDH, and BCKD. As tensions escalate and the three substrates battle for the right of way, mitochondria are confronted with an ever-increasing nutrient burden.
Figure 3. Nutrient Overload Leads to Mitochondrial Gridlock and Cellular Dysfunction.
Glucose, fatty acids, and branched-chain amino acids are degraded to acyl-CoA intermediates that fuel the mitochondrial tricarboxylic acid cycle (TCAC). All catabolic roadways in the mitochondria, including the TCAC, lead to the production of reducing equivalents (NADH) that feed the electron transport chain (ETC), thereby permitting ATP regeneration to support cellular work and energy expenditure. In healthy states, carbon traffic at the crossroads marked by PDH, CPT1, and BCKD is coordinately and reciprocally regulated by an intricate network of metabolic signals that match energy supply to ATP demand. High rates of fat oxidation inhibit glucose and BCAA catabolism and vice versa, thereby preventing mitochondrial congestion when ATP consumption is low. Chronic overnutrition causes metabolic confusion and signal failure, resulting in unabated influx of surplus fuel and an ensuing traffic jam at several critical bottlenecks where the roadways converge. As mitochondrial traffic reaches a state of grid-lock, membrane potential rises and accumulating electrons and acyl-CoAs are diverted toward ROS generation and PTMs such as glutathionylation and lysine acetylation, which further disrupts nutrient sensing and signaling. If these road hazards are not sufficiently managed by the mitochondrial buffering and repair systems, mounting irreversible damage to cellular macromolecules leads to organ dysfunction.
Based on first-order kinetics, the overfed mitochondria continue to degrade incoming carbon substrates. Although rates of fuel catabolism might be low, each molecule of acetyl-CoA produced by the processes of glucose, fat, and amino acid oxidation is accompanied by the generation and delivery of reducing equivalents to the ETC. Because OXPHOS is a demand-driven process regulated by ADP availability, an increase in electron delivery does not necessitate a proportional increase in ATP production. When electron supply to the Q cycle exceeds demand for ATP synthesis, mitochondrial membrane potential rises and proton pumping at complexes I, III, and IV is met with increasing back pressure. Under these circumstances, the main escape route for incoming electrons occurs via the reduction of molecular oxygen and generation of the superoxide anion, followed by its rapid conversion to hydrogen peroxide (H2O2) by superoxide dismutase. Recent studies also identify PDH, BCKD, and α-ketoglutarate dehydrogenase as important sites of ROS production (Fisher-Wellman et al., 2013). Consequently, as the NADH/NAD(+) redox pair shifts to a more reduced state, the microenvironments surrounding the ETC and the 2-oxoacid dehydrogenase complexes becomes more conducive to H2O2 production and emission (Anderson et al., 2009).
Hydrogen peroxide and other reactive oxygen species are now well recognized as bona fide signaling molecules that modulate reversible oxidation/reduction of sulfur atoms within critical cysteine residues of numerous proteins. The interconversion of these so-called “sulfur switches” from protein thiols (reduced form) to their corresponding disulfides (oxidized form) affects the activities and/or functions of an expansive network of redox-sensitive metabolic enzymes and signaling proteins (Brandes et al., 2009). This link implies that perturbations in the frequency, amplitude, and/or duration of the mitochondrial H2O2 pulse could have far-reaching effects on redox circuitry, nutrient flux, and energy homeostasis (Mailloux et al., 2013). Additionally, as oxidative stress builds, H2O2 and other ROS are more likely to collide with and damage cellular constituents via irreversible reactions such as protein carbonylation and lipid peroxidation (Frohnert and Bernlohr, 2013), further compromising the integrity and plasticity of the network.
Meanwhile, as delivery of carbons persists and the amount of excess fuel mounts, the NADH/NAD(+) ratio within the mitochondrial lumen increases and redox inhibition of several TCAC enzymes limits flux through this major metabolic beltway. The ensuing mismatch between the early steps of carbon degradation and TCAC flux can lead to intramitochondrial accumulation of acetyl-CoA and other acyl-CoAs at several crucial bottlenecks, particularly at sites where catabolism of the three fuels converge (Figure 3) (Koves et al., 2008; Newgard, 2012). Also notable is that citrate synthase, the main entry point into the TCAC, does not bind acetyl-CoA without first binding to its other substrate, oxaloacetate. Additionally, amino-acid-derived succinyl-CoA acts as a potent inhibitor of citrate synthase. As such, a limitation at the level oxaloacetate and/or accumulation of succinyl-CoA adds further pressure on the expanding mitochondrial pool of acetyl-CoA.
Acetyl-CoA and other reactive thioesters not only act as allosteric regulators of mitochondrial enzymes, but also serve as acyl donors for protein modifications (PTMs) such as lysine acetylation, succinylation, and palmitoylation via either enzymatic or nonenzymatic mechanisms (Choudhary et al., 2014; Wagner and Payne, 2013). Lysine acetylation is a reversible PTM in which a two carbon acetyl group is covalently attached to the ε-amino group of a lysine residue. Mass-spectrometry-based acetyl-proteomic analyses have led to the estimate that more than one-third of the mitochondrial proteome is acetylated on at least one lysine residue, apparently affecting nearly every major pathway of intermediary metabolism. Moreover, global acetylation in liver and muscle increases in response to diet-induced obesity in mice. Although the functional relevance of these PTMs is largely undefined, a growing number of mitochondrial enzymes have been shown to be negatively regulated by lysine acetylation (Choudhary et al., 2014; Gao et al., 2014; Hirschey et al., 2010; Jing et al., 2011; Jing et al., 2013). Yet unclear is whether these PTMs represent bona fide signaling events and/ or a form of protein damage that comes as a cost of traveling the mitochondrial roadways during rush hour traffic. Either way, a substantive shift in the mitochondrial acylome might perturb carbon and electron flow, leading to sluggish metabolic responses to nutritional and/or hormonal stimuli.
Metabolic Countermeasures and Mitochondrial Damage Control
Considering that most routes connecting food consumption to ATP production travel through the mitochondria and because reactive lysine and cysteine residues of mitochondrial proteins are more vulnerable to nucleophilic attack due to the alkaline environment of the matrix (Ghanta et al., 2013; Mailloux et al., 2013; Wagner and Payne, 2013), it is not surprising that this organelle has developed various countermeasures to buffer excess traffic and repair the damage caused by inadvertent molecular collisions. For example, the redox circuits modulated by H2O2 are buffered by the interdependent glutathione- and thioredoxin-reducing systems (Mailloux et al., 2013). Both use the reducing power of NADPH to mitigate oxidative stress and to modulate reversible oxidation/reduction of protein thiols/disulfides. Additionally, oxidized glutathione molecules (GSSG) can form disulfide linkages with reactive cysteine residues within the redox-sensitive proteome (Mailloux et al., 2013). This interaction produces a PTM known as S-glutathionylation (PSSG), which is thought to protect proteins from permanent oxidative damage. S-glutathionylation of mitochondrial proteins under normal conditions is cysteine thiol specific, reversible, sensitive to changes in redox environment, and enzyme driven (Mailloux et al., 2013). Thus, the S-glutathionylation cycle, catalyzed by a family of protein redoxins, acts as a redox rheostat that modulates the function of a large number of protein targets, including mitochondrial and antioxidant enzymes and several protein kinases and phosphatases (Mailloux et al., 2013). Relevant to this area of study, numerous reports show that circulating and/ or tissue levels of GSH decline in the context of obesity and metabolic disease (Anderson et al., 2009), suggesting diminished redox buffering capacity.
Another buffering system that plays a key role in energy homeostasis utilizes a family of mitochondrial carnitine acyltransferase (CAT) enzymes that catalyze the exchange of acyl groups between CoA and L-carnitine (Ramsay and Zammit, 2004). Unlike their acyl-CoA precursors, acylcarnitine esters are readily transported across cellular membranes. Accordingly, this system permits shuttling of carbon fuels between compartments and thereby acts to offset nutrient-induced expansion of the mitochondrial pools of acetyl- and other reactive acyl-CoA moieties. With the advent and broadening applications of metabolomics technologies, acylcarnitine metabolites have emerged as strong biomarkers of nutrient stress, mitochondrial dysfunction, and metabolic disease (Koves et al., 2008; Muoio and Neufer, 2012; Newgard, 2012; Noland et al., 2009). The most abundant acylcarnitine species, acetylcarnitine, is synthesized by carnitine acetyltransferase (CrAT), a member of the CAT family that localizes to the mitochondrial matrix and strongly prefers acetyl- and other short-chain acyl-CoA end products of fatty acid, glucose, and amino acid catabolism (Muoio et al., 2012). Notably, muscle-specific ablation of crat in mice elevates tissue levels of acetyl-CoA, lowers PDH activity, increases acetylation of several mitochondrial proteins, and diminishes metabolic flexibility (Muoio et al., 2012). Conversely, dietary L-carnitine supplementation promotes glucose tolerance and enhances metabolic flexibility in concert with increased circulating levels of the main CrAT product, acetylcarnitine (Muoio et al., 2012; Noland et al., 2009). Likewise, muscle CrAT activity correlates positively with insulin sensitivity in rodents and humans (Lindeboom et al., 2014). These studies establish an important link between acylcarnitine efflux, mitochondrial acyl-CoA balance, and fuel selection and strongly imply that mitochondrial carbon load directly impacts glucose tolerance and insulin action.
Whereas the carnitine system relieves substrate push on mitochondrial protein acylation, the sirtuin family of NAD+-dependent deacylases performs damage control by removing acyl groups from lysine residues. Importantly, however, the sirtuins do not act on all acyl-lysine residues; thus, it appears that only a specific subset of these PTMs is reversible. SIRT3, SIRT4, and SIRT5 are found in the mitochondrial matrix. SIRT3 is the main mitochondrial deacetylase and the best characterized of the mitochondrial sirtuins (Newman et al., 2012). SIRT5 has robust desuccinylase, demalonylase, and deglutarylase activities, whereas most sirtuins (SIRT1–6) appear to also possess long-chain deacylation activity (Feldman et al., 2013). Interestingly, Sirt3 knockout mice have increased acetylation of several metabolic enzymes, including PDH and superoxide dismutase, which is accompanied by elevated acylcarnitines and increased mitochondrial ROS production (Hirschey et al., 2010; Jing et al., 2011; Jing et al., 2013). These animals are less metabolically flexible than their wild-type counterparts and also exhibit multiple features of the metabolic syndrome as they age. Because the lysine deacylation reactions consume NAD+, which is limiting for sirtuin activity, obesity-induced lowering of NAD+ is thought to constrain the sirtuin system (Peek et al., 2013). Thus, the combination of increased acetyl donors and reduced deacylating capacity might underlie the elevation in mitochondrial protein acetylation observed in response to chronic nutrient overload. These findings have fueled strong interest in the use of nicotinamide riboside and other NAD+ precursors as an antidiabetic strategy to boost sirtuin activity and restore metabolic function (Cantóet al., 2012).
Insulin Resistance Viewed as a Case of Mixed Signals
Aberrant transitions between fuel types at both the cellular and systemic levels link to phenotypes associated with metabolic co-morbidities, including insulin resistance. An important question is whether insulin resistance causes metabolic inflexibility or vice versa. One view suggests that blunted glucose oxidation in response to a meal simply reflects a consequence of impaired insulin signaling. On the other hand, studies in rodents show that metabolic inflexibility occurs early in the course of glucose intolerance, and obesity-induced perturbations in substrate switching are evident in isolated mitochondria and tissue homogenates (Muoio et al., 2012; Noland et al., 2009). These findings suggest that derangements in fuel selection are at least partly independent of and might actually precede and contribute to insulin resistance.
It is important to reiterate that the models developed by Randle and McGarry center on the concepts of reciprocation, cooperation, and communication between substrates. In a healthy state, crosstalk between metabolic pathways is mediated by robust, concise, and decisive changes in cellular levels of metabolites such as fatty acids, pyruvate, citrate, and malonyl-CoA, which in turn regulate incoming mitochondrial traffic (Figure 1). By contrast, chronic overnutrition leads to a state of metabolic confusion, wherein excessive carbon supply and heightened substrate competition give rise to a set of muted and/or conflicting signals. As a result, the gateways that control mitochondrial traffic are never fully open or shut, and the continuous influx of carbon fuel from multiple sources and directions interferes with efficient substrate switching. Thus, metabolic inflexibility can be viewed as both a cause and indicator of mitochondrial congestion, which in turn influences insulin action. Transitions between highly insulin-sensitive and more insulin-resistant states appear to operate on a continuum as a function of carbon and electron supply relative to ATP demand (Figure 3), such that a shift in the positive direction triggers a set of mitochondrial-derived stress signals that oppose cellular glucose uptake as an attempt to limit traffic volume.
From an evolutionary perspective, it seems probable that more primitive and firmly hardwired intracellular nutrient-sensing networks of the cell override hormonal stimuli when the signals disagree. One example of this hierarchy comes from studies of exercise and muscle contraction. Although the systemic nutrient and hormonal environment of exercise mimics a starvation state on many levels, glucose flux into muscle during contraction proceeds unimpeded. The energy charge of working muscles stimulates translocation of the resident glucose transporter, Glut4, from an intracellular compartment to the cell surface, thereby permitting glucose uptake. Whereas Glut4 functions as the classical insulin-responsive glucose transporter, its translocation during exercise occurs via an insulin-independent pathway involving short-acting signals such as an elevated AMP/ATP ratio and consequent activation of AMPK (Jessen and Goodyear, 2005). Similarly, numerous studies have shown that a single bout of vigorous exercise enhances insulin action for up to 24 hr in muscles of obese rodents or humans (Thyfault, 2008). These observations underscore two important points. First, obesity does not cause insulin resistance. Instead, increased adiposity is merely a symptom of chronic (positive) energy imbalance, presumably the true culprit. Second, even brief episodes of increased energy expenditure and accelerated carbon combustion can reset the energy charge of the muscle and enhance insulin action. The precise molecular mechanisms underlying exercise-mediated enhancement of insulin responsiveness have evaded scientists for decades. Interestingly, however, recent studies have established a strong positive association between exercise training and metabolic flexibility (Bergouignan et al., 2013; Koves et al., 2013), suggesting that physical activity enhances mitochondrial traffic control and that persistent substrate competition is a key component of insulin resistance.
Also noteworthy is that a number of reports have found that glucose intolerance precedes overt defects in the canonical insulin signaling cascade leading to Akt/PKB (protein kinase B) phosphorylation (Muoio and Neufer, 2012; Muoio et al., 2012). Thus, intracellular cues dissuading glucose uptake appear to take precedence over Akt activation. This state might be partly attributable to lipid-induced accumulation of G6P and/or flux limitations at HK2, as proposed by Randle and others (Randle, 1998; Wasserman et al., 2011), but likely involves additional feedback signals that further antagonize glucose disposal (Muoio and Neufer, 2012). In this scenario, the pancreatic β cells should compensate for diminished rates of peripheral glucose disposal by releasing more insulin, resulting in hyperinsulinemia. Over time, this compensatory response could lead to insulin-mediated insulin resistance whereby insulin itself is responsible for desensitizing the insulin receptor and its proximal targets (Copps and White, 2012).
Additionally, heightened and persistent competition between oxidative substrates has the potential to impact several other extra-mitochondrial signaling molecules known or presumed to influence insulin action. For instance, diminished flux through PDH could result in re-routing of glycolytic intermediates toward gluconeogenesis in liver or the generation of lipid-signaling molecules such as diacylglycerol and ceramide, all of which have been strongly implicated in insulin resistance (Muoio and Newgard, 2008). In general, excessive production of these lipid species has been attributed to fatty acid toxicity. Notably, however, de novo synthesis of both molecules requires glucose-derived carbon intermediates—namely, glycerol-3-phosphate and serine, respectively. In other words, “it takes two to tango”; therefore, cellular synthesis of diacylglycerols and ceramides diminishes when one of the two precursor fuels is fully committed to the mitochondria for the purpose of ATP production.
As noted earlier, heighted substrate competition promotes ROS production. Mitochondrial-derived H2O2 can oxidize critical cysteine residues within the catalytic sites of several protein phosphatase enzymes that modulate insulin signaling in both directions, including the phosphoinositide phosphatase, PTEN, and several dual specificity protein tyrosine phosphatases. Thus, perturbations in the pattern of H2O2 generation could lead to over activation and/or desensitization of the insulin signaling network. Along with alterations in protein function, major shifts in the redox proteome and/or other PTMs, including carbonylation, glutathionlylation, and lysine acylation, might disrupt protein turnover and/or folding (Frohnert and Bernlohr, 2013; Gao et al., 2014; Mailloux et al., 2013). Perturbations in cellular proteostasis trigger the unfolded protein response (UPR) and endoplasmic reticulum stress, another candidate mediator of insulin resistance (Muoio and Newgard, 2008). Moreover, emerging evidence suggests that hyperacylation of the mitochondrial proteome influences mitophagy, mitochondrial dynamics, and the mitochondrial UPR (Gao et al., 2014; Papa and Germain, 2014). Lastly, several of the foregoing PTMs have also been implicated in the regulation/dysregulation of various mitochondrial membrane organic acid carrier proteins and other metabolite transporters, including the citrate carrier, the adenine nucleotide translocase, and the carnitine acylcarni-tine translocase. Thus, these modifications have the potential to obstruct crosstalk between mitochondria and other cellular compartments, which could dampen meal-induced shifts in nutrient partitioning, glucose flux, and retrograde signaling.
Strategies for Decongestion
The idea that mitochondrial carbon overload and metabolic inflexibility lie at the core of insulin resistance implies that maneuvers to prevent oxidative catabolism of at least one of the three major fuel sources might alleviate substrate competition and restore glucose control. This strategy has, in fact, proven beneficial in mouse models with genetically engineered inhibition of CPT-1 or other proximal steps in fatty acid oxidation (Koves et al., 2008; Li et al., 2014; Muoio and Neufer, 2012). Although fewer studies have targeted glucose oxidation, there is one report showing that cardiac-specific overexpression of PDK4 caused marked suppression of heart PDH activity and glucose oxidation but without the expected deleterious functional and metabolic consequences in response to an ischemia-reperfusion challenge (Chambers et al., 2011). Numerous provocative studies in animals and humans show that very low-carbohydrate (ketogenic) diets produce favorable metabolic outcomes when compared to a traditional mixed macronutrient diet (Hu et al., 2012). Whereas these findings have sparked intense controversy over dietary recommendations for disease prevention, perhaps the salient observation is that a dietary regimen that effectively limits mitochondrial substrate competition produces marked improvements in metabolic control. Alternatively, several lines of evidence suggest that boosting or restoring the buffering capacities of the systems that mitigate mitochondrial carbon stress, including antioxidant defense, glutathione regeneration, carnitine-mediated acyl group buffering, and sirtuin-mediated protein deacylation, also improves whole-body energy homeostasis and glucose control in association with enhanced metabolic flexibility (Anderson et al., 2009; Cantóet al., 2012; Muoio et al., 2012; Noland et al., 2009).
Although blocking mitochondrial influx of carbons and/or building buffering capacity might alleviate the metabolic load on this particular organelle, these strategies do not address the fundamental problem of energy imbalance and the broader implications of nutrient surplus. Thus, approaches targeting the cause rather than the symptoms of nutrient overload should yield more desirable outcomes. Moving beyond the standard recommendation to “eat less and move more,” recent studies employing unconventional behavior modification interventions have produced intriguing results, including intermittent fasting regimens in which rodents or human subjects fast for extended periods followed by ad libitum eating (Azevedo et al., 2013; Trepanowski et al., 2011), as well as “exercise snacking” regimens in which individuals exercise vigorously for brief (~5 min) periods before consuming a standard-size meal (Francois et al., 2014). Interestingly, these routines were found to improve glucose homeostasis more than isoenergetic regimens comprised of traditional feeding and exercise patterns. Likewise, interruption of prolonged sedentary behavior with repeated bouts of low-grade activities of daily living (e.g., post-meal strolling) has been shown to improve postprandial glucose handling in patients with type 2 diabetes (van Dijk et al., 2013). These observations are consistent with the premise that periodic episodes of accelerated carbon combustion, in a manner resembling the lifestyle of our hunter/gatherer ancestors, facilitate rapid and efficient nutrient partitioning.
Relevant to this discussion is the prominent connection between caloric restriction, lifespan, and healthspan. In model organisms, lifelong caloric restriction increases longevity and delays age-related metabolic decline. Similar outcomes have been reported in nonhuman primates, and epidemiological studies have revealed a strong association between energy balance and healthspan in humans (Trepanowski et al., 2011). The caloric restriction literature implies that ad libitum feeding is inevitably damaging to metabolic health and that surplus fuel, beyond that required for optimal cellular function, accelerates biological decay. Energy balance is typically considered at a macro level over periods of days, weeks, or months, with body weight and adiposity targeted as the primary readouts. Alternatively, tracking of nutrient balance at the cellular level on a minute-by-minute or hourly basis might represent the more biologically relevant scale. In theory, each meal tips carbon balance of each individual cell in a positive direction. Whereas the incoming nutrients are ultimately destined for either biosynthetic or catabolic fates, slow or misregulated decisions on how to appropriate the extra energy (i.e., metabolic inflexibility) can result in collateral damage. This paradigm aligns with emerging evidence that larger, less frequent meals are more detrimental to nutrient sensing, retrograde signaling, and metabolic control than smaller, more frequent meals (Fuse et al., 2012; Trepanowski et al., 2011).
Concluding Remarks
In summary, this discussion speaks to the longstanding question of whether fuel selection matters to metabolic health. Historically, this area of study has been divided into opposing viewpoints that argue for or against the idea that it is healthier to burn fat than glucose. In recent years, these two camps have found common ground in the concept of metabolic flexibility, which posits that cells function optimally when they retain their capacity to switch freely between oxidative substrates in response to nutritional and physiological cues. The foregoing network model of metabolic flexibility further suggests that, during conditions of inactivity and low ATP demand, mitochondria function best when acetyl-CoA is produced from one fuel at a time. Robust and decisive shifts in substrate choice are predicted to limit mitochondrial congestion and damaging molecular collisions while also producing strong and clearly interpretable metabolic signals that guide efficient nutrient partitioning to maintain energy homeostasis. This model provides an explanatory context for viewing the severe costs of excessive food consumption and the benefits of habitual physical activity and lifelong caloric restriction.
Acknowledgments
The author is supported by grants from the United States Public Health Service: R01DK089312, P01DK058398, and R01HL101189.
References
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asterholm IW, McDonald J, Blanchard PG, Sinha M, Xiao Q, Mistry J, Rutkowski JM, Deshaies Y, Brekken RA, Scherer PE. Lack of “immunological fitness” during fasting in metabolically challenged animals. J Lipid Res. 2012a;53:1254–1267. doi: 10.1194/jlr.M021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asterholm IW, Mundy DI, Weng J, Anderson RG, Scherer PE. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 2012b;15:171–185. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo FR, Ikeoka D, Caramelli B. Effects of intermittent fasting on metabolism in men. Rev Assoc Med Bras. 2013;59:167–173. doi: 10.1016/j.ramb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Bergouignan A, Antoun E, Momken I, Schoeller DA, Gauquelin-Koch G, Simon C, Blanc S. Effect of contrasted levels of habitual physical activity on metabolic flexibility. J Appl Physiol (1985) 2013;114:371–379. doi: 10.1152/japplphysiol.00458.2012. [DOI] [PubMed] [Google Scholar]
- Brandes N, Schmitt S, Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal. 2009;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers KT, Leone TC, Sambandam N, Kovacs A, Wagg CS, Lopaschuk GD, Finck BN, Kelly DP. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J Biol Chem. 2011;286:11155–11162. doi: 10.1074/jbc.M110.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. The growing landscape of lysine acetylation links metabolism and cell signalling. Nat Rev Mol Cell Biol. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]
- Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sarra D, Tosi F, Bonin C, Fiers T, Kaufman JM, Signori C, Zambotti F, Dall’Alda M, Caruso B, Zanolin ME, et al. Metabolic inflexibility is a feature of women with polycystic ovary syndrome and is associated with both insulin resistance and hyperandrogenism. J Clin Endocrinol Metab. 2013;98:2581–2588. doi: 10.1210/jc.2013-1161. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Baeza J, Denu JM. Activation of the protein de-acetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 2013;288:31350–31356. doi: 10.1074/jbc.C113.511261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Wellman KH, Gilliam LA, Lin CT, Cathey BL, Lark DS, Neufer PD. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic Biol Med. 2013;65:1201–1208. doi: 10.1016/j.freeradbiomed.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois ME, Baldi JC, Manning PJ, Lucas SJ, Hawley JA, Williams MJ, Cotter JD. ‘Exercise snacks’ before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. 2014;57:1437–1445. doi: 10.1007/s00125-014-3244-6. [DOI] [PubMed] [Google Scholar]
- Frohnert BI, Bernlohr DA. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Adv Nutr. 2013;4:157–163. doi: 10.3945/an.112.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse Y, Hirao A, Kuroda H, Otsuka M, Tahara Y, Shibata S. Differential roles of breakfast only (one meal per day) and a bigger breakfast with a small dinner (two meals per day) in mice fed a high-fat diet with regard to induced obesity and lipid metabolism. J Circadian Rhythms. 2012;10:4. doi: 10.1186/1740-3391-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AW, Canto C, Houtkooper RH. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol Med. 2014;6:580–589. doi: 10.1002/emmm.201303782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta S, Grossmann RE, Brenner C. Mitochondrial protein acetylation as a cell-intrinsic, evolutionary driver of fat storage: chemical and metabolic logic of acetyl-lysine modifications. Crit Rev Biochem Mol Biol. 2013;48:561–574. doi: 10.3109/10409238.2013.838204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme de-acetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS, Jr, Kelly TN, He J, Bazzano LA. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176(Suppl 7):S44–S54. doi: 10.1093/aje/kws264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans A, Sparks LM, van Hees AM, Gjelstad IM, Tierney AC, Risérus U, Drevon CA, Roche HM, Schrauwen P, Blaak EE. Transcriptional metabolic inflexibility in skeletal muscle among individuals with increasing insulin resistance. Obesity (Silver Spring) 2011;19:2158–2166. doi: 10.1038/oby.2011.149. [DOI] [PubMed] [Google Scholar]
- Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol. 2005;99:330–337. doi: 10.1152/japplphysiol.00175.2005. [DOI] [PubMed] [Google Scholar]
- Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci USA. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing E, O’Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes. 2013;62:3404–3417. doi: 10.2337/db12-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Koves TR, Sparks LM, Kovalik JP, Mosedale M, Arumugam R, DeBalsi KL, Everingham K, Thorne L, Phielix E, Meex RC, et al. PPARγ coactivator-1α contributes to exercise-induced regulation of intramuscular lipid droplet programming in mice and humans. J Lipid Res. 2013;54:522–534. doi: 10.1194/jlr.P028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivera-Vega M, Alsayed HM, Boesch C, Libman I. Metabolic inflexibility and insulin resistance in obese adolescents with non-alcoholic fatty liver disease. Pediatr Diabetes. 2014 doi: 10.1111/pedi.12141. Published online April 23, 2014 http://dx.doi.org/10.1111/pedi.12141. [DOI] [PMC free article] [PubMed]
- Li LO, Grevengoed TJ, Paul DS, Ilkayeva O, Koves TR, Pascual F, Newgard CB, Muoio DM, Coleman RA. Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes. 2014 doi: 10.2337/db13-1070. Published online July 28, 2014 http://dx.doi.org/10.2337/db13-1070. [DOI] [PMC free article] [PubMed]
- Lindeboom L, Nabuurs CI, Hoeks J, Brouwers B, Phielix E, Kooi ME, Hesselink MK, Wildberger JE, Stevens RD, Koves T, et al. Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. J Clin Invest. 2014;124:4915–4925. doi: 10.1172/JCI74830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 2012;287:25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, Jin X, Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2013;2:123–139. doi: 10.1016/j.redox.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012;15:595–605. doi: 10.1016/j.cmet.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, DeBalsi KL, Ilkayeva OR, Stevens RD, Kheterpal I, Zhang J, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15:764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, He W, Verdin E. Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease. J Biol Chem. 2012;287:42436–42443. doi: 10.1074/jbc.R112.404863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34:699–710. doi: 10.1128/MCB.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior SJ, Ryan AS, Stevenson TG, Goldberg AP. Metabolic inflexibility during submaximal aerobic exercise is associated with glucose intolerance in obese older adults. Obesity (Silver Spring) 2014;22:451–457. doi: 10.1002/oby.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay RR, Zammit VA. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol Aspects Med. 2004;25:475–493. doi: 10.1016/j.mam.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Obayashi M, Murakami T, Harris RA. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care. 2001;4:419–423. doi: 10.1097/00075197-200109000-00013. [DOI] [PubMed] [Google Scholar]
- Sparks LM, Ukropcova B, Smith J, Pasarica M, Hymel D, Xie H, Bray GA, Miles JM, Smith SR. Relation of adipose tissue to metabolic flexibility. Diabetes Res Clin Pract. 2009;83:32–43. doi: 10.1016/j.diabres.2008.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006;112:139–149. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- Thyfault JP. Setting the stage: possible mechanisms by which acute contraction restores insulin sensitivity in muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1103–R1110. doi: 10.1152/ajpregu.00924.2007. [DOI] [PubMed] [Google Scholar]
- Trepanowski JF, Canale RE, Marshall KE, Kabir MM, Bloomer RJ. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: a summary of available findings. Nutr J. 2011;10:107. doi: 10.1186/1475-2891-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer AT, Malloy CR, Newgard CB, Podgoreanu MV. Energetics and metabolism in the failing heart: important but poorly understood. Curr Opin Clin Nutr Metab Care. 2010;13:458–465. doi: 10.1097/MCO.0b013e32833a55a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray GA, Smith SR. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007;56:720–727. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- Vadvalkar SS, Baily CN, Matsuzaki S, West M, Tesiram YA, Humphries KM. Metabolic inflexibility and protein lysine acetylation in heart mitochondria of a chronic model of type 1 diabetes. Biochem J. 2013;449:253–261. doi: 10.1042/BJ20121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk JW, Venema M, van Mechelen W, Stehouwer CD, Hartgens F, van Loon LJ. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care. 2013;36:3448–3453. doi: 10.2337/dc12-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GR, Payne RM. Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem. 2013;288:29036–29045. doi: 10.1074/jbc.M113.486753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DH, Kang L, Ayala JE, Fueger PT, Lee-Young RS. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol. 2011;214:254–262. doi: 10.1242/jeb.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]