Abstract
Background
Transcription factor 7-like 2 gene (TCF7L2), fat mass and obesity-associated gene (FTO), and ectonucleotide pyrophosphatase/phosphodiesterase gene (ENPP1) are known risk loci for type 2 diabetes (T2DM) mostly in European populations.
Objectives
To assess the association of these genes with T2DM risk in a South African mixed-ancestry population.
Methods
Five hundred and sixty six participants were genotyped for ENPP1-rs997509 and -rs1044498, FTO-9941349 and -rs3751812, TCF7L2-rs12255372 and -rs7903146 polymorphisms using Taqman genotyping assays and validated by automated sequencing to assess the association of the polymorphisms with cardiometabolic traits.
Results
In logistic regression models adjusted for age, sex, body mass index (BMI) and insulin resistance, minor allele of rs997509 was associated with a higher risk of prevalent T2DM under a recessive model [odd ratio 4.60 (95% confidence interval: 1.07 to 19.86); p = 0.040].Under additive model, the rs7903146 [1.43 (1.00 to 2.04); p= 0.053] and rs9941349 [1.43 (1.00 to 2.04); p = 0.052] minor alleles showed marginally significant associations with a high risk of T2DM. However, only the rs7903146 alleles (p=0.011) and genotypes (p=0.025) distributions were statistically significantly different between diabetic and non-diabetic individuals.
Conclusion
Our findings demonstrate that ENPP1, TCF7L2, and FTO may predispose to T2DM in the mixed-ancestry population.
Keywords: Type 2 diabetes, genetics, ENPP1, TCF7L2, FTO, Africa
Introduction
The completion of the human genome a decade ago has paved a way for a better understanding of the genetic basis of common and complex diseases. Through candidate gene and linkage analyses, and recently genome wide association studies (GWAS), several single nucleotide polymorphisms (SNPs) have been reported as risk loci for type 2 diabetes in European (39 loci) and Asian (19 loci) population groups1,2. The majority of these loci seem to influence beta-cell function and insulin sensitivity, although the mechanisms by which they impair such functions are yet to be determined. Among these loci are transcription factor 7-like 2 gene (TCF7L2), fat mass and obesity-associated gene (FTO), and ectonucleotide pyrophosphatase/phosphodiesterase gene (ENPP1).
Transcription factor 7-like 2 gene is the first T2DM risk locus to be identified through large-scale association analyses in European populations3. The protein encoded by the TCF7L2 is a transcription factor of the Wnt signalling pathway, which plays a crucial role in beta-cell function, insulin secretion, and proglucagon gene expression4, 5. The first surge of T2DM GWA identified among others the fat mass and obesity-associated gene (FTO), which is the strongest obesity genetic risk factor reported so far6,7. It has been shown to confer susceptibility to T2DM in the presence8,9 and absence of obesity10,11. The most widely studied SNP of the ENPP1, K121Q (rs1044498), has produced conflicting results in relation to T2DM susceptibility12–14. The ENPP1-encoded protein (also known as plasma cell-1, PC-1) is a class II transmembrane glycoprotein that interacts with insulin receptor and inhibits subsequent insulin-signalling through its impaired beta-subunit autophosphorylation, thus causing insulin resistance15.
Although few number of T2DM susceptibility genes discovered through candidate gene, linkage analyses, and GWA studies elsewhere have been replicated in African studies16–21, it must also be emphasized that many other association studies failed to show significant and replicable findings22–24. Therefore in this study we investigated three genes, namely, TCF7L2, FTO, and ENPP1 as risk factors for T2DM in a South African ethnic population group of Mixed-ancestry (Coloureds) with a unique genetic architecture. Structure analysis conducted in this population revealed that its origin is predominantly Khoesan (32–43%), Bantu-speaking African (20–36%), European (21–28%), and a small proportion Asian (9–11%)25.
Materials and Methods
Ethical approval of the study
The study was approved by the Faculty of Health and Wellness Sciences Ethics Committee of the Cape Peninsula University of Technology (CPUT) NHREC: REC-230408-014, and was conducted according to the code of ethics of the World Medical Association (Declaration of Helsinki). All participants who were recruited for the study voluntarily signed written consent after the procedures had been fully explained in the language of their choice. Permission to conduct the study was granted by relevant authorities such as city and community authorities.
Study design and population
The present study was cross-sectional by design, involving participants from a mixed-ancestry ethnic population group residing in Bellville South township in Cape Town, South Africa. The population group has lived in Bellville South since late 1950s. A detailed description of the survey and procedures conducted in the study are available elsewhere26,27.
Clinical data
Clinical data was collected in the form of a standardized questionnaire. During this time, physical examination was conducted with data collection on blood pressure according to World Health Organisation (WHO) guidelines28 using a semi-automatic digital blood pressure monitor (Rossmax PA, USA) on the right arm in sitting position, and anthropometric measurements; body weight was measured to the nearest 0.1 kg with a Sunbeam EB710 digital bathroom scale, which was calibrated and standardized using a weight of known mass. Measurements were recorded with each subject wearing light clothing, without shoes and socks. Waist circumference was determined using a non-elastic tape at the level of the narrowest part of the torso, as seen from the anterior view. All anthropometric measurements were performed three times and their average used for analysis. Participants with no history of doctor diagnosed diabetes mellitus underwent a 75 g oral glucose tolerance test (OGTT) as recommended by the WHO29.
Biochemical analysis
Blood samples were collected from participants after an overnight fast. Blood glucose level and glycated haemoglobin (HbA1c) were measured, respectively, by enzymatic hexokinase method and turbidimetric inhibition immunoassay (Cobas 6000, Roche Diagnostics, Germany). Insulin was determined by a microparticle enzyme immunoassay (Axsym, Abbot). High-density lipoprotein cholesterol (HDL-C) and triglycerides (TG) were estimated by enzymatic colorimetric methods (Cobas 6000, Roche Diagnostics). Low-density lipoprotein cholesterol (LDL-C) was calculated using Friedwald's formula.
Definitions and calculations
Body mass index (BMI) was calculated as weight per square meter (kg/m2) and waist-hip-ratio (WHR) as waist/hip circumferences(cm). Diabetes was based on a history of doctor-diagnosis, fasting blood glucose concentration ≥ 7.0 mmol/L (or 126 mg/dL) and/or 2-hour post-OGTT plasma glucose ≥ 11.1mmol/L (or 200mg/dL). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to the formula: HOMA-IR= [fasting insulin concentration (mIU/L) × fasting plasma glucose (mmol/L]/ 22.5; while functional β-cells (HOMA-B %) were estimated using the formula: 20 × fasting insulin (µIU/ml)/fasting glucose (mmol/ml) − 3.5. The fasting insulin resistance index (FIRI) was calculated with the formula: [fasting insulin (µU/ml) × fasting glucose (mM)]/25 and the quantitative insulin-sensitivity check index (QUICKI) as: 1/ [log (fasting insulin (µU/ml) × log (fasting glucose (mg/dl)].
Genotyping
Genomic DNA was extracted from peripheral blood using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. Briefly, white blood cells were lysed, thereafter cellular proteins were removed by salt precipitation, and high molecular weight genomic DNA left in solution was then concentrated and desalted by isopropanol precipitation. A total of 566 participants (11.7% males) who consented to genetic analyses were genotyped for six single nucleotide polymorphisms (SNPs) that have been shown to confer susceptibility to T2DM in previous studies elsewhere: ENPP1-rs997509 and -rs1044498, FTO-9941349 and -rs3751812, TCF7L2-rs12255372 and -rs7903146. The SNPs were genotyped using their corresponding Taqman genotyping assays (Applied Biosystems, USA) on a BioRad Optica (Biorad, USA) and validated by sequencing, giving 100% genotyping concordance for all samples that were repeated.
Statistical analysis
General characteristics of the study group are summarized as count and percentage for dichotomous traits, mean and standard deviation (SD) or median and 25th–75th percentiles for quantitative traits. Traits were log-transformed to approximate normality, where necessary, prior to analysis. SNPs were tested for departure from Hardy-Weindberg Equilibrium (HWE) expectation via a chi square goodness of fit test. Linkage disequilibrium (LD) was estimated using the D' statistic. Logistic regression models were used for the analysis of dichotomous traits, assuming both recessive and log-additive models for the SNPs. We investigated the association of each SNP with each trait, overall and tested for heterogeneity by major subgroups by adding the interaction term of major grouping variables and each SNP to a model that contained the main effects of grouping variable and the relevant SNP. Results corresponding to p-values below 5% are described as significant. Adjustment for multiple testing was conducted via Bonferroni methods. All analyses used the statistical software R (version 3.0.0 [2013-04-03], The R Foundation for statistical computing, Vienna, Austria). SNPs analyses used the packages ‘genetic’, ‘gap’, ‘SNPassoc’ and ‘hapassoc’.
Results
Baseline characteristics
Of 566 participants that consented for genetic studies, 480 had complete data for analysis and their clinical characteristics are summarized in Table 1. One hundred and fifty eight (32%) individuals had T2DM. As expected, T2DM-related traits differed significantly according to diabetes status. For example, significant differences in the distribution of insulin resistance/sensitivity indicators were observed between the two groups (all p < 0.0001, except for glucose/insulin ratio (p = 0.009). Participants with diabetes compared to non-diabetics, were older (p < 0.0001), had higher body mass index (p= 0.023), higher waist circumference and waist-to-hip ratio (both p < 0.0001), higher systolic blood pressure (130 vs. 121 mmHg, p < 0.0001), and consisted of a higher proportion of individuals with hypertension (77.6% vs. 67.1%, p = 0.018). Furthermore, diabetic individuals had higher triglycerides (p < 0.0001).
Table 1.
Baseline characteristics overall and by body mass index and diabetes status
| Characteristics | No diabetes |
Diabetes | p-value | Normal weight |
overweight | obese | p-value | Overall |
| N | 328 | 152 | 118 | 129 | 233 | 480 | ||
| Gender, male n (%) | 18 (5.5) | 34 (22.4) | <0.000 1 |
16 (13.6) | 17 (13.2) | 19 (8.1) | 0.185 | 52 (10.8) |
| Mean Age, year (SD) | 53.2 (13.5) |
60.1 (11.8) | <0.000 1 |
54.7 (15.0) |
55.9 (12.8) |
55.5 (12.9) |
0.793 | 55.4 (13.4) |
| Mean Systolic blood pressure, mmHg (SD) |
121 (18) | 130 (22) | <0.000 1 |
119 (22) | 122 (17) | 127 (20) | 0.0007 | 124 (20) |
| Mean Diastolic blood pressure, mmHg (SD) |
74 (12) | 77 (16) | 0.076 | 73 (18) | 74 (11) | 77 (12) | 0.016 | 75 (14) |
| Hypertension, n (%) | 220 (67.1) |
118 (77.6) | 0.018 | 64 (54.2) | 93 (72.1) | 181 (77.7) | <0.000 1 |
338 (70.4) |
| Mean Body mass index, kg/m2 (SD) |
30.1 (7.2) | 31.9 (7.2) | 0.012 | 22.0 (2.3) | 27.7 (1.4) | 36.5 (5.8) | 1 <0.000 1 |
30.6 (7.4) |
| Mean Waist circumference, cm (SD) |
96 (15) | 103 (14) | <0.000 1 |
81 (10) | 93 (6) | 109 (11) | <0.000 1 |
98 (15) |
| Mean Hip circumference, cm (SD) |
111 (15) | 112 (16) | 0.558 | 96 (9) | 106 (6) | 122 (13) | <0.000 1 |
111 (15) |
| Mean Waist/hip ratio, (SD) | 0.86 (0.08) |
0.92 (0.08) | <0.000 1 |
0.84 (0.07) |
0.89 (0.07) |
0.89(0.08) | <0.000 1 |
0.88 (0.08) |
| Mean HbA1c, % (SD) | 5.7 (0.4) | 7.8 (1.9) | <0.000 1 |
5.8 (0.9) | 6.6 (1.8) | 6.4 (1.3) | <0.000 1 |
6.3 (1.4) |
| Mean Fasting blood glucose, mmol/l (SD) |
5.3 (0.7) | 9.5 (3.8) | <0.000 1 |
5.9 (2.1) | 7.0 (3.8) | 6.8 (2.8) | 0.007 | 6.6 (3.0) |
| Mean 2h glucose, mmol/l (SD) | 6.8 (1.7) | 13.4 (4.7) | <0.000 1 |
7.2 (2.5) | 7.7 (3.6) | 8.7 (3.9) | 0.0008 | 8.1 (3.5) |
| Mean Triglycerides, mmol/l (SD) |
1.4 (1.0) | 1.8 (0.9) | <0.000 1 |
1.2 (1.0) | 1.6 (1.1) | 1.6 (0.8) | ||
| Mean HDL cholesterol, mmol/l (SD) |
1.3 (0.3) | 1.2 (0.3) | 0.0001 | 1.4 (0.4) | 1.3 (0.3) | 1.2 (0.3) | <0.000 1 |
1.3 (0.3) |
| Mean LDL cholesterol, mmol/l (SD) |
3.8 (1.0) | 3.8 (1.1) | 0.984 | 3.6 (0.9) | 3.8 (1.0) | 3.8 (1.0) | 0.075 | 3.8 (1.0) |
| Mean Total cholesterol, mmol/l (SD) |
5.7 (1.2) | 5.8 (1.2) | 0.582 | 5.5 (1.1) | 5.8 (1.2) | 5.8 (1.2) | 0.084 | 5.7 (1.2) |
| Median Insulin mmol/l(25th–75th percentiles) |
6.9 [2.9–12.2] | 8.7 [2.8–14.8] | 0.043 | 3.7 [1.5–8.0] | 7.0 [3.2–11.1] | 9.1 [4.9–15.0] | <0.000 1 |
7.4 [2.9–13.2] |
| Median 2h insulin mmol/l(25th–75tg percentiles) |
39.5 [21.7–67.4] |
54.9 [21.8–116.1] | 0.026 | 28.8 [17.1–45.2] |
36.7 [22.7–61.0] |
56.5 [31.0–106.4] |
<0.000 1 |
|
| Median Glucose/insulin (25th–75th percentiles) | 0.75 [0.44–1.74] |
1.03 [0.53–2.71] | 0.025 | 1.37 [0.66–3.63] |
0.81 [0.53–1.95] |
0.67 [0.40–1.49] | <0.000 1 | 0.78[0.48–1.95] |
| Median HOMA-IR (25th–75th percentiles) |
1.56 [0.60–2.91] |
3.11 [1.21–5.89] |
<0.000 1 |
0.95 [0.35–2.11] |
1.90 [0.80–3.35] |
2.62 [1.20–4.69] |
<0.000 1 |
1.85 [0.76–3.71] |
| Median HOMA-B% (25th–75th percentiles) |
75.4 [35.6–149.0] |
34.8 [9.4–71.9] | <0.000 1 |
43.0 [15.2–97.1] |
56.2 [15.1–96.8] |
75.4 [31.2–142.9] |
0.001 | 60.0 [20.0–117.5] |
| Median QUICKI (25th–75th percentiles) |
0.15 [0.14–0.18] |
0.14[0.13–0.16] | <0.000 1 |
0.17 [0.15–0.20] |
0.15 [0.14–0.17] |
0.14 [0.13–0.16] |
<0.000 1 |
0.15 [0.14–0.17] |
| Median FIRI (25th–75th percentiles) |
1.40 [0.54–2.62] |
2.80 [1.09–5.30] |
<0.000 1 |
0.85 [0.31–1.90] |
1.71 [0.72–3.01] |
2.36 [1.08–4.22] |
<0.000 1 |
1.66 [0.68–3.34] |
Abbreviations: CRP, C-reactive protein; eGFR, estimated glomerularfiltration rate; FIRI, fasting insulin resistance index; HbA1c, glycated haemoglobin; HDL, High Density Lipoproteins; HOMA-B%, functional β-cells; GGT, γ-glutamyltransferase; LDL, Low Density Lipoproteins; HOMA-IR, homeostatic model assessment of insulin resistance; QUICKI, the quantitative insulin-sensitivity check index; SD, standard deviation.
Allele distribution and association test
Table 2 shows the allele and genotype distribution of SNPs investigated in the present study. None of the SNPs investigated deviated from Hardy Weinberg Equilibrium (HWE) in the overall sample (p ≥ 0.103).
Table 2.
Genotype distributions, minor allele frequencies, and unadjusted p-values for comparing genotype distributions according to diabetes and BMI status
| No diabetes | Diabetes | p-value | Normal weight |
overweight | Obese | p-value | Overall | |
| N | 328 | 152 | 118 | 129 | 233 | 532 | ||
| ENPP1-rs997509 | ||||||||
| C/C, n (%) | 254 (77.4) | 121 (79.6) | 0.186 | 97 (82.2) |
94 (72.9) | 184 (79.0) |
0.342 | 375 (78.1) |
| C/T, n (%) | 70 (21.3) | 26 (17.1) | 28 (15.2) |
33 (25.6) | 45 (19.3) |
96 (20.0) |
||
| T/T, n (%) | 4 (1.2) | 5 (3.3) | 3 (2.5) | 2 (1.5) | 4 (1.7) | 9 (1.9) | ||
| T, n (%) | 78 (11.9) | 36 (11.8) | 0.920 | 34 (14.4) |
37 (14.3) | 53 (11.4) |
0.383 | 114 (11.9) |
| HWE (p-value) | >0.999 | 0.038 | 0.095 | >0.999 | 0.511 | 0.379 | ||
| ENPP1-rs1044498 | ||||||||
| C/C, n (%) | 81 (24.7) | 41 (27.0) | 0.548 | 33 (28.0) |
35 (25.6) | 56 (24.0) |
0.852 | 122 (25.0) |
| C/A, n (%) | 175 (53.4) | 73 (48.0) | 62 (52.5) |
66 (51.2) | 120 (51.5) |
248 (51.7) |
||
| A/A, n (%) | 72 (21.9) | 38 (25.0) | 23 (19.5) |
30 (23.3) | 57 (24.5) |
110 (22.9) |
||
| A, n (%) | 319 (48.6) | 149 (49.0) | >0.999 | 108 (45.8) |
126 (48.8) | 234 (50.2) |
0.538 | 468 (48.8) |
| HWE (p-value) | 0.269 | 0.629 | 0.582 | 0.861 | 0.695 | 0.522 | ||
| FTO-9941349 | ||||||||
| C/C, n (%) | 187 (57.0) | 73 (48.0) | 0.185 | 63 (53.4) |
64 (49.6) | 133 (57.1) |
0.595 | 260 (54.2) |
| C/T, n (%) | 118 (36.0) | 66 (43.4) | 44 (37.3) |
56 (43.4) | 84 (36.0) |
184 (38.3) |
||
| T/T, n (%) | 23 (7.0) | 13 (8.5) | 11 (9.3) | 9 (7.0) | 16 (6.9) |
36 (7.5) | ||
| T, n (%) | 164 (25.0) | 92 (30.3) | 0.102 | 66 (28.0) |
74 (28.7) | 116 (24.9) |
0.475 | 256 (26.7) |
| HWE (p-value) | 0.463 | 0.848 | 0.492 | 0.666 | 0.599 | 0.642 | ||
| FTO-rs3751812 | ||||||||
| G/G, n (%) | 218 (66.5) | 93 (61.2) | 0.516 | 75 (63.6) |
79 (61.2) | 157 (67.4) |
0.761 | 311 (64.8) |
| G/T, n (%) | 100 (30.5) | 53 (34.9) | 38 (32.2) |
46 (35.7) | 69 (29.6) |
153 (31.9) |
||
| T/T, n (%) | 10 (3.0) | 6 (3.9) | 5 (4.2) | 4 (3.1) | 7 (3.0) | 16 (3.3) | ||
| T, n (%) | 120 (18.3) | 65 (21.4) | 0.299 | 48 (20.3) |
54 (20.9) | 83 (17.8) |
0.530 | 185 (19.3) |
| HWE (p-value) | 0.854 | 0.810 | >0.999 | 0.593 | >0.999 | 0.662 | ||
| TCF7L2-rs12255372 | ||||||||
| G/G, n (%) | 221 (67.4) | 92 (60.5) | 0.305 | 72 (61.0) |
88 (68.2) | 153 (65.7) |
0.623 | 313 (65.2) |
| G/T, n (%) | 92 (28.0) | 50 (32.9) | 37 (31.6) |
36 (27.9) | 69 (29.6) |
142 (29.6) |
||
| T/T, n (%) | 15 (4.6) | 10 (6.6) | 9 (7.6) | 5 (3.9) | 11 (4.7) |
25 (5.2) | ||
| T, n (%) | 122 (18.6) | 70 (23.0) | 0.131 | 55 (23.3) |
46 (17.8) | 91 (19.5) |
0.295 | 192 (20.0) |
| HWE (p-value) | 0.200 | 0.364 | 0.197 | 0.552 | 0.403 | 0.115 | ||
| TCF7L2-rs7903146 | ||||||||
| C/C, n (%) | 184 (56.1) | 66 (43.4) | 0.025 | 57 (48.3) |
66 (51.2) | 127 (54.5) |
0.330 | 250 (52.1) |
| C/T, n (%) | 129 (39.3) | 74 (48.7) | 52 (44.0) |
53 (41.1) | 98 (42.1) |
203 (42.3) |
||
| T/T, n (%) | 15 (4.6) | 12 (7.9) | 9 (7.6) | 10 (7.7) | 8 (3.4) | 27 (5.6) | ||
| T, n (%) | 159 (24.2) | 98 (32.2) | 0.011 | 70 (29.7) |
73 (28.3) | 114 (24.5) |
0.275 | 257 (26.8) |
| HWE (p-value) | 0.231 | 0.196 | 0.661 | >0.999 | 0.050 | 0.103 |
Abbreviation: ENPP1, ectonucleotide pyrophosphatase/phosphodiesterase gene; FTO, fat mass and obesity-associated gene; TCF7L2, transcription factor 7-like 2 gene; HWE, Hardy-Weindberg Equilibrium (HWE p-values are from exact tests)
Within each gene, the SNPs were in linkage disequilibrium (LD) in all sub-groups of the study population (D' ≥ 0.614) (online Figure 1). The SNPs investigated showed no differences in the distribution of alleles and genotypes between the study population sub-groups according to T2DM and BMI status, except rs7903146. The TCF7L2-rs7903146 minor allele was prevalent in diabetic compared to non-diabetic individuals (32.2% vs. 24.2 %, p = 0.011).
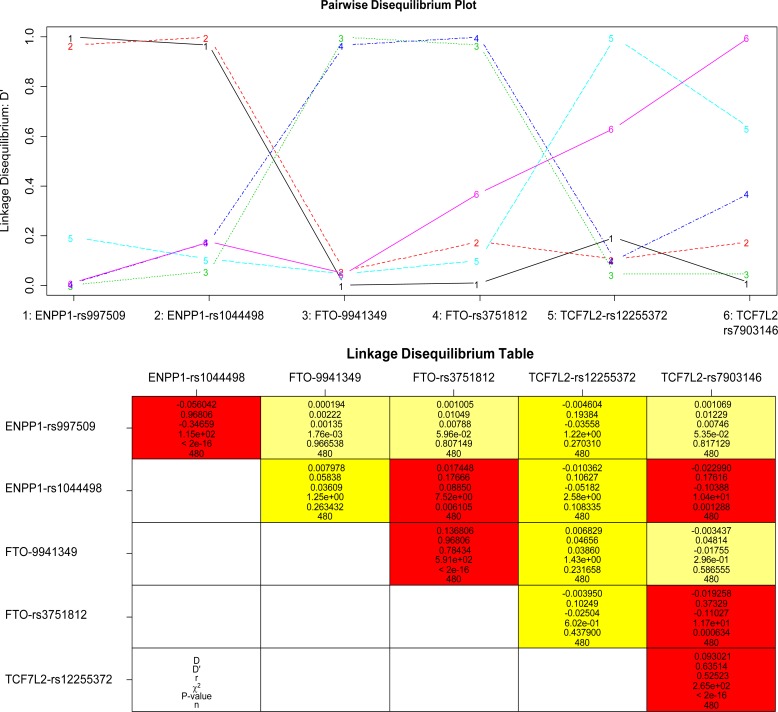
Online Figure 1.
linkage disequilibrium plot (upper panel) and linkage disequilibrium table (lower panel) for the six SNP in 480 participants. ENPP1-rs997509 is in linkage disequilibrium (LD) with ENPP1-rs1044498 overall (D'=0.968), in participants without diabetes (D'=0.950), in those with diabetes (D'=0.999), in normal weight (D'=0.998), overweight (D'=0.999) and obese participants (D'=0.939). FTO-9941349 is in linkage disequilibrium (LD) with FTO-rs3751812 overall (D'=0.968), in participants without diabetes (D'=0.952) and in those with diabetes (D'>0.999), in normal weight (D'=0.937), overweight (D'=0.943) and obese participants (D'=0.999). TCF7L2-rs12255372 is in linkage disequilibrium (LD) with TCF7L2-rs7903146 overall (D'=0.635), in participants without diabetes (D'=0.648) and in those with diabetes (D'=0.602), in normal weight (D'=0.614), overweight (D'=0.648) and obese participants (D'=0.641).
In linear analyses adjusted for age, sex, BMI and HOMA IR (Table 3), minor allele (T) of ENPP1-rs997509 was associated with a higher risk of prevalent type 2 diabetes under a recessive model [odds ratio (95% confidence interval), 4.60 (1.07 to 19.86); p = 0.040], but with no evidence of significant statistical interaction with BMI categories. The TCF7L2-rs7903146 minor allele showed a borderline significant association with a high risk of T2DM [1.43 (1.00 to 2.04); p= 0.053] in log-additive models (Table 3).
Table 3.
logistic regression models showing the effects of genes on prevalent diabetes risk.
| Overall | BMI categories*SNP interaction | |||
| SNP | Allele | Effects size (95%CI) | p | |
| Recessive model | ||||
| ENPP1-rs997509 | T/T | 4.60 (1.07 to 19.86) | 0.040 | 0.173 |
| ENPP1-rs1044498 | A/A | 1.48 (0.89 to 2.47) | 0.134 | 0.059 |
| FTO-9941349 | T/T | 1.10 (0.49 to 2.43) | 0.822 | 0.155 |
| FTO-rs3751812 | T/T | 0.85 (0.26 to 2.81) | 0.937 | 0.374 |
| TCF7L2-rs12255372 | T/T | 1.56 (0.61 to 4.00) | 0.361 | 0.160 |
| TCF7L2-rs7903146 | T/T | 1.57 (0.64 to 3.86) | 0.329 | 0.972 |
| Log additive model | ||||
| ENPP1-rs997509 | T | 0.98 (0.62 to 1.54) | 0.915 | |
| ENPP1-rs1044498 | A | 0.99 (0.72 to 1.36) | 0.954 | |
| FTO-9941349 | T | 1.40 (1.00 to 1.96) | 0.052 | |
| FTO-rs3751812 | T | 1.22 (0.83 to 1.80) | 0.307 | |
| TCF7L2-rs12255372 | T | 1.28 (0.89 to 1.83) | 0.191 | |
| TCF7L2-rs7903146 | T | 1.43 (1.00 to 2.04) | 0.053 | |
Models are adjusted for age, sex, BMI and HOMA IR. Effect estimates are odd ratio and 95% confidence intervals. Abbreviation: ENPP1, ectonucleotide pyrophosphatase/phosphodiesterase gene; FTO, fat mass and obesity-associated gene; TCF7L2, transcription factor 7-like 2 gene.
Although the distribution of alleles and genotypes of the FTO-rs9941349 were not significant between the subgroups (Table 2), the minor allele was marginally significantly associated with a high risk of T2DM [1.40 (1.00 to 1.96); p = 0.052] in log-additive models (Table 3). The inferred haplotype association analysis showed a negative effect of the rs997509-rs1044498 (TA) and rs9941349-rs3751812 (CT) on the risk of T2DM, however, the haplotypes occurred at very low frequencies (Table 4).
Table 4.
Logistic regression models showing the haplotype effects of genes on prevalent diabetes risk
| Overall | |||||
| SNP 1 | SNP 2 | Haplotype | Estimated frequency (%) |
Effects size (95%CI) | p |
| ENPP1-rs997509 | ENPP1-rs1044498 | CA | 48.4 | 1 (reference) | |
| CC | 39.6 | 0.98 (0.70 to 1.38) | 0.904 | ||
| TA | 0.2 | 7.213(−10) [7.213(−10) to 7.213(−10)] | <0.000 1 |
||
| TC | 11.8 | 1.01 (0.62 to 1.65) | 0.969 | ||
| Global effects | 0.753 | ||||
| FTO-rs9941349 | FTO-rs3751812 | CG | 73.1 | 1 (reference) | |
| CT | 0.5 | 4.407(−9) [4.407(−9) to 4.407(−9)] | <0.000 1 |
||
| TG | 7.6 | 1.52 (0.88 to 2.64) | 0.135 | ||
| TT | 18.8 | 1.34 (0.90 to 2.00) | 0.150 | ||
| TCF7L2-rs12255372 | TCF7L2-rs7903146 | GC | 67.8 | 1 (reference) | |
| GT | 12.2 | 1.49 (0.91 to 2.45) | 0.110 | ||
| TC | 5.3 | 1.31 (0.64 to 2.73) | 0.455 | ||
| TT | 14.5 | 1.41 (0.90 to 2.20) | 0.129 | ||
Models are adjusted for age, sex, body mass index and HOMA-IR. Effect estimates are odd ratio and 95% confidence intervals for qualitative traits. Abbreviation: ENPP1, ectonucleotide pyrophosphatase/phosphodiesterase gene; FTO, fat mass and obesity-associated gene; TCF7L2, transcription factor 7-like 2 gene.
Discussion
In the present study we demonstrated that, in a recessive model, the minor allele of the ENPP1 rs997509 is associated with a high risk of T2DM. The presence of both ENPP1-rs997509 T alleles conferred a 7% higher risk of prevalent T2DM. However, when analysed as a haplotype with rs1044498 (TA), the direction of association changed and this effect was abolished by the presence of the rs1044498 C allele. The haplotype association should be analysed with caution due to the low frequency of the TA haplotype (0.2%). The rs1044498 minor allele failed to show any association with T2DM risk, similar to studies conducted elsewhere in other ethnic population groups13,16. This is in contrast to findings of other studies conducted in Europeans and Chinese12,14 which demonstrated increased risk of T2DM among carriers of the 121QQ genotype.
The ENPP1-encoded protein inhibits insulin-signalling through interaction with and autophosphorylation of the insulin receptor beta-subunit, thus causing insulin resistance15. However, in the present study we did not find any significant association between ENPP1 SNPs and indicators of insulin resistance/sensitivity. Instead, in a previous study we demonstrated that the PPARGPro12 allele was associated with insulin resistance in this mixed ancestry cohort21. Like ENPP1, PPARG plays a role in insulin sensitivity. Although the role of ENPP1 SNPs in insulin resistance was not evident in the present study, combined the findings of our two studies and based on the functions of the two proteins suggest that insulin resistance rather than a defect in insulin secretion is a primary defect that may lead to the development of T2DM in this population group. Further supporting this hypothesis is the marginal association between TCF7L2 and T2DM without any effect on the measure of beta-cell function (HOMA-B%).
The two TCF7L2 polymorphisms have been reported to influence the development of T2DM across several population groups worldwide30–32. While this is the case in other population groups, some studies reported a weak33,34 or no association35–38. Other studies highlighted the effect of the TCF7L2 polymorphisms on the progression of T2DM35,39, in some ethnic population groups these variants were associated with responses to medication40,41, and in others their effect on the disease was modulated by the type of diet42–44. Our results [1.43 (1.00 to 2.04), p= 0.053] are comparable to those reported for other African ethnic population groups17, 18,20. It is possible that TCF7L2 polymorphisms have different effects in various ethnic population groups depending on the diet.
Insulin resistance is often related to obesity, and several studies have demonstrated that the association of ENPP1 and FTO polymorphisms with T2DM is mediated by obesity8,9,45,46. However, our study failed to show any association between ENPP1, FTO and obesity. The FTO-rs9941349 was marginally associated with an increased risk of T2DM independent of obesity in our study population. The finding that the rs9941349 polymorphism is marginally associated with the risk of T2DM raises the possibility that this SNP is in linkage disequilibrium with a causal locus that is yet to be identified in mixed ancestry population. In African-Americans, this polymorphism was positively associated with BMI47. It is possible that the different effects of the rs9941349 are attributable to different linkage disequilibrium blocks harbouring FTO polymorphisms in these two ethnic population groups. A comprehensive analysis of FTO polymorphisms in a large population of South African mixed-ancestry is warranted to clarify their effect on T2DM and obesity.
Limitations
Our study had limitations. We investigated for the first time the role of ENPP1, FTO and TCF7L2 polymorphisms in a South African mixed ancestry ethnic population with a unique genetic architecture. However, only two SNPs in each gene were studied, and therefore we cannot rule out the possible role of other variants within these genes. Due to financial constraints, ancestry informative markers were not used to account for population admixture, and there is a possibility that population admixture interfered with the association analysis. However, when tested none of the SNPs deviated from HWE. Association of ENPP1, FTO and TCF7L2 polymorphisms with high risk of T2DM may have also been confounded by lifestyle effects as reported elsewhere. For example, Moore and co-workers48 showed that the significant association between ENPP1 and increased incidence of T2DM was mediated by lifestyle or metformin intervention. Ortega-Azorin and co-workers49 also found an interaction between FTO and mediterranean diet in determining T2DM, with carriers of the FTO-rs9939609 minor allele on a low Meddiet having a higher risk of prevalent T2DM than individuals homozygous for the major allele. In a European-American population group, Mattei and co-workers50 observed a significant interaction between the TCF7L2-rs12255372-TT risk genotype and fat intake for changes in BMI, total fat mass, and trunk fat mass at 6 month of lifestyle intervention. The study suggested that this effect may help in inducing better glycemic control for such individuals predisposed to T2DM. It is therefore possible that the marginal association between FTO and TCF7L2 polymorphisms may be strengthened by inclusion of environmental factors in the analysis, which was not possible in our study due to lack of information on these variables. Due to a small sample size, we did not match cases of T2DM and controls by BMI to investigate whether or not obesity modulates the effect of polymorphisms on T2DM.
Conclusion
Our study has conducted a genetic association of the FTO, ENPP1, and TCF7L2 polymorphisms with T2DM and related traits. We found significant association between the rs997509 SNP while the rs7903146 and rs9941349 demonstrated borderline correlation with T2DM in the South African mixed ancestry ethnic population. A genome-wide association analysis in a large sample size is required to identify polymorphisms that may predispose mixed ancestry individuals to T2DM, accounting for ancestry genetic background.
Acknowledgements
We would like to express our gratitude to the Bellville South Community of Cape Town, South Africa. This research was supported by grants from the South African Medical Research Council, and University Research Fund of the Cape Peninsula University of Technology, South Africa.
Disclosure statement
None for all co-authors.
Contribution statement: YYY: conception and design, acquisition and interpretation of data, preparation of the first draft and approval of final draft. JHM acquisition and interpretation of data, and approval of final draft. APK: analysis and interpretation of data, revision for important intellectual content and approval of final draft. RTE: acquisition of data, revision for important intellectual content and approval of final draft. TSP: revision for important intellectual content and approval of final draft. TEM: conception and design, acquisition and interpretation of data, revision for important intellectual content and approval of final draft.
References
- 1.Qi Q, Hu FB. Genetics of type 2 diabetes in European populations. J Diabetes. 2012;4:203–212. doi: 10.1111/j.1753-0407.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu W, Hu C, Jia W. Genetic advances of type 2 diabetes in Chinese populations. J Diabetes. 2012;4:213–220. doi: 10.1111/j.1753-0407.2012.00225.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1074/jbc.M411487200. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- 4.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem. 2005;280:1457–1464. doi: 10.1074/jbc.M411487200. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Damcott CM, Pollin TI, Reinhart LJ, et al. Polymorphisms in the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in the Amish: replication and evidence for a role in both insulin secretion and insulin resistance. Diabetes. 2006;55:2654–2659. doi: 10.2337/db06-0338. PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scuteri A, Sanna S, Chen WM, et al. Genome-Wide Association Scan Shows Genetic Variants in the FTO Gene Are Associated with Obesity-Related Traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renström F, Payne F, Nordström A, et al. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet. 2009;18:1489–1496. doi: 10.1093/hmg/ddp041. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasan SK, Karpe F, Gu HF, et al. FTO genetic variants and risk of obesity and type 2 diabetes: a meta-analysis of 28,394 Indians. Obesity (Silver Spring) 2014;22:964–970. doi: 10.1002/oby.20606. [DOI] [PubMed] [Google Scholar]
- 10.Hertel JK, Johansson S, Sonestedt E, et al. FTO, type 2 diabetes, and weight gain throughout adult life: a meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC, and MPP studies. Diabetes. 2011;60:1637–1644. doi: 10.2337/db10-1340. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rees SD, Islam M, Hydrie MZ, et al. An FTO variant is associated with Type 2 diabetes in South Asian populations after accounting for body mass index and waist circumference. Diabet Med. 2011;28:673–680. doi: 10.1111/j.1464-5491.2011.03257.x. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAteer JB, Prudente S, Bacci S, et al. The ENPP1 K121Q polymorphism is associated with type 2 diabetes in European populations: evidence from an updated meta-analysis I 42,042 subjects. Diabetes. 2008;57:1125–1130. doi: 10.2337/db07-1336. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Seo HJ, Kim SG, Kwon OJ. The K121Q polymorphism in ENPP1 (PC-1) is not associated with type 2 diabetes or obesity in Korean male workers. J Korean Med Sci. 2008;23:459–464. doi: 10.3346/jkms.2008.23.3.459. PubMed. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YY. ENPP1 K121Q polymorphism and type 2 diabetes mellitus in the Chinese population: a meta-analysis including 11,855 subjects. Metabolism. 2012;61:625–633. doi: 10.1016/j.metabol.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Goldfine ID, Maddux BA, Youngren JF, et al. The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities. Endocr Rev. 2008;29:62–75. doi: 10.1210/er.2007-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzidi I, Mtiraoui N, Cauchi S, et al. Contribution of type 2 diabetes associated loci in the Arabic population from Tunisia: a case-control study. BMC Med Genet. 2009;10:33. doi: 10.1186/1471-2350-10-33. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouhaha R, Choquet H, Meyre D, et al. TCF7L2 is associated with type 2 diabetes in nonobese individuals from Tunisia. Pathol Biol (Paris) 2010;58:426–429. doi: 10.1016/j.patbio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Pirie FJ, Motala AA, Pegoraro RJ, Paruk IM, Govender T, Rom L. Variants in PPARG, KCNJ11, TCF7L2, FTO, and HHEX genes in South African subjects of Zulu descent with type 2 diabetes. Afr J Diab Med. 2010;18:12–16. [Google Scholar]
- 19.Cauchi S, Ezzidi I, El Achhab Y, et al. European genetic variants associated with type 2 diabetes in North African Arabs. Diabetes Metab. 2012;38:316–323. doi: 10.1016/j.diabet.2012.02.003. PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Danquah I, Othmer T, Frank LK, Bedu-Addo G, Schulze MB, Mockenhaupt FP. The TCF7L2 rs7903146 (T) allele is associated with type 2 diabetes in urban Ghana: a hospital-based case-control study. BMC Med Genet. 2013;14:96. doi: 10.1186/1471-2350-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vergotine Z, Yako YY, Kengne AP, Erasmus RT, Matsha TE. Proliferator-activated receptor gamma Pro-12Ala interacts with the insulin receptor substrate 1 Gly972Arg and increase the risk of insulin resistance and diabetes in the mixed ancestry population from South Africa. BMC Genet. 2014;15:10. doi: 10.1186/1471-2156-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YS, Chen CH, Hu C, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2011;44:67–7223. doi: 10.1038/ng.1019. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai K, Imamura M, Tanaka Y, et al. Replication study for the association of 9 East Asian GWAS-derived loci with susceptibility to type 2 diabetes in a Japanese population. PLoS One. 2013;8:e76317. doi: 10.1371/journal.pone.0076317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergotine Z, Yako YY, Kengne AP, Erasmus RT, Matsha TE. Insulin Receptor Substrate-1 Gly972Arg variant is not associated with type 2 diabetes in a South African population. S Afri Med J. 2014;104:420–423. doi: 10.7196/samj.7419. [DOI] [PubMed] [Google Scholar]
- 25.de Wit E, Delport W, Rugamika CE, et al. Genome-wide analysis of the structure of the South African coloured population in the Western Cape. Hum Genet. 2010;128:145–153. doi: 10.1007/s00439-010-0836-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Zemlin AE, Matsha TE, Hassan MS, Erasmus RT. HbA1c of 6.5% to diagnose diabetes mellitus - does it work for us?-the Bellville South Africa study. PloS One. 2011;6:e22558. doi: 10.1371/journal.pone.0022558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsha TE, Hassan MS, Kidd M, Erasmus RT. The 30-year cardiovascular risk profile of South Africans with diagnosed diabetes, undiagnosed diabetes, pre-diabetes or normoglycaemia: the Bellville, South Africa pilot study. Cardiovasc J Afr. 2012;23:5–11. doi: 10.5830/CVJA-2010-087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens. 1999;21:1009–1060. doi: 10.3109/10641969909061028. PubMed. [DOI] [PubMed] [Google Scholar]
- 29.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. PMID: 9686693. [DOI] [PubMed] [Google Scholar]
- 30.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 31.Cauchi S, Nead KT, Choquet H, et al. The genetic susceptibility to type 2 diabetes may be modulated by obesity status: implications for association studies. BMC Med Genet. 2008;9:45. doi: 10.1186/1471-2350-9-45. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uma Jyothi K, Jayaraj M, Subburaj KS, et al. Association of TCF7L2 gene polymorphisms with T2DM in the population of Hyderabad, India. PLoS One. 2013;8:e60212. doi: 10.1371/journal.pone.0060212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo T, Hanson RL, Traurig M, et al. TCF7L2 is not a major susceptibility gene for type 2 diabetes in Pima Indians: analysis of 3,501 individuals. Diabetes. 2007;56:3082–3088. doi: 10.2337/db07-0621. PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Ren Q, Han XY, Wang F, et al. Exon sequencing and association analysis of polymorphisms in TCF7L2 with type 2 diabetes in a Chinese population. Diabetologia. 2008;51:1146–1152. doi: 10.1007/s00125-008-1039-3. [DOI] [PubMed] [Google Scholar]
- 35.Chang YC, Chiu YF, Ho LL, et al. TCF7L2 genetic variants and progression to diabetes in the Chinese population: pleiotropic effects on insulin secretion and insulin resistance. J Mol Med (Berl) 2010;88:183–192. doi: 10.1007/s00109-009-0542-4. [DOI] [PubMed] [Google Scholar]
- 36.Kifagi C, Makni K, Boudawara M, et al. Association of Genetic Variations in TCF7L2, SLC30A8, HHEX, LOC387761, and EXT2 with Type 2 Diabetes Mellitus in Tunisia. Genet Test Mol Biomarkers. 2011;15:399–405. doi: 10.1089/gtmb.2010.0199. [DOI] [PubMed] [Google Scholar]
- 37.Zheng X, Ren W, Zhang S, et al. Association of type 2 diabetes susceptibility genes (TCF7L2, SLC30A8, PCSK1 and PCSK2) and proinsulin conversion in a Chinese population. Mol Biol Rep. 2012;39:17–23. doi: 10.1007/s11033-011-0705-6. [DOI] [PubMed] [Google Scholar]
- 38.Park SE, Lee WY, Oh KW, et al. Impact of common type 2 diabetes risk gene variants on future type 2 diabetes in the nondiabetic population in Korea. J Hum Genet. 2012;57:265–268. doi: 10.1038/jhg.2012.16. [DOI] [PubMed] [Google Scholar]
- 39.Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355:241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearson ER, Donnelly LA, Kimber C, et al. Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTs study. Diabetes. 2007;56:2178–2182. doi: 10.2337/db07-0440. [DOI] [PubMed] [Google Scholar]
- 41.'t Hart LM, Fritsche A, Nijpels G, et al. The CTRB1/2 locus affects diabetes susceptibility and treatment via the incretin pathway. Diabetes. 2013;62:3275–3281. doi: 10.2337/db13-0227. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher E, Boeing H, Fritsche A, Doering F, Joost HG, Schulze MB. Whole-grainconsumptionand transcriptionfactor-7-like 2 (TCF7L2) rs7903146: gene-diet interaction in modulating type 2 diabetes risk. Br J Nutr. 2009;101:478–481. doi: 10.1017/S0007114508020369. [DOI] [PubMed] [Google Scholar]
- 43.Cornelis MC, Qi L, Kraft P, Hu FB. TCF7L2, dietarycarbohydrate, and risk of type 2 diabetes in US women. Am J ClinNutr. 2009;89:1256–1262. doi: 10.3945/ajcn.2008.27058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hindy G, Sonestedt E, Ericson U, et al. Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia. 2012;55:2646–2654. doi: 10.1007/s00125-012-2634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bochenski J, Placha G, Wanic K, et al. New polymorphism of ENPP1 (PC-1) is associated with increased risk of type 2 diabetes among obese individuals. Diabetes. 2006;55:2626–2630. doi: 10.2337/db06-0191. [DOI] [PubMed] [Google Scholar]
- 46.Robiou-du-Pont S, Bonnefond A, Yengo L, et al. Contribution of 24 obesity-associated genetic variants to insulin resistance, pancreatic beta-cell function and type 2 diabetes risk in the French population. Int J Obes (Lond) 2013;37:980–985. doi: 10.1038/ijo.2012.175. [DOI] [PubMed] [Google Scholar]
- 47.Hassanein MT, Lyon HN, Nguyen TT, et al. Fine mapping of the association with obesity at the FTO locus in African-derived populations. HumMol Genet. 2010;19:2907–2916. doi: 10.1093/hmg/ddq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore AF, Jablonski KA, Mason CC, et al. The association of ENPP1 K121Q with diabetes incidence is abolished by lifestyle modification in the diabetes prevention program. J Clin Endocrinol Metab. 2009;94:449–455. doi: 10.1210/jc.2008-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortega-Azorín C, Sorlí JV, Asensio EM, et al. Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc Diabetol. 2012;11:137. doi: 10.1186/1475-2840-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattei J, Qi Q, Hu FB, Sacks FM, Qi L. TCF7L2-rs12255372-TT risk genotype and fat intake for changes in BMI, total fat mass, and trunk fat mass at 6 month of lifestyle intervention. Am J Clin Nutr. 2012;96:1129–1136. doi: 10.3945/ajcn.112.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]