Abstract
Background
The burden of stroke worldwide is increasing rapidly. There is paucity of data on post-stroke depression (PSD) among stroke survivors in Uganda, despite the high prevalence of PSD reported elsewhere.
Methods
In a cross-sectional study, we assessed adult participants with confirmed first stroke with a standardized questionnaire. The Patient Health Questionnaire-9 was used to assess for depression among non-aphasic patients while the Aphasic Depression Rating Scale was administered to aphasic patients. Univariable and multivariable analyses performed to describe associations with PSD.
Results
Forty three females (58.9%) and 30 males (41.1%) who had a stroke participated. Fifty eight (79.5%) had ischemic strokes and 12 participants (16.4%) were aphasic. The prevalence of PSD among the study participants was 31.5%. PSD was higher among patients assessed within 6 months after the onset of stroke. PSD was strongly associated with the total Barthel index of activities of daily living (BIADL) score; p=0.001. There was no significant association between demographic characteristics and PSD.
Conclusion
There is a high prevalence of unrecognized post-stroke depression. Post-stroke depression was strongly associated with the patient's inability to undertake activities of daily life. There is urgent need for integration of screening for and management of post-stroke depression among stroke survivors.
Keywords: Stroke, post-stroke depression
Introduction
Stroke is a common neurological problem and one of the leading causes of death in developing countries of the world1. Stroke is also the leading causes of functional impairments, with 20% of survivors requiring institutional care after three months and 15–30% being permanently disabled2. Stroke patients have been consistently noted to experience changes in their emotional status that range from simple emotional reactions such as low motivation, indifference, and frustration to more complicated depressive symptoms like loss of appetite, insomnia, and feelings of worthlessness3. Depression is the most common mood problem after stroke and it can occur within days to several months post-stroke4. Major depression is estimated at 10 – 27% among these stroke survivors while 15 – 40% will experience symptoms of depression within two months following a stroke4. Previous studies have reported prevalence rates that have ranged from 18% to 61%, depending upon patient selection and criteria used5–7. In Nigeria, Oladiji et al found a prevalence of 25.5% among post-stroke patients attending an out-patient setting in Nigeria using the depression anxiety stress scale8. A study in Finland using DSM III-R found a prevalence of 40.1% among adult subjects with ischemic stroke9.
It is widely accepted that the early recognition and management of depression is an important aspect at any stage of stroke rehabilitation10. Important socio-demographic factors such as age above 55 years, female sex and living environment after stroke have been reported to increase the risk of PSD. Neurobiological mechanisms, with the highest risk for depression in cases with a left hemispherical infarction and theories that catecholamines may be depleted have been suggested among these patients3,11,12. Diagnosis of PSD is challenging; therefore, it often remains unrecognized and or under treated. PSD is associated with cognitive impairment, increased mortality and risk of falls13.
Depressed stroke patients may die at a significantly younger age than those without depression. The risk of dying is 3.5 times greater among those depressed within a 10 year period following the acute event14. Depression has been reported to be an independent risk factor for vascular disease and myocardial infarction; this may be the cause of death among this population15,16.
This is the first study to document PSD in Uganda and was aimed at determining the burden of depression and the factors associated with depression among poststroke survivors in our setting. With the increasing burden of stroke and its related complications, this study provides baseline information on the prevalence of factors associated with PSD and severity of depression in post-stroke patients in Uganda. It also provides a basis for the integration of psychiatric care into the chronic care clinics. The adoption of the simple depression screening tool (PHQ-9) to guide health practitioners in our setting would help with early detection and intervention.
Methods
Ethical consideration and informed consent
The written informed consent was obtained from all study participants. The study, its potential risks and benefits to the patients were elaborated to the patients/relatives in simple and concise language. Patient confidentiality was maintained at all times and no patient identifiers were collected. Approval for conducting the study in the two hospitals was provided from the Department of Internal Medicine Mother Kevin Postgraduate Medical School and the School of Medicine, Research and Ethics Committee of Makerere University College of Health Sciences (Ref no -2013-146) and Uganda National Council of Science and Technology.
This was a cross sectional study conducted in two urban hospitals; St. Raphael of St Francis Hospital Nsambya and Mulago National Referral Hospital in Kampala, Uganda. These two hospitals provide specialist services for neurology and are teaching hospitals for medical students. Nsambya hospital is a private not for profit, church aided institution, while Mulago is a government supported institution. Patients with confirmed stroke attending these two hospitals between February 2014 to April 2014, who fulfilled the inclusion criteria and provided written, informed consented were enrolled for the study. The inclusion criteria included the following; age 18 years and above, with a single episode of stroke confirmed by brain computed tomography (CT) scan; and written informed consent. We excluded patients who were medically unstable with concurrent systemic disease, patients who had a prior diagnosis of depression or other psychiatric disorder that could affect cognition prior to the onset of stroke.
Using the stroke registry of St. Raphael of St Francis Hospital Nsambya post-stroke patients were contacted and appointments fixed on routine Medical clinic days of Monday and Thursday; those patients who could not make it on the routine clinic days were scheduled on days of their convenience and met by a study team member. Patients attending the Neurology clinic of Mulago Hospital Complex were enrolled at the weekly Wednesday neurology specialist outpatient clinic as they came for scheduled reviews. The information regarding the study was availed in Luganda a local language and English for those who did not understand Luganda. Patients or their eligible attendants who accepted to volunteer to participate signed written consent forms. Patients' demographics and clinical characteristics were collected using a pre-tested questionnaire. A focused neurological examination was performed. Limb power was graded using the Medical Research Council (MRC) scale17. Patients' admission and follow-up notes were reviewed to complete and confirm clinical variables and CT scan findings. During this study, the PHQ-9 was used and it has been validated in a number of studies with a sensitivity of 80% and specificity of 78% using a cutoff of ≥1018,19. The study participants were then classified according to the PHQ-9 total severity score. The PHQ-9 classifies the severity total score as minimal symptoms 5–9, minor depression, dysthymia, major depression, mild 10–14, major depression, moderately severe 15–19, and major depression, severe >2020,21.
Among aphasic patients, the aphasic depression rating scale (ADRS) was used22. The clinicians ruled out physical causes of depression, normal bereavement and a history of manic or hypo manic episodes. Patients who scored less than 5 on the scale were considered to have no depression while those with scores between 5 and 28 were considered depressed and further assessments performed20. For the aphasic patients, a score below 9 was considered as no depression while a score above 9 was considered as significant depression. For the aphasic patients, a score below 9 was considered as no depression while a score above 9 was considered as significant depression The main outcome was the patient's score on either the PHQ-9 or the ADRS22. The Barthel index of activities of daily living (BIADL) was used to assess the physical disability among the subjects23.
Data management and analysis
The data collected was double entered into EPI-DATA (version 2.1) software to minimize data entry errors. It was then exported to STATA software version 12 (Stata Corporation, College Station, TX, USA) for analysis. Confidentiality was maintained by the use of serial numbers instead of patients' names. Demographic and clinical characteristics were described among the study participants using frequencies and proportions. The variables of age, duration and total BIADL score were compared using Wilcoxon rank sum test. We calculated odds ratios (OR) and corresponding 95 percent confidence intervals (95% CI) to express the odds of post stroke depression.. The extent and severity of PSD were described and categorized from the respective PHQ-9 or ADRS scores of the patients. The prevalence of PSD was calculated as a proportion of the total number of post-stroke patients assessed for PSD. For these analyses, we defined depression as PHQ-9 score >5, and as ARDS-score >9. Patients' performances on the PHQ-9 or ADRS were analyzed using Kruskal-Wallis test to determine association between the severity of PSD and duration since the onset of stroke symptoms. We used these results to inform the choice of factors to include in the multivariable analyses. Final choice of input variables to the model were: age, duration of stroke symptoms, BIADL score, weakness of right upper limb and lower limbs. Associations with p values less than 0.05 were considered significant.
Results
Demographic characteristics of participants
A total of 73 post-stroke participants were enrolled. Nearly sixty percent (43/73) of the study participants were females (Table 1). Forty eight percent (35/73) of the study participants were between the ages sixty and seventy nine. Majority of the study participants, 75% were urban dwellers while the rest came from rural areas. Only 22% (16/73) were unemployed. Nearly forty percent (29/73) of the study participants were enrolled and assessed within 3 months since the onset of stroke symptoms, while for 27% (20/73) their stroke symptoms had lasted for more than 12 months prior to assessment.
Table 1.
Demographic characteristics of the study subjects
| Characteristic | N (%) |
| Sex | |
| Female | 43 (58.9) |
| Age in years | |
| 20 – 39 | 6 (8.2) |
| 40 – 59 | 25 (34.2) |
| 60 – 79 | 35 (47.9) |
| 80 –99 | 7(9.6) |
| Marital status | |
| Single | 10 (13.7) |
| Married | 32 (43.8) |
| Separated /Divorced | 10 (13.7) |
| Widowed | 21 (28.8) |
| Urban residence | 55 (75.3) |
| Employment type | |
| Formal / salaried | 24 (32.9) |
| Informal/wage | 33 (45.2) |
| Unemployed | 16 (21.9) |
| Form of income | |
| Monthly salary | 9 (12.3) |
| Wage | 3 (4.1) |
| Pension | 1 (1.4) |
| Business revenue | 27 (37.0) |
| Dependant | 22 (30.1) |
| More than one source | 11 (15.1) |
| Education level attained | |
| None | 8 (11.0) |
| Primary | 27 (37.0) |
| Secondary | 9 (12.3) |
| Diploma/degree and higher | 29 (39.7) |
| Duration of symptoms since onset in months | |
| Up to 3 months | 29 (39.7) |
| 3 – 6 months | 14 (19.2) |
| 7 – 12 months | 10 (13.7) |
| More than 12 months | 20 (27.4) |
| Number of co-existent co-morbidities | |
| One | 49 (67.1) |
| Two | 13 (17.8) |
| Three or more | 11 (15.1) |
Stroke characteristics of participants
Nearly seventy-nine percent (58/73) of the study participants had ischemic strokes while 21% (15/73) had hemorrhagic strokes. Nearly half of the study participants (36/73) had stroke lesions involving the left hemisphere while 33 involved the right. Only five percent (4/73) had lesions involving both hemispheres. The temporal lobe was the commonest brain lobe involved among the study participants and majority (75%) of these had single lobar involvement. Seventy percent (51/73) had brain lesions that were bigger than 225 mm2 while 30% (22/73) had lesions up to 225 mm2. Only 16% (12/73) were aphasic.
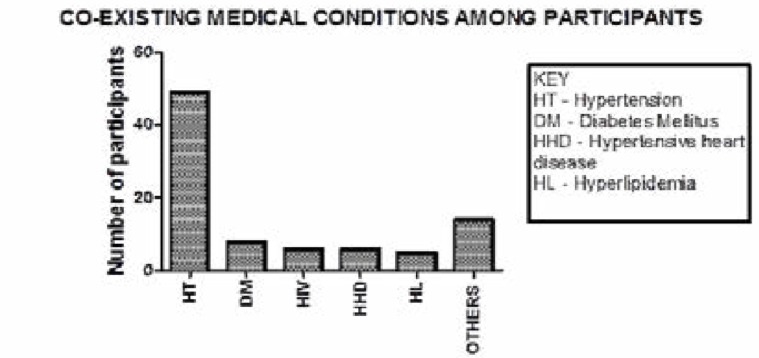
Sixty seven percent of the study participants had pre-existing hypertension, Diabetes was present in 7% (8/73), Human Immunodeficiency virus and Hypertensive heart disease each contributed 5% (6/73) while hyper lipidemia occurred in about 7% (5/73).
Two of the participants had associated atrial fibrillation, rheumatoid arthritis while two had un-explained strokes with no identifiable risk factor. Other associated diagnoses found in the study participants were deep venous thrombosis, temporal arteritis, metabolic syndrome, degenerative valvular heart disease, parkinsonism, Alzheimer's dementia, erectile dysfunction, and benign prostatic hypertrophy (Figure 1).
Figure 1.
Co-existing medical conditions among the study participants.
Majority of the study participants had co-existing hypertension followed by diabetes mellitus. Six study participants had HIV infection and were receiving attendant care from HIV care clinics at Mulago and Nsambya hospitals. Other medical conditions included atrial fibrillation, rheumatoid arthritis, deep venous thrombosis, temporal arteritis, associated Parkinsonism, Alzheimer's dementia, erectile dysfunction and benign prostatic hypertrophy.
Prevalence and severity of post-stroke depression
The prevalence of post-stroke depression was 31.5% (23/73); with a 95% CI: 20.6% – 42.4% among the study participants.
Among the seventeen non-aphasic subjects with depression, twelve (70.6%) had mild major depression, four (23.5%) had minimal symptoms of depression while one (5.9%) had moderately severe depression. None was found to have severe depression. In the aphasic group with depression, six had significant depression using the aphasic depression rating scale.
Only 37% (7/19) of both aphasic and non-aphasic participants were receiving treatment for their depressive illness despite regularly attending the neurology clinic.
Association of post-stroke depression with demographic characteristics
None of the baseline demographic characteristics was significantly associated with post-stroke depression. Participants that had not attended formal education were not associated with PSD; p =0.086. (See Table 2).
Table 2.
Stroke characteristics among the study participants
| Characteristic | N (%) |
| Stroke type | |
| Ischemic stroke | 58 (79.5) |
| Anatomical location of stroke | |
| Right hemisphere | 33 (45.2) |
| Left hemisphere | 36 (49.3) |
| Both hemispheres | 4 (5.5) |
| Dominant hemisphere lesion | 40 (54.8) |
| Brain lobe involvement | |
| Frontal lobe | 19 (26.0) |
| Temporal lobe | 36 (49.3) |
| Parietal lobe | 22 (30.1) |
| Occipital lobe | 2 (2.7) |
| Number of lobes involved | |
| None | 7 (9.6) |
| One | 55 (75.3) |
| Two | 9 (12.3) |
| 3 and more | 2 (2.7) |
| Lesion size | |
| >225mm2 | 51 (69.9) |
| Presence of aphasia | 12 (16.4) |
| MRC grading of power in the limbs | |
| Right upper limb | |
| 0 – 1 | 8 (11.0) |
| 2 – 3 | 14 (19.2) |
| 4 – 5 | 51 (69.9) |
| Right lower limb | |
| 0 – 1 | 8 ( 11.0) |
| 2 – 3 | 15(20.5) |
| 4 – 5 | 50 (68.5) |
| Left upper limb | |
| 0 – 1 | 4 (5.5) |
| 2 – 3 | 12 (16.4) |
| 4 – 5 | 57 (78.1) |
| Left lower limb | |
| 0 – 1 | 1 (1.4) |
| 2 – 3 | 8 (11.0) |
| 4 – 5 | 64 (87.7) |
| Total score on BIADL <19 | 35 (47.9) |
| Right handedness | 69 (94.5) |
Association of post-stroke depression with stroke characteristics
Post-stroke depression was commonly seen among participants who had the assessment done within 3 months after onset of stroke, 13 (56.5%), compared to those beyond 3 months (p=0.047), OR 0.36, 95% CI 0.131–1.00). However multivariable analysis did not reveal any statistical significance (p=0.356, OR 0.54, 95% CI 0.15 – 1.98). The location of strokes was not associated with PSD. Post stroke depression occurred proportionately higher (35.3%) among patients with bigger lesions more than 225 mm2 compared to those with smaller lesions (22.7%), but was not statistically different, p= 0.732; OR 1.85, 95% 0.58 — 5.86). The presence of aphasia was associated with a higher proportion of PSD (50.0%) compared to those without aphasia (27.9%). There was a higher proportion of PSD among participants whose limb power was less than 4 on the MRC scale, with highest significance seen in those with right upper limb weakness, p=0.013. There was no association of PSD with dominant hemisphere among the study participants, p=0.417; OR 0.66, 95%CI 0.25 – 1.79). (Table 3)
Table 3.
Association of post-stroke depression with demographic characteristics
| Characteristic | Depressed N=23(31.5%) |
Not depressed N=50(68.5%) |
p value |
| Age in years, n (%) | |||
| 20–39 | 2(8.7) | 4(54.8) | 0.777 |
| 40–59 | 8(34.8) | 17(23.3) | |
| 60–79 | 12(52.2) | 23(31.5) | |
| 80–99 | 1(4.3) | 6(8.2) | |
| Gender, n (%) | |||
| Male | 6 (26.1) | 24 (48.0) | 0.207 |
| Female | 17 (73.9) | 26 (52.0) | |
| Marital status, n (%) | |||
| Single | 4 (17.4) | 6 (12.0) | 0.401 |
| Married | 10 (43.5) | 22 (44.0) | |
| Divorced | 1 (4.4) | 9 (18.0) | |
| Widowed | 8 (34.8) | 13 (26.0) | |
| Residence status, n (%) | |||
| Rural | 5 (21.7) | 13 (26.0) | 0.695 |
| Urban | 18 (78.3) | 37 (74.0) | |
| Employment status, n (%) | |||
| Formal/salaried | 7 (30.4) | 17 (34.0) | 0.485 |
| Informal/wage | 9 (39.1) | 24 (48.0) | |
| Unemployed | 7 (30.04) | 9 (18.0) | |
| Form of income n (%) | |||
| Monthly salary | 2 (22.2) | 7 (77.8) | 0.561 |
| Wage | 1 (33.3) | 2 (66.7) | |
| Pension | 1 (100.0) | 0 (0) | |
| Business revenue | 7 (25.9) | 20 (74.1) | |
| Dependant | 9 (40.9) | 13 (59.1) | |
| More than one source | 3 (27.3) | 8 (72.3) | |
| Education level, n (%) | |||
| None | 5 (21.7%) | 3 (6.0%) | 0.086 |
| Primary | 5 (21.7%) | 22 (44.0%) | |
| Secondary | 2 (8.7%) | 7 (14.0%) | |
| Higher | 11 (47.8%) | 18 (36.0%) |
The median (IQR) BIADL score for those who were depressed was significantly different from those who were not depressed; 14 (11 – 19) compared to 20 (15–20) respectively with a p-value of 0.001 using Mann-Whitney U test.
Association of PSD severity with demographic characteristics
The demographic characteristics studied included age, gender, marital status, residence, employment status, education level and forms of income. However there was no association of the demographic characteristics with PSD for both the PHQ-9 with p-values of 0.611, 0.849, 0.965, 0.762, 0.285, 0.219 and 0.550 respectively. Even among the aphasic participants using the ADRS score, none of the demographic characteristics was also associated with PSD with p-values of 0.670, 1.00, 0.646, 0.762, 0.285, 0.219, and 0.550 respectively.
Relationship between severity of post-stroke depression and the time period after stroke
Among the non-aphasic patients with PSD, 10 participants out of the 13 with moderate and moderately severe depression (76.9%) had a time period between onset of stroke symptoms and assessment of up to 6 months; there was none in the 6 to 12 month group while 3 had a time period of 12 months and over (23.1%) (p= 0.128, OR 0.721, 95% CI 0.474–1.098). On multivariable analysis, duration of stroke symptoms up to 6 months was associated with more severe PSD only if BIADL score was below full capacity (p= 0.091, OR 1.199, 95% CI 0.290–4.963).
Discussion
This is the first study to describe the burden of post stroke depression in Uganda, although various studies have been conducted worldwide. The study was aimed at determining the burden of depression and the factors associated with post stroke depression and provide baseline data for integration of psychiatric care in the neurology clinics in Uganda.
We found a high prevalence (31.5%) of post-stroke depression and only seven of these subjects were receiving treatment for their depressive illnesses. This illustrates the treatment gap among stroke survivors and highlights the implications of their health outcomes. Earlier studies have reported a prevalence of 25.5% among post-stroke patients attending an out-patient setting in Nigeria using the depression anxiety stress scale8, while using DSM III-R a prevalence of 40.1% was reported9. The variation in prevalence among the various studies may be partly explained by the use of various tools by individual studies, presence of other causes of depression and possible differences in how samples were enrolled. It might also be due to the complexity of stroke patients for whom ordinary tools for depression may not be appropriate24.
Majority of the study participants were elderly females and mostly urban dwellers. This is similar to earlier work describing stroke in Uganda, which noted similar percentages of females25. The greater proportion of women with PSD in this study could be attributed to the generally better health seeking practices when compared to males due to gender differences in medical care utilization26. However, some studies have reported associations of depression and stroke among middle aged women27–30. Also regarding the sex-based differences, it could be attributed to response bias created by the greater tendency for women to remember and report symptoms of depression31. In our study, gender was not found to be an important risk factor of PSD, compared to other studies though the female gender contributed to 73.9% of study subjects with PSD.
Post-stroke depression (PSD) runs a chronic course and this enormously determines the health outcomes, including disability, morbidity and mortality32,33. About one third of all stroke survivors at any time during their follow-up may show depressive symptoms7. PSD may persist for 3–6 months after stroke with highest incidence occurring within 3 months after onset of stroke4. Overall the prevalence of post stroke depression reduces over time with an improvements in activities of daily living if treatment is initiated early3. In this present study, majority of the study participants (76.9%) had developed moderate and moderately severe depression within six months of onset of stroke symptoms. This is much higher than the prevalence of PSD compared to other African countries8,34. This might be due to different study settings and study methods, aphasic stroke subjects were excluded from these studies. Yet aphasia has been reported to be having a profound effect on a patient's life leading to depression35. The exclusion of aphasic patients might have contributed to the low prevalence in these studies. In this study the duration of stroke symptoms up to 6 months was associated with more severe PSD only if BIADL score was below full capacity. The social circumstances and personality issues may be important in prevalence of depression among different communities and races. The time of duration of stroke does not affect PSD occurrence. However, depressive symptoms are frequent and are likely to prevail at longer follow-up times. The duration of the depressive symptoms is associated with stroke severity and functional outcome from 2 months onwards9.
In this present study urban dwellers had higher proportions of PSD compared to rural dwellers; this is similar to earlier work36. This could be due to locations where the two centers where this study was conducted are urban and serve mainly urban dwellers, although one of them is a national referral serving the whole country. In addition the demands of life could be more strenuous coupled with less community support which is more likely to prevail among urban dwellers37. The uncertainties of getting through a day that are associated with unemployment could explain the higher prevalence of PSD among the unemployed38. The higher proportion among the non-educated is most probably coupled with the lack of skills to sustain themselves especially in the presence of such a debilitating condition as stroke39,40.
Notably none of the patients with occipital or brainstem lesions had depression and this is similar to earlier findings41 which could be because posterior circulation areas of the brain are not involved in personality and executive functions. In this present study, the sample size was too small to permit an accurate assessment of PSD in relation to stroke location.
In this present study, the size of lesions involving any of the lobes of the brain as well having lesions bigger than the arbitrary lacunar level of 15 mm was associated with a higher proportion of PSD. A recent biological study has reported that cytokine changes are significantly higher among patients with bigger ischemic lesions, and such patients have been found to have higher levels of PSD and more profound disability42 and therefore the higher prevalence of PSD among the physically impeded subjects. This biological basis could explain why involvement of the dominant side was not associated with higher proportions of PSD in our participants. Depression was also found more commonly among the aphasic patients. Speech is a vital part of human nature and communication and therefore its absence is a big cause of distress and depression; this is similar to previous studies that have noted aphasia as a severely disabling condition that markedly contributes to the severity and persistence of PSD24.
Disability has been recognized as a big impediment to performance of social roles1 and is the focus of current rehabilitation strategies among post-stroke patients. In this present study, there was a statistically significant relationship between PSD and reduced limb power especially when the weakness involved the dominant hand. Among our participants, those with impaired performance of activities of daily living arising from limb weakness demonstrated very high proportions of PSD of up to 51.4%. Previous studies have demonstrated a significant relation between the severity or existence of PSD and severity of impairment in Activities of Daily Living (ADLs) and that depression influences recovery in ADLs3,43. This underlines the fact that there is need to do universal screening for PSD among all stroke survivors but especially those with physical impairment.
The integration of mental health care into chronic care clinics is currently being advocated for; however this still remains a challenge in resource limited settings like Uganda. Post stroke care in Uganda is provided for by neurology specialist clinics and in this present study, we found that only seven of the study subjects with PSD had been initiated on tri-cyclic anti-depressants in the right doses. Instituting mental health liaison teams in primary care would be a long term solution to address these challenges and also help provide holistic care for post stroke sufferers. We would also recommend the utilization of simple screening tools available like PHQ — 9 by the care providers for such patients. Routine sensitization of the care providers as well as highlighting this high prevalence would help us institute measures to mitigate this.
Stroke rehabilitation programs including self-management programs should be instituted into care for poststroke patients so as to help mitigate disability arising from stroke. Further studies are needed to assess the relationship between physical disability and the development of post-stroke depression among our population. More studies are needed to determine if PSD improves with changes in activities of daily living.
Limitations
This study has several limitations because of the nature of the study population: for example, the small sample size, unequal gender distribution, and longer poststroke duration. The major limitation was the preselected nature of the study population; patients with mild or severe strokes are not likely to have been included. The methods of assessing depression were screening measures which reflect depressive symptomatology and there may have been an over diagnosis of depression. The diagnosis of PSD was made by a physician with help of a psychiatrist.
Conclusion
There is a high prevalence of post-stroke depression in stroke patients in two urban hospitals in Uganda. There is a need to integrate mental health liaison services and utilization of already available screening tools in the neurology primary care clinics to improve our patient care. Institution of anti-depressive therapy provides benefit not only for the mood but also for functional recovery. Data from this study will also contribute to development of evidence based local protocols for the assessment of depression in post-stroke patients.
Table 4.
Association of post-stroke depression with stroke characteristics
| Characteristic | Depressed N=23 |
Not depressed N=50 |
P | |
| Duration since onset in months, n (%) | ≤3 | 14 (60.9) | 15 (28.3) | 0.128 |
| 3 –6 | 2 (8.7) | 12 (22.6) | ||
| 6–12 | 1 (4.3) | 9 (17.0) | ||
| ≥12 | 6 (26.1) | 14 (26.4) | ||
| Number of co-morbidities, n (%) | One | 15 (65.2) | 34 (68) | |
| Two | 2 (8.7) | 11 (22) | 0.316 | |
| 3 and more | 6 (26.1) | 5 (10) | ||
| Type of stroke, n (%) | Hemorrhagic | 4 (17.4) | 11 (22.0) | 0.864 |
| Ischemic | 19 (82.6) | 39 (78.0) | ||
| Dominant side | Yes | 11 (47.8%) | 29 (72.5%) | 0.417 |
| No | 12 (52.2%) | 21 (27.5%) | ||
| Frontal lobe involvement, n (%) | Yes | 7 (30.4) | 12 (24.0) | 0.561 |
| No | 16 (69.6) | 38 (76.0) | ||
| Temporal lobe involvement, n (%) | Yes | 13 (56.6) | 23(46.0) | 0.404 |
| No | 10 (43.4) | 27 (54.0) | ||
| Parietal lobe involvement, n (%) | Yes | 7 (30.4) | 15 (30.0) | 0.970 |
| No | 16 (69.6) | 35 (70.0) | ||
| Occipital lobe involvement, n (%) | Yes | 0 (0.0) | 2 (4.0) | 1.000 |
| No | 23 (100) | 48 (96.0) | ||
| Posterior circulation, n (%) | Yes | 0 | 5 (6.8%) | --- |
| No | 0 | 68 (93.2%) | ||
| Number of lobes involved, n (%) | None | 1 (4.3) | 6 (12.0) | 0.722 |
| One | 18 (78.3) | 37 (74.0) | ||
| Two | 3 (13.0) | 6 (12.0) | ||
| ≥3 | 1 (4.3) | 1 (2) | ||
| Size of lesion, n (%) | ≤225 mm2 | 5 (21.7) | 17 (34) | 0.732 |
| > 225 mm2 | 18 (78.3) | 33 (66) | ||
| Presence of aphasia, n (%) | Yes | 6 (26.1) | 6 (12.0) | 0.131 |
| No | 17 (73.9) | 44 (88.0) | ||
| RUL power, n (%) | 0–1 | 2 (8.7) | 6 (12.0) | 0.013 |
| 2–3 | 9 (39.1) | 5 (10.0) | ||
| 4–5 | 12 (52.2) | 39 (78.0) | ||
| RLL power, n (%) | 0–1 | 3 (13.0) | 5 (10.0) | 0.093 |
| 2–3 | 8 (34.8) | 7 (14.0) | ||
| 4–5 | 12 (52.2) | 38 (76.0) | ||
| Total score on BIADL | Up to 19 | 18 (78.3) | 17 (34.0) | 0.001 |
| 20 | 5 (21.7) | 33 (66.0) | ||
| Handedness, n (%) | Right | 22 (95.6) | 47 (94.0) | 0.773 |
| Left | 1 (4.4) | 3 (6.0) | ||
Key: RUL= right upper limb; RLL= right lower limb; LUL: left upper limb; LLL: left lower limb;BIADL: Barthel index of activities of daily living.
Table 5.
Association of severity of PSD with stroke characteristics
| Characteristics | PHQ - 9 score | ADRS | PHQ-9 | ADRS | ||
| Min (5 – 9) |
Mild (10 – 14) |
Mod-Sev (15 –19) |
9–32 | P-value | P-value | |
| Duration since onset in months ≤ 3 3–6 6–12 ≥ 12 |
1 0 1 2 |
8 2 0 2 |
0 0 0 1 |
5 0 0 1 |
0.200 |
0.030 |
| Number of co-morbidities 1 2 ≥3 |
1 1 2 |
8 1 3 |
1 0 0 |
5 0 1 |
0.562 |
0.286 |
| Type of stroke Hemorrhagic Ischemic |
1 3 |
3 9 |
0 1 |
1 5 |
0.849 |
0.857 |
| Anatomical site of lesion Right Left |
2 2 |
5 7 |
1 0 |
3 3 |
0.528 |
1.000 |
| Dominant side Yes No |
2 2 |
6 6 |
0 1 |
3 3 |
---- |
0.350 |
| Lobar involvement Frontal Temporal Parietal |
1 2 1 |
3 5 3 |
0 1 0 |
0 2 3 |
0.801 |
0.072 |
| Number of lobes involved None 1 2 ≥ 3 |
0 4 0 0 |
1 11 0 0 |
0 1 0 0 |
0 2 3 1 |
0.279 |
0.143 |
| Size of lesion ≤ 22.5mm2 ≥ 225mm2 |
1 3 |
3 9 |
1 0 |
0 6 |
0.279 |
0.143 |
| RUL power 0 – 1 2 – 3 4 – 5 |
1 0 3 |
0 7 5 |
0 0 1 |
1 2 3 |
0.124 |
0.459 |
| RLL power 0 – 1 2 – 3 4 – 5 |
0 1 3 |
0 7 5 |
0 0 1 |
1 1 3 |
0.319 |
0.233 |
| Total BIADL score ≤ 19 > 20 |
1 3 |
11 1 |
0 1 |
6 0 |
0.011 |
----- |
KEY: ADRS-Aphasic depression rating scale, BIADL-Barthel index of activities of daily living; RUL= right upper limb; RLL= right lower limb; LUL: left upper limb; LLL: left lower limb;PHQ-9-Nine item Patient health questionnaire
Acknowledgments
This study was supported by the National Institute of Neurological Disorders and Stroke of the National Institute of Health under MEPI — Neurology linked award number R25NS080968. We also thank our patients for participating in this study.
List of abbreviations
- ADRS
Aphasic depression rating scale
- BIADL
Barthel index of activities of daily living
- CT scan
Computerized Tomography scan
- DSM III
Diagnostic and Statistical Manual of Mental Disorders III
- MRC scale
Medical Research Council Scale for Muscle strength
- PHQ
9 - PubMed Patient Health Questionnaire - 9
- PSD
Post Stroke Depression
Competing interests
The authors declare that they have no competing interest.
Author's contributions
JO, ED, MK and RO collected data and managed the patients; MK and JO performed data analyses; MS, KS and EK designed the study; JO, MK, MS and RO wrote the paper. MS, ED, KS and EK revised the manuscript for important intellectual content. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
References
- 1.World Health Organization, author. Global status report on noncommunicable diseases 2010. Geneva: World Health Organisation; 2011. [Google Scholar]
- 2.Fang J, Alderman MH. Trend of stroke hospitalization, United States, 1988–1997. Stroke. 2001;32(10):2221–2226. doi: 10.1161/hs1001.096193. [DOI] [PubMed] [Google Scholar]
- 3.Robinson R G, editor. The clinical neuropsychiatry of stroke: Cognitive, behavioural & emotional disorders following vascular brain injury. 2nd edition. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 4.Kelly-Hayes M, Robertson JT, Broderick JP, Duncan PW, Hershey LA, Roth EJ, Thies WH, Trombly CA. The American Heart Association Stroke OutcomeClassification. Stroke. 1998;29(6):1274–1280. doi: 10.1161/01.str.29.6.1274. [DOI] [PubMed] [Google Scholar]
- 5.House A. Mood disorders after stroke: A review of the evidence. International Journal of Geriatric Psychiatry. 1987;2(4):211–221. [Google Scholar]
- 6.Kong KH, Yang SY. Health-related quality of life among chronic stroke survivors attending a rehabilitation clinic. Singapore Med J. 2006;47(3):213–218. [PubMed] [Google Scholar]
- 7.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(6):1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 8.Oladiji JO, Akinbo SR, Aina OF, Aiyejusunle CB. Risk factors of post-stroke depression among stroke survivors in Lagos, Nigeria. Afr J Psychiatry (Johannesbg) 2009;12(1):47–51. doi: 10.4314/ajpsy.v12i1.30278. [DOI] [PubMed] [Google Scholar]
- 9.Berg A, Palomaki H, Lehtihalmes M, Lonnqvist J, Kaste M. Poststroke depression: an 18-month follow-up. Stroke. 2003;34(1):138–143. doi: 10.1161/01.str.0000048149.84268.07. [DOI] [PubMed] [Google Scholar]
- 10.Turner-Stokes L, Hassan N. Depression after stroke: a review of the evidence base to inform the development of an integrated care pathway. Part 1: Diagnosis, frequency and impact. Clin Rehabil. 2002;16(3):231–247. doi: 10.1191/0269215502cr487oa. [DOI] [PubMed] [Google Scholar]
- 11.Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry. 1983;24(6):555–566. doi: 10.1016/0010-440x(83)90024-x. [DOI] [PubMed] [Google Scholar]
- 12.Robinson RG, Lipsey JR, Rao K, Price TR. Two-year longitudinal study of post-stroke mood disorders: comparison of acute-onset with delayed onset depression. Am J Psychiatry. 1986;143(10):1238–1244. doi: 10.1176/ajp.143.10.1238. [DOI] [PubMed] [Google Scholar]
- 13.Parikh RM, Robinson RG, Lipsey JR, Starkstein SE, Fedoroff JP, Price TR. The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol. 1990;47(7):785–789. doi: 10.1001/archneur.1990.00530070083014. [DOI] [PubMed] [Google Scholar]
- 14.Robinson RG. Neuropsychiatric consequences of stroke. Annu Rev Med. 1997;48:217–229. doi: 10.1146/annurev.med.48.1.217. [DOI] [PubMed] [Google Scholar]
- 15.House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32(3):696–701. doi: 10.1161/01.str.32.3.696. [DOI] [PubMed] [Google Scholar]
- 16.Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry. 2004;161(6):1090–1095. doi: 10.1176/appi.ajp.161.6.1090. [DOI] [PubMed] [Google Scholar]
- 17.Medical Research Council, author. Aids to the examination of the peripheral nervous system, Memorandum no. 45. London: Her Majesty's Stationery Office; 1981. [Google Scholar]
- 18.Arroll B, Goodyear-Smith F, Crengle S, Gunn J, Kerse N, Fishman T, Falloon K, Hatcher S. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 8(4):348–353. doi: 10.1370/afm.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Steenbergen-Weijenburg KM, de Vroege L, Ploeger RR, Brals JW, Vloedbeld MG, Veneman TF, Hakkaart-van Roijen L, Rutten FF, Beekman AT, van der Feltz-Cornelis CM. Validation of the PHQ-9 as a screening instrument for depression in diabetes patients in specialized outpatient clinics. BMC Health Serv Res. 10:235. doi: 10.1186/1472-6963-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. scale. Stroke 2004, 35(7):1692–1696. [DOI] [PubMed] [Google Scholar]
- 22.Benaim C, Cailly B, Perennou D, Pelissier J. Validation of the aphasic depression rating scale. Stroke. 2004;35(7):1692–1696. doi: 10.1161/01.STR.0000130591.95710.20. [DOI] [PubMed] [Google Scholar]
- 23.Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988;10(2):64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 24.Kauhanen M, Korpelainen JT, Hiltunen P, Brusin E, Mononen H, Maatta R, Nieminen P, Sotaniemi KA, Myllyla VV. Poststroke depressioncorrelates with cognitive impairment and neurological deficits. Stroke. 1999;30(9):1875–1880. doi: 10.1161/01.str.30.9.1875. [DOI] [PubMed] [Google Scholar]
- 25.Nakibuuka Jane An, Namale Alice, Blondin Nicholas A, Ddumba Edward. A descriptive Epidemiological study on stroke in Kampala, Uganda: a hospital-based study. African Journal of Neurological Sciences. 2012;31(1):54–58. [Google Scholar]
- 26.Cleary PD, Mechanic D, Greenley JR. Sex differences in medical care utilization: an empirical investigation. J Health Soc Behav. 1982;23(2):106–119. 44(6):1555–1560. [PubMed] [Google Scholar]
- 27.Jackson CA, Mishra GD. Depression and risk of stroke in midaged women: a prospective longitudinal study. Stroke. 2004;35(4):936–941. doi: 10.1161/STROKEAHA.113.001147. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson M, Asplund K, Glader EL, Norrving B, Stegmayr B, Terent A, Asberg KH, Wester PO. Self-reported depression and use of antidepressants after stroke: a national survey. Stroke. doi: 10.1161/01.STR.0000121643.86762.9a. [DOI] [PubMed] [Google Scholar]
- 29.Desmond DW, Remien RH, Moroney JT, Stern Y, Sano M, Williams JB. Ischemic stroke and depression. J Int Neuropsychol Soc. 2003;9(3):429–439. doi: 10.1017/S1355617703930086. [DOI] [PubMed] [Google Scholar]
- 30.Paolucci S, Gandolfo C, Provinciali L, Torta R, Sommacal S, Toso V. Quantification of the risk of post stroke depression: the Italian multicenter observational study DESTRO. Acta Psychiatr Scand. 2005;112(4):272–278. doi: 10.1111/j.1600-0447.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm K, Parker G. Sex differences in lifetime depression rates: fact or artefact? Psychol Med. 1994;24(1):97–111. doi: 10.1017/s0033291700026878. [DOI] [PubMed] [Google Scholar]
- 32.Ayerbe L, Ayis S, Crichton S, Wolfe CD, Rudd AG. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2013;85(5):514–521. doi: 10.1136/jnnp-2013-306448. [DOI] [PubMed] [Google Scholar]
- 33.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306(11):1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojagbemi A, Owolabi M, Atalabi M, Baiyewu O. Stroke lesions and post-stroke depression among survivors in Ibadan, Nigeria. Afr J Med Sci. 42(3):245–251. [PubMed] [Google Scholar]
- 35.Spaccavento S, Craca A, Del Prete M, Falcone R, Colucci A, Di Palma A, Loverre A. Quality of life measurement and outcome in aphasia. Neuropsychiatr Dis Treat. 2014;10:27–37. doi: 10.2147/NDT.S52357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava A, Taly AB, Gupta A, Murali T. Poststroke depression: prevalence and relationship with disability in chronic stroke survivors. Ann Indian Acad Neurol. 2010;13(2):123–127. doi: 10.4103/0972-2327.64643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsouna-Hadjis E, Vemmos KN, Zakopoulos N, Stamatelopoulos S. First-stroke recovery process: the role of family social support. Arch Phys Med Rehabil. 2000;81(7):881–887. doi: 10.1053/apmr.2000.4435. [DOI] [PubMed] [Google Scholar]
- 38.Rachpukdee S, Howteerakul N, Suwannapong N, Tang-Aroonsin S. Quality of life of stroke survivors: a 3-month follow-up study. J Stroke Cerebrovasc Dis. 2013;22(7):e70–e78. doi: 10.1016/j.jstrokecerebrovasdis.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Sturm JW, Donnan GA, Dewey HM, Macdonell RA, Gilligan AK, Srikanth V, Thrift AG. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2004;35(10):2340–2345. doi: 10.1161/01.STR.0000141977.18520.3b. [DOI] [PubMed] [Google Scholar]
- 40.Kwok T, Lo RS, Wong E, Wai-Kwong T, Mok V, Kai-Sing W. Quality of life of stroke survivors: a 1-year follow-up study. Arch Phys Med Rehabil. 2006;87(9):1177–1182. doi: 10.1016/j.apmr.2006.05.015. quiz 1287. [DOI] [PubMed] [Google Scholar]
- 41.Starkstein SE, Robinson RG, Price TR. Comparison of cortical and subcortical lesions in the production of poststroke mood disorders. Brain. 1987;110:1045–1059. doi: 10.1093/brain/110.4.1045. (Pt 4) [DOI] [PubMed] [Google Scholar]
- 42.Spalletta G, Bossu P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11(11):984–991. doi: 10.1038/sj.mp.4001879. [DOI] [PubMed] [Google Scholar]
- 43.Robinson RG, Spalletta G. Poststroke depression: a review. Can J Psychiatry. 55(6):341–349. doi: 10.1177/070674371005500602. [DOI] [PMC free article] [PubMed] [Google Scholar]