Abstract
The development of precise connectivity patterns during the establishment of the nervous system depends on the regulated action of diverse, conserved families of guidance cues and their neuronal receptors. Determining how these signaling pathways function to regulate axon growth and guidance is fundamentally important to understanding wiring specificity in the nervous system and will undoubtedly shed light on many neural developmental disorders. Considerable progress has been made in defining the mechanisms that regulate the correct spatial and temporal distribution of guidance receptors and how these receptors in turn signal to the growth cone cytoskeleton to control steering decisions. This review focuses on recent advances in our understanding of the mechanisms mediating growth cone guidance with a particular emphasis on the control of guidance receptor regulation and signaling.
Keywords: growth cone, Rho GTPases, cytoskeleton, protein trafficking, endocytosis, proteolysis
INTRODUCTION: THE FOUR CLASSIC LIGAND/RECEPTOR SYSTEMS MEDIATING AXON GUIDANCE
To ensure correct and efficient wiring of the nervous system, an intricately choreographed sequence of events must take place. First, neurons and their surrounding target tissues must be specified to express the correct complement of receptors and guidance cues, respectively. Second, receptors must be assembled into the appropriate complexes and localized to the axonal or dendritic growth cones, whereas guidance cues must be correctly trafficked to and localized within the extracellular environment. Third, signaling mechanisms must be in place to integrate and transmit signals from the surface receptors into changes in the growth cone actin cytoskeleton, resulting in stereotyped steering decisions. Each of these steps provides many potential levels for the regulation of axon guidance decisions, and although recent work has enriched our understanding of the complexities of guidance regulation, many questions remain. Here we highlight recent advances in our understanding of guidance receptor signaling and regulation, with a particular emphasis on findings in vivo.
During development, neuronal growth cones, the specialized structures at the tips of extending axons, follow specific pathways and navigate a series of intermediate choice points to find their correct targets. At each decision point, growth cones encounter a number of guidance cues in their extracellular environments (Dickson 2002, Yu & Bargmann 2001). Researchers have discovered several phylogenetically conserved families of guidance cues and receptors, including (a) semaphorins (semas) and their plexin (Plex) and neuropilin receptors (Pasterkamp & Kolodkin 2003), (b) netrins and their deleted in colorectal carcinoma (DCC) and UNC5 receptors (Kennedy 2000), (c) Slits and their roundabout (Robo) receptors (Brose & Tessier-Lavigne 2000), and (d) ephrins and their Eph receptors (Kullander & Klein 2002). A common theme from studies of these guidance molecules is that it is the type of receptor, or receptor complex, expressed on the growth cone’s surface, rather than a given guidance cue, that determines the direction of axon growth (Garbe & Bashaw 2004, Huber et al. 2003). More recently, additional protein families previously recognized for other developmental functions have been implicated in growth cone guidance including sonic hedgehog (Shh) (Charron et al. 2003), bone morphogenetic proteins (BMPs) (Butler & Dodd 2003), and Wingless-type (Wnt) proteins (Lyuksyutova et al. 2003, Yoshikawa et al. 2003). In this review, we focus our discussion on the four major developmental ligand/receptor systems listed previously, drawing examples from other signaling pathways when they illuminate or reinforce general principles that have emerged from the study of the signaling and regulatory mechanisms of the Slit, netrin, semaphorin, and ephrin families. This review is divided into two major sections. First we discuss posttranslational mechanisms that regulate the localization and distribution of guidance receptors in the growth cone plasma membrane. This level of regulation has emerged as a potent strategy to control guidance responses, and as we shall see, many signaling molecules typically considered to act exclusively as downstream effectors can influence guidance receptor distribution. In the second section, we consider how guidance receptors transmit their signals to the actin/microtubule cytoskeleton to control steering decisions.
AXON GUIDANCE RECEPTOR REGULATION
The expression of guidance cues and receptors is exquisitely tailored to allow growth cones to make appropriate path-finding decisions at specific times and places throughout development. A wide variety of mechanisms are in place to ensure the correct presentation and receipt of guidance signals, ranging from spatially and temporally restricted transcriptional regulation of cues and receptors to their specific posttranslational trafficking. There are doubtless additional regulatory mechanisms awaiting discovery. Transcriptional control of axon guidance, and in particular of guidance cues and receptors, has been reviewed recently (Polleux et al. 2007), so here we focus on posttranslational regulation with an emphasis on the strategies used by neurons to regulate receptor localization and function, including control of guidance receptor trafficking to the plasma membrane, regulated endocytosis, and regulated proteolysis.
Trafficking of Guidance Receptors to the Growth Cone Plasma Membrane
In principle, regulating surface levels of axon guidance receptors could provide a potent mechanism to regulate guidance responses. Indeed, work over the past several years has documented several in vitro and in vivo examples of this level of regulation, suggesting that this strategy will prove to be a widespread and general mechanism for controlling axon path finding. Here we discuss the following three recent examples: first, the regulation of Robo receptor trafficking by Commissureless (Comm) in Drosophila, second, the regulation of DCC trafficking by protein kinase A (PKA) in rodents, and third, the regulation of UNC-40 (DCC), UNC-5, and SAX-3 (Robo) trafficking in Caenorhabditis elegans.
At the fly midline, Comm controls midline crossing by negatively regulating the repulsive Robo receptor, thereby preventing commissural neurons from prematurely responding to the midline repellant Slit. comm mRNA is detected both in midline glia as well as in a temporally restricted window in commissural neurons as they approach the midline. Although cell transplantation and gain-of-function genetic experiments have been interpreted to suggest that Comm functions predominantly cell autonomously in neurons, other genetic data, including mosaic rescue experiments, also supported an additional role for Comm in midline glia (Georgiou & Tear 2002, Keleman et al. 2002). This issue has been further clarified by a more recent quantitative analysis of the tissue-specific requirement for Comm function, in which the findings strongly support the hypothesis that Comm expression in midline glia does not contribute to its function in midline guidance (Keleman et al. 2005).
How does Comm function to regulate Robo? Several lines of evidence, including sub-cellular localization experiments and transgenic expression of mutant forms of comm, indicate that Comm can recruit Robo receptors directly to endosomes for degradation before they ever reach the cell surface and that this sorting function is important for regulating midline repulsion (Keleman et al. 2002) (Figure 1c). In addition, Comm’s ability to regulate surface levels of Robo has been suggested to depend on its interaction with the Nedd 4 ubiquitin ligase; mutation in either the dNedd4 binding site or the ubiquitin acceptor sites in Comm disrupts its ability to regulate Robo (Myat et al. 2002). More recently, the importance of Nedd4 and Comm ubiquitination for its midline regulatory function has been challenged by the observation that a mutant version of Comm lacking all ubiquitin acceptor sites retains full activity in an in vivo rescue assay. Perhaps the previous biochemical and gain-of-function genetic interactions between Nedd4 and Comm could indicate a role for Nedd4 in another Commdependent process that is distinct from its role in midline guidance.
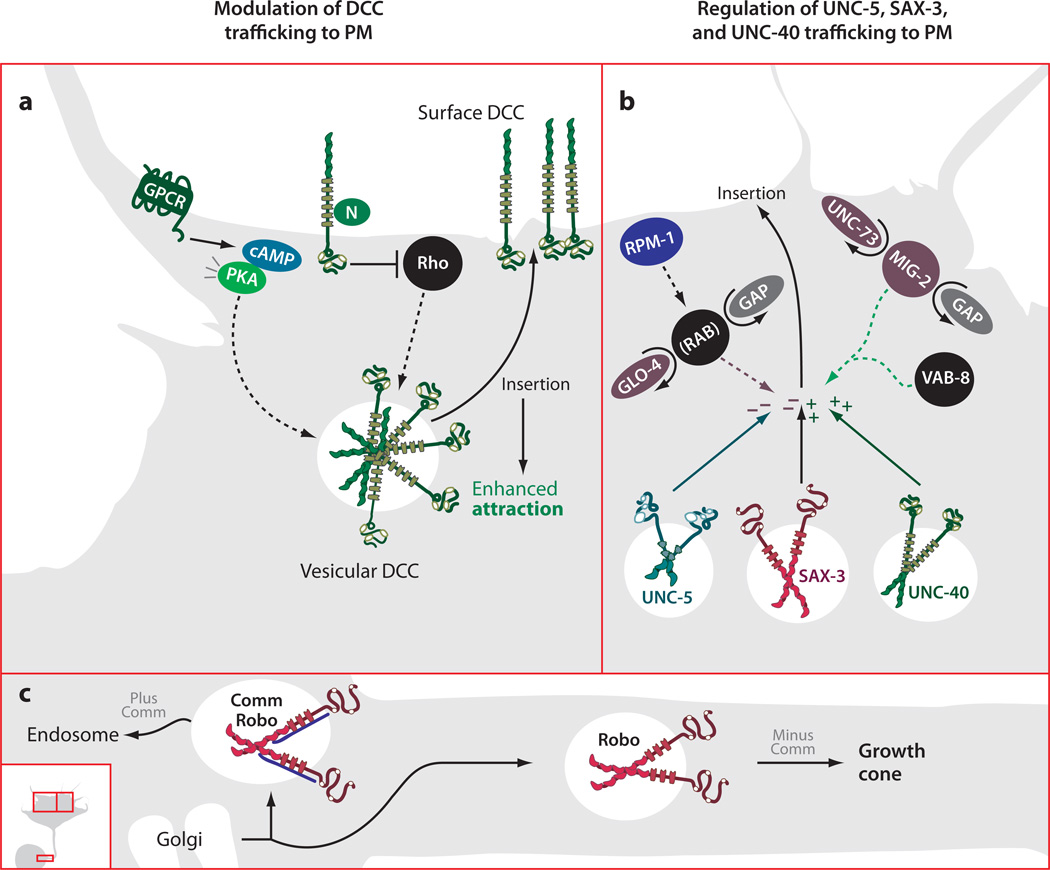
Figure 1.
Trafficking guidance receptors to the growth cone plasma membrane (PM). (a) Both PKA activation and Rho inhibition positively regulate mobilization of an intracellular, vesicular pool of DCC. Increases in surface DCC lead to increases in Netrin responsiveness. (b) Positive and negative regulation of membrane expression of UNC-5, SAX-3, and UNC-40. RPM-1 activates GLO-4, a RAB GEF, which negatively regulates surface levels of UNC-5 and SAX-3. Activation of UNC-73, a MIG-2 (Rac) GEF, as well as activation of VAB-8, positively regulates surface levels of UNC-40 and SAX-3. Vesicles leaving the trans-Golgi network containing Robo are subjected to differential trafficking depending on the presence or absence of Comm. Vesicles containing Comm along with Robo are sorted to the endosome, whereas those containing Robo alone are trafficked down the axon toward the growth cone.
The endosomal sorting model has been extended to show that Comm can prevent Robo delivery to the growth cone surface in living embryos (Keleman et al. 2005). Expression of green fluorescent protein (GFP) tagged Robo in sensory axons provides investigators with live visualization of the anterograde axonal transport of Robo positive vesicles. When Comm is genetically introduced into these RoboGFP-positive neurons, the transport of Robo positive vesicles is almost completely abolished, providing strong evidence for the in vivo significance of Comm-directed endosomal targeting of Robo (Keleman et al. 2005) (Figure 1c).
Despite significant progress in the understanding of Comm function, many questions remain. Are there vertebrate Comm homologs that serve similar functions during commissural axon guidance in the spinal cord, or instead do other molecules play this role? So far, no vertebrate Comm proteins have been found; however, compelling genetic evidence indicates that another molecule may have an analogous function in the spinal cord. Rig-1/Robo3, a divergent Robo family member, is required in precrossing commissural neurons to downregulate the sensitivity to midline Slit proteins, although this function is achieved by a distinct mechanism (Sabatier et al. 2004). Another interesting question is how comm mRNA expression is regulated during midline crossing to ensure a pulse of expression just as axons cross. Which signal activates Comm expression as the growth cone approaches the midline? How is Comm repression of Robo relieved in postcrossing neurons? Identifying the signals that regulate comm mRNA expression and dissecting the comm promoter and regulatory sequences should begin to answer these questions.
DCC family members, attractive receptors for netrin that play important roles in many developmental contexts, in particular in promoting midline crossing of commissural axons in the spinal cord, are also regulated by trafficking to the growth cone plasma membrane. DCC resides in two distinct pools in embryonic rat commissural axons: a surface pool and an intracellular vesicular pool. Netrin stimulation leads to an increase in DCC surface levels, and this effect is enhanced by PKA activation. Specifically, PKA mobilizes the intracellular pool of DCC, leading to netrin-dependent increases in both surface expression and axon outgrowth (Bouchard et al. 2004). Blocking adenylate cyclase, PKA activity, or exocytosis prevents the increase in DCC surface levels and blunts netrin-induced axon outgrowth. Significantly, in contrast with earlier findings in cultured Xenopus neurons, netrin did not directly influence PKA activity in these experiments, suggesting that in rat commissural neurons other signals are likely required to activate PKA, which can in turn potentiate netrin responses by upregulating surface levels of DCC (Figure 1a).
In addition to PKA’s role in regulating translocation of the DCC receptor to the growth cone plasma membrane, recent findings indicate that netrin-dependent inhibition of Rho activity also contributes to DCC mobilization (Moore et al. 2008a). Although the effects of manipulating Rho on surface DCC levels do not seem to be as profound as those seen with PKA manipulations, the data suggest a more complex role for Rho in regulating guidance responses than previously acknowledged. Specifically, these effects indicate that, in addition to constituting a major signaling output of guidance receptor activation, the Rho GTPases (guanosine triphosphatases) may also have an upstream or feedback role in regulating the surface levels of guidance receptors (Figure 1a). Together these studies offer new insight that may help to explain how changing cyclic AMP (adenosine monophosphate) (cAMP), PKA, and Rho activity promotes netrin-mediated chemoattraction. The implications of these findings for how we conceptualize the signaling mechanism underlying netrin attraction, as well as the mechanisms underlying other ligand-receptor systems where PKA and Rho function are involved, are discussed in more detail below.
Genetic approaches in C.elegans have also offered substantial support for the importance of regulating receptor trafficking to control axon growth and guidance, and as we have seen in the case of Robo regulation and DCC regulation, both negative and positive regulatory strategies have significant impact on axon responses. Several studies have established that the trafficking and polarized localization of netrin and Slit receptors are critical for proper direction of axon outgrowth. Specifically, mutations in the UNC-73 Trio-family RacGEF or the VAB-8 kinesin-related protein disrupt the normal localization of the SAX-3 (Robo) and UNC-40 (DCC) receptors, and in the case of UNC-40, regulation of localization also requires the MIG-2 Rac small GTPase (Levy-Strumpf & Culotti 2007, Watari-Goshima et al. 2007). These perturbations in normal receptor localization lead to significant defects in Slit and netrin-dependent posterior oriented cell and growth cone migration and further emphasize important upstream regulatory roles for Rho GTPases in the control of axon guidance receptor localization (Levy-Strumpf & Culotti 2007, Watari-Goshima et al. 2007). Again, this rather unexpected upstream regulatory role must be carefully weighed when considering the outcome of Rho GTPase manipulations on axon guidance, especially because many earlier studies have assumed that, by their very nature as regulators of the actin cytoskeleton, the Rho GTPases function exclusively as downstream effectors of guidance signaling (Figure 1b).
In addition to these positive regulatory mechanisms, the trafficking of SAX-3 (Robo) and UNC-5 can also be negatively regulated with important outcomes for axon growth. A genetic screen in C. elegans for genes that could modulate UNC-6 (netrin) signaling identified mutations in rpm-1, the C. elegans member of the conserved Pam/Highwire/RPM protein family that plays important roles in presynaptic differentiation (Li et al. 2008a). In genetic backgrounds where sax-3 and unc-5 function is partially reduced, rpm-1 mutants lead to specific axon overgrowth and branching phenotypes, and SAX-3 and UNC-5 proteins show increased expression levels and altered localization. In the context of axon termination prior to synapse formation, RPM mediates two distinct outputs: one through a MAP (mitogenactivated protein kinase) kinase pathway and a second through GLO-4, a RAB (Ras-related in brain) GEF (guanine nucleotide exchange factor) implicated in vesicle trafficking (Grill et al. 2007). Genetic analysis indicates that the role of RPM-1 in regulating SAX-3 and UNC-5 function is dependent on GLO-4, again indicating an important role for protein trafficking in axon growth regulation (Li et al. 2008a) (Figure 1b).
Regulated Endocytosis and Axon Guidance Receptor Function
As detailed above, regulating the delivery of guidance receptors to the growth cone plasma membrane can have profound influences on axon growth and guidance; therefore, it is not surprising that the regulation of receptor expression at the cellular level is not confined strictly to surface expression, but also includes regulated removal by endocytosis. In several cases, receptor endocytosis appears to be an obligate step in receptor activation that is evoked by ligand binding, whereas other examples point to the modulation of guidance responses by receptor endocytosis that is triggered by an independent pathway. Here we briefly consider a few examples of endocytosis as a prerequisite for receptor signaling; in particular, we discuss the role of the Rac specific GEF Vav2 in the regulation of Eph receptor endocytosis. In addition, we will highlight the role of protein kinase C (PKC) activation in the regulation of responses to netrin through the specific endocytosis of the UNC5 receptor (Figure 2).
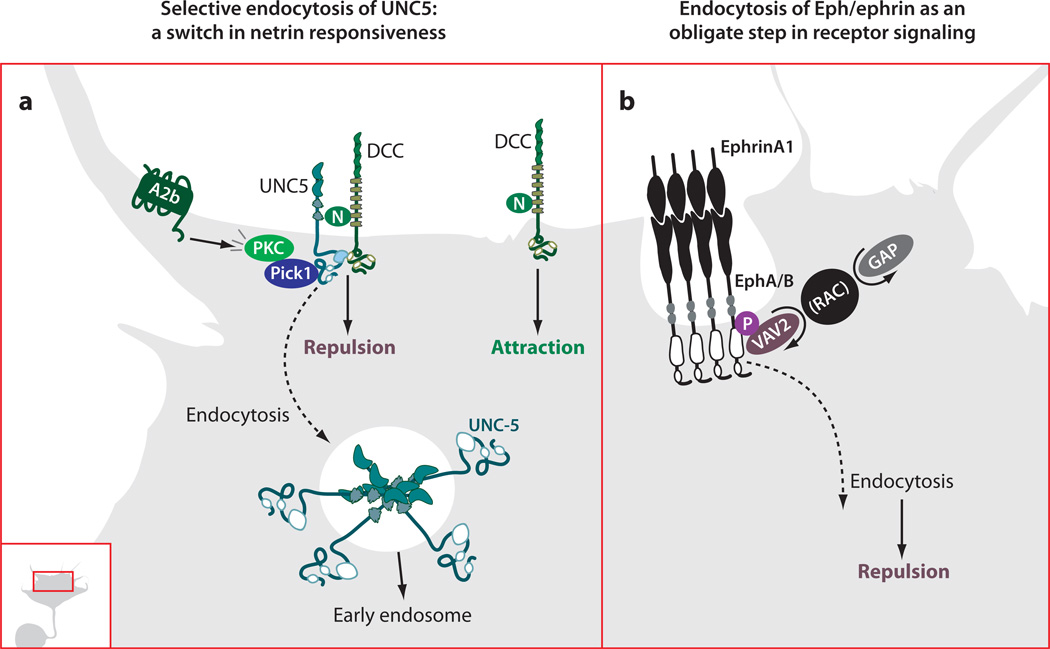
Figure 2.
Regulated endocytosis in axon guidance. (a) Adenosine2b receptor (A2b) activity leads to PKC-dependent endocytosis of UNC5, which requires a physical interaction between PKC, Pick1, and the cytoplasmic domain of UNC5. This change in receptor composition at the plasma membrane leads to a switch in responsiveness to netrin from repulsion mediated by UNC5 alone, or by an UNC5/DCC complex, to attraction mediated by DCC. N, netrin. (b) The Vav family of Rac GEFs is required for endocytosis of ephrin ligand/Eph receptor complexes in retinal ganglion cell growth cones. Vav2 is recruited to the ephrin-stimulated juxtamembrane phosphorylated tyrosine of EphA and EphB receptors and then stimulates endocytosis. This endocytotic event is an obligate step in the forward signaling leading to growth cone retraction or repulsion.
Ephrin ligands and Eph receptors contribute to the guidance of retinal ganglion cell (RGC) axons in the visual system; specifically, EphB receptor mutations in mice result in a reduction in the ipsilateral projection to the dorsal lateral geniculate nucleus. Disruption of vav-2 and vav-3, members of the Vav family of Rac GEFs, leads to similar defects in the targeting of ipsilateral RGC axons in mice (Cowan et al. 2005). Unlike wild-type RGCs, growth cones of RGCs cultured from vav-deficient mice do not collapse in response to ephrin. Surface labeling of Eph receptors in vav-deficient RGC growth cones reveals a selective deficit in Eph receptor endocytosis in response to preclustered ephrin-A1 treatment, suggesting that endocytosis of activated Eph receptors at the growth cone is necessary to allow for proper forward signaling, leading to growth cone retraction (Cowan et al. 2005) (Figure 2b). A similar dependency on endocytosis to trigger axon retraction is observed in neurons responding to sema 3A, where the L1 IgCAM, a component of the sema receptor complex, mediates endocytosis of the sema 3A holoreceptor in response to ligand binding (Castellani et al. 2004).
In addition to contributing to receptor signaling, endocytosis can also modulate axon responses by regulating which receptors are expressed at the surface of the growth cone. This type of mechanism is best exemplified by regulated endocytosis of the repulsive netrin receptor UNC5 in vertebrate neurons. Here, activation of protein kinase C (PKC) triggers the formation of a protein complex including the cytoplasmic domain of UNC5H1, protein interacting with C-kinase 1 (Pick1), and PKC and leads to the specific removal of UNC5H1 (but not DCC) from the growth cone surface; reducing surface levels of UNC5H1 correlates with the inhibition of the netrin-dependent collapse of cultured hippocampal growth cones (Williams et al. 2003). Furthermore, PKC activation leads to colocalization of UNC5A with early endosomal markers, supporting the idea that the observed inhibition of growth cone collapse is due to UNC5A endocytosis (Bartoe et al. 2006). Thus, PKC-mediated removal of surface UNC5 provides a means to switch netrin responses from repulsion, mediated by either UNC5 alone or an UNC5-DCC complex, to attraction mediated by DCC. How then is this switch activated, or which signals lead to the activation of PKC? Interestingly, recent evidence supports the model that the G protein-coupled Adenosine 2B (A2b) receptor is a likely mediator of PKC activation because activation of A2b leads to the PKC-dependent endocytosis of UNC5 (McKenna et al. 2008). A2b is a netrin receptor that, together with DCC, appears to be required to mediate axon attraction (Corset et al. 2000), although this proposal has been quite controversial, and other evidence indicates either that A2b plays no role in netrin signaling (Bouchard et al. 2004, Stein et al. 2001) or that its role in netrin signaling is to modulate netrin responses (Shewan et al. 2002).In the context of UNC5 regulation, A2b acts independently of netrin, and its ability to regulate UNC5 surface levels supports its role as a potent modulator of netrin responses (Figure 2a).
Regulated Proteolytic Processing and Axon Guidance
Another emergent theme in axon guidance is that proteolytic processing of both guidance ligands and receptors can have profound impacts on path finding. A role for proteolysis in axon guidance was supported by a number of early studies demonstrating that growth cones secrete proteases, and investigators proposed that cleavage of extracellular matrix components is required to advance through the extracellular environment (Krystosek & Seeds 1981, Schlosshauer et al. 1990). Later, genetic screens for defects in axonal navigation at the midline in Drosophila, and subsequent cloning and characterization of mutated genes, implicated the Kuzbanian ADAM family transmembrane metalloprotease in the regulation of axon extension and guidance at the midline (Fambrough et al. 1996). Several additional studies have implicated ADAM metalloproteases as well as matrix metalloproteases in contributing to axon guidance in vivo in both invertebrate and vertebrate nervous systems (Chen et al. 2007, Hehr et al. 2005). Here, we focus our discussion on emerging links between these proteases, in particular Kuzbanian/ADAM10, and the regulated proteolysis of axon guidance receptors and their ligands.
Several studies have implicated Kuzbanian/ADAM10 activity in the signaling pathways of guidance receptors. For example, in Drosophila, mutations in kuzbanian (kuz) exhibit dose-dependent genetic interactions with Slit, the midline repulsive ligand for Robo receptors. Specifically, ectopic midline crossing of ipsilateral interneurons, a hallmark of defective midline repulsion, is observed in kuz zygotic mutant embryos and in embryos where both slit and kuz activity are partially reduced. This dose-dependent interaction supports the idea that Kuz may be a positive regulator of Slit-Robo signaling (Schimmelpfeng et al. 2001). Antibody staining for Robo1 in kuz mutants reveals that the midline phenotype is accompanied by a failure to exclude Robo1 protein expression from the midline-crossing portions of axons, which suggests that kuz activity may be necessary for exclusion from, but more likely clearance of Robo from, axons. Galko & Tessier-Lavigne (2000) observed a similar effect on receptor expression in the context of metalloprotease-dependent ectodomain shedding of DCC. Specifically, blocking the function of metalloprotease activity results in enhanced DCC receptor expression at the membrane, suggesting that proteolytic cleavage regulates clearance of receptors from the plasma membrane. The outcome of preventing metalloprotease function in these two examples is opposite: Elevated levels of DCC potentiate DCC’s ability to mediate netrin-induced axon outgrowth, whereas Robo expression in axon commissures evidently reflects impaired receptor function. Together, the alteration in Robo receptor expression and the reduction in midline repulsion in kuz mutants raise the intriguing possibility that Kuz may regulate guidance by regulating the cleavage of Robo (Figure 3).
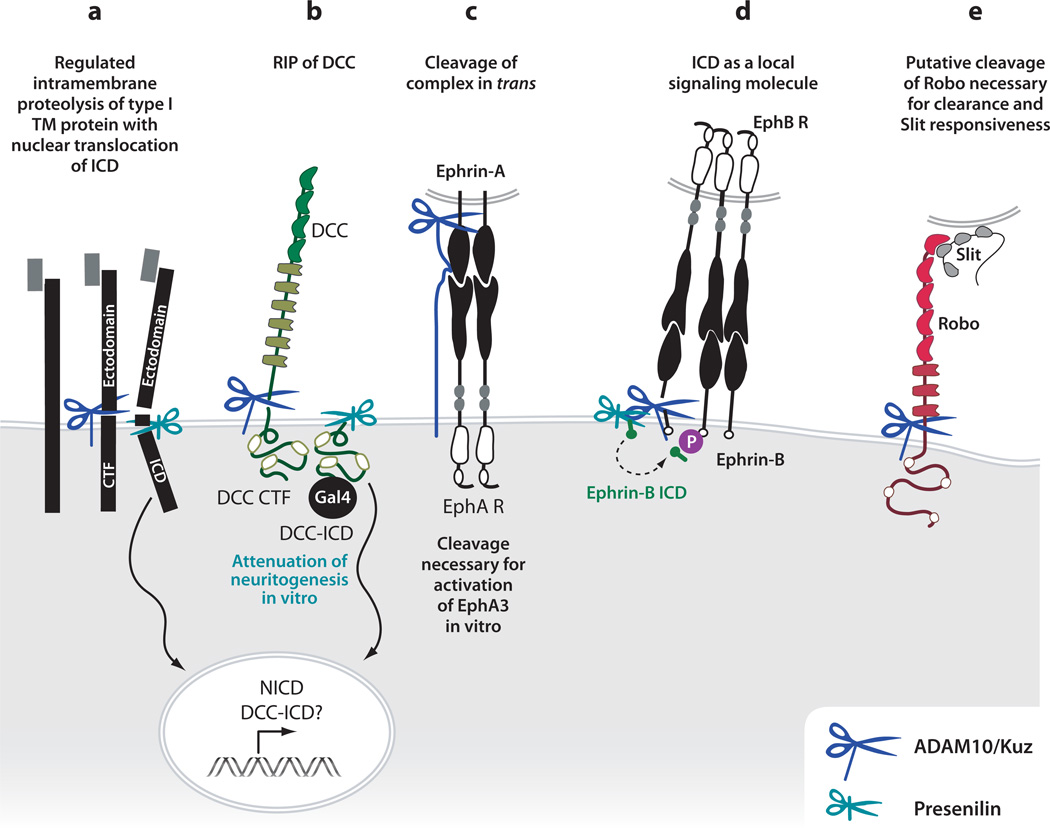
Figure 3.
Regulated proteolysis regulates guidance receptor function. (a) Processive proteolysis of a prototypical type I transmembrane (TM) protein, such as Notch or APP. Upon ligand binding, cleavage by an ADAM10 in the juxta-membrane region causes release of an N-terminal fragment into the extracellular space (ectodomain) and generates a C-terminal fragment (CTF) with a small extracellular stub. A second, constitutive cleavage by the gamma-secretase complex within the plane of the plasma membrane releases the intracellular domain (ICD). In the case of Notch, the ICD translocates to the nucleus, where it regulates transcription. (b) Regulated proteolysis of DCC occurs by ADAM10-mediated creation of a CTF, followed by gamma-secretase-mediated intramembraneous cleavage releasing DCC ICD. This ICD is competent to translocate to the nucleus when fused to Gal4. The cleavage event by ADAM10 leads to attenuation of neuritogenesis in vitro. (c) Following ligand-receptor complex formation, ADAM10 cleaves the ephrin-A5 ligand. This regulated proteolytic event leads to release from the initial cell-cell adhesion, allowing for growth cone retraction, and is necessary for the transduction of the EphA3 forward signal. (d) Processive cleavage in the ephrinB/ephB system indicates that the released ephrinB ICD may activate SRC-family kinases to contribute to reverse signaling. On the other hand, cleavage of the EphB2 receptor, in this case by matrix metalloproteases, is required for activation in vitro. (e) Kuzbanian appears to act positively in the Slit-Robo signaling pathway. On the basis of genetic observations and the abnormal presence of Robo protein on the commissural portions of axons in kuz mutants, we speculate that Kuz may cleave Robo to regulate receptor activity.
Investigators have detailed more direct links between Kuz/ADAM10 and guidance molecule cleavage of Eph receptors and ephrin-A2 ligands. Eph receptors and their ephrin ligands are both capable of transmitting signals in the cell in which they are expressed: Eph receptor signaling is termed forward signaling, and ephrin ligand signaling is termed reverse signaling (reviewed in Egea & Klein 2007). ADAM10 forms a stable complex with ephrin-A2, and upon EphR interaction with ephrin-A2, the resulting ligand-receptor complex is clipped by selective ADAM10-dependent cleavage of ephrin-A2 (Hattori et al. 2000) (Figure 3). This model has been extended through the study of additional EphR/ephrin receptor/ligand pairs, and Janes et al. (2005) have beautifully elucidated the molecular and structural basis for how cleavage events are restricted to only those ephrin ligands that are engaged by receptors. Ligand/receptor binding and formation of an active complex expose a new recognition sequence for ADAM10, resulting in the optimal positioning of the protease domain with respect to the substrate (Janes et al. 2005). The ligand dependence of the cleavage event provides an elegant explanation for how an initially adhesive interaction can be converted to repulsion and offers an efficient strategy for axon detachment and attenuation of signaling. Emerging evidence indicates that the matrix metalloprotease family can play a similar role in converting ephrinB/EphB adhesion into axon retraction by specific cleavage of the EphB2 receptor (Lin et al. 2008). Thus, both ephrin ligands and Eph receptors can be substrates for regulated proteolysis, and these proteolytic events appear to be critical in mediating axon retraction. It will be interesting to see how widespread this mechanism is in controlling axon and dendrite retraction. For example, the Down syndrome cell adhesion molecule (Dscam) family of homophilic and adhesive axon and dendrite guidance receptors would be prime candidates for this mechanism of converting stable adhesion into retraction.
Processive Proteolysis: Gamma-Secretase and Guidance Receptors
Kuzbanian (Kuz) was originally identified in Drosophila for its role in regulating Notch signaling during neurogenesis (Pan & Rubin 1997, Rooke et al. 1996). Kuz-directed cleavage of Notch releases the extracellular domain and triggers the subsequent cleavage and release of the Notch intracellular domain (ICD) by the gamma-secretase complex. This second cleavage event releases Notch ICD from the membrane, allowing it to translocate to the nucleus where it acts as a transcriptional regulator (Mumm & Kopan 2000). This well-characterized model of processive proteolytic cleavage of Notch is becoming increasingly relevant to an expanding list of type I transmembrane receptors, including axon guidance molecules (Beel & Sanders 2008). More specifically, evidence is mounting for a common regulatory mechanism for DCC and a number of ephrin ligands in which metalloprotease-mediated ectodomain shedding is followed by intramembraneous gamma-secretase cleavage (Figure 3). These sequential cleavage events produce an ectodomain fragment which is shed into the extracellular space and a C-terminal fragment (CTF) that is subsequently cleaved within the membrane to release the ICD (Selkoe & Wolfe 2007).
In the case of DCC, metalloprotease-dependent proteolytic fragments are detected in endogenous tissue and explant cultures (Galko & Tessier-Lavigne 2000). Furthermore, detection of DCC fragments in mouse brain lysates that correspond in size to fragments engineered to estimate the size of presumptive DCC CTF is enhanced in Presenilin-1 (PS1) knockout mice (Parent et al. 2005, Taniguchi et al. 2003). Accordingly, in primary neural cultures from PS1 mutant mice, accumulation of surface DCC is enhanced. The functional significance of these processing events is underscored by the fact that accumulation of transmembrane forms of DCC in neuronal cells transfected with both full-length DCC and DCC-CTF is correlated with enhanced neurite outgrowth in the presence of a gamma-secretase inhibitor. This observation suggests a role for presenilin-mediated cleavage of DCC-CTF in attenuating the intracellular signaling process that drives neurite outgrowth (Parent et al. 2005). In addition to DCC, several ephrin ligands and Eph receptors appear to undergo a similar ADAM10/gamma-secretase sequential proteolysis (Georgakopoulos et al. 2006, Litterst et al. 2007, Tomita et al. 2006). As in the case of DCC, in vitro evidence supports the idea that these cleavage events lead to functional consequences for ephrin-EphR-dependent process extension (Figure 3).
What is the in vivo significance of these processing events, and what is the fate of the released extracellular and ICD domains? Although in vivo evidence supporting physiological roles for these gamma-secretase-directed cleavage events has yet to emerge, several observations from in vitro studies hint at potentially important regulatory activities of released receptor ICDs. In the case of Notch and APP, the ICD generated by gamma-secretase cleavage is translocated to the nucleus to control gene transcription (Selkoe & Wolfe 2007). A chimeric version of DCC with a Gal4 DNA-binding domain inserted in its intracellular domain can initiate transcription in a gamma-secretase-dependent manner, suggesting that like Notch ICD, DCC-ICD could be acting as a transcriptional regulator in mammalian cells (Taniguchi et al. 2003). In the case of ephrin’s ICD, in vitro evidence supports an additional model in which the released ICD can bind to and activate Src family kinases, thereby contributing to ephrin-dependent cytoskeletal rearrangement (Georgakopoulos et al. 2006) (Figure 3). An alternative possibility is that these cleavage events represent a mechanism to limit the duration of receptor signaling because, once the ICD is released from the full-length receptor, the spatial regulation of signaling conferred by directional detection of ligand would presumably be rapidly lost. If and how these processing events contribute to in vivo receptor function will be an important area of future research.
AXON GUIDANCE RECEPTORS: DOWNSTREAM SIGNALING MECHANISMS
Once guidance cues and receptors are correctly deployed and assembled into the appropriate combinations and complexes, they must activate signaling pathways to steer the growth cone. Although guidance receptor signaling mechanisms are incompletely understood, they are likely to act locally to modulate actin cytoskeletal dynamics in the growth cone, rather than by signaling to the cell body (Figure 4). Activation of specific signaling pathways can promote attraction or repulsion, result in growth cone collapse, or affect the rate of axon extension. How a given guidance signal is interpreted also depends on the activities of a number of second-messenger pathways within the cell, and these pathways are potent modulators of axon responses in vivo. Here we review recent insights into how specific guidance receptors from each of the four classic guidance pathways engage downstream regulators of the growth cone cytoskeleton with an emphasis on links to the Rho GTPases. This coverage is not intended to be an exhaustive treatment of this immense topic, and we frequently refer the reader to more in-depth reviews of particular aspects of guidance receptor signaling, especially in instances where direct links to guidance receptors are unclear, as is the case for many key actin regulatory proteins (Pak et al. 2008) (Figure 4). In addition, the important role of calcium signaling in directing growth cone responses has been recently reviewed and is not addressed in detail here (Gomez & Zheng 2006, Zheng & Poo 2007).
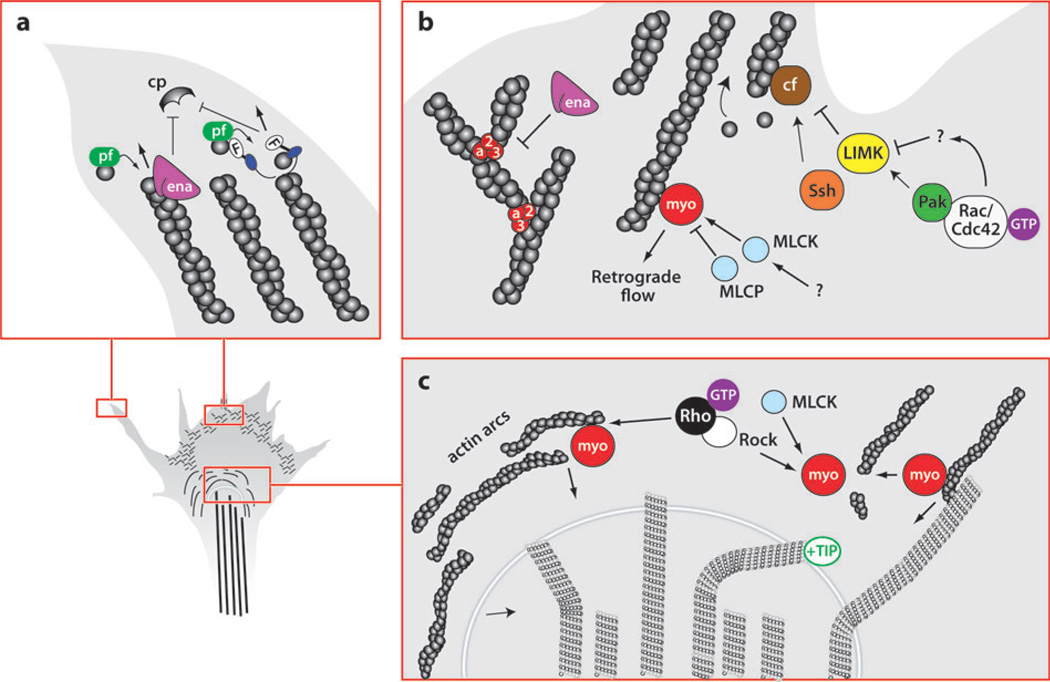
Figure 4.
The growth cone cytoskeleton: structural components and regulatory proteins. (a) Filopodial actin dynamics. Guidance cues (netrin and Slit) may regulate the activity of Ena/VASP (ena) proteins, which in turn promote filament elongation either by antagonizing capping protein (CP) or by barbed-end G-actin addition (gray circles, bound to profilin, pf). Diaphanous-related formins (F) act as actin nucleators and promote barbed-end addition of G-actin, in addition to inhibiting CP. (b) Actin dynamics in the lamellipodium. Ena/VASP antagonizes Arp2/3-dependent filament branching, promoting filopodia formation. Cofilin severs actin filament pointed ends, providing a fresh pool of actin monomers. Rac/Cdc42 inhibit cofilin function through Pak/LIMK, whereas Ssh activates cofilin. Myosin-II (myo)-dependent retrograde actin flow toward the central domain is regulated by myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP). (c) Actin/microtubule dynamics in the growth cone central domain. Rho/ROCK regulate myosin-dependent actin arc contractility. Myosin also promotes actin filament severing, as well as microtubule bundling. Microtubule advancement is regulated by retrograde actin flow in addition to microtubule-associated proteins (MAPs), such as the +TIP protein, orbit/CLASP.
Rho GTPases in Axon Growth and Guidance
Rho-family GTPases, a subgroup of the Ras superfamily of small GTPases, have been extensively studied for their role in cell motility and regulation of cytoskeletal structures (Hall 1998). Members of the Rho (Rac homology) family include well-studied members Rac, Cdc42, and RhoA, as well as several additional members whose roles in cytoskeletal dynamics are not as well understood. Seminal work in fibroblasts demonstrated that Cdc42 and Rac activity are associated with formation of protrusive structures, filopodia and lamellopodia, respectively, whereas RhoA promotes formation of stress fibers and focal adhesions (Hall 1998). These classifications represent a simplistic view of the function of the GTPases in cytoskeletal dynamics, and considerable cross talk between these pathways occurs (Yuan et al. 2003). Rho family GTPases catalyze the hydrolysis of bound GTP to GDP, switching from active (GTP-bound) and inactive (GDP-bound) states. The activity of these GTPases has profound effects on actin cytoskeletal and microtubule dynamics. Considerable evidence has demonstrated the importance of Rho family GTPases in mediating axon guidance receptor signaling output. In this section, we discuss recent advances in our understanding of the role of Rho-family GTPases in axon growth and guidance, highlighting the many examples that challenge the overly simplistic view that Rho promotes repulsion and Rac and Cdc42 promote attraction. For more extensive discussion of the role of Rho GTPases in neuronal development, we refer the reader to several excellent reviews (Govek et al. 2005, Luo 2000, Yuan et al. 2003).
Guidance cues including Slits, netrins, ephrins, and semaphorins can all influence the activity of Rho-family GTPases (Table 1). Slits, acting through Robo receptors, lead to decreased levels of active Cdc42 and increased RhoA and Rac activity (Fan et al. 2003, Wong et al. 2001). Ephrins, through Eph receptors, result in increased RhoA activity as well, but they also cause transient, decreased Rac activity in RGCs (Jurney et al. 2002, Wahl et al. 2000). Sema treatment via plexin-B1 activates RhoA (Swiercz et al. 2002) and sequesters active Rac (Hu et al. 2001, Vikis et al. 2000); however, sema3A via plexin-A activates Rac but not RhoA (Turner et al. 2004). There is no general consensus for how Rho GTPases mediate repulsion because each of these repulsive guidance pathways influences RhoA, Rac, and Cdc42 activity in distinct ways. Netrin, through DCC, increases Rac activity in fibroblasts (Li et al. 2002b), increases Rac and Cdc42 activity in rat commissural neurons (Shekarabi et al. 2005), and inhibits RhoA activity (Moore et al. 2008a) (Table 1).
Table 1.
Regulation of Rho-GTPase activity by the four classic guidance pathways during axon guidance. This table summarizes known links between guidance receptors and Rho-GTPase regulation in axon guidance, primarily through Rho GEFs and GAPs. Examples from other cell types or processes, as well as ligands and receptors whose influence on Rho-GTPases is unknown, are not included here
| Ligand/receptor family |
Effect on Rho-GTPase activitya |
Organism(s) | Mediated by |
|||||
|---|---|---|---|---|---|---|---|---|
| Ligand(s) | Receptor(s) | Rho | Rac | Cdc42 | GEFs/GAPs (substrate)d |
Additional regulation | References | |
|
Slit/Robo |
||||||||
| Slit | Robo | (+) | (+) | (−) | Drosophila | Sos-GEF(Rac) Vilse/crGAP-GAP(Rac) |
Yang & Bashaw 2006, Hu et al. 2005, Lundstrom et al. 2004 | |
|
Netrin/DCC |
||||||||
| Netrin | DCC | (−) | (+) | (+) | Vertebrates | DOCK180-GEF(Rac) Trio-GEF(Rac) |
Li et al. 2008 Briançon-Marjollet et al. 2008 | |
| NetA,B | Frazzled | Drosophila | Trio-GEF(unknown) | Forsthoefel et al. 2005 | ||||
| Unc-6 | Unc-40 | C. elegans | ? | Unc-40 P2 motif regulation of CED-10/Rac and Unc-115/AbLIM |
Gitai et al. 2003 | |||
|
Netrin/Unc5 |
||||||||
| Netrin | Unc-5 | ? | ? | ? | ||||
|
Semaphorin/Plexin |
||||||||
| Sema3 | Plexin- A/Npn-1/2 |
? | (+) | ? | Vertebrates | FARP2-GEF(Rac1) | Toyofuku et al. 2005 | |
| Sema4 | Plexin-B | (+) | (−)c | ? | Vertebrates | PDZ-RhoGEF/LARG- GEF(RhoA) |
Direct Plexin-B binding (Rac) |
Aurandt et al. 2002; Driessens et al. 2001; Swiercz et al. 2002; Vikis et al. 2000, 2002 |
| Sema2a | PlexB | Drosophila | ? | Direct PlexB binding (Rac, Rho) |
Hu et al. 2001 | |||
|
Ephrin/Eph receptors |
||||||||
| Ephrin A | EphAb | (+) | (−/+) | ? | Vertebrates | α-Chimaerin- GAP(Rac1) |
Beg et al. 2007, Iwasato et al. 2007, Shi et al. 2007, Wegmeyer et al. 2007 | |
| Ephexin-GEF(RhoA) | Tyrosine phosphorylation of Ephexin |
Sahin et al. 2005, Shamah et al. 2001 | ||||||
| Vavl/2-GEF(Rac) | Vav-dependent Eph endocytosis |
Cowan et al. 2005 | ||||||
| Ephrin B | EphB | (+) | (−/+) | ? | Vertebrates | Vavl/2-GEF(Rac) | Vav-dependent Eph endocytosis |
Cowan et al. 2005 |
As demonstrated biochemically; (+), increased Rho-GTP; (−), decreased Rho-GTP; (−/+), transient decrease in Rho-GTP. See text for references.
Exception is EphA4, which binds to both class A/B ephrins.
Activated Rac is sequestered from PAK via the PlexB receptor.
GEF or GAP primary specificity for a particular Rho-GTPase or subfamily of Rho-GTPases during axon guidance is indicated in parentheses, see references on right.
How are these diverse guidance signals coupled to the Rho GTPases to affect their activity, and how do different patterns of Rho, Rac, and Cdc42 activation evoked by distinct repulsive signals all result in repulsion? To begin to answer these questions, mechanisms directly linking these receptors to activation of the GTPases need to be elaborated. Additionally, detailed analysis of the spatial and temporal activation of Rho GTPases using new methods that allow in vivo visualization of active GTPases promise to be particularly informative. The origins of distinct outputs may lie in the specificity of downstream Rho/Rac/Cdc42 effectors that modulate peripheral retrograde actin flow, myosin contractility, and microtubule dynamics. Our understanding of the precise mechanisms by which Rho GTPases exert control over growth cone dynamics through these effectors is rapidly advancing. In the following sections we discuss how guidance receptors are linked to Rho GTPase activation, and how these pathways in turn result in modulation of cytoskeletal dynamics.
Linking Guidance Receptors to Regulation of Rho GTPases: Rho GEFs and GAPs
The primary regulators of Rho GTPase cycling and activity are the Rho family GEFs (guanine nucleotide exchange factors) and GAPs (GTPase activating proteins). The number of GEFs and GAPs encoded in most genomes far exceeds the number of Rho GTPases, suggesting that upstream regulation is likely to provide tissue-specific, as well as temporal control of Rho GTPase signaling during growth cone guidance. Guidance receptors can directly regulate Rho GTPases: For instance, plexin-B binds directly to Rac, presumably sequestering Rac from its effector Pak, thereby inhibiting its activity (Vikis et al. 2000, 2002). However, this mechanism of direct regulation of GTPases through interaction with receptors appears to be more of an exception than the rule. Thus, identifying the GEFs and GAPs that function downstream of a given guidance receptor is critical to understanding the mechanism of guidance receptor signal transduction. Recent insights into Eph/ephrin regulation of Rho GTPases in the mouse and Slit/Robo regulation of Rho GTPases in Drosophila have suggested common themes in how repulsive signals coordinately regulate multiple Rho GTPases.
α-Chimaerin in Eph Receptor Signaling
Identification of individual Rho GTPase regulators that are essential mediators of guidance receptor signaling pathways is complicated by at least three major factors: (a) Redundancy can obscure important functions, (b) individual GEFs and GAPs can act in multiple signaling pathways, and (c) GEFs and GAPs often contribute to only part of any given signaling output. However, in at least one instance, a single Rac GAP functions as a critical downstream component of a guidance receptor pathway. Work from several groups identified the Rac-GAP α-chimaerin as an essential mediator of the ephrinB3/EphA4 guidance pathway in vivo. Mutations in either the ligand ephrinB3 or the receptor EphA4 result in mice with a characteristic hopping gait phenotype that occurs at least in part because of misrouting of interneuron axons of the mammalian locomotory central pattern generator (CPG), the circuit necessary for coordinating alternating limb movement (Kullander et al. 2003). These wiring defects are caused by a loss of ephrinB3/EphA4 forward signaling and can be attributed to a failure of axons to respond to midline ephrins, resulting in abnormal midline recrossing (Kullander et al. 2001). Incredibly, mutations in α-chimaerin result in phenotypes almost identical to ephrinB3 −/− or EphA4 −/− mice, including the locomotory behavior phenotype (Beg et al. 2007, Shi et al. 2007, Wegmeyer et al. 2007). Tract-tracing experiments in α-chimaerin mutants reveal that the corticospinal tract axons that control voluntary movements and commissural interneurons aberrantly cross the midline, whereas structures that require ephrinB3 reverse signaling, such as the corpus callosum, are unaffected. Together, the similarities of phenotypes among ephrin B3 −/−, EphA4−/−, and α-chimaerin mutants, the observation that mice lacking one copy of EphA4 and one copy of α-chimaerin exhibit α-chimaerin mutant phenotypes, and the demonstration that α-chimaerin is a necessary mediator of ephrinB3/EphA4-induced growth cone collapse in cultured neurons strongly argue that this GAP functions as a necessary mediator of ephrin forward signaling (Beg et al. 2007, Shi et al. 2007, Wegmeyer et al. 2007).
How does α-chimaerin function in EphA4 repulsion? The α2-chimaerin isoform contains two interaction domains for EphA4, the N-terminal SH2 domain, which can interact with phosphorylated juxtamembrane tyrosines of EphA4, and a second region in the C-terminus that constitutively interacts with the kinase domain of EphA4. EphA4-dependent tyrosine phosphorylation of α2-chimaerin occurs in response to ephrin B3, and this treatment increases the Rac-GAP activity of α-chimaerin (Shi et al. 2007). In addition, α-chimaerin’s diacylglycerol (DAG) binding C1 domain is very likely to regulate the GAP activity of α2-chimaerin, as indicated by the crystal structure of the closely related β2-chimaerin. The GAP domain in β2-chimaerin is occluded by the N-terminal SH2 motif, mediated by intramolecular interactions with the C1 domain, and ligand binding to the C1 domain is predicted to result in exposure of the Rac-GAP domain (Canagarajah et al. 2004). Thus, increases in DAG production (by phospholipase signaling, for instance) would be expected to increase the Rac-GAP activity of α2-chimaerin. Similarly, SH2-mediated interactions with receptors may free the GAP domain for Rac inhibition. It remains to be determined how interaction with EphA4 influences α2-chimaerin GAP activity, and to resolve whether input from kinase or phospholipase signaling plays a role in refining signaling downstream of Eph receptors, thereby influencing connectivity of the CPG.
Although a reduction in Rac activity is clearly required to mediate ephrin-A-induced collapse, Rac activation also appears, paradoxically, to be necessary for responses to ephrins. Interference with Rac signaling blocks growth cone collapse in response to both semaphorins and ephrins (Jin & Strittmatter 1997, Jurney et al. 2002, Kuhn et al. 1999, Vastrik et al. 1999). Although decreases in Rac activity are observed following ephrin stimulation, reactivation of Rac is temporally correlated with growth cone collapse. Rac activity appears to be required for endocytosis; semaphorin 3A or ephrin treatment of retinal growth cones results in Rac-dependent endocytosis, which appears to mediate contact repulsion. Specifically, for class B Eph/ephrins, bidirectional endocytosis occurs as the ephrin ligand and the Eph receptor are each internalized in trans to neighboring cells in a process that depends on their cytoplasmic domains as well as Rac activity (Marston et al. 2003, Zimmer et al. 2003). As discussed earlier, the conserved Vav subfamily of Dbl GEFs plays a central role in this process, which appears to be instrumental for growth cone retraction (Cowan et al. 2005).
Ephrins also function through Rho activation, and this activation appears to be mediated by the Dbl family Rho GEF ephexin. Ephexin activates RhoA, Rac, and Cdc42, but activation of the EphA receptor results in preferential activity toward RhoA (Shamah et al. 2001). In mice, the ephexin family has five members, two of which—ephexin1 and ephexin5 (Vsm-Rho-GEF)—are expressed in the mouse brain (Ogita et al. 2003, Sahin et al. 2005). Ephexin1−/− mice have no phenotypic abnormalities, but cultured RGC axons derived from these mice are deficient in growth cone collapse in response to ephrin-A1 and exhibit axon outgrowth deficits as well. Additionally, in chick lateral motor column neurons, which normally stall in response to ephrin-A5 prior to entering limb mesoderm, sh-RNA-mediated knockdown of chick ephexin (c-ephexin) results in premature entry of these axons into the limb mesoderm. c-Ephexin being the only ephexin family member expressed in these neurons suggests that ephexin is required to trigger the response to ephrin-A5, and that redundancy could explain the lack of guidance defects in ephexin1−/− mice. Ephexin1/ephexin5 double mutants could help to address this question, although the positive role of ephexin in axon outgrowth could confound the interpretation of these experiments.
GEFs and GAPs Linking Slit-Robo Signaling to the Regulation of Rac Activity
Although inhibition of Rac is often thought to accompany repulsive guidance decisions, recent evidence suggests that activation of Rac may also be involved in mediating responses to repulsive cues, as we have seen for the role of Rac in ephrin/EphR endocytosis and growth cone retraction. In the context of Slit-Robo-mediated repulsion, for example, recent biochemical and genetic evidence suggests that activation of Robo receptors by Slit leads to activation of Rac (Fan et al. 2003, Wong et al. 2001) and that limiting Rac function reduces the efficiency of Slit-Robo signaling (Fan et al. 2003, Hakeda-Suzuki et al. 2002). Recent evidence indicating that both Rac GAPs and GEFs regulate Robo repulsion gives insight into the complex regulation of Rho GTPases needed for effective guidance decisions.
A conserved Rho family GAP, Vilse/CrGAP, was identified in Drosophila as a regulator of Slit-dependent guidance decisions in both CNS axons at the midline and in tracheal cells (Hu et al. 2005, Lundstrom et al. 2004). Loss of function of vilse/crGap enhances both tracheal and axon guidance defects in genetic backgrounds where slit and robo functions are limited, indicating that it normally functions as a positive regulator of Slit repulsion. Vilse/crGAP, however, specifically antagonizes Rac function both in vivo and in vitro. Overexpression of Vilse/crGAP suppresses the gain of function phenotype of Rac, but not Rho, in the Drosophila compound eye and enhances midline guidance defects caused by expression of a dominant-negative Rac, but not by a dominant-negative Cdc42 (Hu et al. 2005). In axons, high levels of Vilse/crGAP overexpression cause similar defects to those present in robo loss-of-function mutants, and low levels of overexpression cause dosage-dependent defects in Slit-Robo repulsion similar to loss of function of vilse/crGap (Hu et al. 2005). Thus, the consequences of increasing or decreasing vilse/crGap function are similar: Both lead to a compromise in the efficiency of Slit-Robo midline repulsion.
How can Vilse/crGAP act as both a positive and a negatve regulator of Slit-Robo axon repulsion? The interaction of Vilse/crGAP with Robo may be regulated in different subcellular contexts or during distinct stages of Slit-Robo repulsion. A clue may come from the localization of Vilse/crGAP in response to Slit. Treatment of Robo and Vilse/crGAP-expressing cells with Slit causes Vilse/crGAP to leave the cell membrane and localize to the cytoplasm, thus this relocalization may relieve Rac inhibition at the receptor and allow subsequent activation of Rac by GEFs (M. Li & G. Bashaw, unpublished results). Although regulation of GAP activity of Vilse/crGAP could account for the observed increase in Rac-GTP following Robo receptor activation, loss-of-function mutants for vilse/crGap lead to only very subtle defects in midline repulsion. Considering that Rac activity is required for midline repulsion in the Drosophila CNS, additional regulators should link Robo to Rac activation in these neurons. The dual Ras-Rho GEF Sos is a likely candidate, on the basis of its CNS expression, its genetic interaction with slit and comm mutants (Fritz & VanBerkum 2000), and its interaction with the adaptor protein Nck (Dock in Drosophila), which binds to Robo and recruits Pak in Drosophila (Fan et al. 2003).
It has recently been demonstrated that sos zygotic mutants display mild defects in midline repulsion, and these defects can be significantly enhanced through genetic removal of one copy of slit or robo. sos functions primarily through Rac in midline axon guidance; heterozygosity for one of three rac-like genes significantly enhances sos mutant defects in midline repulsion, whereas rho-homozygous mutants only mildly enhance this phenotype (Yang & Bashaw 2006). Sos interacts with Robo by binding to the adaptor, Dock. In response to Slit treatment, the normally cytoplasmic Sos is recruited to the plasma membrane, where it colocalizes with Robo. This presumably leads to Rac activation because membrane ruffling and lamellipodia formation, which are hallmarks of Rac activation, occur in cultured human 293T cells treated with Slit (Yang & Bashaw 2006).
On the basis of this work and that described for ephrins/EphRs, we can draw considerable parallels in how these repulsive guidance pathways regulate Rac activity. Each pathway uses a Rac GAP (Vilse/crGAP for Robo and α-chimaerin for Ephs) and a Rac GEF (Sos for Robo and Vav for Ephs) to mediate repulsion. In the context of repulsion, Rac cycling appears to be more important than the overall levels of Rac-GTP in a responding growth cone (reminiscent of the observation that cycling is required for malignant transformation, GEFs can transform cells, whereas constitutively active Rho GTPases cannot). Alternatively, these GAPs and GEFs may represent distinct steps in the repulsive signal transduction output, as in the example of Eph receptors where Vavfamily GEFs likely mediate endocytosis of the ligand-receptor complex through Rac activation (Cowan et al. 2005). Rac activation in the case of Robo receptors may precede internalization, and intracellular accumulations of Sos and Robo have been observed in cultured cells (L. Yang & G. Bashaw, unpublished observations). Thus, parallel mechanisms of repulsion may exist in these distinct ligand-receptor systems, although unlike Eph/ephrin repulsion via ephexin, no Rho GEF has yet been described for Slit/Robo repulsion.
Plexin A1 and the Activation of Rac For Repulsion
In contrast to the mechanism of plexin-B1 activation via Rac sequestration and RhoA activation (Hu et al.2001, Swiercz et al. 2002), growth cone collapse induced by sema3A requires activation of Rac. Plexin-A1, together with neuropilin, transduces guidance signals from class 3 semaphorins, leading to Rac activation (Turner et al. 2004), Rnd1 recruitment (Zanata et al. 2002), and reduction in R-Ras activity (Toyofuku et al. 2005). A recent study has implicated the FERM (protein 4.1, Ezrin, Radixin, Moesin domain) domain-containing Rac GEF, FARP2, in mediating sema3A-induced Rac activation and growth cone collapse in dorsal root ganglia neurons (DRGs) (Toyofuku et al. 2005). In the absence of semaphorins, FARP2 interacts with plexin-A1. Sema3A treatment of transfected HEK293 cells causes dissociation of FARP2 from plexin-A and coincident recruitment of Rnd1, as well as increases in active Rac and reduction in active R-Ras through a process dependent on FARP2 GEF activity. Inhibiting FARP2 function, by siRNA or expression of dominant-negative forms, blocks sema3A-induced Rac activation, growth cone collapse, and repulsion of DRG axons (Toyofuku et al. 2005). Sema3A also appears to regulate cell adhesion through FARP2-mediated sequestration of PIPKIγ661 from talin and reduction of PtdIns(4,5)P2, resulting in suppression of adhesion. It is unclear at present whether Rac-GTP binds to plexin-A1, as it does in the case of plexin-B1, and thus how activation of Rac results in Rnd1 recruitment and R-Ras downregulation. We await future work to elaborate this pathway using in vivo studies to determine whether loss of function for FARP2 mimics phenotypes of sema3A–plexin-A1 deficiency.
Netrin Attraction: GEFs Linking DCC to the Rho GTPases
Several lines of evidence indicate that netrins induce outgrowth and attractive turning via the DCC family of receptors at least in part by regulating Rho GTPases. Outgrowth of commissural axons in response to netrin requires Rho GTPase activity, and DCC-dependent neurite outgrowth in N1E-115 cells requires the activity of both Rac1 and Cdc42 (Li et al. 2002b). In addition, mutations in the Rac gene ced-10 in C. elegans partially suppress defects caused by expression of a constitutively active form of the DCC receptor homolog, Unc-40 (Gitai et al. 2003). Netrin induces rapid activation of Rac1, Cdc42, and Pak 1, which may occur in a complex containing the constitutive components DCC and Nck-1, as well as netrin-induced components, Rac1, Cdc42, Pak1, and N-WASP (Li et al. 2002a, Shekarabi et al. 2005, Shekarabi & Kennedy 2002). Activation of this complex by netrin causes profound changes in growth cone morphology, leading to increased surface area and a greater number of filopodia. Netrin treatment increases the amount of a nonhydrolyz-able GTP analog, GTPγS, bound to Rac and Cdc42 in commissural neurons, suggesting that one or more GEFs may be associated with this complex to drive the observed increases in Rac and Cdc42 activity.
Although investigators have not identified a candidate GEF that is committed to netrin-DCC signaling in all contexts, recent work suggests that in at least certain cell types, the Trio GEF may fulfill this function. Trio is an important regulator of axon guidance decisions in several contexts (Liebl et al. 2000, Newsome et al. 2000); however, direct evidence that Trio functions downstream in a particular pathway has been elusive. Trio contains 2 Rho GEF domains, one with specificity for Rac and RhoG and another that activates RhoA. Trio positively contributes to midline axon crossing in the embryonic CNS in Drosophila and can physically interact with Frazzled (Forsthoefel et al. 2005) and with mammalian DCC (Briançon-Marjollet et al. 2008), although in the latter case the interaction is likely mediated through binding to PAK-1. Genetic removal of Trio in mice reduces netrin-dependent outgrowth responses in both cortical and dorsal spinal neurons. In cortical neurons from Trio −/− mice, netrin stimulation does not result in Rac activation, whereas in WT mice these neurons normally display DCC-dependent Rac activation in response to netrin (Briançon-Marjollet et al. 2008). Trio −/− mice also display a variety of CNS axon guidance defects, which partially overlap with defects seen in netrin or DCC −/− mice, suggesting that Trio can account for a portion of the function of netrin-DCC signaling in midline axon guidance (Briançon-Marjollet et al. 2008). However, the commissural axon guidance defects in Trio−/− mice are considerably milder than those seen in netrin or DCC −/− mice, indicating that at least one or more additional factors must be present in these neurons to mediate netrin-dependent midline attraction.
The CZH (CDM, zimzimin homology containing) family GEF, DOCK180, also appears to contribute to netrin-DCC attraction in mouse cortical and commissural neurons by mediating Rac activation. Through siRNA knockdown approaches, Li et al. demonstrated that DOCK180 is required for dissociated cortical neuron outgrowth in response to netrin and for commissural neuron turning in explant assays (Li et al. 2008b). Knockdown of DOCK180 in chick spinal cords also reduces commissural axon crossing. Netrin can induce both axon outgrowth and attractive axon turning; therefore, it is unclear in these assays whether commissural neuron turning defects are a secondary consequence of defects in axon outgrowth (Li et al. 2008b). It is also unclear whether Trio and DOCK180 function in the same or a parallel pathway to mediate netrin-dependent Rac activation downstream of DCC. Each could act in independent contexts to activate Rac because both Trio and DOCK180 can interact with DCC. Because Rac is recruited to DCC in response to netrin, interaction of either Trio or DOCK180 with DCC may be sufficient to activate Rac upon recruitment to the complex. Additionally, because Cdc42 activation is also required for attraction via DCC, identifying additional GEFs that regulate this GTPase will add to our understanding of netrin-DCC regulation of Rac and Cdc42 activity.
Rho-Family Effectors: p21 Activated Kinase (PAK)
In motile cells, activation of Rho GTPases results in modulation of cytoskeletal dynamics via effector proteins, and one of the best characterized of these is the dual Cdc42/Rac effector, p21-activated kinase (Bokoch 2003). A well-established pathway of PAK activation via Cdc42 or Rac results in inhibition of the actin depolymerizing factor cofilin by activating its inhibitor, LIM kinase (Dan et al. 2001). Other notable targets of PAK include the myosin activator, myosin light chain kinase (MLCK), and the microtubule destabilizing protein, Op18/stathmin, which are each inhibited by PAK phosphorylation (Daub et al. 2001, Sanders et al. 1999). PAK is required for Drosophila photoreceptor axon targeting in conjunction with the Rac/Rho GEF Trio and the SH2/SH3 domain-containing adaptor protein, Dock (Nck) (Hing et al. 1999, Newsome et al. 2000). Dock and Pak function in a common pathway and are required cell autonomously in Drosophila olfactory neurons for proper glomerular axon targeting (Ang et al. 2003).
GTP-bound Rac and Cdc42 regulate Pak activity through binding to its Cdc42/Rac interactive binding (CRIB) domain, relieving auto-inhibition of Pak by its N-terminal domain (Buchwald et al. 2001, Lei et al. 2000). A few examples suggest Pak likely functions downstream of Rac/Cdc42 in axon guidance. Drosophila pak, dock, and rac each function in midline axon repulsion and interact genetically with the Slit/Robo pathway. Expression of a constitutively membrane-targeted Pak suppresses defects caused by rac loss of function (Fan et al. 2003), which suggests that these Rac-dependent defects likely occur through loss of PAK regulation. PAK overexpression in Drosophila mushroom body neurons also results in axon growth and guidance defects, and these can be suppressed through genetic removal of one copy of cdc42 or one copy of each of two Drosophila rac genes (Ng & Luo 2004). In C. elegans, there are three rac genes (mig-2, ced-10, and rac-2) and three pak genes, two of which, pak-1 and max-2, are expressed in the nervous system. Mutation of max-2 results in misrouting of ventral cord commissural motor neurons, and removal of pak-1 enhances these defects. Expression of a constitutively active version of the Rac, Mig-2, results in misrouting of these axons, and this phenotype is suppressed in pak-1 mutants, suggesting that pak-1 functions in the same pathway as Rac in dorsal guidance of these neurons (Lucanic et al. 2006).
Both Cdc42 and Rac likely also function through pathways independently of PAK, particularly in axon growth; expression of Rac mutants that are unable to bind Pak rescues axon growth defects and partially rescues axon guidance defects caused by rac loss of function in Drosophila mushroom body neurons (Ng et al. 2002). Overexpression of Rac or Cdc42 in Drosophila motor neurons results in outgrowth or guidance defects, respectively. Whereas the guidance defects caused by Cdc42 overexpression are suppressed by mutating the Pak interaction motif of Cdc42, the growth defects caused by Rac gain of function are not (Kim et al. 2003). Taken together, these in vivo studies suggest that regulation of outgrowth via Rac can occur through a PAK-independent mechanism; however, guidance mediated through Rac and Cdc42 at least partly involves PAK function.
Rho-Family Effectors: LIM Kinase
How does regulation of Pak lead to modulation of actin dynamics in axon growth and guidance? Recent evidence, in agreement with biochemical studies and evidence from fibroblasts, suggests that this occurs by regulating the actin depolymerizing factor, cofilin, by modulating the activity of the serine/threonine kinase, LIMK (lin-11, Isl-1, and Mec-3 kinase) (Sarmiere & Bamburg 2004). Cofilin destabilizes F-actin through pointed-end severing of actin filaments (Figure 4), although this activity may be necessary to maintain the supply of monomeric G-actin, thus promoting actin polymerization. This activity is inhibited by phosphorylation at the N-terminal Ser3: Phosphorylation at this site is reciprocally regulated by the LIM and TES kinases and by the Slingshot phosphatase (Ssh) (Arber et al. 1998, Niwa et al. 2002, Yang et al. 1998). Evidence suggests that in some cases the rate of cycling between phosphorylated and nonphosphorylated states, rather than the absolute level of either species, can determine the influence of cofilin on actin dynamics (Aizawa et al. 2001, Meberg 1998). In Drosophila, cells deficient for ssh display dramatic increases in F-actin, as well as morphological defects (Niwa et al. 2002). How LIM kinase and slingshot function in concert to regulate growth cone dynamics by regulating cofilin is of great interest in understanding receptor-mediated guidance.
Ng & Luo (2004) recently addressed this question through an in vivo analysis of the pathways regulating axon growth in Drosophila mushroom body neurons. In neurons lacking the sole Drosophila cofilin homolog, twinstar (tsr), axons frequently stall, and axon shafts in these neurons have excessive protrusions reminiscent of filopodia and lamellipodia. These findings suggest that cofilin function, likely through actin depolymerization, is required to limit these structures and, in turn, promotes axon growth. Neither a non-phosphorylatable (S3A) nor a phosphomimetic (S3E) version of tsr, nor both in combination, rescues these axon growth phenotypes as effectively as does WT tsr, suggesting that cycling of cofilin is required for promoting axon growth. LIMK antagonizes, while Slingshot promotes, tsr/cofilin function in these neurons. LIMK, in turn, is activated in these neurons by both the Rho1/ROCK pathway as well as the Cdc42/Rac/Pak pathway because genetic reduction of components of either of these pathways suppresses growth defects resulting from LIMK overexpression or ssh loss of function. This finding suggests that these pathways converge to regulate twinstar/cofilin, consistent with previous reports that morphological changes in growth cones associated with changes in cofilin phosphorylation occur via ROCK- (Gehler et al. 2004) or ROCK/PAK-dependent (Aizawa et al. 2001) pathways.
In apparent contrast to the negative role in axon outgrowth described above, LIMK also appears to mediate both axon outgrowth and attraction in certain contexts. RNAi-mediated in-hibition of LIMK1/LIMK2 blocks NGF (nerve growth factor)-induced neurite outgrowth of PC12 cells and axon outgrowth of chick DRG neurons (Endo et al. 2007). Furthermore, BMP7-induced Xenopus spinal growth cone turning requires LIMK activity; a cell-permeable peptide (S3) containing serine 3 of Xenopus ADF/Cofilin blocks the normal attractive response in these neurons (Wen et al. 2007). A gradient of phosphorylated cofilin accompanies the attractive response to BMP7, and repulsive responses from the same ligand are mediated by Ssh activity, demonstrating that distinct responses can be generated through activities’ converging on a single actin regulator. These results also imply that inhibition of cofilin via LIMK is required for growth cone attraction in certain contexts (Wen et al. 2007). However, at present, the precise role of guidance-receptor pathways in regulation of cofilin through LIMK and Ssh remains unresolved.
Rho-Family Effectors: Rho Kinase (ROCK)
Stimulation of RhoA results in activation of Rho kinase. Rho kinases (ROCK or Rok) are serine/threonine kinases that, similar to PAK, regulate LIMK. Additionally, Rho kinases can regulate myosin activity through the phosphorylation of myosin light chain (MLC), which results in activation and increased actin-myosin contractility. Inhibition of Rho-kinase blocks growth cone turning induced by a gradient of lysophosphatidic acid (LPA), which acts through G protein–coupled receptors to activate RhoA (Yuan et al. 2003). Also, a gradient of the Rho-kinase inhibitor, Y-27632, is sufficient to induce growth cone turning, and this action is blocked in the presence of an MLC kinase inhibitor, ML-7, suggesting that these pathways cooperate to regulate myosin-dependent turning. In contrast, ML-7 switches LPA-induced repulsion to attraction, indicating that activation of a myosin-independent pathway by LPA/RhoA results in attraction. This Rho-dependent attractive pathway may be mediated by regulating diaphanous, a Formin family member (a family of linear actin filament nucleating proteins) (Arakawa et al. 2003) (Figure 4). Rho kinase also indirectly regulates myosin activity by phosphorylating and inhibiting the MLC phosphatase (MLCP) (Feng et al. 1999). This and other studies suggest that ROCK activity is necessary for RhoA-induced retraction, likely through regulation of myosin II (Zhang et al. 2003). In contrast to proposed models of Rho/ROCK regulation of Myosin in growth cone retraction (Huber et al. 2003), modulation of Rho or ROCK do not appear to affect retrograde flow of actin in the peripheral domain of a growth cone. Instead, inhibition of Rho or ROCK prevents the stability and contraction of actin arcs, which are filamentous actin structures that form in the transition zone of growth cones and affect microtubule bundling and dynamics (Schaefer et al. 2002, Zhang et al. 2003) (Figure 4).
Are MLC and MLCP the only relevant targets of ROCK in growth cone turning? Although ROCK can phosphorylate LIMK to regulate cofilin activity, it has yet to be demonstrated in the context of an axon guidance decision. ROCK also phosphorylates the collapsin response mediator protein-2 (CRMP-2) after LPA or ephrin-A5 stimulation, inhibiting its ability to bind tubulin heterodimers. CRMP-2 normally promotes axon outgrowth and branching, presumably by nucleating and promoting microtubule assembly. The same residue in CRMP-2 that is targeted by ROCK downstream of LPA and ephrin A5 is phosphorylated by Cdk5 downstream of sema3A-induced growth cone collapse (Arimura et al. 2005, Brown et al. 2004), suggesting that independent signaling pathways can converge on the regulation of CRMP-2 phosphorylation.
Cyclic Nucleotides and the Modulation of Guidance Responses
More than ten years ago, Mu Ming Poo and colleagues made the striking finding that reducing the levels of the cyclic nucleotide cAMP or inhibiting PKA in the growth cones of cultured Xenopus spinal neurons could convert attraction toward sources of brain-derived neurotrophic factor and acetylcholine into repulsion (Song et al. 1997). Additional studies in the Xenopus culture system demonstrated that cyclic nucleotide (cAMP or cGMP)-dependent response conversion could also be observed for other attractive guidance cues such as netrin (Ming et al. 1997), as well as a number of repulsive cues, including semaphorins (Song et al. 1998). The general picture that emerged from these studies is that high cyclic nucleotide levels favor attraction, whereas low levels favor repulsion. More recently Nishiyama and colleagues (2003) have demonstrated that it is the ratio of cAMP/cGMP that is important in determining the polarity of the guidance response and have implicated calcium channel modulation in the control of guidance responses.
A clear demonstration that cyclic nucleotides and their downstream effectors can convert receptor responses from attraction to repulsion and vice versa during axon guidance in vivo is still lacking; however, a growing body of evidence supports a potent role for cyclic nucleotide signaling in modulating the strength of receptor responses. For example, a recent study of motor axon guidance in Drosophila has shown that cAMP signaling through PKA can modulate axon repulsion. Specifically, the Drosophila A-kinase anchoring protein (AKAP), Nervy, links the Plexin-A receptor to PKA to inhibit sema repulsion (Terman & Kolodkin 2004). The simplest interpretation of nervy and sema/plexin genetic interactions is that rather than switching repulsion into attraction, Nervy and PKA weaken the strength of the repulsive response. Together with a number of recent studies in vertebrate neuronal culture and zebrafish that suggest a similar inhibitory effect of cAMP/PKA on the strength of various repulsive guidance signals, the role of nervy in Sema signaling suggests that rather than a whole-sale conversion of guidance responses, cyclic nucleotide signaling modulates the strength of guidance outputs (Chalasani et al. 2003, 2007; Dontchev & Letourneau 2002). On the basis of the role of PKA in regulating surface levels of DCC, which we discussed earlier (Bouchard et al. 2004), it is tempting to speculate that cAMP/PKA signaling may be influencing the strength of other receptor outputs in a similar way, namely by controlling surface receptor levels.
In these examples, the signals mediating changes in cyclic nucleotide levels are thought to be independent of the guidance receptors whose responses they modulate; however, a more direct role of guidance receptor signaling in activating cAMP signaling has been suggested in the case of netrin. Specifically, experiments in cultured Xenopus neurons have shown that netrin acting through DCC (or A2b) leads to elevation of cAMP and activation of PKA, and these events have been proposed to be instrumental in promoting netrin-mediated axon outgrowth and attraction (Corset et al. 2000, Hopker et al. 1999). More recently, Wu et al. (2006) proposed that soluble adenylyl cyclase plays an essential role in netrin-dependent axonal development by triggering elevation of cAMP in response to netrin in rat neurons. However, several other studies have proposed a rather different role for cAMP/PKA in contributing to netrin responses and have convincingly demonstrated, at least in rodent commissural neurons, that (a) netrin treatment does not lead to elevations in cAMP or activation of PKA; that (b) PKA is not required for netrin attraction, but rather can regulate the potency of netrin responses through promoting DCC recruitment to the plasma membrane; and that (c) mutations in soluble adenylyl cyclase do not reveal a requirement in mediating netrin signaling during commissural axon guidance in mice (Bouchard et al. 2004, Moore et al. 2008b, Moore & Kennedy 2006). Although these observations do not preclude a role for direct netrin-dependent cAMP signaling in other cellular contexts such as in vitro steering of cultured Xenopus neurons, they do argue against the generality of this mechanism for netrin-directed axon path finding in vivo. Together the preponderance of evidence favors an important role for cyclic nucleotides in modulating the strength of guidance responses in vivo rather than switching the polarity of responses. The challenge now is to define the signals and receptors that regulate cyclic nucleotide signaling in vivo and to define specific contexts where their modulatory effects influence axon guidance.
CONCLUSIONS AND FUTURE DIRECTIONS
The past several years have seen substantial progress in defining some of the mechanisms required to regulate receipt and transduction of axon guidance signals. In particular, a number of recent studies have given new emphasis to the importance of the posttranslational regulation of guidance receptors in determining axon responsiveness. Indeed, many molecules that previously had been almost exclusively thought to be effectors of receptor signaling, such as PKA and Rho family GTPases, clearly can play important roles in regulating surface expression of guidance receptors. These observations require investigators in this field to exercise caution in interpreting the effects of manipulating these molecules and suggest the importance of considering potential upstream effects. We predict that exciting insights into the role of proteolytic processing in guidance molecule signaling and signal termination will soon be forthcoming; it will be particularly interesting to learn the in vivo roles of gamma-secretase-dependent cleavage of axon guidance receptors.
Although details of signaling pathways continue to emerge, our understanding of the key ligand-regulated events that control receptor activation and signaling is still fragmented. Progress in this area will rely on the development of biochemical and optical strategies to reveal the dynamic changes in multi-protein signaling complexes that are set in motion by guidance receptor activation. For example, genetically encoded optical reporters for the activated forms of Rho GTPases and their effectors will likely prove to be instrumental in understanding the spatial and temporal dynamics of receptor signaling in vivo. It is also clear that many signaling and additional regulatory components await discovery, and molecular and genetic approaches, including sensitized genetic screens in Drosophila and C. elegans, will continue to identify these missing components.
Acknowledgments
We apologize in advance to all the investigators whose research could not be appropriately cited owing to space limitations. We thank Long Yang and Tim Evans for valuable discussions and critical comments on the manuscript.
Glossary
- DCC
deleted in colorectal carcinoma
- Robo
Roundabout
- Comm
Commissureless
- PKA
protein kinase A
- cAMP
cyclic AMP (adenosine monophosphate)
- GEF
guanine nucleotide exchange factor
- PKC
protein kinase C
- ICD
intracellular domain
- CTF
C-terminal fragment
- GAP
GTPase activating protein
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Michael O’Donnell, Email: odonnel2@mail.med.upenn.edu.
Rebecca K. Chance, Email: chancek@mail.med.upenn.edu.
Greg J. Bashaw, Email: gbashaw@mail.med.upenn.edu.
LITERATURE CITED
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Ang LH, Kim J, Stepensky V, Hing H. Dock and Pak regulate olfactory axon pathfinding in Drosophila. Development. 2003;130:1307–1316. doi: 10.1242/dev.00356. [DOI] [PubMed] [Google Scholar]
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, et al. Control of axon elongation via an SDF-1α/Rho/mDia pathway in cultured cerebellar granule neurons. J. Cell Biol. 2003;161:381–391. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Arimura N, Menager C, Kawano Y, Yoshimura T, Kawabata S, et al. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol. Cell Biol. 2005;25:9973–9984. doi: 10.1128/MCB.25.22.9973-9984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoe JL, McKenna WL, Quan TK, Stafford BK, Moore JA, et al. Protein interacting with C-kinase 1/protein kinase Calpha-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J. Neurosci. 2006;26:3192–3205. doi: 10.1523/JNEUROSCI.3469-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell Mol. Life Sci. 2008;65:1311–1334. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Sommer JE, Martin JH, Scheiffele P. alpha2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron. 2007;55:768–778. doi: 10.1016/j.neuron.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bouchard JF, Moore SW, Tritsch NX, Roux PP, Shekarabi M, et al. Protein kinase A activation promotes plasma membrane insertion of DCC from an intracellular pool: a novel mechanism regulating commissural axon extension. J. Neurosci. 2004;24:3040–3050. doi: 10.1523/JNEUROSCI.4934-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briançon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, et al. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol. Cell Biol. 2008;28:2314–2323. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose K, Tessier-Lavigne M. Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr. Opin. Neurobiol. 2000;10:95–102. doi: 10.1016/s0959-4388(99)00066-5. [DOI] [PubMed] [Google Scholar]
- Brown M, Jacobs T, Eickholt B, Ferrari G, Teo M, et al. Alpha2-chimaerin, cyclin-dependent Kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse. J. Neurosci. 2004;24:8994–9004. doi: 10.1523/JNEUROSCI.3184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald G, Hostinova E, Rudolph MG, Kraemer A, Sickmann A, et al. Conformational switch and role of phosphorylation in PAK activation. Mol. Cell Biol. 2001;21:5179–5189. doi: 10.1128/MCB.21.15.5179-5189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38:389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- Canagarajah B, Leskow FC, Ho JY, Mischak H, Saidi LF, et al. Structural mechanism for lipid activation of the Rac-specific GAP, beta2-chimaerin. Cell. 2004;119:407–418. doi: 10.1016/j.cell.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Castellani V, Falk J, Rougon G. Semaphorin 3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol. Cell Neurosci. 2004;26:89–100. doi: 10.1016/j.mcn.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. Achemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J. Neurosci. 2003;23:1360–1371. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Sabol A, Xu H, Gyda MA, Rasband K, et al. Stromal cell-derived factor-1 antagonizes slit/robo signaling in vivo. J. Neurosci. 2007;27:973–980. doi: 10.1523/JNEUROSCI.4132-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chen YY, Hehr CL, Atkinson-Leadbeater K, Hocking JC, McFarlane S. Targeting of retinal axons requires the metalloproteinase ADAM10. J. Neurosci. 2007;27:8448–8456. doi: 10.1523/JNEUROSCI.1841-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chédotal A, Mehlen P. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J. Biol. Chem. 2001;276:32115–32121. doi: 10.1074/jbc.M100871200. [DOI] [PubMed] [Google Scholar]
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J. Biol. Chem. 2001;276:1677–1680. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dontchev VD, Letourneau PC. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J. Neurosci. 2002;22:6659–6669. doi: 10.1523/JNEUROSCI.22-15-06659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohashi K, Mizuno K. LIM kinase and slingshot are critical for neurite extension. J. Biol. Chem. 2007;282:13692–13702. doi: 10.1074/jbc.M610873200. [DOI] [PubMed] [Google Scholar]
- Fambrough D, Pan D, Rubin GM, Goodman CS. The cell surface metalloprotease/disintegrin Kuzbanian is required for axonal extension in Drosophila. Proc. Natl. Acad. Sci. USA. 1996;93:13233–13238. doi: 10.1073/pnas.93.23.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Labrador JP, Hing H, Bashaw GJ. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40:113–127. doi: 10.1016/s0896-6273(03)00591-9. [DOI] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, et al. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J. Biol. Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development. 2005;132:1983–1994. doi: 10.1242/dev.01736. [DOI] [PubMed] [Google Scholar]
- Fritz JL, VanBerkum MF. Calmodulin and son of sevenless dependent signaling pathways regulate midline crossing of axons in the Drosophila CNS. Development. 2000;127:1991–2000. doi: 10.1242/dev.127.9.1991. [DOI] [PubMed] [Google Scholar]
- Galko MJ, Tessier-Lavigne M. Function of an axonal chemoattractant modulated by metalloprotease activity. Science. 2000;289:1365–1367. doi: 10.1126/science.289.5483.1365. [DOI] [PubMed] [Google Scholar]
- Garbe DS, Bashaw GJ. Axon guidance at the midline: from mutants to mechanisms. Crit. Rev. Biochem. Mol. Biol. 2004;39:319–341. doi: 10.1080/10409230490906797. [DOI] [PubMed] [Google Scholar]
- Gehler S, Shaw AE, Sarmiere PD, Bamburg JR, Letourneau PC. Brain-derived neurotrophic factor regulation of retinal growth cone filopodial dynamics is mediated through actin depolymerizing factor/cofilin. J. Neurosci. 2004;24:10741–10749. doi: 10.1523/JNEUROSCI.2836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, et al. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou M, Tear G. Commissureless is required both in commissural neurones and midline cells for axon guidance across the midline. Development. 2002;129:2947–2956. doi: 10.1242/dev.129.12.2947. [DOI] [PubMed] [Google Scholar]
- Gitai Z, Yu TW, Lundquist EA, Tessier-Lavigne M, Bargmann CI. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 2003;37:53–65. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Grill B, Bienvenut WV, Brown HM, Ackley BD, Quadroni M, Jin Y. C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1. Neuron. 2007;55:587–601. doi: 10.1016/j.neuron.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, et al. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- Hehr CL, Hocking JC, McFarlane S. Matrix metalloproteinases are required for retinal ganglion cell axon guidance at select decision points. Development. 2005;132:3371–3379. doi: 10.1242/dev.01908. [DOI] [PubMed] [Google Scholar]
- Hing H, Xiao J, Harden N, Lim L, Zipursky SL. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Hu H, Li M, Labrador JP, McEwen J, Lai EC, et al. Cross GTPase-activating protein (CrossGAP)/Vilse links the Roundabout receptor to Rac to regulate midline repulsion. Proc. Natl. Acad. Sci. USA. 2005;102:4613–4618. doi: 10.1073/pnas.0409325102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Marton TF, Goodman CS. Plexin b mediates axon guidance in Drosophila by simultaneously inhibiting active rac and enhancing rhoa signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, et al. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurney WM, Gallo G, Letourneau PC, McLoon SC. Rac1-mediated endocytosis during ephrin-A2-and semaphorin 3A-induced growth cone collapse. J. Neurosci. 2002;22:6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- Keleman K, Ribeiro C, Dickson BJ. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat. Neurosci. 2005;8:156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- Kennedy TE. Cellular mechanisms of netrin function: long-range and short-range actions. Biochem. Cell Biol. 2000;78:569–575. [PubMed] [Google Scholar]
- Kim MD, Kamiyama D, Kolodziej P, Hing H, Chiba A. Isolation of Rho GTPase effector pathways during axon development. Dev. Biol. 2003;262:282–293. doi: 10.1016/s0012-1606(03)00393-2. [DOI] [PubMed] [Google Scholar]
- Krystosek A, Seeds NW. Plasminogen activator release at the neuronal growth cone. Science. 1981;213:1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. J. Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, et al. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, et al. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, et al. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Levy-Strumpf N, Culotti JG. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat. Neurosci. 2007;10:161–168. doi: 10.1038/nn1835. [DOI] [PubMed] [Google Scholar]
- Li H, Kulkarni G, Wadsworth WG. RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity. J. Neurosci. 2008a;28:3595–3603. doi: 10.1523/JNEUROSCI.5536-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gao X, Liu G, Xiong W, Wu J, Rao Y. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat. Neurosci. 2008b;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Meriane M, Triki I, Shekarabi M, Kennedy TE, et al. The adaptor protein Nck-1 couples the netrin-1 receptor DCC (deleted in colorectal cancer) to the activation of the small GTPase Rac1 through an atypical mechanism. J. Biol. Chem. 2002a;277:37788–37797. doi: 10.1074/jbc.M205428200. [DOI] [PubMed] [Google Scholar]
- Li X, Saint-Cyr-Proulx E, Aktories K, Lamarche-Vane N. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J. Biol. Chem. 2002b;277:15207–15214. doi: 10.1074/jbc.M109913200. [DOI] [PubMed] [Google Scholar]
- Liebl EC, Forsthoefel DJ, Franco LS, Sample SH, Hess JE, et al. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio’s role in axon pathfinding. Neuron. 2000;26:107–118. doi: 10.1016/s0896-6273(00)81142-3. [DOI] [PubMed] [Google Scholar]
- Lin KT, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2 induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J. Biol. Chem. 2008;283:28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, et al. Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J. Biol. Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucanic M, Kiley M, Ashcroft N, L’etoile N, Cheng HJ. The Caenorhabditis elegans P21-activated kinases are differentially required for UNC-6/netrin-mediated commissural motor axon guidance. Development. 2006;133:4549–4559. doi: 10.1242/dev.02648. [DOI] [PubMed] [Google Scholar]
- Lundstrom A, Gallio M, Englund C, Steneberg P, Hemphala J, et al. Vilse, a conserved Rac/Cdc42 GAP mediating Robo repulsion in tracheal cells and axons. Genes Dev. 2004;18:2161–2171. doi: 10.1101/gad.310204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD. Rac-dependent transendocytosis of ephrin Bs regulates Ephephrin contact repulsion. Nat. Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- McKenna WL, Wong-Staal C, Kim GC, Macias H, Hinck L, Bartoe JL. Netrin-1-independent adeno-sine A2b receptor activation regulates the response of axons to netrin-1 by controlling cell surface levels of UNC5 A receptors. J. Neurochem. 2008;104:1081–1090. doi: 10.1111/j.1471-4159.2007.05040.x. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil. Cytoskelet. 1998;39:172–190. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Moore SW, Correia JP, Lai Wing Sun K, Pool M, Fournier AE, Kennedy TE. Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development. 2008a;135:2855–2864. doi: 10.1242/dev.024133. [DOI] [PubMed] [Google Scholar]
- Moore SW, Kennedy TE. Protein kinase A regulates the sensitivity of spinal commissural axon turning to netrin-1 but does not switch between chemoattraction and chemorepulsion. J. Neurosci. 2006;26:2419–2423. doi: 10.1523/JNEUROSCI.5419-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SW, Sun KL, Xie F, Barker PA, Conti M, Kennedy TE. Soluble adenylyl cyclase is not required for axon guidance to netrin-1. J. Neurosci. 2008b;28:3920–3924. doi: 10.1523/JNEUROSCI.0547-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev. Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- Myat A, Henry P, McCabe V, Flintoft L, Rotin D, Tear G. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron. 2002;35:447–459. doi: 10.1016/s0896-6273(02)00795-x. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, et al. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–294. doi: 10.1016/s0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;424:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, et al. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Ogita H, Kunimoto S, Kamioka Y, Sawa H, Masuda M, Mochizuki N. EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ. Res. 2003;93:23–31. doi: 10.1161/01.RES.0000079310.81429.C8. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Harada A, Negishi M. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells. J. Biol. Chem. 2003;278:25671–25677. doi: 10.1074/jbc.M303047200. [DOI] [PubMed] [Google Scholar]
- Pak CW, Flynn KC, Bamburg JR. Actin-binding proteins take the reins in growth cones. Nat. Rev. Neurosci. 2008;9:136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Parent AT, Barnes NY, Taniguchi Y, Thinakaran G, Sisodia SS. Presenilin attenuates receptor-mediated signaling and synaptic function. J. Neurosci. 2005;25:1540–1549. doi: 10.1523/JNEUROSCI.3850-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr. Opin. Neurobiol. 2003;13:79–89. doi: 10.1016/s0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Polleux F, Ince-Dunn G, Ghosh A. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat. Rev. Neurosci. 2007;8:331–340. doi: 10.1038/nrn2118. [DOI] [PubMed] [Google Scholar]
- Rooke J, Pan D, Xu T, Rubin GM. KUZ, a conserved metalloprotease-disintegrin protein with two roles in Drosophila neurogenesis. Science. 1996;273:1227–1231. doi: 10.1126/science.273.5279.1227. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, et al. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Sarmiere PD, Bamburg JR. Regulation of the neuronal actin cytoskeleton by ADF/cofilin. J. Neurobiol. 2004;58:103–117. doi: 10.1002/neu.10267. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 2002;158:139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmelpfeng K, Gogel S, Klambt C. The function of leak and kuzbanian during growth cone and cell migration. Mech. Dev. 2001;106:25–36. doi: 10.1016/s0925-4773(01)00402-6. [DOI] [PubMed] [Google Scholar]
- Schlosshauer B, Walter J, Bonhoeffer F. Is guidance of chick retinal axons in vitro influenced by proteases? Neurosci. Lett. 1990;113:333–338. doi: 10.1016/0304-3940(90)90607-b. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131:215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Kennedy TE. The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell Neurosci. 2002;19:1–17. doi: 10.1006/mcne.2001.1075. [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Moore SW, Tritsch NX, Morris SJ, Bouchard JF, Kennedy TE. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 2005;25:3132–3141. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan D, Dwivedy A, Anderson R, Holt CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat. Neurosci. 2002;5:955–962. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- Shi L, Fu WY, Hung KW, Porchetta C, Hall C, et al. Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc. Natl. Acad. Sci. USA. 2007;104:16347–16352. doi: 10.1073/pnas.0706626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Ming GL, He Z, Lehmann M, McKerracher L, et al. Conversion of neuronal growth cones responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001;291:1976–1982. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC) J. Biol. Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- Terman JR, Kolodkin AL. Nervy links protein kinase A to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- Tomita T, Tanaka S, Morohashi Y, Iwatsubo T. Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol. Neurodegener. 2006;1:2. doi: 10.1186/1750-1326-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, et al. FARP2 triggers signals for Sema3A–mediated axonal repulsion. Nat. Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- Turner LJ, Nicholls S, Hall A. The activity of the plexin-A1 receptor is regulated by Rac. J. Biol. Chem. 2004;279:33199–3205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- Vastrik I, Eickholt BJ, Walsh FS, Ridley A, Doherty P. Sema3A-induced growth-cone collapse is mediated by Rac1 amino acids 17–32. Curr. Biol. 1999;9:991–998. doi: 10.1016/s0960-9822(99)80447-3. [DOI] [PubMed] [Google Scholar]
- Vikis HG, Li W, Guan KL. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 2002;16:836–845. doi: 10.1101/gad.966402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikis HG, Li W, He Z, Guan KL. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc. Natl. Acad. Sci. USA. 2000;97:12457–12462. doi: 10.1073/pnas.220421797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. C.elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat. Neurosci. 2007;10:169–176. doi: 10.1038/nn1834. [DOI] [PubMed] [Google Scholar]
- Wegmeyer H, Egea J, Rabe N, Gezelius H, Filosa A, et al. EphA4-dependent axon guidance is mediated by the RacGAP alpha2-chimaerin. Neuron. 2007;55:756–767. doi: 10.1016/j.neuron.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J. Cell Biol. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Wu SC, McKenna WL, Hinck L. Surface expression of the netrin receptor UNC5H1 is regulated through a protein kinase C-interacting protein/protein kinase-dependent mechanism. J. Neurosci. 2003;23:11279–11288. doi: 10.1523/JNEUROSCI.23-36-11279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat. Neurosci. 2006;9:1257–1264. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Bashaw GJ. Son of sevenless directly links the robo receptor to rac activation to control axon repulsion at the midline. Neuron. 2006;52:595–607. doi: 10.1016/j.neuron.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- Yu TW, Bargmann CI. Dynamic regulation of axon guidance. Nat. Neurosci. 2001;4(Suppl.):1169–1176. doi: 10.1038/nn748. [DOI] [PubMed] [Google Scholar]
- Yuan XB, Jin M, Xu X, Song YQ, Wu CP, et al. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 2003;5:38–45. doi: 10.1038/ncb895. [DOI] [PubMed] [Google Scholar]
- Zanata SM, Hovatta I, Rohm B, Puschel AW. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J. Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, Forscher P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron. 2003;40:931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annu. Rev. Cell Dev. Biol. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Kohler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat. Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]