Abstract
Background and purpose
Many dose-limiting normal tissues in radiotherapy (RT) display considerable internal motion between fractions over a course of treatment, potentially reducing the appropriateness of using planned dose distributions to predict morbidity. Accounting explicitly for rectal motion could improve the predictive power of modelling rectal morbidity. To test this, we simulated the effect of motion in two cohorts.
Materials and methods
The included patients (232 and 159 cases) received RT for prostate cancer to 70 and 74 Gy. Motion-inclusive dose distributions were introduced as simulations of random or systematic motion to the planned dose distributions. Six rectal morbidity endpoints were analysed. A probit model using the QUANTEC recommended parameters was also applied to the cohorts.
Results
The differences in associations using the planned over the motion- inclusive dose distributions were modest. Statistically significant associations were obtained with four of the endpoints, mainly at high doses (55–70 Gy), using both the planned and the motion-inclusive dose distributions, primarily when simulating random motion. The strongest associations were observed for GI toxicity and rectal bleeding (Rs=0.12–0.21; Rs=0.11–0.20). Applying the probit model, significant associations were found for tenesmus and rectal bleeding (Rs=0.13, p=0.02).
Conclusion
Equally strong associations with rectal morbidity were observed at high doses (>55 Gy), for the planned and the simulated dose distributions including in particular random rectal motion. Future studies should explore patient-specific descriptions of rectal motion to achieve improved predictive power.
Keywords: organ motion, morbidity, rectum, prostate cancer, radiotherapy
Introduction
The process of establishing dose/volume parameters being predictive for normal tissue morbidity following radiotherapy (RT) is likely to be influenced by the geometric uncertainties of the concerned organ, with potential implications for the estimated dose/volume parameters [1–4]. One of the key organs at risk (ORs) in RT of prostate cancer, the rectum, displays considerable motion during the course of treatment [2–5]. Studies of the dose/volume response of rectal morbidity have usually associated ‘static’ dose distributions, obtained from the treatment planning computer tomography (CT) scan, with rectal bleeding or gastro-intestinal (GI) toxicity [2]. For RTOG GI toxicity [6] we have previously investigated the associations instead using motion-inclusive dose distributions [3, 4, 7, 8]. In one of these studies motion-inclusive dose distributions were obtained by introducing simulations of (rigid, translational) motion of the planning CT organ geometry, moving within the planned dose distribution [8]. Different associations compared to the associations observed using the static dose distribution were found [8]. However accurate interpretation of these results would likely require investigation in independent patient series [1, 2, 9].
In this study we further investigate whether accounting for rectal motion improves the predictive power of modelling rectal morbidity. More specifically we explore the associations between motion-inclusive rectal dose distributions, obtained from a refined version of our previously proposed motion analysis, and several late rectal morbidity endpoints in two cohorts of patients treated with RT for prostate cancer.
Material and Methods
Motion model
Organ motion was simulated as rigid translations of random or systematic errors/“shifts”, assumed being normally distributed. Parameters chosen for the motion analyses in this study were based on the findings from our previous investigation, as applied to the rectum [8]. In the present study motion was repeatedly simulated as multiple pseudo trials (n=10) over the treatment fractions (35 and 37 fractions corresponding to the fractionation scheme in each cohort; see Patient cohorts below) for each patient in the anterior-posterior direction. Small, intermediate and large motion magnitudes were explored by altering the standard deviation (SD, σ = 0.2, 0.5 and 0.8 cm) of the related normal distribution. The simulations were performed with the Computational Environment for Radiotherapy Research software [10] using a 2.4 GHz Intel Core 2 Duo processor Macintosh HD.
Patient cohorts
The motion model was applied to the rectum (the original delineations extending from the recto-sigmoid flexure to the anal verge, including contents) in two series of patients previously treated with photon RT for prostate cancer. One of these cohorts included 232 patients treated at Haukeland University Hospital (HUH) in Bergen, Norway from 2000 to 2001 [7, 8, 11]. These patients received RT to either the prostate only, to the prostate and seminal vesicles or to also the pelvic lymph nodes (initial 50 Gy), to a total dose of 70 Gy delivered in 35 fractions of 2 Gy. In addition we applied the motion model to another cohort including 159 patients that received RT to 74 Gy to the prostate only or to also the pelvic lymph nodes (44 or 46 Gy) over 37 fractions of 2 Gy from 2002 to 2006 at the Fraser Valley Centre, British Columbia Cancer Agency (BCCA), Canada [12] (Table S1, Supplementary material). In the following, the cohorts are referred to as the HUH and the BCCA cohort. The rectal morbidity endpoints registered in the HUH cohort included RTOG GI toxicity [6], stool frequency, mucosal loss, tenesmus, sphincter control and rectal bleeding [13] while only rectal bleeding data were available in the BCCA cohort [14] (Table 1; Table S2, Supplementary material). For the RTOG GI toxicity endpoint only the locally treated subset of the HUH patients (192 patients) were analysed since a distinction between toxicity attributed to the small bowel and the rectum is limited using this morbidity definition [6]. The morbidity scoring was performed based on physician assessments (Table S2, Supplementary material) and the median follow-up time was 60 months for the HUH patients (follow-up: every 6 months the first year and then annually) and 32 months for the BCCA patients (follow-up: every 6 months the three first years and then annually).
Table 1.
The number (%) of patients with ≥ Grade 2 morbidity for each endpoint for the HUH and the BCCA cohort.
| Endpoints
| ||||||
|---|---|---|---|---|---|---|
| ≥ Grade 2 | RTOG GI | Stool frequency | Mucosal loss | Tenesmus | Sphincter control | Rectal bleeding |
| HUH | 14 (7%) | 25 (11%) | 24 (10%) | 14 (6%) | 10 (4%) | 4 (2%) |
| BCCA | - | - | - | - | - | 12 (7.5%) |
|
| ||||||
| Grade 3 | ||||||
| HUH | - | 1 (0.4%) | 4 (2%) | - | 3 (1%) | - |
| BCCA | - | - | - | - | - | 4 (3%) |
| Grade 4 | ||||||
| HUH | 1 (0.4%) | - | - | - | - | - |
| BCCA | - | - | - | - | - | 1 (0.6%) |
Model application and statistics
For both the cohorts, the simulated motion-inclusive dose-volume histograms (simDVHs) and the planned DVH (pDVH), i.e. with no motion, were analysed. The morbidity cut-off was chosen as Grade 2 or higher (the maximum assessed grade for each of the morbidity endpoints); cf. Table 1 and Table S2, Supplementary material. Spearman’s rank correlation (Rs) was used to investigate differences between rectal dose-volume parameters, i.e. the percentage rectum volume receiving more than x Gy (Vx (%)), sampled in 5 Gy intervals from 5 Gy up to the prescribed dose. The reported Spearman’s rank correlation coefficients (Rs) were calculated as one median Rs for each patient obtained from bootstrap resampling (1000 samples). Bootstrap was also applied to assess the 95% Confidence Interval (CI) of the Rs and to correct the p-values for multiple testing in the simultaneously investigated dose/volume parameters. Following our previous study the Rs was corrected for ties and the p-values resulting from the pseudo trials were corrected for the increased certainty resulting from the increased patient population [8]. All tests were considered statistically significant on a two-sided 5% significance level (p≤0.05). The statistical analyses were performed in the Dose Response Explorer System [15].
A probit normal tissue complication probability (NTCP), the Lyman Kutcher Burman (LKB), model was applied to the pDVHs and the simDVHs, obtained from the motion pattern resulting in the largest number of Vx values being significantly associated with the investigated endpoint.
The NTCP values given in the rectal Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) paper were used for this purpose (the 50% chance of complications for uniform irradiation, TD50=76.9 Gy; the parameter describing the steepness of the dose-response, m=0.13; the volume dependence parameter, n=0.09) [2]. In a further analysis the influence of altered gEUD n values (n=0.01–1.00) on the associations with these endpoints was investigated.
Results
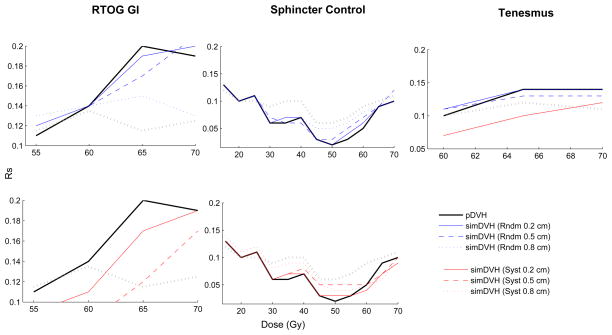
Across all investigated endpoints and motion patterns the strongest associations between the simDVHs and rectal morbidity was obtained with RTOG GI toxicity (V70 (Rs=0.21, σrandom=0.5cm)) and rectal bleeding (V35, V40 (Rs=0.20, σsystematic=0.5cm) and V65 (Rs=0.20, σrandom=0.2cm; Table 2)). Simulations of random motion resulted in a slightly broader range of dose levels being significantly associated with morbidity as compared to the use of the pDVH only: four vs. three dose levels for RTOG GI toxicity and three vs. two dose levels for tenesmus and sphincter control). The associations with morbidity were generally stronger applying random shifts relative to systematic shifts (Figure 1; Table 2; Table S3, Supplementary material).
Table 2.
Upper panel: The Rs coefficients (95% CI) and p values for the dose levels at which significant associations (p≤0.05) were obtained using the pDVH or the simDVHs (HUH cohort). Numbers in bold indicate the dose level at which the significant associations were the strongest for each motion pattern. Lower panel: Application of a probit NTCP model, using the QUANTEC recommended parameters, to the pDVH and the simDVHs (HUH cohort).
| Endpoint | Vx (Gy) | Rs (95% CI) | P |
|---|---|---|---|
| RTOG GI | |||
| pDVH | 60, 65, 70 | 0.14 (0.10–0.18), 0.20 (0.15–0.24), 0.19 (0.15–0.24) | 0.03, 0.003, 0.004 |
| simDVH (Rndm 0.2 cm) | 55, 60, 65, 70 | 0.12 (0.07–0.17), 0.14 (0.10–0.19), 0.19 (0.15–0.24), 0.20 (0.16–0.24) | 0.05, 0.02, 0.004, 0.003 |
| simDVH (Rndm 0.5 cm) | 55, 60, 65, 70 | 0.12 (0.07–0.17), 0.14 (0.10–0.19), 0.17 (0.12–0.21), 0.21 (0.17–0.26) | 0.05, 0.02, 0.009, 0.002 |
| simDVH (Rndm 0.8 cm) | 55, 60, 65, 70 | 0.13 (0.08–0.17), 0.14 (0.10–0.19), 0.15 (0.10–0.20), 0.13 (0.08–0.17) | 0.04, 0.03, 0.02, 0.04 |
| Tenesmus | |||
| pDVH | 65, 70 | 0.14 (0.11–0.18), 0.14 (0.10–0.17) | 0.01, 0.02 |
| simDVH (Rndm 0.2 cm) | 60, 65, 70 | 0.11 (0.08–0.14), 0.14 (0.11–0.18), 0.14 (0.10–0.17) | 0.05, 0.02, 0.02 |
| simDVH (Rndm 0.5 cm) | 60, 65, 70 | 0.11 (0.07–0.14), 0.13 (0.09–0.16), 0.13 (0.10–0.16) | 0.05, 0.03, 0.02 |
| Sphincter control | |||
| pDVH | 15, 25 | 0.13 (0.10–0.16), 0.11 (0.07–0.15) | 0.02, 0.04 |
| simDVH (Rndm 0.2 cm) | 15, 25 | 0.13 (0.10–0.16), 0.11 (0.07–0.15) | 0.02, 0.04 |
| simDVH (Rndm 0.5 cm) | 15, 25, 70 | 0.13 (0.11–0.16), 0.11 (0.07–0.16), 0.12 (0.08–0.16) | 0.02, 0.04, 0.03 |
| simDVH (Rndm 0.8 cm) | 15, 25, 70 | 0.13 (0.11–0.16), 0.11 (0.07–0.15), 0.12 (0.08–0.17) | 0.02, 0.05, 0.03 |
| Rectal bleeding | |||
| pDVH | 30, 35, 40, 45, 50 | 0.16 (0.12–0.20), 0.19 (0.15–0.23), 0.18 (0.13–0.23), 0.16 (0.11–0.20), 0.15 (0.10–0.19) | 0.01, 0.001, 0.003, 0.01, 0.01 |
| 55, 60, 65, 70 | 0.17 (0.13–0.21), 0.19 (0.15–0.23), 0.20 (0.15–0.24), 0.16 (0.12–0.20) | 0.01, 0.002, 0.001, 0.01 | |
| simDVH (Rndm 0.2 cm) | 30, 35, 40, 45, 50 | 0.16 (0.12–0.20), 0.19 (0.15–0.23), 0.18 (0.14–0.23), 0.16 (0.12–0.21), 0.16 (0.12–0.20) | 0.01, 0.002, 0.003, 0.01, 0.01 |
| 55, 60, 65, 70 | 0.17 (0.13–0.22), 0.19 (0.15–0.24), 0.20 (0.16–0.24), 0.16 (0.12–0.20) | 0.01, 0.002, 0.001, 0.01 | |
| simDVH (Rndm 0.5 cm) | 30, 35, 40, 45, 50 | 0.15 (0.11–0.19), 0.19 (0.14–0.23), 0.18 (0.13–0.22), 0.17 (0.13–0.22), 0.17 (0.12–0.21) | 0.01,0.002, 0.003, 0.004, 0.01 |
| 55, 60, 65, 70 | 0.18 (0.13–0.22), 0.19 (0.14–0.23), 0.19 (0.14–0.23), 0.14 (0.09–0.18) | 0.004, 0.002, 0.002, 0.02 | |
| simDVH (Rndm 0.8 cm) | 30, 35, 40, 45, 50 | 0.15 (0.11–0.19), 0.18 (0.14–0.22), 0.18 (0.14–0.23), 0.18 (0.13–0.22), 0.17 (0.12–0.22) | 0.01, 0.003, 0.003, 0.003, 0.004 |
| 55, 60, 65, 70 | 0.18 (0.13–0.22), 0.19 (0.14–0.23), 0.19 (0.14–0.23), 0.14 (0.10–0.19) | 0.003, 0.002, 0.002, 0.01 | |
| LKB (QUANTEC) | Rs (95% CI) | P | |
|
| |||
| Tenesmus | |||
| pDVH | 0.13 (0.09–0.18) | 0.02 | |
| simDVH (Rndm 0.2 cm) | 0.13 (0.09–0.17) | 0.02 | |
| Rectal bleeding | |||
| pDVH | 0.13 (0.09–0.17) | 0.02 | |
| simDVH (Rndm 0.2 cm) | 0.13 (0.09–0.17) | 0.02 | |
| simDVH (Rndm 0.8 cm) | 0.13 (0.09–0.17) | 0.02 | |
Figure 1.
The associations between the pDVH or the simDVHs and RTOG GI toxicity, sphincter control and tenesmus (Rs) plotted for the dose levels (Gy) at which significant associations were obtained. Grey dashed line indicates significance (p≤0.05 for all curves above this line).
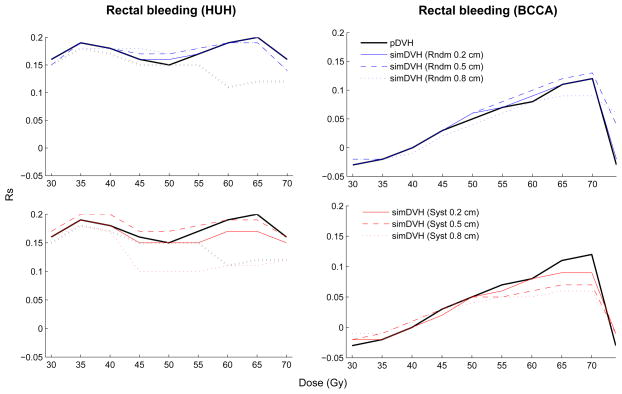
Considering the different dose levels, significant associations were found between the simDVHs and RTOG GI toxicity for random shifts at 55–70 Gy (Rs=0.13–0.21, Table 2). Significant associations (p≤0.05) were also seen at V65 and V70 (σ=0.2 cm, 0.5 cm) applying systematic shifts (Table S3, Supplementary material). For tenesmus significant associations (p≤0.05) with the simDVHs were obtained at V60, V65 and V70 (Rs=0.12–0.14) for small and intermediate random shifts and at V70 for small systematic shifts. For sphincter control associations were seen at low doses (V15–V25, Rs=0.11–0.14) for all simulated motion patterns and at one high dose level (V70, Rs=0.12) using intermediate or large random shifts. The largest number of dose levels being significantly associated both with the pDVH and the simDVHs were seen for rectal bleeding within the HUH cohort (V30–V70). No associations were obtained for rectal bleeding in the BCCA cohort (Figure 2, Table 2).
Figure 2.
The associations between the investigated dose levels and rectal bleeding using the pDVH or the simDVHs. Grey dashed line indicates significance (p≤0.05 for all curves above this line).
Introducing the probit NTCP model to the data the strongest associations were observed for tenesmus and rectal bleeding within the HUH cohort (Rs=0.13, p=0.02) and were equally strong using either the simDVHs or the pDVH (Table 2). Investigating the influence of altered gEUD n values significant associations were found for n values ranging from 0.01 to 0.16 for tenesmus and from 0.05 to 1.00 for rectal bleeding. The associations with rectal bleeding were similar using the pDVH and the simDVHs in the near vicinity of the QUANTEC recommended n value (n=0.09) whereas for tenesmus the association curves for the pDVH and the simDVHs converged at n=0.05 (Figure S1, Supplementary material).
Discussion
In this study we have applied a previously developed motion model in the setting of morbidity prediction following RT. The rectum was used as an example and the associations between the resulting simulated motion-inclusive dose distributions, simDVHs, and various rectal morbidity endpoints were explored in two patient cohorts. Associations were mainly observed in the high dose region (55–70 Gy) using the planned, pDVH, or the simDVHs resulting from simulations of random (in particular small and intermediate magnitude) motion. Among all investigated endpoints the strongest associations were obtained for RTOG GI toxicity and rectal bleeding. The most pronounced, although modest, differences in associations relative to the pDVH were also seen for RTOG GI toxicity. Overall, inclusion of simulated systematic fraction-to-fraction variations, in particular the large motion magnitudes, had no improved influence on the associations with any of the investigated morbidity endpoints.
Associations between pDVHs and late RTOG GI toxicity or rectal bleeding have mainly been observed in the high dose region (≥60 Gy) [2]. For both the pDVH and the simDVHs we observed associations between high doses and GI toxicity in our study, although using the simDVHs resulted in a somewhat broader range of dose levels being significantly associated with this endpoint. For rectal bleeding we established significant associations at both intermediate and high dose levels, however the strongest associations with the simDVHs that resulted from simulations of random motion were found at the higher dose levels, within the HUH cohort. A related association could not be established using either the simDVHs or the pDVH for the BCCA cohort. The morbidity rates for this endpoint were sparse in both cohorts yet in the high dose region fairly high Rs-values were obtained also for the BCCA cohort (strongest for V70: Rs=0.12, 0.13, p=0.06 for σrandom=0.2 cm and 0.5 cm). Applying the probit NTCP model, with the QUANTEC recommended parameters, to tenesmus or rectal bleeding (HUH cohort), equally strong associations were observed using the simDVHs and the pDVHs (Rs=0.13, p=0.02). Altering the volume dependence parameter the associations with rectal bleeding were slightly stronger using the simDVHs. Introducing the probit model with the QUANTEC recommended parameters to rectal bleeding for the BCCA cohort the strongest relationship, yet not on a 5% significance level, were seen with the simDVHs as compared to the pDVH (Rs=0.11, p=0.08 using σrandom=0.5 cm relative to Rs=0.10, p=0.11 using the pDVH). For the BCCA cohort Liu et al [12] applied a probit NTCP model to the pDVH with the QUANTEC recommended parameters and found an equivalent association (Rs=0.10, p=0.11).
In our previous study on this topic using an initial implementation of our motion model we compared the DVHs resulting from the simulated motion patterns to actually re-calculated DVHs using a repeat CT material. It was found that the difference in relative volumes averaged over the whole DVH between the simDVHs and the re-calculated DVHs ranged from 1 to 10%. The most pronounced differences were found for the simulations of the largest motion magnitudes and were additionally explained by extensive re-allocations between the pCT and the repeat CT scans [8]. Subsequently we investigated the simDVHs relative to late RTOG GI toxicity in the complete HUH cohort (including patients who received also pelvic lymph node irradiation). For the same motion patterns as applied in this study the strongest associations (Rs=0.03–0.10) were obtained for doses >55 Gy although no significant associations was found in the complete cohort [8]. We obtained associations also for doses >55 Gy for this endpoint in the current study but were here able to disclose a stronger and significant relationship (Rs=0.13–0.21; p<0.05). Using the RTOG GI toxicity definition a distinction between small bowel and rectal toxicity is difficult [6] and its intrinsic non-specificity might result in loss of information [16, 17], which might have had an impact on the results obtained in our previous study.
For the more specific morbidity endpoints associations were obtained for high doses (60–70 Gy) as well. Using the simDVHs rather than the pDVH a broader range of dose levels were significantly associated with two of the endpoints (tenesmus, sphincter control). For tenesmus significant associations with the probit NTCP model were observed for volume dependence parameters, n, up to the QUANTEC recommended n value. To date, there have been only few studies associating rectal dose distributions with these endpoints [2, 17, 18], and none where motion-inclusive dose distributions have been used. Due to recent developments enabling both image-guided and intensity-modulated RT being used routinely the morbidity rates of historically higher prioritised endpoints may decrease [2, 19–21] opening up for studying the whole spectrum of rectal morbidity endpoints that are affecting quality of life.
For sphincter control significant associations were established at two intermediate dose levels (V15 and V25) for the pDVH whereas for the simDVHs we also established significant associations at one high dose level (V70, σrandom=0.2 cm and 0.8 cm). Using the same morbidity definition for patients treated with comparable RT techniques, Gulliford et al [22] found a relation with the rectal pDVHs at V65 for this endpoint. In a study by Peeters et al [16] the strongest association between incontinence for blood/mucus/stools was found at V65, using planned DVHs of the anorectal wall. For stool frequency, Peeters et al [16] also found an association at V40 using pDVHs of the anorectum. A similar trend of association at this particular dose level was not seen in our study (Rs=0.08, p=0.11) and overall no strictly significant associations were obtained for this endpoint.
The associations with several specific rectal morbidity endpoints have been shown to be stronger utilising the DVHs calculated for different rectal sub volumes as compared to the associations with DVHs being based on the whole rectum [16, 17]. However in order to disclose the underlying three dimensional dose-response relationship, the use of spatial dose distributions within these sub volumes should probably be investigated [17, 20, 23].
It should clearly be pointed out that the motion model investigated in this study – using a series of rigid organ translations – is obviously a crude representation of the actual motion of the rectum. In each of the motion simulations, we also assumed the magnitude of the motion to be the same in the whole cohort. The resulting simDVHs are therefore (still) only surrogates of the actually delivered DVHs. Actual rectal motion patterns are caused mainly by individual deformations due to variations in organ filling, varying both between patients as well as fractions [2, 3, 5, 24] and may additionally be correlated to the motion patterns of the adjacent organs [25]. In a previous study we have compared accumulated dose distributions, based on re-calculations on repeat CT images following either rigid or deformable image registration, to planned dose distributions. We found that the planned and delivered doses may diverge considerably, in particular when large volume changes are seen [26]. Based on re-calculations on repeat cone-beam CT scans of twelve prostate cancer patients Hatton et al similarly found that the dose distributions assessed from the initial treatment plan deviated from the treatment fractions DVHs [27]. In another recent study comparing the associations with morbidity between dose/volume parameters derived from planned vs. actually delivered motion-inclusive dose distributions (obtained from re-calculations on a median of nine repeat CTs/patient in a series of 38 patients), associations were seen using the motion-inclusive parameters only [3]. This indicates that studies investigating the dose-response relationship are most likely to benefit from accounting for the deviations between planned and delivered dose/volume parameters on a patient specific level [1–3]. The present study should therefore rather be seen as an attempt to anticipate what we might expect from studies investigating actual motion-inclusive dose distributions with morbidity.
Conclusion
In this study we have shown that associating simulated motion-inclusive rectal dose distributions, primarily simulated as random motion, with late rectal morbidity lead to significant associations for four of the studied endpoints in the high dose region (55–70 Gy), although there were no obvious improvements in predictive power over the use of planned dose distributions. Among all studied endpoints the strongest associations were obtained for RTOG GI toxicity and rectal bleeding. Accounting for rectal motion is likely to improve the accuracy of future rectal morbidity prediction studies however such studies should include patient-specific descriptions of rectal motion over the course of therapy to achieve optimal predictive power.
Supplementary Material
Acknowledgments
This study has been supported by research grants from CIRRO-The Lundbeck Foundation Center for Interventional Research in Radiation Oncology, the Danish Cancer Society, FSS (The Danish Council for Independent Research) as well as the Danish Council for Strategic Research. This study was also supported by NIH grant R01 CA85181.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaffray DA, Lindsay PE, Brock KK, Deasy JO, Tomé WA. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):135–9. doi: 10.1016/j.ijrobp.2009.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S123–9. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thor M, Bentzen L, Hysing LB, et al. Prediction of rectum and bladder morbidity following radiotherapy of prostate cancer based on motion-inclusive dose distributions. Radiother Oncol. 2013;107:147–52. doi: 10.1016/j.radonc.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Muren LP, Karlsdottir A, Kvinnsland Y, Wentzel-Larsen T, Dahl O. Testing the new ICRU 62 ‘Planning Organ At Risk Volume’ concept for the rectum. Radiother Oncol. 2005;75:293–302. doi: 10.1016/j.radonc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Thor M, Petersen JB, Bentzen L, Høyer M, Muren LP. Deformable image registration for contour propagation from CT to cone-beam CT scans in radiotherapy of prostate cancer. Acta Oncol. 2011;50:918–25. doi: 10.3109/0284186X.2011.577806. [DOI] [PubMed] [Google Scholar]
- 6.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;5:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 7.Thor M, Væth M, Karlsdottir A, Muren LP. Rectum motion and morbidity prediction: Improving correlation between late morbidity and DVH parameters through use of rectum planning organ at risk volumes. Acta Oncol. 2010;49:1061–8. doi: 10.3109/0284186X.2010.505200. [DOI] [PubMed] [Google Scholar]
- 8.Thor M, Apte A, Deasy JO, Muren LP. Statistical simulations to estimate motion-inclusive dose-volume histograms for prediction of rectal morbidity following radiotherapy. Acta Oncol. 2013;52:666–75. doi: 10.3109/0284186X.2012.720382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deasy JO, Bentzen SM, Jackson A, et al. Improving normal tissue complication probability models: the need to adopt a “data-pooling” culture. Int J Radiat Oncol Biol Phys. 2012;76(3 Suppl):151–4. doi: 10.1016/j.ijrobp.2009.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deasy JO. CERR: A computational environment for radiotherapy research. Med Phys. 2003;30:979–85. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 11.Karlsdottir A, Muren LP, Wenzel-Larsen T, Dahl O. Late gastrointestinal morbidity after three-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys. 2008;70:1478–86. doi: 10.1016/j.ijrobp.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Moiseenko V, Agranovich A, et al. Normal Tissue Complication Probability (NTCP) modeling of late rectal bleeding following external beam radiotherapy for prostate cancer: A Test of the QUANTEC-recommended NTCP model. Acta Oncol. 2010;49:1040–4. doi: 10.3109/0284186X.2010.509736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LENT SOMA tables. Radiother Oncol. 1995;35:17–60. [PubMed] [Google Scholar]
- 14.Liu M, Pickles T, Agranovich A, et al. Impact of neoadjuvant androgen ablation and other factors on late toxicity after external beam prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58:59–67. doi: 10.1016/s0360-3016(03)00777-6. [DOI] [PubMed] [Google Scholar]
- 15.El Naqa I, Suneja G, Lindsay PE, et al. Dose response explorer: an integrated open-source tool for exploring and modelling radiotherapy dose-volume outcome relationships. Phys Med Biol. 2006;51:5719–35. doi: 10.1088/0031-9155/51/22/001. [DOI] [PubMed] [Google Scholar]
- 16.Peeters ST, Lebesque JV, Heemsbergen WD, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1151–61. doi: 10.1016/j.ijrobp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Buettner F, Gulliford SL, Webb S, Sydes MR, Dearnaley DP, Partridge M. The dose-response of the anal sphincter region-an analysis of data from the MRC RT01 trial. Radiother Oncol. 2012;103:347–52. doi: 10.1016/j.radonc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Gulliford SL, Partridge M, Sydes MR, Webb S, Evans PM, Dearnaley DP. Parameters for the Lyman Kutcher Burman (LKB) model of Normal Tissue Complication Probability (NTCP) for specific rectal complications observed in clinical practice. Radiother Oncol. 2012;102:347–51. doi: 10.1016/j.radonc.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Zelefsky MJ, Kollmeier M, Cox B, et al. Improved Clinical Outcomes With High-Dose Image Guided Radiotherapy Compared With Non-IGRT for the Treatment of Clinically Localized Prostate Cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–9. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 20.Maeda Y, Høyer M, Lundby L, Norton C. Faecal incontinence following radiotherapy for prostate cancer: a systematic review. Radiother Oncol. 2011;98:145–53. doi: 10.1016/j.radonc.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Crechange G, Mirjolet C, Gauthier M. Clinical impact of margin reduction on late toxicity and short-term biochemical control for patients treated with daily on-line image guided IMRT for prostate cancer. Radiother Oncol. 2012;103:244–6. doi: 10.1016/j.radonc.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Gulliford SL, Partridge M, Sydes MR, Andrejev J, Dearnaley DP. A comparison of dose-volume constraints derived using peak and longitudinal definitions of late rectal toxicity. Radiother Oncol. 2010;94:241–7. doi: 10.1016/j.radonc.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Trott KR, Doerr W, Facoetti A, et al. Biological mechanisms of normal tissue damage: importance for the design of NTCP models. Radiother Oncol. 2012;105:79–85. doi: 10.1016/j.radonc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Anderson NS, Yu JB, Peschel RE, Decker RH. A significant decrease in rectal volume and diameter during prostate IMRT. Radiother Oncol. 2011;98:187–91. doi: 10.1016/j.radonc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Onishi H, Kuriyama K, Komiyama T, et al. Large prostate motion produced by anal contraction. Radiother Oncol. 2012;104:390–4. doi: 10.1016/j.radonc.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Andersen ES, Muren LP, Sørensen TS, et al. Bladder dose accumulation based on a biomechanical deformable image registration algorithm in volumetric modulated arc therapy for prostate cancer. Phys Med Biol. 2012;57:7089–100. doi: 10.1088/0031-9155/57/21/7089. [DOI] [PubMed] [Google Scholar]
- 27.Hatton JA, Greer PB, Tang C, et al. Does the planning dose-volume histogram represent treatment doses in image-guided prostate radiation therapy? Assessment with cone-beam computerised tomography scans. Radiother Oncol. 2011;98:162–8. doi: 10.1016/j.radonc.2011.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.