Summary
Lung transplantation survival remains significantly impacted by infections and the development of chronic rejection manifesting as bronchiolitis obliterans syndrome (BOS). Traditional microbiologic data has provided insight into the role of infections in BOS. Now, new non-culture-based techniques have been developed to characterize the entire population of microbes resident on the surfaces of the body, also known as the human microbiome. Early studies have identified that lung transplant patients have a different lung microbiome and have demonstrated the important finding that the transplant lung microbiome changes over time. Furthermore, both unique bacterial populations and longitudinal changes in the lung microbiome have now been suggested to play a role in the development of BOS. In the future, this technology will need to be combined with functional assays and assessment of the immune responses in the lung to help further explain the microbiome’s role in the failing lung allograft.
Keywords: Lung transplant, bronchiolitis obliterans syndrome, microbiome, bacterial infections, viral infections, virome, fungome
Introduction
Lung transplantation is an increasingly common procedure to treat intractable, end-stage lung disease in appropriate patients. In 2011, 3,640 lung transplants were performed, representing the largest number in any given year[1–3]. Transplantation for chronic obstructive pulmonary disease (COPD), interstitial lung disease including idiopathic pulmonary fibrosis (IPF), and cystic fibrosis (CF) accounts for nearly 75% of the procedures[1]. Despite this growth, lung transplantation remains limited by a high mortality rate relative to other solid organ transplants. The median reported survival was just 5.6 years in the Thirtieth Official Lung and Heart-Lung Transplant report of the Registry of the International Society for Heart and Lung Transplantation, released in 2013. The majority of mortality within the first year after transplant is due to infection. In contrast, late mortality in lung transplantation is largely due to chronic rejection manifested as bronchiolitis obliterans syndrome (BOS)[1]. Improvement in survival in lung transplantation over time has been largely due to improvements in early mortality without significant change in the development of BOS over time[4]. With this in mind, a breakthrough is needed in prevention of BOS to provide meaningful and substantial impact on long-term survival after lung transplant.
The role of microbial infection in the development of BOS has been long suspected and evaluated with traditional microbiologic studies[5–13]. Recently, non-culture based techniques have been developed that utilize high-throughput DNA sequencing to broadly characterize the microbiome, the community of bacteria that occupies the surfaces of the human body[14,15]. With these techniques, entire populations of bacteria can be characterized where traditional cultures may have appeared sterile. As such, the role of commensal organisms, dysbiosis, and bacterial colonization on disease states such as BOS can be better established. Research into the microbiome and lung disease is in its early stages and has begun to be applied to lung transplantation, where the potential impact of microbiota on the allograft is compelling.
Basics of lung microbiome analysis
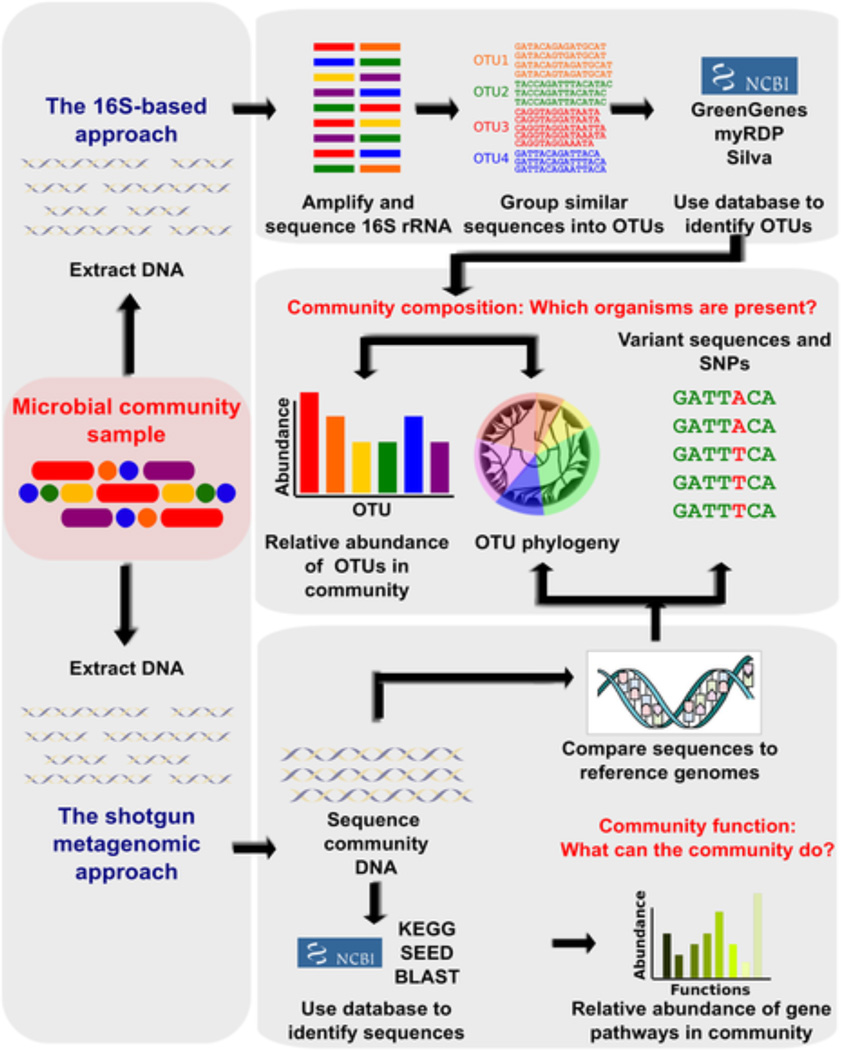
The microbiome consists of the entire community of microorganisms and their products that reside within the human body. Evaluation of the microbiome has become possible due to proliferation of increasingly inexpensive and high-throughput DNA sequencing technology[15]. Bacteria contain the highly conserved 16S rRNA gene known, which, when amplified and sequenced, can be used to identify resident bacteria by comparison to databases of known sequences[16]. In addition, functional metagenomic analysis may be performed to identify which biological tasks may be performed by microbial communities (Figure 1)[15]. The amplification of 16S RNA gene, harnessed in much of the bacterial microbiome work to date, allows bacterial sequences to be selected from samples also containing eukaryotic DNA. Further techniques are available to evaluate the “fungome” and “virome”, but as these techniques are less developed, little has been published with regard to lung disease. Consequently, the focus of most research has been on the bacterial microbiome of the lung.
Figure 1.
- OTU: Operational Taxonomic Unit, a cluster of organisms similar at the sequence level beyond some threshhold (e.g. 95%) used in place of species, genus, etc
- SNP: single-nucleotide polymorphism
Reprinted under the open access Crea4ve Commons A;ribu4on License from Morgan XC, Hu;enhower C (2012) Chapter 12: Human Microbiome Analysis. PLoS Comput Biol 8(12): e1002808. doi:10.1371/journal.pcbi.1002808 http://www.ploscompbiol.org/ar4cle/info:doi/10.1371/journal.pcbi.1002808
Sampling the lung - a unique challenge
The human lung has posed unique sampling and analysis challenges for microbiome research, summarized by Dickson et al [17]. In contrast to the relatively easy acquisition of samples from skin, oral compartments, vaginal compartments, and fecal samples as performed for the National Institute of Health’s Human Microbiome Project[14], sampling the lung compartment is more difficult. Although the upper and lower respiratory tracts are contiguous, previous studies have noted a difference between sputum and lower airway microbiota in patients with lung disease. As a result, bronchoscopy is required for determination of the lower airway microbiome [18,19]. However, since bronchoscopy requires traversing the oral cavity, important concerns arise regarding the distinction between carryover from the upper respiratory tract that occurs during procedures and clinically significant lower airway microbiota that may be present due to the continuous nature of the aerodigestive tract. Different sampling techniques as well as novel statistical analysis have been used to address this challenge[20,21]. Furthermore, the lung microbiome may not be uniform, as suggested by studies in COPD demonstrating regional heterogeneity in microbial populations[22]. The majority of published studies have utilized bronchoalveolar lavage (BAL), which may not reflect segmental variation in the airway microbiome. In addition, low biomass of microorganisms results in low yield of bacteria for sequencing [23] and, therefore, increased importance (or interference) of background and environmental bacteria such as can be detected in sampling equipment and reagents[20,21]. Finally, the sequences obtained may reflect dead bacteria[24]. Thus, important questions remain unanswered about what should be the gold standard method of determining the lung microbiome in human subjects.
Sampling the lung allograft
The nature of transplantation also provides substantial benefits in studying the microbiome in this population. In part, this is due to increased sampling of the post transplant allograft with both routinely scheduled surveillance and clinically indicated bronchoscopies. As a result, this has allowed the transplant population to be the first to have longitudinal bronchoscopic microbiome studies. Frequent healthcare follow-up allows for detailed tracking of phenotypes and infectious complications. However, there are several additional challenges in the interpretation of the post lung allograft microbiome that include the native lung microbiome, the recipient residual microbiome and the host-donor interaction and its effect on the allograft microbiome. As a further complication, the effect of immunosuppressive drugs on microbial populations in the lung allograft is unknown.
Microbiota present in lung transplant recipients
Where does the lung allograft microbiome come from?
Microbiota present in the lung allograft have many potential sources, including: environmental acquisition during the course of normal respiration; carryover from the upper respiratory tract of the recipient; carryover from the remaining native lung in single lung transplant; retained microbiota from the donor lung; and spillover from the aerodigestive tract.
Pre-transplant lung disease
Pre-transplant, candidates for lung transplant have severe, underlying lung disease. With non-culture based techniques, current literature, reviewed by Dickson et al [17], demonstrates that patients with conditions that lead to transplant, such as COPD[19,22,25,26], IPF[27,28], and CF[18,29,30] have different microbiota when compared to patients with healthy lungs. For patients with suppurative lung diseases, such as cystic fibrosis, it is known that existing infection identified with traditional cultures may present a risk post-transplant. Although bilateral transplantation is the rule for suppurative lung disease, other areas of continuity in the aerodigestive tract, such as the upper airways and sinuses, serve as reservoirs for colonized bacteria and may repopulate donor lungs[31]. For example, the presence of Burkholderia cenocepacia pre-transplant has been so consistently linked with excess mortality after transplantation that its presence excludes patients from transplant at most centers[32–36]. Microbiome studies have begun to address this question. Dickson et al reported no effect of pre-transplant diagnosis on lung allograft microbial populations [37], while Willner and colleagues demonstrated that the lung microbiome of lung transplant recipients with CF were distinct from those with other pre-transplant diagnoses[38]. The extent to which differences represent carryover of existing microbiota or an underlying immune profile in each disease remains unclear.
Donor lung and native lung microbiota
Healthy lungs have been detected to have a native microbiome, although the degree to which this represents spillover from the upper respiratory tract or unique taxa remains an analytical challenge[20–22]. The healthy donor lung will carry this native microbiome to the recipient. To date no published studies evaluate donor lungs pre-transplant. As a result, the impact of the donor microbiota and the interaction between the donor and the recipient microbiota is unclear. Similarly, the effect of the remaining native lung in recipients of single lung transplants is also unclear. Future research must be directed at determining the native and donor lung microbiota, their longitudinal change, and interaction.
Spillover from aerodigestive tract
Gastroesophageal reflux (GERD) has been associated with several different lung diseases including IPF. In addition, recent data has suggested that GERD is associated with worse outcomes after lung transplant [39–43]. While GERD and aspiration could lead to sterile inflammation, clearly deposition from the digestive tract may cause shifts in the airway microbiome. Seeding from the nasal and oral cavities, discussed in more detail below (“The role of the upper airway microbiome”), is an additional source of microbiota. Further characterization of this interaction on the lung allograft microbiome is needed.
Initial characterization of the lung allograft
Bacterial communities
Microbiome studies in lung transplant recipients have begun with the fundamental demonstration that the bacterial microbiome of lung transplant recipients appears to be different from that of healthy control patients. Charlson et al[44] evaluated the oral and lung microbiome of 21 lung transplant recipients compared with healthy controls. Transplanted patients had overall higher bacterial load in bronchoalveolar lavage fluid (BALF). While the BALF of healthy patients had a microbial content that mirrored the oral flora, a subset of lung transplant recipients (three with CF, one with IPF) had BALF that was dominated by other organisms, namely Pseudomonas, Staphylococcus, or Achromobacter, which were also demonstrated on traditional culture. In contrast, one subject with late BOS had BALF dominated by Sneathia, which was unable to be cultured[44]. These results suggest that non-culture techniques may complement traditional cultures in the detection of certain bacterial species.
Additional studies have confirmed that the lung transplant microbiome appears to be distinct. Borecwicz et al in a small sampling of 4 lung transplant recipients and 2 controls, confirmed a significant difference in the bacterial populations between transplant and healthy lungs[25]. Furthermore, Luna et al have found that transplanted CF patients differed in microbial populations from non-transplanted CF patients. While non-transplanted CF patients demonstrated predominance of Firmicutes phylum, transplanted CF patients had the greatest abundance of the phylum Proteobacteria with predominant genus Acinetobacter [45]. In a separate analysis of 33 lung transplant recipients, Dickson et al found significant difference between lung transplant recipients and controls, with the phylum Proteobacteria favored over Bacteroidetes and Firmicutes [37]. Lastly, Rogers et al found that transplanted patients were more likely to have Pseudomonas, Staphylococcus, Proprionobacterium, and Veillonella [23]. With these additional studies, it has been consistently shown that the microbiota in transplanted lungs are distinct from non-transplanted lungs.
Measures of bacterial community diversity, in other words the taxonomic distribution within a community[15], have varied in lung transplant recipients. Rogers et al found that transplanted patients had different bacteria but similar diversity in BALF when compared to control patients[23]. In contrast, Dickson et al have noted decreased diversity in BALF of 46 lung transplant recipients compared with 26 controls. In this analysis, the loss of diversity mostly occurred in lung transplant recipients who had symptom-triggered bronchoscopy associated with clinical infection, as opposed to routine surveillance bronchoscopy [46]. Charlson and colleagues found that both richness and diversity were lower in transplant patients, with the strongest effect in those transplanted for suppurative lung disease[44]. These results suggest that a loss of diversity may be tied to the emergence of a predominant organism during periods of infection.
Fungal populations
There has only been one published study evaluating fungal populations in oral wash and BALF of lung transplant recipients[44]. This study by Charlson and colleagues showed that fungal population in the oral wash were predominantly environmental fungi and Candida species. Fungal populations in BALF were distinct in the transplant population compared to healthy controls. Transplant patients demonstrated Candida, Aspergillus, and Cryptococcus species notably often discordant from oral samples. This initial study demonstrates the potential of further studying the fungal populations in the allograft.
Longitudinal analysis
The post-transplant period is unique in that surveillance bronchoscopy is routinely performed, allowing for repeating sampling of the lung microbiota. For this reason, lung transplantation has been the first area of lung microbiome research that has produced longitudinal invasive evaluation of an individual’s lung microbiome. In a longitudinal evaluation of four transplant patients, Borewicz et al found substantial variation in the microbial population in each transplant recipient such that fewer than 10% of the population was retained over time[25]. Luna et al also demonstrated temporal changes in bacterial diversity, with diversity increasing until 9 months and then decreasing between 9 and 12 months[45]. Further, Willner et al [47] undertook “holistic” sampling at multiple body sites in lung transplant recipients, using BALF, lung tissue, nasopharyngeal, oral, and rectal swabs. BALF samples were strongly affected by antibiotic pressure with a gradual and significant decrease in Shannon diversity index. Willner and colleagues separately showed that 16 lung transplant recipients with CF (six with BOS) demonstrated a dynamic microbiota over time. Antibiotic pressure played an important role in the dynamics of the microbiome, and impacted the presence of Pseudomonas[38]. Notably, these investigations have largely found that the microbiome of the allograft is dynamic. While antibiotics are one important factor, other influences have not yet been identified.
Clinical impact of the microbiome in lung transplantation
Bronchiolitis obliterans syndrome (BOS)
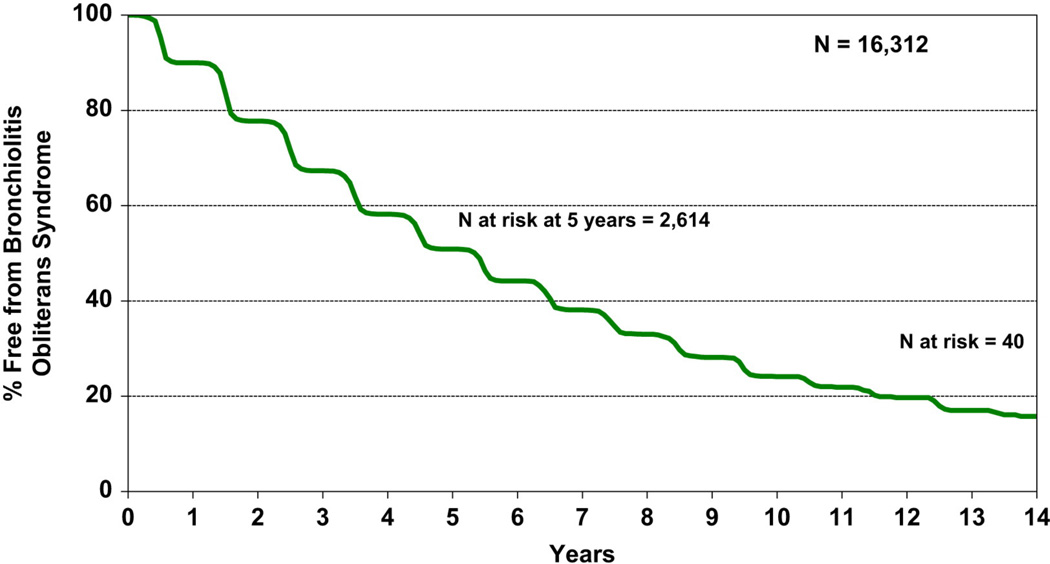
BOS is the physiological correlate of obliterative bronchiolitis. It is defined by the progressive development of airflow obstruction without alternative explanation such as infection or acute rejection[48]. Approximately 50% of lung transplant recipients will develop BOS within 5 years of transplantation, and 76% by ten years (Figure 2)[1]. BOS mortality is high and accounts for the majority of deaths after 1 year post-transplant (Table 1)[1].
Figure 2.
Adult lung transplant recipient freedom from bronchiolitis obliterans syndrome (BOS), conditional on survival to 14 days (transplant follow-ups: April 1994–June 2012).
Reprinted from The Journal of Heart and Lung Transplantation, Vol 32, Issue 10, Roger D. Yusen, Jason D. Christie, Leah B. Edwards, Anna Y. Kucheryavaya, Christian Benden, Anne I. Dipchand, Fabienne Dobbels, Richard Kirk, Lars H. Lund, Axel O. Rahmel, Josef Stehlik, The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report—2013; Focus Theme: Age, Page 974, Copyright © 2013 with permission from Elsevier.
Table 1.
Causes of Deaths for Adult Lung Transplant Recipients (Deaths: January 1992-June 2012)[1]
| Cause of Death | 0–30 days | 31 days-1 year |
>1–3 years | >3–5 years | >5–10 years | >10 years |
|---|---|---|---|---|---|---|
| (n = 2,725) | (n = 4,737) | (n = 4,315) | (n = 2,449) | (n = 2,892) | (n = 899) | |
| No. (%)a | No. (%)a | No. (%)a | No. (%)a | No. (%)a | No. (%)a | |
| Bronchiolitis | 8 (0.3) | 216 (4.6) | 1,119 (25.9) | 710 (29.0) | 734 (25.4) | 188 (20.9) |
| Acute rejection | 94 (3.4) | 85 (1.8) | 63 (1.5) | 16 (0.7) | 17 (0.6) | 2 (0.2) |
| Malignancy | ||||||
| Lymphoma | 1 (0.0) | 110 (2.3) | 78 (1.8) | 36 (1.5) | 56 (1.9) | 31 (3.4) |
| Non-lymphoma | 5 (0.2) | 134 (2.8) | 329 (7.6) | 266 (10.9) | 379 (13.1) | 113 (12.6) |
| Infection | ||||||
| CMV | 0 | 112 (2.4) | 42 (1.0) | 7 (0.3) | 4 (0.1) | 1 (0.1) |
| Non-CMV | 535 (19.6) | 1,687 (35.6) | 971 (22.5) | 471 (19.2) | 523 (18.1) | 154 (17.1) |
| Graft failure | 672 (24.7) | 790 (16.7) | 807 (18.7) | 440 (18.0) | 515 (17.8) | 156 (17.4) |
| Cardiovascular | 298 (10.9) | 228 (4.8) | 179 (4.1) | 120 (4.9) | 148 (5.1) | 58 (6.5) |
| Technical | 301 (11.0) | 162 (3.4) | 38 (0.9) | 14 (0.6) | 24 (0.8) | 8 (0.9) |
| Other | 811 (29.8) | 1,213 (25.6) | 689 (16.0) | 369 (15.1) | 492 (17.0) | 188 (20.9) |
Reprinted from The Journal of Heart and Lung Transplantation, Vol 32, Issue 10, Roger D. Yusen, Jason D. Christie, Leah B. Edwards, Anna Y. Kucheryavaya, Christian Benden, Anne I. Dipchand, Fabienne Dobbels, Richard Kirk, Lars H. Lund, Axel 0. Rahmel, Josef Stehlik, The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report—2013; Focus Theme: Age, Page 971, Copyright © 2013 with permission from Elsevier.
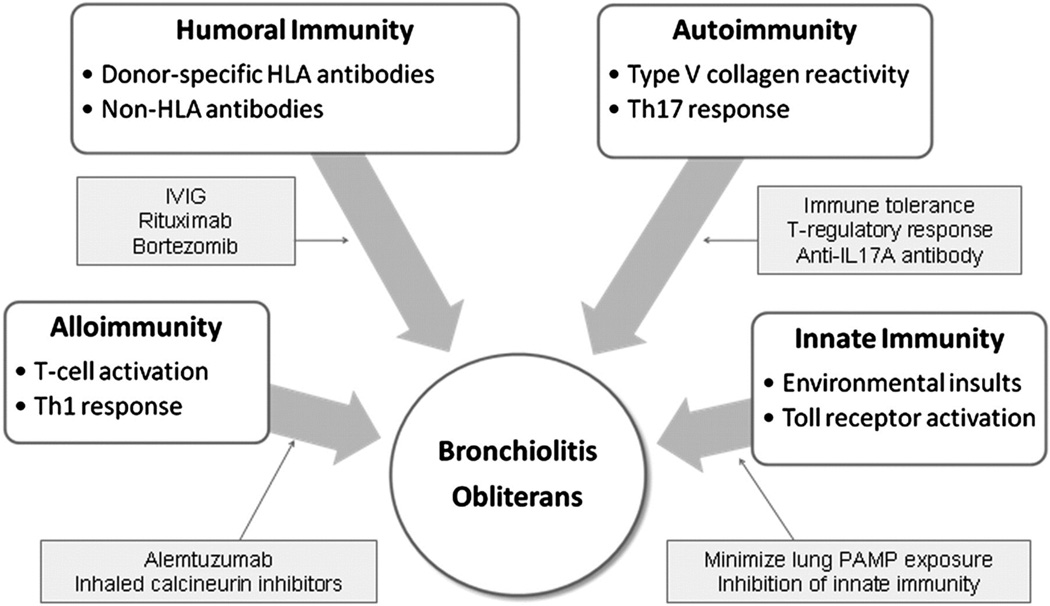
Proposed mechanisms for BOS include activation of the innate immune system, immune activity against the allograft, humoral and autoimmunity, and response to external factors such as aspiration and infection (Figure 3)[48]. The role of infections in the development of BOS has been studied with traditional methods and is now being studied with non-culture based methods.
Figure 3.
Multiple immune mechanisms contribute to the development of BO. Potential therapeutic targets are highlighted. HLA = human leukocyte antigen; IVIG = IV immunoglobulin; PAMP = pathogen-associated molecular pattern; Th = T helper.
Reproduced with permission from the American College of Chest Physicians. Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest, 140(2), 502–508 (2011).
Microbiology of BOS: the role of bacteria
While infections are a common cause of death early in lung transplant course[1], there have also been compelling links between certain infections and the subsequent development of BOS. These studies have been performed with traditional microbiology, serum antibody detection techniques, and more recently, non-culture-based microbiome analytic techniques focusing on the role of bacteria.
Microbiome studies in BOS
There have been several studies in lung transplant recipients that suggest that the lung microbiome is distinct in patients who develop BOS. . Dickson et al found, that the microbiome was significantly different between those with and without BOS at the time of bronchoscopy [37]. Willner et al also noted that lung transplant recipients with CF who developed BOS had more co-infections with other recognized CF pathogens (Stenotrophomonas, Achromobacter, Haemophilus) than those who did not develop BOS[49]. In addition, changes in the lung microbiome over time may be associated with BOS. Poroyko and colleagues found that patients who eventually developed BOS had a predominance of Firmicutes, while non-BOS patients had a predominance of Proteobacteria. The patients with BOS also demonstrated increased variability of the microbiome over time compared to patients without BOS [50].
Changes in microbial community diversity with relation to BOS have differed between studies. Willner et al found that BOS was associated with a significant decrease in Shannon index of diversity[38]. In contrast, Dickson et al did not find such an association[46]. The different findings of microbial community diversity may depend on several other clinical factors that have yet to be defined.
Are particular bacteria risk factors for BOS?
Pseudomonas
Microbiome analysis and traditional culture studies have overlapped in investigating the role of Pseudomonas. The post-transplant presence of Pseudomonas aeruginosa colonization, unlike other gram negative bacteria[50], has been associated with the development of BOS in a strong body of literature utilizing traditional culture. In a retrospective single-center study, Vos et al evaluated 92 lung transplant recipients and found that post-operative colonization with Pseudomonas was a risk factor for BOS and was associated with worse BOS stage and survival in univariate analysis. In multivariate analysis, a trend toward colonization as a risk for BOS was still seen[51].
Further studies have looked the impact of pre-transplant and post-transplant Pseudomonas airway colonization. The fate of this colonization and the timing of Pseudomonas establishment in the airway post-transplant have been shown to play a role in BOS development. A prospective study of 59 adult CF patients demonstrated that those patients whose transplanted lungs remained free of Pseudomonas were less likely to develop BOS[52]. In a separate study of recipients with and without CF, Botha et al evaluated 155 patients for new development or persistence of pre-operative colonization with P. aeruginosa. After transplantation, 64 patients (41.3%) became culture positive for P. aeruginosa. Interestingly, patients with de novo colonization had a higher likelihood of developing BOS compared to those who were either never colonized of colonized pre- transplant with Pseudomonas[5].
Further statistical analysis has emphasized the impact of timing of Pseudomonas infection on outcome after lung transplantation. Gregson et al suggest that the interaction between the presence of Pseudomonas and the immune system that leads to BOS may be dependent on the patient’s state at the time of Pseudomonas establishment in the allograft. Using a Cox semi-Markovian approach, the likelihood of transitioning between three states (lung transplant, BOS, and death) was assessed. In this model, Pseudomonas clinical infection increased the risk of developing new BOS and of death prior to the development of BOS. In patients who had elevated BAL fluid chemokines and Pseudomonas infection, this risk was further amplified. In addition, once the diagnosis of BOS was established, both Pseudomonas infection and colonization led to increased risk of death [8].
Similarly, using microbiome analysis, Willner and colleagues also demonstrated a link between the reestablishment of Pseudomonas that had been present pre-transplant in the lungs of CF recipients and a decreased risk of BOS [38]. In this study, they evaluated the BAL microbiome of 57 transplant patients, 17 of whom had BOS and 29 of whom had cystic fibrosis as an underlying transplant etiology. Patients with CF with BOS had non-typical pathogens, including Streptococcus, Lactobacillus, Enterococcus, Neisseria, and Haemophilus. CF patients without BOS were more likely to demonstrate Pseudomonas, Burkholderia, and Staphylococcus. In the non-CF population, patients with BOS demonstrated a shift in population from “normal respiratory flora” to new pathogens including Pseudomonas, Burkholderia and Staphylococcus [38]. These results imply that the relationship between pre- and post- transplant microbiota may impact the development of BOS. In sum, these data suggest that further research combining both traditional culture methods as well as non-culture techniques will identify both global lung microbial populations as well as individual bacteria that may contribute to health as well as lung disease.
Traditional culture studies of Pseudomonas colonization have prompted the first attempts at modification of a specific bacterial organism to influence BOS development. Given that the upper airway is the likely reservoir of Pseudomonas in cystic fibrosis, Vital et al performed endoscopic frontal-spheno-ethoidectomy in the majority of patients with CF who underwent lung transplantation. The goal was to treat bacterial colonization of the sinuses immediately post transplantation. Of 66 patients who had Pseudomonas airway colonization pre-transplant, 65% of patients (38% pulmonary colonization, 27% nasal colonization only) had persistent colonization after transplantation despite a trial of eradication with multiple antibiotics active against Pseudomonas. Compared to patients with persistent Pseudomonas airway colonization, those patients without Pseudomonas had improved 5-year survival, and a decreased incidence of BOS. Patients with Pseudomonas were also more likely to have neutrophilic alveolitis, an indication that the presence of Pseudomonas leads to airway inflammation and immune activation[31]. This study, a window into potential therapeutic use of microbiome studies, demonstrated both the difficulty in eradicating some bacterial inhabitants and the importance of pre-transplant microbiota on the post-transplant allograft.
Treatments for BOS and the bacterial microbiome
Azithromycin
Azithromycin, a macrolide antibiotic, has been shown to protect against BOS and death. Most notably, a retrospective analysis of 178 lung transplant recipients with BOS demonstrated patients who received azithromycin had a survival advantage, particularly when treatment was initiated during BOS stage 1[53]. The mechanism of azithromycin in this setting is uncertain and may be due to its immunomodulatory role[54]. An alternative explanation is that the antibiotic properties of azithromycin may alter the lung microbiota in a beneficial way. A systematic evaluation of azithromycin on the allograft microbiome has not been done, but in one instance, Moraxella was decreased after treatment with azithromycin [38]. Clearly, this will be an area of deep interest in future research.
Microbiome of BOS: Viruses and Fungi
While the bacterial microbiome data is becoming more robust, there has yet to be published literature regarding the role of other microorganism communities in relation to BOS. However, traditional literature provides evidence that viruses and fungi have importance.
Viruses
The role of community-acquired respiratory viruses has been reliably shown to be associated with the development of BOS. In a retrospective cohort study of 259 consecutive adult lung transplant patients, 21 community-acquired upper or lower respiratory viral illnesses were identified including respiratory syncytial virus (RSV), parainfluenza, influenza, and adenovirus. The group with viral infection was more likely to develop BOS, death, and death-from-BOS, with lower respiratory tract infections enhancing risk[10]. In a subsequent prospective single-center study, 388 lung transplant recipients at various times post-transplant were screened by nasopharyngeal and oropharyngeal swabs for 12 different community-acquired respiratory viruses. Thirty- four viruses were detected in 30 patients, including parainfluenza, RSV, metapneumovirus, coronavirus, rhinovirus, and influenza. In patients in whom a virus was detected, the one-year incidence of new BOS was 25% compared to 9% in virus-negative patients (P=0.01)[7]. Influenza pneumonia has also been associated with progressive loss of lung function [55] as well as the risk of graft dysfunction in lung transplant recipients[56]. Similarly, a prospective evaluation by Kumar et al showed that in 93 transplant recipients, a respiratory virus was documented in 51.6% of patients on at least one BAL. Acute rejection or decline in FEV1 occurred within three months in 33% of patients who had a documented respiratory virus compared with 6.7% of those without a respiratory viral infection. Chronic rejection defined as obliterative bronchiolitis histologically or clinical BOS was diagnosed in 62.5% of patients within 1 year of infection[57]. RSV infection has also been associated with the onset of rapid development of BOS [9]. Other viruses implicated in BOS have been HHV-6[61] and EBV[58]. Thus, viral infections and their interaction with other microbial communities will be an important target as research expands into this realm.
Human cytomegalovirus
The relationship between CMV and BOS has been inconsistent in the published literature. A series of studies demonstrated a positive relationship between CMV detection in the lung transplant recipient and development of BOS. The presence of human CMV DNA in the blood within the first six months post-transplant[59], CMV replication in the allograft[60], and treatment for CMV pneumonitis within the first six months after transplant[13] have all been associated with increased risk of BOS. Further, Fiser et al found CMV to be a risk factor for progression of BOS[61]. In contrast to these studies, other literature has found that CMV detection in bronchoalveolar lavage[62] and histologically diagnosed CMV pneumonia treated with ganciclovir[63] had no relationship to the subsequent development of BOS or survival. Likewise, the presence of beta-herpesviruses including CMV and human herpesvirus (HHV)-6 and -7) have been associated with acute rejection but not with the development of BOS[64]. Despite these conflicting results, prophylaxis against CMV has been associated with decreased risk of BOS and survival[65][66]. Thus, the role of this specific viral infection remains unclear. It is unknown whether the presence of other baseline microbial communities explains the variance in these studies.
Fungi
Aspergillus
Detection of Aspergillus has been associated with BOS in traditional cultures. Aspergillus colonization, described as positive cultures without evidence of invasive disease, was associated with BOS and mortality from BOS in a study of 201 patients at a single center[67]. Furthermore, Gregson et al found that Aspergillus isolation was associated with increased risk of developing BOS and an increased risk of death after BOS[8]. [38]
Co-infection
The interaction between viral and fungal illnesses with bacterial infection may enhance risk for BOS. In multivariate analysis of 49 patients with documented respiratory viral illnesses, coexistence of bacteria at the time of initial viral illness was associated with graft dysfunction at 2 years after viral infection[56]. Using non-culture-based techniques, Willner et al found that Aspergillus-positive BALF cultures never occurred in conjunction with Pseudomonas-dominated communities, suggesting “Pseudomonas-Aspergillus antagonism”[38]. As both of these organisms have been associated with BOS, the implications of these finding in relationship to BOS are not clear. It is, at minimum, a striking example of the importance of looking at whole microbial communities (viral, bacterial, and fungal) in future research.
The role of the upper airway microbiome
The upper airway microbiome is connected to the lung microbiome
The upper airway microbiome, including residents of the nasopharynx and oropharynx, are likely an important reservoir for the lung microbiome. Willner et al also noted that all transplanted patients and controls shared a background lung microbiome characterized by oral and upper respiratory tract flora, including Streptococcus, Veillonella, and Prevotella [49]. Important supporting evidence for this has come from topographical sampling of the respiratory tract in both healthy patients and those with lung disease, including transplanted lungs[20,38,44]. Charlson and colleagues evaluated oral microbiota in additional to that in BALF, and found that each subject’s BALF remained more closely linked to his or her own oral wash than to other patients; the divergence between oral wash and BALF was strongest in transplant patients[44]. Likewise, Willner et al also determined that changes in BALF communities were mirrored by changes in the nasopharyngeal communities[38]. In contrast, Charlson et al found that in normal lungs, the nasopharyngeal community appeared distinct from the lower respiratory tract[20].
Transplantation may change the oral microbiome
Evidence for the effect of immunosuppression and transplant status on the oral microbiome may be found in longitudinal studies of other organ transplant recipients. Remarkably, in early post-transplant periods the oral microbiome is similar to the oral microbiome from the pre-transplant state. Ames et al performed a longitudinal analysis of the oral microbiota of patients who underwent allogeneic stem cell transplantation for hematologic malignancy. Oral microbiota were largely unchanged in patients immediately after transplantation, with the majority of organisms from the expected genera Streptococcus and Veillonella. Patients who developed respiratory signs and symptoms had the most notable shifts in oral microbiota populations. It is unclear if the respiratory infection and subsequent antibiotic treatment led to the shift in the oral microbiome or if the microbiome predisposed to the respiratory symptoms[68]. Likewise, the oral microbiome of pediatric liver transplant patients was shown to initially change at 3 days but then revert to a pre-transplant state by 100 days[69]. In contrast, the oral microbiome late after transplant may be altered. Diaz et al found that the oral microbiome differed between recipients of renal or cardiac transplant over one year post-transplant compared with non-transplant controls. Transplanted patients were more likely to have bacteria in phyla Gammaproteobacteria (Klebsiella, Pseudomonas, Acinetobacter, Vibrio, Enterobacteriacea) and the presence of these organisms was positively correlated with the doses of prednisone and mycophenolate mofetil[70]. These studies suggest that the oral microbiota is a dynamic entity that may change due to post transplant immunosuppression, antibiotics and other clinical factors associated with transplant. The impact of the oral microbiota on the lung microbiota is still unclear.
Lessons from other organ transplantation
Stem cell transplantation
Early mouse studies have suggested that the presence of microbiota increase the risk of gut graft-versus-host disease (GVHD) in the stem-cell transplant recipient, leading to the practice of gut decontamination before conditioning for transplant. While the direct role of the microbiota is unknown, murine studies have shown modulation of innate immune pathways leads to differences in GVHD. However, studies in the modern era of microbiome analysis are yet to be published[71]. Given similarities of BOS after lung transplantation and GVHD of the lung after stem cell transplantation, further data may provide important information regarding the development of BOS after lung transplantation.
Small bowel and liver transplantation
Studies in patients with small bowel and liver recipients have provided some important clues about the changing nature of microbiota in the setting of transplantation. Recipients of small bowel transplantation have been found have shifts in the ileostomy microbiota that is directly related to increased exposure to oxygen in the environment[72], a finding that emphasizes the role of anatomic microenvironments that may also occur in lung transplantation and diseased lungs. Animal and human studies in cirrhosis have demonstrated stool dysbiosis associated with underlying cirrhosis that then normalized after transplantation [73,74], highlighting the potential importance of the underlying disease on the state of the microbiome. Finally, studies in both liver and small bowel have demonstrated a potential clinical correlate of rejection associated with disrupted microbiota. Oh et al found that the bacterial fingerprints in ileal effluents could distinguish between rejecting and non-rejecting recipients of small bowel transplantation[75]. In murine studies of liver transplantation, acute rejection has been associated with alteration in gut microbiota[76] while microbial-associated molecule patterns may lead to increased susceptibility to liver preservation or reperfusion injury after transplantation[77]. Whether similar patterns could become a predictive tool or treatment target in lung transplantation remains to be seen.
Expert commentary
Research into the lung microbiome is in its nascent stages. Basic questions about sampling technique and analysis are still being answered. Lung transplantation provides a unique opportunity to sequentially sample the lung microbiome as transplant pulmonologists routinely perform bronchoalveolar lavage and transbronchial biopsies at several time points after transplantation. With that in mind, lung transplant has tremendous promise to be an early area of advancement in the understanding of the lung allograft microbiome. Current data suggest that the lung transplant microbiome differs from “healthy” lung microbiome. In addition, while the majority of studies suggest that the lung transplant microbiome has decreased diversity and richness, some suggest that there is no difference in diversity. Importantly, longitudinal changes in bacterial flora have been described, raising the question of whether change in microbiota over time is of more importance than individual pathogens in the development of BOS. The future of microbiome research is exciting as we transition from these very necessary descriptive studies to a mechanistic understanding of the microbiome in transplantation.
Five-year view
Over the next five years, we expect the field of lung transplant microbiome research to standardize sample collection and establish the relationship between donor lung microbiota and the post-transplant state, as well as to determine the effects of immunosuppression and antibiotics on the lung microbiome. Further research will then move beyond the initial stages of descriptive studies to look at the function of microbial communities within the lung. Microbiome studies must be linked to assessments of innate and adaptive immune function within the lung allograft. Metabolic products of lung microbial communities must be identified to determine their role in disease development. Assessment of the microbiome may ultimately be used as a predictor for patients at risk for rejection/ BOS and may provide therapeutic targets. Finally, as technology evolves, the evaluation of the fungome and virome[78,79] must also be included in the analysis of microorganisms in lung transplantation, adding another dimension to this already complex system as bacterial, fungal, and viral communities interact.
Key issues.
New non-culture based techniques use DNA sequencing to identify entire microbial populations on the surfaces of the body, and these techniques are beginning to be applied to the lung transplant recipients.
Infections and bronchiolitis obliterans syndrome (BOS) are the major causes of death in lung transplant recipients. Studies of the lung allograft microbiome may provide a new understanding of these complications and a target for therapy.
The microbiota in the lung transplant recipient differs from that of healthy control patients. However, the driving force behind this difference and the prognostic implications of these differences are unclear.
The development of BOS has been strongly linked to bacteria, viral, and fungal infections in traditional microbiological studies. Current analyses of the lung microbiome suggest that there are important differences between lung transplant recipients with and without BOS. The significance of these differences need further investigation.
Major future directions include understanding the interaction between different microbial populations and the human immune system and harnessing microbiome analysis as a prognostic tool and therapeutic target.
Contributor Information
Julia B. Becker, Fellow, Section of Pulmonary and Critical Care Medicine, Department of Medicine, University of Chicago, 5841 S. Maryland Ave, Chicago, IL 60637.
Valeriy Poroyko, Department of Pediatrics, University of Chicago, 5841 S. Maryland Ave, Chicago, IL 60637.
Sangeeta Bhorade, Division of Pulmonary and Critical Care, Department of Medicine, Northwestern University Feinberg School of Medicine, McGaw Pavilion Suite M-300, 240 E Huron, Chicago IL 60611, Phone (312) 908-8163, sbhorade@nmff.org.
References
- 1.Yusen RD, Christie JD, Edwards LB, et al. The registry of the international society for heart and lung transplantation: thirtieth adult lung and heart-lung transplant report-2013; focus theme: age. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32(10):965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31(10):1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(10):1104–1122. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Orens JB, Garrity ER., Jr General overview of lung transplantation and review of organ allocation. Proceedings of the American Thoracic Society. 2009;6(1):13–19. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- 5.Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85(5):771–774. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 6.Chambers DC, Davies B, Mathews A, Yerkovich ST, Hopkins PM. Bronchiolitis obliterans syndrome, hypogammaglobulinemia, and infectious complications of lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32(1):36–43. doi: 10.1016/j.healun.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb J, Schulz TF, Welte T, et al. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87(10):1530–1537. doi: 10.1097/TP.0b013e3181a4857d. [DOI] [PubMed] [Google Scholar]
- 8.Gregson AL, Wang X, Weigt SS, et al. Interaction between Pseudomonas and CXC chemokines increases risk of bronchiolitis obliterans syndrome and death in lung transplantation. American journal of respiratory and critical care medicine. 2013;187(5):518–526. doi: 10.1164/rccm.201207-1228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes D, Jr, Mansour HM, Kirkby S, Phillips AB. Rapid acute onset of bronchiolitis obliterans syndrome in a lung transplant recipient after respiratory syncytial virus infection. Transplant infectious disease : an official journal of the Transplantation Society. 2012;14(5):548–550. doi: 10.1111/j.1399-3062.2012.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. American journal of respiratory and critical care medicine. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 11.Kroshus TJ, Kshettry VR, Savik K, John R, Hertz Ml, Bolman RM., 3rd Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. The Journal of thoracic and cardiovascular surgery. 1997;114(2):195–202. doi: 10.1016/S0022-5223(97)70144-2. [DOI] [PubMed] [Google Scholar]
- 12.Shalhoub S, Husain S. Community-acquired respiratory viral infections in lung transplant recipients. Current opinion in infectious diseases. 2013;26(4):302–308. doi: 10.1097/QCO.0b013e3283630e85. [DOI] [PubMed] [Google Scholar]
- 13.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. American journal of respiratory and critical care medicine. 2010;181(12):1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aagaard K, Petrosino J, Keitel W, et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012 doi: 10.1096/fj.12-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan XC, Huttenhower C. Chapter 12: human microbiome analysis. PLoS computational biology. 2012;8(12):e1002808. doi: 10.1371/journal.pcbi.1002808. A clear, concise, and illuminating chapter that explains human microbiome analysis to the uninitiated.
- 16.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and environmental microbiology. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert review of respiratory medicine. 2013;7(3):245–257. doi: 10.1586/ers.13.24. A clear review looking at lung microbiome research from a broad perspective.
- 18.Goddard AF, Staudinger BJ, Dowd SE, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(34):13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrera-Rubio R, Garcia-Nunez M, Seto L, et al. Microbiome diversity in the bronchial tracts of patients with chronic obstructive pulmonary disease. Journal of clinical microbiology. 2012;50(11):3562–3568. doi: 10.1128/JCM.00767-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. American journal of respiratory and critical care medicine. 2011;184(8):957–963. doi: 10.1164/rccm.201104-0655OC. A seminal paper looking at both the technique of collecting lung transplant samples and is the first in a line of studies working to establish the healthy lung microbiome.
- 21.Morris A, Beck JM, Schloss PD, et al. Comparison of the Respiratory Microbiome in Healthy Non-Smokers and Smokers. American journal of respiratory and critical care medicine. 2013 doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PloS one. 2011;6(2):e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers G, Van Der Gast C, Hopkins P, Yerkovich S, Bruce K. C14. CLINICAL AND TRANSLATIONAL ADVANCES IN LUNG TRANSPLANTATION. American Thoracic Society; 2013. The Lower Airway Bacterial Microbiota In Patients With And Without Bronchiolitis Obliterans Syndrome Following Lung Transplantation; pp. A3780–A3780. [Google Scholar]
- 24.Pezzulo AA, Nassar B, Gansemer ND, et al. C56. BENCH TO BEDSIDE ADVANCES IN BRONCHIECTASIS. American Thoracic Society; A Diverse Landscape Of DNA From Live And Dead Bacteria In The Airways; pp. A6406–A6406. [Google Scholar]
- 25.Borewicz K, Pragman AA, Kim HB, Hertz M, Wendt C, Isaacson RE. Longitudinal analysis of the lung microbiome in lung transplantation. FEMS microbiology letters. 2012 doi: 10.1111/1574-6968.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2012;185(10):1073–1080. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han MK, Erb-Downward J, Zhou Y, et al. C13. INFLUENCE OF THE MICROBIOME ON AIRWAY MUCOSAL RESPONSES IN HEALTH AND DISEASE. American Thoracic Society; 2013. The IPF Microbiome: Analysis From The COMET Study; pp. A3769–A3769. [Google Scholar]
- 28.Molyneaux PL, Russell AM, Cox MJ, Moffatt MF, Cookson WO, Maher TM. C103. PATHOGENESIS, BIOMARKERS, AND RISK FACTORS FOR INTERSTITIAL LUNG DISEASE: FROM BENCH TO BEDSIDE. American Thoracic Society; 2012. The Respiratory Microbiome In Idiopathic Pulmonary Fibrosis; pp. A5174–A5174. [Google Scholar]
- 29.Fodor AA, Klem ER, Gilpin DF, et al. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PloS one. 2012;7(9):e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. American journal of respiratory and critical care medicine. 2013;187(10):1118–1126. doi: 10.1164/rccm.201210-1937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vital D, Hofer M, Benden C, Holzmann D, Boehler A. Impact of sinus surgery on pseudomonal airway colonization, bronchiolitis obliterans syndrome and survival in cystic fibrosis lung transplant recipients. Respiration; international review of thoracic diseases. 2013;86(1):25–31. doi: 10.1159/000339627. [DOI] [PubMed] [Google Scholar]
- 32.Alexander BD, Petzold EW, Reller LB, et al. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(5):1025–1030. doi: 10.1111/j.1600-6143.2008.02186.x. [DOI] [PubMed] [Google Scholar]
- 33.Aris RM, Routh JC, LiPuma JJ, Heath DG, Gilligan PH. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. American journal of respiratory and critical care medicine. 2001;164(11):2102–2106. doi: 10.1164/ajrccm.164.11.2107022. [DOI] [PubMed] [Google Scholar]
- 34.Chaparro C, Maurer J, Gutierrez C, et al. Infection with Burkholderia cepacia in cystic fibrosis: outcome following lung transplantation. American journal of respiratory and critical care medicine. 2001;163(1):43–48. doi: 10.1164/ajrccm.163.1.9811076. [DOI] [PubMed] [Google Scholar]
- 35.De Soyza A, Meachery G, Hester KL, et al. Lung transplantation for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single-center experience. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29(12):1395–1404. doi: 10.1016/j.healun.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. American journal of epidemiology. 2001;153(4):345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickson RP, Erb-Downward J, Britt ZE, Walker N, Lama VN, Huffnagle GB. A104. LUNG TRANSPLANTATION: CLINICAL AND TRANSLATIONAL ADVANCES. American Thoracic Society; 2013. The Lung Microbiota Is Distinct Following Lung Transplantation And Is Associated With Bronchiolitis Obliterans Syndrome; pp. A2209–A2209. [Google Scholar]
- 38. Willner DL, Hugenholtz P, Yerkovich ST, et al. Reestablishment of recipient-associated microbiota in the lung allograft is linked to reduced risk of bronchiolitis obliterans syndrome. American journal of respiratory and critical care medicine. 2013;187(6):640–647. doi: 10.1164/rccm.201209-1680OC. The most robust published literature on the longitudinal change in microbial populations and the risk for BOS.
- 39.Fisichella PM, Davis CS, Kovacs EJ. A review of the role of GERD-induced aspiration after lung transplantation. Surgical endoscopy. 2012;26(5):1201–1204. doi: 10.1007/s00464-011-2037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrity ER., Jr Gastroesophageal reflux disease and bronchiolitis obliterans syndrome: where are we today? The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32(6):579–580. doi: 10.1016/j.healun.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Hartwig MG, Davis RD. Gastroesophageal reflux disease-induced aspiration injury following lung transplantation. Current opinion in organ transplantation. 2012;17(5):474–478. doi: 10.1097/MOT.0b013e328357f84f. [DOI] [PubMed] [Google Scholar]
- 42.Hoppo T, Komatsu Y, Jobe BA. Gastroesophageal reflux disease and patterns of reflux in patients with idiopathic pulmonary fibrosis using hypopharyngeal multichannel intraluminal impedance. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / I. S.D.E. 2012 doi: 10.1111/j.1442-2050.2012.01446.x. [DOI] [PubMed] [Google Scholar]
- 43.Murthy SC, Nowicki ER, Mason DP, et al. Pretransplant gastroesophageal reflux compromises early outcomes after lung transplantation. The Journal of thoracic and cardiovascular surgery. 2011;142(1):47–52. e43. doi: 10.1016/j.jtcvs.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 44. Charlson ES, Diamond JM, Bittinger K, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. American journal of respiratory and critical care medicine. 2012;186(6):536–545. doi: 10.1164/rccm.201204-0693OC. The first study looking at both bacterial and fungal populations in the lung allograft.
- 45.Luna RA, Sagar M, Crabtree SA, et al. Characterization of the Lung Microbiome in Pediatric Lung Transplant Recipients. The Journal of Heart and Lung Transplantation. 2013;32(4, Supplement):S291. [Google Scholar]
- 46.Dickson RP, Britt ZE, Erb-Downward J, et al. C14. CLINICAL AND TRANSLATIONAL ADVANCES IN LUNG TRANSPLANTATION. American Thoracic Society; 2013. Lung Microbiota Diversity Is Decreased Among Lung Transplant Recipients; pp. A3781–A3781. [Google Scholar]
- 47.Willner D, Ab-Ghani NA, Yerkovich S, et al. Longitudinal Holistic Profiling of the Lung Transplant Microbiome. The Journal of Heart and Lung Transplantation. 2013;32(4, Supplement):S10–S11. [Google Scholar]
- 48.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140(2):502–508. doi: 10.1378/chest.10-2838. [DOI] [PubMed] [Google Scholar]
- 49.Willner D, Hugenholtz P, Tan ME, Yerkovich ST, Hopkins PM, Chambers DC. 68 Distinct Microbial Signatures of Healthy and Failing Lung Allografts. The Journal of Heart and Lung Transplantation. 2012;31(4, Supplement):S32. [Google Scholar]
- 50.Hayes D, Jr, Weiland A, Kirkby S, Galantowicz M, McConnell PI, Tobias JD. Gram-negative infection and bronchiectasis in lung transplant recipients with bronchiolitis obliterans syndrome. The Thoracic and cardiovascular surgeon. 2013;61(3):240–245. doi: 10.1055/s-0032-1322619. [DOI] [PubMed] [Google Scholar]
- 51.Vos R, Vanaudenaerde BM, Geudens N, Dupont LJ, Van Raemdonck DE, Verleden GM. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? The European respiratory journal. 2008;31(5):1037–1045. doi: 10.1183/09031936.00128607. [DOI] [PubMed] [Google Scholar]
- 52.Gottlieb J, Mattner F, Weissbrodt H, et al. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respiratory medicine. 2009;103(5):743–749. doi: 10.1016/j.rmed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Jain R, Hachem RR, Morrell MR, et al. Azithromycin is associated with increased survival in lung transplant recipients with bronchiolitis obliterans syndrome. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29(5):531–537. doi: 10.1016/j.healun.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy DM, Forrest IA, Corris PA, et al. Azithromycin attenuates effects of lipopolysaccharide on lung allograft bronchial epithelial cells. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27(11):1210–1216. doi: 10.1016/j.healun.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 55.Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest. 2001;119(4):1277–1280. doi: 10.1378/chest.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 56.Halloran K, Chang J, Kapasi A, Weinkauf J, Lien D, Nador R. The International Society for Heart and Lung Transplantation 33rd Annual Meeting and Scientific Sessions. Montreal, QC, CA: 2013. Predictors of chronic lung allograft dysfunction (CLAD) following respiratory viral infection (RVI) Ed.^(Eds) [Google Scholar]
- 57.Kumar D, Husain S, Chen MH, et al. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89(8):1028–1033. doi: 10.1097/TP.0b013e3181d05a71. [DOI] [PubMed] [Google Scholar]
- 58.Engelmann I, Welte T, Fuhner T, et al. Detection of Epstein-Barr virus DNA in peripheral blood is associated with the development of bronchiolitis obliterans syndrome after lung transplantation. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2009;45(1):47–53. doi: 10.1016/j.jcv.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Westall GP, Michaelides A, Williams TJ, Snell Gl, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75(12):2064–2068. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- 60.Paraskeva M, Bailey M, Levvey BJ, et al. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(10):2190–2196. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- 61.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. The Annals of thoracic surgery. 2002;73(4):1041–1047. doi: 10.1016/s0003-4975(01)03606-2. discussion 1047–1048. [DOI] [PubMed] [Google Scholar]
- 62.Neurohr C, Huppmann P, Leuchte H, et al. Human herpesvirus 6 in bronchalveolar lavage fluid after lung transplantation: a risk factor for bronchiolitis obliterans syndrome? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(12):2982–2991. doi: 10.1111/j.1600-6143.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- 63.Tamm M, Aboyoun CL, Chhajed PN, Rainer S, Malouf MA, Glanville AR. Treated cytomegalovirus pneumonia is not associated with bronchiolitis obliterans syndrome. American journal of respiratory and critical care medicine. 2004;170(10):1120–1123. doi: 10.1164/rccm.200310-1405OC. [DOI] [PubMed] [Google Scholar]
- 64.Manuel O, Kumar D, Moussa G, et al. Lack of association between beta-herpesvirus infection and bronchiolitis obliterans syndrome in lung transplant recipients in the era of antiviral prophylaxis. Transplantation. 2009;87(5):719–725. doi: 10.1097/TP.0b013e3181963262. [DOI] [PubMed] [Google Scholar]
- 65.Chmiel C, Speich R, Hofer M, et al. Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(6):831–839. doi: 10.1086/528689. [DOI] [PubMed] [Google Scholar]
- 66.Ruttmann E, Geltner C, Bucher B, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81(10):1415–1420. doi: 10.1097/01.tp.0000209439.27719.ed. [DOI] [PubMed] [Google Scholar]
- 67.Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(8):1903–1911. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ames NJ, Sulima P, Ngo T, et al. A characterization of the oral microbiome in allogeneic stem cell transplant patients. PloS one. 2012;7(10):e47628. doi: 10.1371/journal.pone.0047628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheehy EC, Beighton D, Roberts GJ. The oral microbiota of children undergoing liver transplantation. Oral microbiology and immunology. 2000;15(3):203–210. doi: 10.1034/j.1399-302x.2000.150309.x. [DOI] [PubMed] [Google Scholar]
- 70. Diaz PI, Hong BY, Frias-Lopez J, et al. Transplantation-associated long-term immunosuppression promotes oral colonization by potentially opportunistic pathogens without impacting other members of the salivary bacteriome. Clinical and vaccine immunology: CVI. 2013;20(6):920–930. doi: 10.1128/CVI.00734-12. The first suggestion in the literature that immunosuppresive medications may alter microbial populations.
- 71.Murphy S, Nguyen VH. Role of gut microbiota in graft-versus-host disease. Leukemia & lymphoma. 2011;52(10):1844–1856. doi: 10.3109/10428194.2011.580476. [DOI] [PubMed] [Google Scholar]
- 72.Hartman AL, Lough DM, Barupal DK, et al. Human gut microbiome adopts an alternative state following small bowel transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(40):17187–17192. doi: 10.1073/pnas.0904847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu ZW, Ling ZX, Lu HF, et al. Changes of gut bacteria and immune parameters in liver transplant recipients. Hepatobiliary & pancreatic diseases international: HBPD INT. 2012;11(1):40–50. doi: 10.1016/s1499-3872(11)60124-0. [DOI] [PubMed] [Google Scholar]
- 74.Xie YR, Liu SL, Liu X, et al. Intestinal microbiota and innate immunity-related gene alteration in cirrhotic rats with liver transplantation. Transplantation proceedings. 2011;43(10):3973–3979. doi: 10.1016/j.transproceed.2011.08.113. [DOI] [PubMed] [Google Scholar]
- 75.Oh PL, Martinez I, Sun Y, Walter J, Peterson DA, Mercer DF. Characterization of the ileal microbiota in rejecting and nonrejecting recipients of small bowel transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(3):753–762. doi: 10.1111/j.1600-6143.2011.03860.x. [DOI] [PubMed] [Google Scholar]
- 76.Xie Y, Luo Z, Li Z, et al. Structural shifts of fecal microbial communities in rats with acute rejection after liver transplantation. Microbial ecology. 2012;64(2):546–554. doi: 10.1007/s00248-012-0030-1. [DOI] [PubMed] [Google Scholar]
- 77.Corbitt N, Kimura S, Isse K, et al. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation-reperfusion injury after orthotopic liver transplantation. The American journal of pathology. 2013;182(1):180–191. doi: 10.1016/j.ajpath.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wylie KM, Weinstock GM, Storch GA. Emerging view of the human virome. Translational research : the journal of laboratory and clinical medicine. 2012;160(4):283–290. doi: 10.1016/j.trsl.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willner D, Thurber RV, Rohwer F. Metagenomic signatures of 86 microbial and viral metagenomes. Environmental microbiology. 2009;11(7):1752–1766. doi: 10.1111/j.1462-2920.2009.01901.x. [DOI] [PubMed] [Google Scholar]