Abstract
Platforms that are sensitive and specific enough to assay low-abundance protein biomarkers, in a high throughput multiplex format, within a complex biological fluid specimen, are necessary to enable protein biomarker based diagnostics for diseases such as cancer. The signal from an assay for a low-abundance protein biomarker in a biological fluid sample like blood is typically buried in a background that arises from the presence of blood cells and from high-abundance proteins that make up 90% of the assayed protein mass. We present an automated on-chip platform for the depletion of cells and highly abundant serum proteins in blood. Our platform consists of two components, the first of which is a microfluidic mixer that mixes beads containing antibodies against the highly abundant proteins in the whole blood. This complex mixture (consisting of beads, cells, and serum proteins) is then injected into the second component of our microfluidic platform, which comprises a filter trench to capture all the cells and the beads. The size-based trapping of the cells and beads into the filter trench is significantly enhanced by leveraging additional negative dielectrophoretic forces to push the micron sized particles (cells and beads which have captured the highly abundant proteins) down into the trench, allowing the serum proteins of lower abundance to flow through. In general, dielectrophoresis using bare electrodes is incapable of producing forces beyond the low piconewton range that tend to be insufficient for separation applications. However, by using electrodes passivated with atomic layer deposition, we demonstrate the application of enhanced negative DEP electrodes together with size-based flltration induced by the filter trench, to deplete 100% of the micron sized particles in the mixture.
Keywords: Microfluidics, Particle actuation, Dielectrophoresis, Sample preparation, Cell depletion
1. Introduction
A major challenge in point-of-care diagnostics is the detection of biomarkers in complex biological fluids. These problems arise both in spectroscopy based techniques such as mass spectrometry and also in antibody based techniques like ELISA [1,2], electrical immunoassays [3–13], and fluorescent antibody arrays [14,15]. For spectroscopy based techniques, one of the main difficulties in assaying blood for low abundance proteins in multiplex format is the high level of background arising from the presence of blood cells and also from highly abundant proteins [16–20] (~1–1000 μM) such as albumin, Immunoglobulin G (IgG), Immunoglobulin A (IgA), transferrin, haptoglobin and cx-1-antitrypsin that make up 90% of the protein mass. The dynamic range of the mass of proteins in blood plasma alone spans nine orders of magnitude, resulting in difficulties for quantification and spectroscopic analysis. For antibody based techniques, where selectivity is achieved using antibody pairing, the presence of high abundance non-targeted proteins results in high levels of background signal. This is because the non-targeted proteins interfere and cross-react with the primary species involved in the binding reactions. Furthermore, the presence of numerous cell types including platelets, white blood cells, and red blood cells makes injection of whole blood onto a protein sensor surface impossible due to the interference caused by the cells as they sediment onto the sensor surface and interfere with binding of target proteins to the probe antibodies. Depletion of cells from blood is normally done by centrifugation, which is unsuitable for point-of-care diagnostics and as a result blood work generally is outsourced from the clinic to an external laboratory.
Various on-chip sample preparation techniques for the filtration of high-abundance proteins have been proposed and demonstrated. Several promising techniques have been demon-strated where either the target proteins are pre-concentrated or the non-targeted proteins are depleted. This can be done either electrokinetically or hydrodynamically. Various electrokinetic techniques such as isoelectric focusing [21] and isotachophoresis [22–27] have demonstrated pre-concentration of target proteins in complex mixtures. Hydrodynamic methods for pre-concentration of target biomolecules have also been demonstrated such as the bead based assay demonstrated by Toner et al. [28]. Here a Herringbone mixer was utilized to mix whole blood with antibody coated magnetic beads which were subsequently sorted by the application of a magnetic field. The bead-based sorting strategy was used to capture and pre-concentrate HIV virus molecules on-chip. On-chip microfluidic techniques have also been applied to the separation of cells from whole blood. Heath et al. [29] performed separation of cells from whole blood using the Zweifach-Fung technique. They also demonstrated an integrated platform for separation of cells from blood with downstream analysis based on inertial focusing [30]. Lee et al. [31] demonstrated a passively pumped platform for separation of serum from whole blood, by depletion of cells using a filter trench. Voldman et al. [32] presented an electrokinetic method for cell separation where positive dielectrophoresis was used to trap cells at the base of a channel as the supernatant fluid passes through. All of the above mentioned techniques, perform either cell depletion or protein depletion. An automated technique capable of performing both on-chip would be highly desirable.
We describe a two-component microfluidic platform in this paper that depletes cells with 100% efficiency and abundant serum proteins from blood with 95% efficiency. Our platform consists of two components, the first of which is a microfluidic mixer, which mixes beads containing antibodies against the highly abundant proteins with the whole blood (Fig. 1A) and a second component for large particle depletion (Fig. 1B). In the first component of our platform, whole blood is mixed with beads coated with antibodies for capture and depletion of highly abundant serum proteins. The contents of the first component are fed into the second component, which consists of a filter trench with interdigitated electrodes at the top of the channel where a negative dielectrophoretic (DEP) force is applied to push down all large particles (cells and beads) into the filter trench, allowing the filtered serum to pass through. In this paper, we present the proof of concept for both the components separately. For the first component, we demonstrate the depletion of IgG molecules, and for the second component, we demonstrate the enhanced depletion of beads in the filter trench using enhanced negative dielectrophoresis. We also demonstrate a proof of concept for an integrated device that uses a herringbone structure [33] to induce chaotic mixing of beads with proteins in the channel. This complex mixture (consisting of beads, cells, and serum proteins) is then injected into the second component, which uses interdigitated electrodes using DEP to push all of the cells and beads down into a filter trench.
Fig. 1.
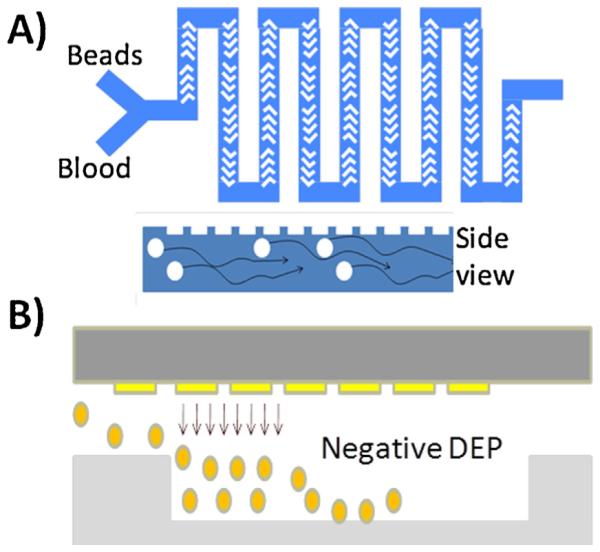
(A) Microfluidic structure for mixing antibody coated beads with blood sample to deplete the highly abundant serum proteins. (B) The output of the mixer is fed into the microchannel with a negative DEP enhanced filter trench to trap all of the beads, allowing the serum to pass through.
2. Protein depletion component
As mentioned, depletion of highly abundant proteins from the initial sample is performed by mixing the sample blood with beads coated with antibodies against the highly abundant proteins. Micro-scale fluid flow is typically laminar, which results in diffusion-limited mixing between reagents. A microfluidic herringbone mixer, as described by Whitesides et al. [33], was fabricated to induce chaotic mixing at the micro-scale, thereby significantly improving the mixing efficiency.
3. Materials and methods for protein depletion component
We fabricated the herringbone mixer using a two-layer soft lithography process. The channel mold was fabricated using SU-8 photoresist and stamped into a PDMS slab. Fig. 2A shows a bird’s eye view of a close up of a 300 μm wide by 100 μm tall micro-channel with herringbone corrugations at the top. A long mixing channel length (163 cm effective length) resulted in a large mixing residence time (1 h) and the channel was packed into a serpentine configuration to minimize on-chip real estate.
Fig. 2.
(A) (Top) Optical micrograph of bird’s eye view of microfluidic herringbone structure (300 μm wide). (Bottom) Beads coated with anti-mouse IgG molecules are mixed with fluorescent mouse IgG molecules. Image taken before and after mixing. (B) Comparison between IgG depletion results for microfluidic mixer and anti-IgG bead column.
The target protein that we depleted in this study as a proof of concept is Immunoglobulin G. The beads we used were 2.8 μm Dynal Beads (Invitrogen) functionalized with anti-goat IgG antibodies. The beads were washed thrice in Phosphate Buffered saline to ensure that there was no contamination from detergents and miscellaneous reagents in the buffer solution that was injected into the fluidic device. We injected the beads-containing buffer solution into the channel and allowed the supernatant in the buffer solution to dry to deposit the beads in the channel. By allowing the supernatant from the bead solution to dry, the final concentration of fluorescent IgG obtained post-separation is from IgG molecules depleting by binding to the antibody-functionalized beads and is not a consequence of dilution. Fluorescent goat IgG molecules (Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA) were used as a test bed platform and the fluorescence signal intensity served as the measure for quantifying the amount of antibody present before and after mixing. The initial concentration of the goat IgG molecules was 666 nM.
4. Protein depletion component results and discussion
Fig. 2A (bottom two figures) shows the beads, coated with anti-goat IgG, mixing with the solution of goat IgG molecules, both before and after mixing, respectively. The aggregation of the beads is an indicator of successful binding of goat IgG molecules to the surface of the beads. The accumulation of IgG molecules on the bead surface results in cross-linking of the beads to each other. Qualitatively, the fluorescence intensity of the fluid itself also decreases as we move further downstream the mixer as seen when comparing the background intensity of Fig. 2A.
In order to quantify the effectiveness of the depletion using micromixing, we developed a fluorescent readout test bed using a 96 well plate and a fluorescent plate reader. We performed titration of the fluorescent goat IgG to obtain a standard curve. Using the adapted herringbone serpentine channel (after removing the beads), we achieved depletion of Immunoglobulin G down to 35 nM, yielding a depletion efficiency of 95%.
As a control experiment, we compared the depletion efficiency from the bead-based separation to the depletion achievable using standard bead based anti-IgG columns. For this control procedure, the beads were loaded in an Eppendorf tube, and magnetic separation was used to remove the initial supernatant. The starting IgG solution was then injected into the Eppendorf tube and thoroughly mixed with the beads for at least 3 h to ensure sufficient time for maximum capture of target protein. Subsequently magnetic separation was used again to separate the beads from the supernatant. Final IgG levels were quantified using fluorescent readout as described earlier (Fig. 2B).
Depletion of the cells and the protein-coated beads is the next step in the sample preparation. A filter trench is used to trap the cells and beads from the first component as the serum passes through. The idea of using a filter trench for extracting cells from blood was previously presented by Lee et al. [31]. Here, we aim to significantly enhance the trapping of the cells and beads in the filter trench by integrating a pair of interdigitated electrodes at the top of the channel above the trench (Fig. 1B) to use negative DEP to push all of the micron sized particles (cells and beads that have captured the highly abundant proteins) down into the trench. Lowabundance serum proteins are able to flow through the trench and are unaffected by the negative dielectrophoretic forces. For a given trench and channel geometry the presence of the negative DEP force will enhance the particle filtration, allowing for higher flow rates and thus higher throughput.
5. Enhanced dielectrophoresis (DEP) for particle depletion
Traditionally, electrokinetic techniques such as dielectrophoresis are characterized by relatively weak forces (less than 10 pN) [34,35] which are, often insufficient for separation processes. The DEP force is given by the following equation to first order:
where εm is the relative permittivity of the surrounding medium, r is the particle radius, and ERMS is the root-mean-square value of the electric field. fCM in the above equation is the Clausius–Mossotti (CM) factor which is a function of the polarizability of the particle and the polarizability of the medium. fCM is of the form
| (2) |
where are the relative complex permittivities of the particle and the medium, respectively. In order to achieve negative dielectrophoresis, to push the particles or cells downward, we operate in a region where the Clausius–Mossotti factor is negative. Depending on the conductivity of the buffer, this will vary for both cells and beads. In our previous work [35], we calculated the DEP spectrum and determined that for a buffer conductivity greater than 5 × 10−4 S/m for polystyrene beads (dielectric constant of 2.5), the CM factor will be negative across the whole frequency spectrum. Castellarnau et al. [36] also calculated the DEP spectrum for bacterial cells in buffers of various conductivities, and showed that for conductivities greater than 0.1 S/m, at f = 1 MHz, the CM factor will be negative. Here for proof of concept, we focus on demonstrating depletion of 6.8 μm for polystyrene beads (dielectric constant 2.5, conductivity 0.2 mS/m) from DI water (1 × 10−3 S/m).
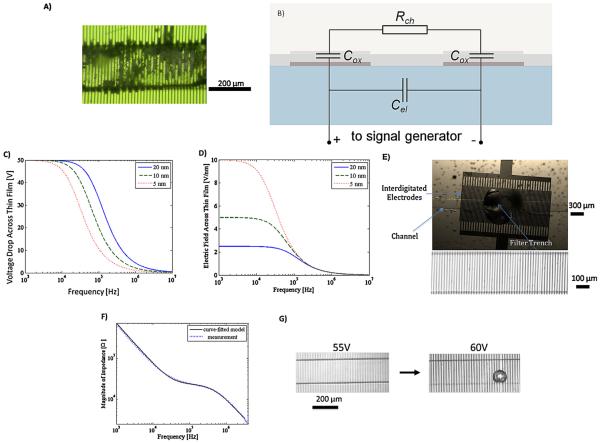
A fourfold increase in the DEP force in the microchannel is possible by merely doubling the voltage applied between the electrodes, in accordance with the quadratic relationship between the DEP force and ERMS. There is, however, a limit to how much static voltage at an electrode–electrolyte interface can be applied before the electrodes become corroded and damaged, especially if the electrodes lack a passivating layer. The range of applied voltages can be extended somewhat by the use of a time varying signal. For example, we have determined through experimentation that at a frequency of 1 MHz, the electrochemical breakdown of gold electrodes occurs at 20 V peak-to-peak voltage for bare gold electrodes. Fig. 4A shows an image of damaged bare gold electrodes where a 15 V AC signal has been applied. Platinum microelectrodes have shown to improve electrochemical stability compared to gold electrodes over a broad frequency range [37].
Fig. 4.
(A) Figure of bare interdigitated electrodes showing corrosion after applying 15 V 1 MHz AC voltage. (B) Equivalent circuit model of electrode/electrolyte interface. (C) Plot of the calculated percentage of voltage drop across the oxide. (D) Plot of the calculated electric field level across the oxide. At high frequencies (>1 MHz), the majority of the drop is across the electrolyte, and the electric field level decreases to a level below the oxide breakdown level independent of the oxide thickness. (E) (Top) Micrograph of bottom side view of 300 μm diameter trench embedded in micro-channel with interdigitated electrodes integrated on top wall of micro-channel. (Bubbles are from the glue that was used to bond the back of the glass chip to the microscope slide and do not interfere with the experiment.) The filter trench looks like a big bubble, however this is not a bubble. The filter trench is a hole that is punched through the PDMS substrate. (Bottom figure) Micrograph of channel with interdigitated electrodes (7 μm wide) with 10 nm layer of SiO2 patterned on glass chip. (F) Impedance spectrum measured vs. modeled for interdigitated electrodes, confirming that above 100 kHz, the insulative layer is electrically shorted, and DEP degradation is minimized. (G) Microscope image of ALD passivated interdigitated electrodes withstanding voltages as high as 60 V. Beyond 60 V, bubbles emerge at the surface of the electrode, most likely due to outgassing of CO2.
Deposition of a passivation layer onto the electrode surface helps protect the underlying metallic electrode from electrochemical corrosion and damage, thereby increasing the useful lifespan of the electrode. The passivation layer thickness should be minimized to reduce the voltage drop in the passivation film. However, deposition of pinhole-free, ultra-thin insulating films on metallic substrates using conventional deposition techniques such as Plasma Enhanced Chemical Vapor Deposition (PECVD) has proven challenging.
Here we explore atomic layer deposition (ALD) to deposit pinhole-free oxides as thin as 10 nm. The main concern with applying high electric fields across an extremely thin layer is the occurrence of oxide breakdown. This is generally the case when applying DC fields where 100% of the voltage drop occurs across the oxide. However in the case of an AC signal, the voltage drop occurs over the oxide film and across the electrolyte double layer, with the ratio of the fields in the two regions determined by the frequency of the AC signal. In order to quantify the voltage drop across each region, we have developed an equivalent circuit model of the interdigitated electrodes/insulator/electrolyte interface as shown in Fig. 4B. The impedance of the bulk conducting medium in the electrolyte has been modeled as a resistance (R). The impedance of the electrode/insulator/electrolyte system is represented as a parasitic capacitance (Cox). The fringing field in between the electrodes also results in an additional parasitic capacitance (Cel). Thus, a voltage divider forms at the oxide capacitances at each terminal with the solution buffer resistance resulting in an unwanted voltage drop across the oxide films at each end. This results in a drop in the available DEP force being exerted in the channel. The parasitic oxide capacitance shorts at a high frequency AC signal such that the majority of the voltage drop occurs across the electrolyte resistor. This condition becomes feasible when the oxide is sufficiently thin, yielding a large Cox value. In Fig. 4C, the percentage of voltage drop across the oxide is plotted as a function of frequency for different oxide thicknesses. The relative dielectric constant for SiO2 is 3.9 and we assume the conductivity to be negligible. As frequency increases, the voltage drop across the oxide increases to the extent that beyond 1 MHz, less than 5% of the voltage drops across a 10 nm oxide. The other condition, which is equally important, requires that the field across the oxide is minimal, to prevent oxide breakdown from occurring. As shown in Fig. 4D, where electric field across the oxide verses frequency is plotted, at sufficiently high frequency the electric field for different oxide thicknesses converges to a minimal value (0.2 V/nm), which is well below our ALD oxide (SiO2) breakdown field of 1 V/nm [38]. Therefore, oxide breakdown would not directly set the lower limit on the required thickness.
Both Fig. 4C and D indicate that high frequency operation is desirable. However, if the operating frequency is too large (>10 MHz) then the electrode capacitance (Cel) will also be short, resulting in no voltage drop across the solution. Given the strong electric fields created in the system, local temperature rise along the microelectrodes due to joule heating will occur [39,40]. We have also discussed the thermal behavior and have performed detailed thermal analysis of uDEP microelectrodes in a separate work [41]. For this work, to facilitate microscopic imaging for characterization of bead depletion, we were limited to use glass as our substrate because of its transparency. In this case where we apply 40 V across our electrodes, the result is a rise in temperature of less than 30 °C, which we measured using infrared (IR) microscopy. However, in the practical setting we can use a more thermally conductive substrate such as silicon, thus reducing the undesirable temperature increase to a few degrees Celsius.
6. Materials and methods for uDEP for particle depletion
6.1. Fabrication of chips
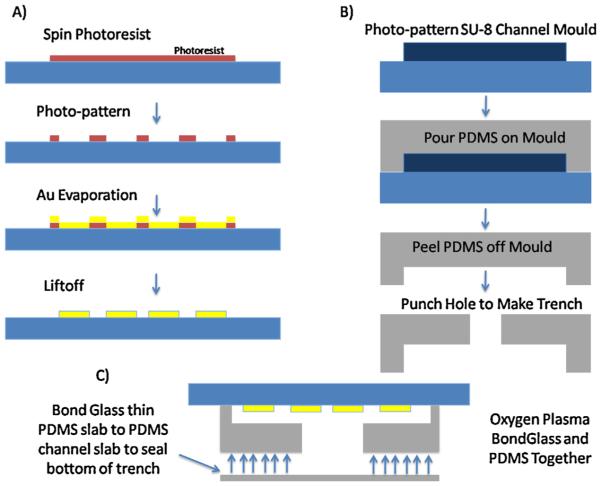
The electrodes depicted in Fig. 4E are fabricated using the process flow depicted in Fig. 3A. The photoresist is micro-patterned on a glass wafer using standard photolithography techniques. Thin films of chromium (50 Å ) and gold (1500 Å ) are deposited on the glass wafer by evaporation using the photoresist pattern as a mask. Liftoff removes the extra photoresist and preserves the interdigitated pattern of the electrodes on the glass wafer surface. Atomic layer deposition is used to coat the electrodes with a 10 nm layer of passivating SiO2. The bond pads for electrical connection to the electrodes are exposed via subsequent photolithography and dry etch of the underlying oxide film. Finally, the wafer is then diced into individual chips using a wafer saw.
Fig. 3.
Fabrication process for cell and particle depletion component of sample preparation platform. (A) Process flow diagram for fabricating interdigitated electrodes on glass substrate. Photoresist is micropatterned on glass. Gold and chromium (for adhesion) are deposited on the surface using e-beam evaporation. Liftoff is performed to remove unwanted electrode regions. (B) Soft lithography used to pattern channel in PDMS. Hole is punched through the top of the channel to make a filter trench. (C) PDMS and glass are treated with oxygen plasma, then aligned, and bonded together.
6.2. Microchannel fabrication
The microchannels are fabricated using standard soft lithography processing (Fig. 3B). The master mold of the microchannel is fabricated using SU-8. PDMS, mixed with primer (10:1 ratio), is poured onto the master mold and cured for 2 h at 70 °C, and then peeled off. Inlet and outlet holes are punched into the PDMS chip using a hole puncher tool (Technical Innovations, LLC, Brazoria, TX, USA). Another 300 μm hole was punched into the center of the channel to create a hole for the filter trench. The PDMS chips and the electrode/glass chips are both treated with oxygen plasma and then aligned under a microscope and covalently bonded together (Fig. 3C). Another thin slab of PDMS was bonded on top of the PDMS channel layer in order to seal the bottom of the filter trench. Fig. 4E shows a micrograph of the interdigitated electrodes integrated with single channel PDMS microfluidic device, where a filter trench had been manually punched into the PDMS chip. Fluid flow is provided in the channel using a syringe pump (Harvard Apparatus Pump 11 Plus).
6.3. Particle preparation
For demonstrating proof of concept of using ultra-DEP for particle depletion, we use 6.8 μm polystyrene beads (Spherotech, IL, USA). The beads were extracted from the stock solution, washed three times, and resuspended in DI water.
7. Results and discussion for particle depletion
The electrochemical response of the fabricated interdigitated electrode structures was characterized using impedance spectroscopy (Fig. 4F). AC signals were generated using Agilent 33220a function generator in conjunction with a Trek 2100 HF high voltage amplifier (Trek Inc., New York, USA). Below 10 kHz, the impedance of the system is predominantly capacitive and the oxide capacitance dominates the impedance since the majority of the voltage drop is across the oxide. Between 50 and 700 kHz, the oxide capacitance is shorted and the primary voltage drop occurs across the electrolyte. Beyond 700 kHz the electrode capacitance shorts, resulting in a decrease in the system impedance with frequency. We choose 1 MHz as an optimum frequency of operation since the frequency dependence of the dielectric permittivity of the beads and the medium indicates that around 1 MHz the CM factor is negative. This meets both requirements of achieving DEP in its negative form and electrically shorting the undesired oxide capacitance. The ALD passivated interdigitated electrodes remained stable up to 60 Vrms/pp AC voltage (Fig. 4G). The formation of the bubble may be attributed to the generated heat in the channel, which results in outgassing or evaporation phenomena, as the temperature approaches the boiling point.
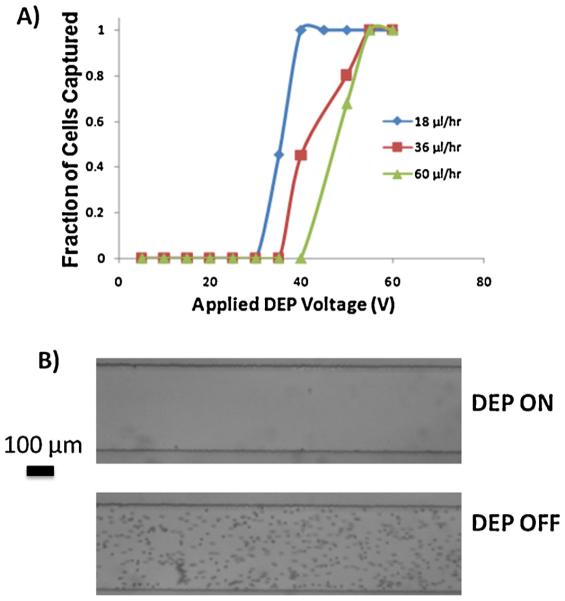
In order to test the operation of the enhanced DEP filter trench, we injected 6.8 μm polystyrene beads into the channel at various flow rates to test the capture efficiency of the filter trench. We did this over a wide range of voltages (0–60 V) until 100% capture of beads in the trench was achieved (Fig. 5A). Fig. 5B shows two images of the channel downstream from the filter trench. Application of 60 V at 1 MHz to a 210 μ/h flow rate results in 100% particle depletion (Fig. 5B, top image), whereas all of the particles pass through the capture region when the field is off (Fig. 5B, bottom image).
Fig. 5.
(A) Fraction of beads captured in the filter trench as a function of applied nDEP voltage for various flow rates. (B) Optical micrograph of channel downstream of the pore. Turning DEP on results in 100% depletion of beads, whereas when DEP is off all beads pass over the pore.
8. Conclusion
We have demonstrated the proof of concept for a two-component sample preparation platform for depleting cells and high abundance proteins from blood. The advantage of this approach lies in the ability to make this miniaturized, handheld, and integrated onto a single chip with a readout platform. This approach displaces the need for bulky laboratory equipment such as centrifuges and protein purification columns. The mixing and depletion of proteins were performed using a herringbone mixer and the process of cell and bead depletion was achieved using a negative DEP enhanced filter trench. The use of negative DEP for enhancement of particle trapping would not have been possible were it is not for the 36× increase in DEP strength resulting from the use of ALD protected electrodes. The work performed in this study can be extended to various biological applications such as the multiplexed detection of protein biomarkers and also point-of-use detection of bio-threat agents. Further work can be performed to develop new optimized structures to further optimize capture rate and operate at even higher flow rates and thus higher throughput. Further work will be carried out to improve protein depletion levels by experimenting with various bead sizes, flow rates, and herringbone geometries. Also, as mentioned, given the portability and miniaturized nature of this sample prep platform, one can envision developing a platform integrated with an optical or electronic biosensor for a fully micro total analysis system. We believe that our sample preparation platform results strongly motivate such effort, given the advantages of this platform over standard laboratory based instrumentation. This work provides a strong starting point for a new class of handheld point-of-use diagnostic platforms.
His research interests include micro electromechanical system (MEMS) design, micro/nanomachining processes, and self-assembly processes. A major focus of his research from the early 1980s until recently was technologies for integrated microsystems, which incorporate both silicon integrated circuits and micromechanical structures. Recently, his research has shifted to nanoelectromechanical systems (NEMS), for applications ranging from chemical sensors to relays and logic devices. Prof. Howe has made contributions to the design of MEMS accelerometers, gyroscopes, electrostatic actuators, and microresonators. He was elected an IEEE Fellow in 1996, was co-recipient of the 1998 IEEE Cledo Brunetti Award, and was elected to the U.S. National Academy of Engineering in 2005 for his contributions to MEMS processes, devices, and systems. He was a co-founder of Silicon Clocks, Inc., a start-up company that commercialized poly-SiGe integrated MEMS-on-CMOS for timing applications, which was acquired by Silicon Laboratories, Inc., in April 2010.
In December 2009, he became the Faculty Director of the Stanford Nanofabrication Facility. In February 2011, he became the Stanford Site Director of the National Nanotechnology Infrastructure Network (NNIN) and in September 2011, the Director of the NNIN.
Acknowledgements
This research was supported by the Department of Defense Grant: DARPAICLF10-56. Fabrication of the devices was performed in the Stanford Nanofabrication Facility.
Biographies
Mehdi Javanmard is a Senior Research Engineer at the Stanford Genome Technology Center (SGTC) in the Department of Biochemistry at Stanford University. He received his BS (2002) from Georgia Institute of Technology where he received the Outstanding Undergraduate Research Award. He received his MS in Electrical Engineering at Stanford University (2004) working at Stanford Linear Accelerator Center researching the use of photonic nanostructures for high energy physics. In 2008, he received his PhD in Electrical Engineering at Stanford University working on development of electronic microfluidic platforms for low cost genomic and proteomic biomarker detection. At SGTC, he worked as a postdoctoral scholar from 2008 to 2009, and then as a staff engineering research associate from 2009 to 2012. He was the recipient of the IEEE Sensors Conference 2013 Best Paper Award. His interests lie in the exploitation of emerging micro- and nanotechnologies, electronic, micromechanical and photonic techniques for developing rapid and low cost technologies for point-of-care diagnostics, proteomic biomarker discovery, global health, and drug screening.
Sam Emaminejad received his BASc (2009) and MS (2011) degrees in electrical engineering from the University of Waterloo and Stanford University, respectively. He is pursuing his PhD in electrical engineering at Stanford University where he is working toward his thesis at Stanford Genome Technology Center. His current research is focused on design and implementation of a low-cost ultra-sensitive electronic multiplexed protein biosensor and he is involved with development of a quantum tunneling spectroscopy platform for label- and probe-free biomolecular sensing. Sam has previously worked as an ASIC and Analog Designer in semi-conductor companies such as STMicroelectronics and Analog Devices and is the co-author of Supplemental Problems and Solutions Manual for Microelectronic Circuits (Sedra/Smith, 2013). Sam was the recipient of the Best Paper Award of the IEEE Sensors conference in 2013.
Chaitanya Gupta received his BTech in Chemical Engineering in 2001 from the Indian Institute of Technology in 2001. After a brief stint in industry, he joined the PhD program at the University of Illinois at Urbana-Champaign, and received his MS and PhD in Chemical Engineering in 2007 and 2009, respectively. He holds a MS in Physics, also from the University of Illinois (received in 2009). Currently, he is a research associate at Stanford University and his research interests are in engineering meso-scale platforms for applications in sensing and electronics.
J. Provine received his BA in physics in 1998, the BS and MS degrees in electrical engineering from Rice University, Houston, TX, in 1999, and the PhD in electrical engineering from Cornell University, Ithaca, NY, in 2005. He is currently a Senior Research Associate in the Department of Electrical Engineering at Stanford University, Stanford, CA. His research interests include atomic layer deposition, nanofabrication, surface-mode photonics, NEMS, and surface force probes.
Ronald Davis received his BS in Mathematics, Physics, Chemistry, and Botany from East Illinois University in 1964, and the PhD in Chemistry from California Institute of Technology in 1970. He is considered to be a world leader in biotechnology, and the development and application of recombinant DNA and genomic methodology to bio-logical systems. His laboratory has developed many of the techniques currently used in academic and industrial biotechnology laboratories. He is also considered to be a world expert in the electron microscopy of nucleic acids and has developed many of the mapping methods for which he received the Eli Lilly Award in Microbiology in 1976. His laboratory was also instrumental in the development of lambda vectors, which were commonly used for the primary cloning of DNA molecules in E. coli. His laboratory also developed many of the yeast vectors and helped to develop yeast as a host for recombinant DNA for which he received the United States Steel Award in 1981, presented by the National Academy of Sciences. In 1983 he became a member of The National Academy of Sciences. He was a co-author on a publication that first described a new approach for conducting human genetics and for the construction of a human genetic linkage map for which he received the Rosenstiel Award for Work in Basic Medical Research. His laboratory is now conducting genomic analysis of Saccharomyces cerevisiae for which he received the 2004 Lifetime Achievement Award from the Yeast Genetics Society. His laboratory is developing many new technologies for the genetic, genomic, and molecular analysis of model organisms and humans with a focus on clinical medicine for which he received the 2004 Sober Award from the American Society for Biochemistry and Molecular Biology (ASBMB/IUBMB).
Roger T. Howe is the William E. Ayer Professor in the Department of Electrical Engineering at Stanford University, as well as the Faculty Director of the Stanford Nanofabrication Facility. He earned a BS degree in physics from Harvey Mudd College, Claremont, California, and an MS and PhD in electrical engineering from the University of California, Berkeley in 1981 and 1984. After faculty positions at Carnegie-Mellon University in 1984–1985 and the Massachusetts Institute of Technology in 1985–1987, he returned to Berkeley where he was a professor until 2005.
Footnotes
Selected Paper Presented at The 17th International Conference on Solid-State Sensors, Actuators and Microsystems, June 16–20, 2013, Barcelona, Spain.
References
- [1].Bracke S, Speeckaert R, Van Geel N, De Bacquer D, Lambert J. Evaluation of commercially available ELISA assays as a tool for monitoring and managing pemphigus patients: a prospective study. European Journal of Dermatology. 2013;23(1):33–39. doi: 10.1684/ejd.2012.1901. [DOI] [PubMed] [Google Scholar]
- [2].Chang C, Middeldorp J, Yu KJ, Juwana H, Hsu WL, Lou PJ, Wang CP, Chen JY, Liu MY, Pfeiffer RM, Chen CJ, Hildesheim A. Characterization of ELISA detection of broad-spectrum anti-Epstein-Barr virus antibodies associated with nasopharyngeal carcinoma. Journal of Medical Virology. 2012;85(3):524–529. doi: 10.1002/jmv.23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Esfandyarpour R, Esfandyarpour H, Javanmard M, Harris JS, Davis RW. MRS Proceedings. Vol. 1414. Cambridge Univ Press; 2012. Electrical detection of protein biomarkers using nanoneedle biosensors. [Google Scholar]
- [4].Esfandyarpour R, Esfandyarpour H, Javanmard M, Harris JS, Davis RW. Microneedle biosensor: a method for direct label-free real time protein detection. Sensors and Actuators B: Chemical. 177(2012):848–855. doi: 10.1016/j.snb.2012.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Esfandyarpour R, Javanmard M, Koochak Z, Esfandyarpour H, Harris JS, Davis RW. MRS Proceedings. Vol. 1572. Cambridge Univ Press; 2013. Thin film nanoelectronic probe for protein detection. [Google Scholar]
- [6].Javanmard M, Esfandyarpour H, Pease F, Davis RW. Electrical detection of proteins and DNA using bioactivated microfluidic channels: theoretical and experimental considerations. Journal of Vacuum Science & Technology B. 2009;27(6):3099–3103. doi: 10.1116/1.3264675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Javanmard M, Talasaz AH, Nemat-Gorgani M, Huber DE, Pease F, Ronaghi M, Davis RW. A microfluidic platform for characterization of protein–protein interactions. Sensors Journal IEEE. 2009;9(8):883–891. doi: 10.1109/JSEN.2009.2022558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Javanmard M, Talasaz AH, Nemat-Gorgani M, Pease F, Ronaghi M, Davis RW. Targeted cell detection based on microchannel gating. Biomicrofluidics. 2007;1:044103. doi: 10.1063/1.2815760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Javanmard M, Talasaz AH, Nemat-Gorgani M, Pease F, Ronaghi M, Davis RW. Electrical detection of protein biomarkers using bioactivated microfluidic channels. Lab on a Chip. 2009;9(10):1429–1434. doi: 10.1039/b818872f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Javanmard M, Talasaz AH, Nemat-Gorgani M, Ronaghi M, Pease F, Davis RW. Early diagnosis of cancer by electrical detection of nucleic acid biomarkers; Solid-State Sensors, Actuators and Microsystems Conference 2009. TRANSDUC-ERS 2009, International, IEEE.2009. pp. 947–950. [Google Scholar]
- [11].Esfandyarpour R, Javanmard M, Koochak Z, Esfandyarpour H, Harris JS, Davis RW. Label-free electronic probing of nucleic acids and proteins at the nanoscale using the nanoneedle biosensor. Biomicrofluidics. 2013;7:044114. doi: 10.1063/1.4817771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Esfandyarpour R, Javanmard M, Koochak Z, Harris JS, Davis RW. Nanoelectronic impedance detection of target cells. Bioengineering and Biotechnology. 2013 doi: 10.1002/bit.25171. http://dx.doi.org/10.1002/bit.25171 [DOI] [PubMed] [Google Scholar]
- [13].Esfandyarpour R, Esfandyarpour H, Harris JS, Davis RW. Simulation and fabrication of a new novel 3D injectable biosensor for high throughput genomics and proteomics in a lab-on-a-chip device. Nanotechnology. 2013;24(46):465301. doi: 10.1088/0957-4484/24/46/465301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].MacBeath G. Proteomics comes to the surface. Nature Biotechnology. 2001;19(9):828–829. doi: 10.1038/nbt0901-828. [DOI] [PubMed] [Google Scholar]
- [15].MacBeath G. Protein microarrays and proteomics. Nature Genetics. 2002;32(Suppl.):526–532. doi: 10.1038/ng1037. [DOI] [PubMed] [Google Scholar]
- [16].Andersen JD, Boylan KL, Xue FS, Anderson LB, Witthuhn BA, Markowski TW, Higgins L, Skubitz AP. Identification of candidate biomarkers in ovarian cancer serum by depletion of highly abundant proteins and differential in-gel electrophoresis. Electrophoresis. 2010;31(4):599–610. doi: 10.1002/elps.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mahn A, Ismail M. Depletion of highly abundant proteins in blood plasma by ammonium sulfate precipitation for 2D-PAGE analysis. Journal of Chromatography B. 2011;879(30):3645–3648. doi: 10.1016/j.jchromb.2011.09.024. [DOI] [PubMed] [Google Scholar]
- [18].Mahn A, Reyes A, Zamorano M, Cifuentes W, Ismail M. Depletion of highly abundant proteins in blood plasma by hydrophobic interaction chromatography for proteomic analysis. Journal of Chromatography B. 2010;878(15–16):1038–1044. doi: 10.1016/j.jchromb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- [19].Ringrose JH, van Solinge WW, Mohammed S, O’Flaherty MC, van Wijk R, Heck AJ, Slijper M. Highly efficient depletion strategy for the two most abundant erythrocyte soluble proteins improves proteome coverage dramatically. Journal of Proteome Research. 2008;7(7):3060–3063. doi: 10.1021/pr8001029. [DOI] [PubMed] [Google Scholar]
- [20].Serohijos AW, Rimas Z, Shakhnovich EI. Protein biophysics explains why highly abundant proteins evolve slowly. Cell Reports. 2012;2(2):249–256. doi: 10.1016/j.celrep.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Herr AE, Molho JI, Drouvalakis KA, Mikkelsen JC, Utz PJ, Santiago JG, Kenny TW. On-chip coupling of isoelectric focusing and free solution electrophoresis for multidimensional separations. Analytical Chemistry. 2003;75(5):1180–1187. doi: 10.1021/ac026239a. [DOI] [PubMed] [Google Scholar]
- [22].Bahga SS, Chambers RD, Santiago JG. Coupled isotachophoretic preconcentration and electrophoretic separation using bidirectional isotachophoresis. Analytical Chemistry. 2011;83(16):6154–6162. doi: 10.1021/ac200268f. [DOI] [PubMed] [Google Scholar]
- [23].Bahga SS, Kaigala GV, Bercovici M, Santiago JG. High-sensitivity detection using isotachophoresis with variable cross-section geometry. Electrophoresis. 2011;32(5):563–572. doi: 10.1002/elps.201000338. [DOI] [PubMed] [Google Scholar]
- [24].Bercovici M, Kaigala GV, Mach KE, Han CM, Liao JC, Santiago JG. Rapid detection of urinary tract infections using isotachophoresis and molecular beacons. Analytical Chemistry. 2011;83(11):4110–4117. doi: 10.1021/ac200253x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bercovici M, Kaigala GV, Santiago JG. Method for analyte identification using isotachophoresis and a fluorescent carrier ampholyte assay. Analytical Chemistry. 2010;82(5):2134–2138. doi: 10.1021/ac9025658. [DOI] [PubMed] [Google Scholar]
- [26].Bercovici M, Lele SK, Santiago JG. Compact adaptive-grid scheme for high numerical resolution simulations of isotachophoresis. Journal of Chromatography A. 2010;1217(4):588–599. doi: 10.1016/j.chroma.2009.11.072. [DOI] [PubMed] [Google Scholar]
- [27].Chambers RD, Santiago JG. Imaging and quantification of isotachophoresis zones using nonfocusing fluorescent tracers. Analytical Chemistry. 2009;81(8):3022–3028. doi: 10.1021/ac802698a. [DOI] [PubMed] [Google Scholar]
- [28].Chen GD, Alberts CJ, Rodriguez W, Toner M. Concentration and purification of human immunodeficiency virus type 1 virions by microfluidic separation of superparamagnetic nanoparticles. Analytical Chemistry. 2010;82(2):723–728. doi: 10.1021/ac9024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fan R, Vermesh O, Srivastava A, Yen BK, Qin L, Ahmad H, Kwong GA, Liu CC, Gould J, Hood L, Heath JR. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nature Biotechnology. 2008;26(12):1373–1378. doi: 10.1038/nbt.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang J, Ahmad H, Ma C, Shi QH, Vermesh O, Vermesh U, Heath J. A self-powered, one-step chip for rapid, quantitative and multiplexed detection of proteins from pinpricks of whole blood. Lab on a Chip. 2010;10(22):3157–3162. doi: 10.1039/c0lc00132e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dimov IK, Basabe-Desmonts L, Garcia-Cordero JL, Ross BM, Park Y, Ricco AJ, Lee LP. Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS) Lab on a Chip. 2011;11(5):845–850. doi: 10.1039/c0lc00403k. [DOI] [PubMed] [Google Scholar]
- [32].Gadish N, Voldman J. High-throughput positive-dielectrophoretic bioparticle microconcentrator. Analytical Chemistry. 2006;78(22):7870–7876. doi: 10.1021/ac061170i. [DOI] [PubMed] [Google Scholar]
- [33].Stroock AD, Dertinger SK, Ajdari A, Mezic I, Stone HA, White-sides GM. Chaotic mixer for microchannels. Science. 2002;295(5555):647–651. doi: 10.1126/science.1066238. [DOI] [PubMed] [Google Scholar]
- [34].Voldman J, Braff RA, Toner M, Gray ML, Schmidt MA. Holding forces of single-particle dielectrophoretic traps. Biophysical Journal. 2001;80(1):531–541. doi: 10.1016/S0006-3495(01)76035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Javanmard M, Emaminejad S, Dutton RW, Davis RW. Use of negative dielectrophoresis for selective elution of protein-bound particles. Analytical Chemistry. 2012;84(3):1432–1438. doi: 10.1021/ac202508u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Castellarnau M, Errachid A, Madrid C, Juarez A, Samitier J. Dielectrophoresis as a tool to characterize and differentiate isogenic mutants of Escherichia coli. Biophysical Journal. 2006;91(10):3937–3940. doi: 10.1529/biophysj.106.088534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sukhorukov VL, Kürschner M, Dilsky S, Lisec T, Wagner B, Schenk WA, Benz R, Zimmermann U. Phloretin-induced changes of lipophilic ion transport across the plasma membrane of mammalian cells. Biophysical Journal. 2001;81(2):1006–1013. doi: 10.1016/S0006-3495(01)75758-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Won S-J, Suh S, Huh MS, Kim HJ. High-quality low-temperature silicon oxide by plasma-enhanced atomic layer deposition using a metal–organic silicon precursor and oxygen radical, Electron Device Letters. IEEE. 2010;31(8):857–859. [Google Scholar]
- [39].Khoshmanesh K, Zhang C, Tovar-Lopez FJ, Nahavandi S, Baratchi S, Mitchell A, Kalantar-Zadeh K. Dielectrophoretic-activated cell sorter based on curved microelectrodes. Microfluidics and Nanofluidics. 2010;9(2–3):411–426. [Google Scholar]
- [40].Wlodkowic D, Khoshmanesh K, Sharpe JC, Darzynkiewicz Z, Cooper JM. Apoptosis goes on a chip: advances in the microfluidic analysis of programmed cell death. Analytical Chemistry. 2011;83(6):2133–2144. doi: 10.1021/ac200588g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Emaminejad S, Javanmard M, Dutton RW, Davis RW. Ultra dielectrophoresis using atomic layer deposited films: electrothermal analysis. Electrochemical Society Transactions. 2013;58(10):67–72. [Google Scholar]