Abstract
Background
The pathogenesis of breast cancer remains unclear.
Aims
To investigate the pathogenesis of breast cancer through targeted metabolomics of amino acids components in serum of patients with breast cancer.
Methods
Patients with breast cancers were enrolled in our hospital between year January 1st, 2013 and December 31st, 2014. Targeted analysis of amino acids was performed using ESI-QTOF-MS instrument. In vitro experiment was performed to determine the influence of tryptophan towards interleukin-10 (IL-10) secretion by CD4+ T cell.
Results
Targeted metabolomics of amino acids showed that the level of tryptophan significantly (p<0.05) increased in patients with breast cancer. Furthermore, the biological function of tryptophan was determined through determining the influence of tryptophan towards IL-10 secretion using in vitro method. The addition of tryptophan (100 uM) in the cell medium can significantly inhibited the secretion of IL-10 by CD4+ T cells, as indicated by the mRNA level and protein concentration.
Conclusion
The inhibition of IL-10 secretion by CD4+ T cells is a potential pathogenesis of breast cancer.
Keywords: breast cancer, interleukin-10 (IL-10), pathogenesis, T cell
Introduction
Breast cancer, developing from breast tissue, remains the top reason for death of women. Unclear target information leads to the limited therapeutic drugs. Therefore, more and more studies are carried out to find new therapeutic targets. The long noncoding RNA SPRY4-IT1 has been demonstrated to be a novel prognostic biomarker and a potential therapeutic candidate for breast cancer1. The experiment performed by Finlay et al. showed that RNA-based TWIST1 inhibition can reduce breast cancer metastasis through dendrimer complex2. However, all these reasons can not completely explain the pathogenesis of breast cancer. The elucidation of a new mechanism for breast cancer pathogenesis is important and necessary.
Metabolomics analysis is an art-of-work technology for profiling all the small molecules in the biosamples (e.g., urine, serum, etc.). Metabolomics has been successfully applied to explain the pathogenesis of diseases, and the efficiency, pharmacokinetic behavior, and toxicity of xenobiotics3. Metabolomics studies indicated the importance of intestinal farnesoid X receptor signaling in the pathogenesis of nonalcoholic fatty liver disease4. The experiment performed by Zhang et al. showed that persistent organic pollutants can modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation5.
The present study aims to compare the metabolomics profile of serum between health volunteers and patients withbreast cancer to find the key inducing factor for breast cancer.
Materials and methods
Patients and biosamples preparation
Healthy volunteers (n=50, age, 18–70 years; body weight, 45–90kg) and patients with breast cancers (n=20, age, 18–68 years; body weight, 40–85kg) were enrolled in our hospital between year January 1st, 2013 and December 31st, 2014. The patients were confirmed using histological and cytological examination methods. The patients with a second primary malignancy, distant metastasis, or any serious concomitant systemic disorders were excluded from this study. The blood samples were taken 3 months from the beginning, and stored at −80°C.
Targeted analysis of amino acids components
10 uL of serum was added in the 190 uL 95% acetonitrile to deproteinization. After 20-min centrifugation at 14,000*g, the aliquots (10 uL) were dropped on the filter paper. The filter paper was put into a 96-well polypropylene micro titer plates. 100 µL of methanol solution containing isotope labeled amino acid and acylcarnitine internal standards (NSK-A, NSK-B, Cambridge Isotope Laboratories, Andover, USA). 30 min gentle shaking at room temperature was performed. 60 µL of 3n butanolic-HCL was added for derivatisation at 65°C after evaporation at 70°C for 50 min. After a second evaporation step the samples were reconstituted with 150 µL of the mobile phase (1/1 v/v isopropanol/water) and analyzed by flow injection analysis (FIA)-MS/MS using a API 4000 triple quadrupole mass spectrometer (AB SCIEX, Darmstadt, Germany). The mobile phase is 80/20 (v/v) acetonitrile/water, and changed flow rate was used as followed: 0.01–0.09 min, 0.26 mL/min; 0.11–0.45 min, 0.03 mL/min; 1–1.13 min, 0.1–0.6 mL/min; 1.13–1.4 min, 0.6 mL/min; 1.41–2 min, 0.26 mL/min. The following mass conditions were used: Curtain gas, 20 psi; Collision gas, 8 psi; Ionspray voltage, 4500 V; Temperature, 350°C; Ion source gas (GS) 1 and 2, 35 psi. Neutral loss (NL) of 102.1 Da and precursor ion monitoring of 85.1 Da were used to monitoring the amino acids and their corresponding internal standard.
Determination of tryptophan-induced IL-10 secretion
CD4+CD25− T cells for cell culture were separated by EasySep™ Mouse CD4+CD25+ Regulatory T Cell Isolation Kit were from Stemcell Technologies, and were cultured with plated bound anti-CD3 (5 µg/ml) and soluble anti-CD28 (2 µg/ml) antibodies in the presence or absence of tryptophan for 24 hours. After that, cells were collected for IL-10 mRNA expression. IL-10 protein in culture supernatants was determined by ELISA analysis.
Quantitative polymerase chain reaction (qPCR) analysis
A total of liver RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), and cDNA was generated from 1 µg RNA with a SuperScript IITM Reverse Transcriptase kit and random oligonucleotides (Invitrogen, Grand Island, NY). The primer pairs were designed using qPrimerDepot, and messenger RNA quantitation was performed using the comparative cycle threshold (CT) method. The expression of mRNA was normalized to mouse 18S rRNA.
Results
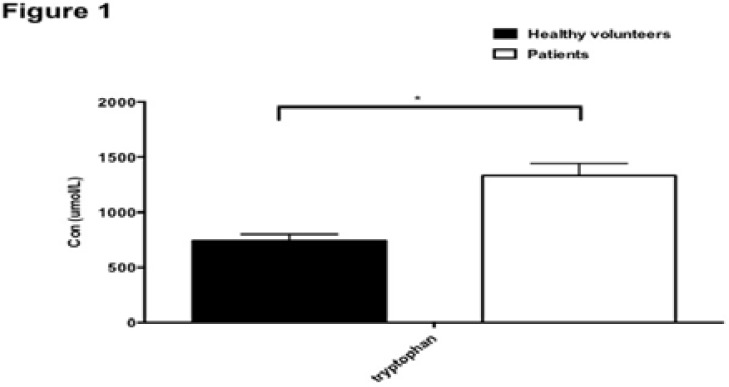
Targeted metabolomics of amino acids showed that the level of tryptophan significantly (p<0.05) increased in patients with breast cancer (Figure 1).
Figure 1.
Comparison of concentration of tryptophan in patients with breast cancer. *, p<0.05.
The blood sample was taken 3 months since the beginning of the experiment.
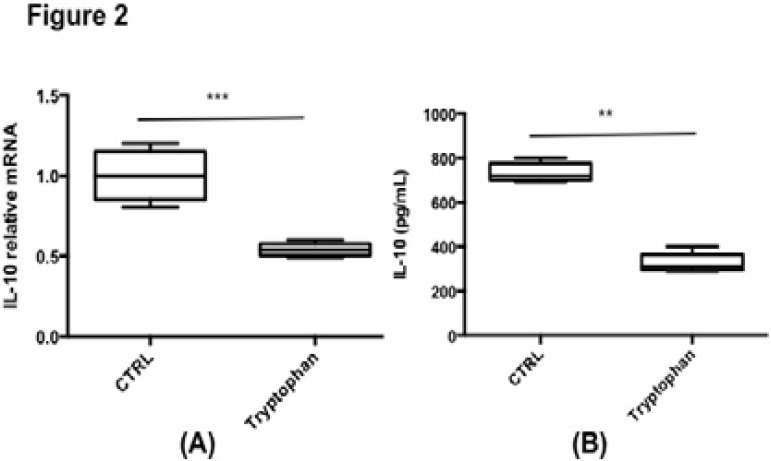
Furthermore, the biological function of tryptophan was determined through determining the influence of tryptophan towards IL-10 secretion using in vitro method. The addition of tryptophan (100 uM) in the cell medium can significantly inhibited the secretion of IL-10 by CD4+ T cells, as indicated by the mRNA level (Figure 2A) and protein concentration (Figure 2B).
Figure 2.
The inhibition of tryptophan towards IL-10 secretion by CD4+ T cells.
CD4+ Naive T cells were isolated from mice splenocytes and cultured with anti-CD3 plus anti-CD28 for 48hrs. (A) Bar graph shows IL-10 mRNA expression in T cells cultured with or without tryptophan. (B) Bar graph shows IL-10 production in the culture media. **, p<0.01.
Discussion
Interleukin-10 (IL-10) is a multifunctional cytokine which exerts the ability to inhibit the activation and effector function of T cells. Generally speaking, the function of IL-10 is to limit and even terminate the immune reaction6. The relationship between IL-10 and pathogenesis of cancers has been reported in previous literature. For example, the experiment performed by Chard et al. has shown that a vaccinia virus armed with IL-10 is a promising therapeutic agent for pancreatic cancer7. IL-10 promoter genotypes are reported to be associated with lung cancer8.
The present study used metabolomics method to find the significant elevation of tryptophan in serum, and no change for other amino acids. In vitro experiment showed the inhibition of tryptophan towards the IL-10 by T cells, indicating that inhibition of IL-10 secretion by elevated tryptophan was a potential pathogenesis of breast cancer. This phenomenon has been previously reported in previous literature9.
Conclusion
The present study gives a short communication on the pathogenesis of breast cancer, which will be beneficial for the understanding of mechanism of breast cancer and the discovery of new drugs for breast cancer.
References
- 1.Shi Y, Li J, Liu Y, Dong J, Fan Y, Tian Y, Wang L, Lian Y, Wang K, Shu Y. The long noncoding RNA SPRY4-IT1 increases the proliferation of human breast cancer cells by upregulating ZNF703 expression. Mol Cancer. 2015;14(1):51. doi: 10.1186/s12943-015-0318-0. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finlay J, Roberts CM, Lowe G, Loeza J, Rossi JJ, Glackin CA. RNA-based TWIST1 inhibition via dendrimer complex to reduce breast cancer cell metastasis. Biomed Res Int. 2015;2015:382745. doi: 10.1155/2015/382745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang ZZ, Gonzalez FJ. LC-MS-based metabolomics: an update. Arch Toxicol. 2014;88(8):1491–502. doi: 10.1007/s00204-014-1234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. J Clin Invest. 2015;125(1):386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Nichols RG, Correll J, Murray IA, Tanaka N, Smith P, Hubbard TD, Sebastian A, Albert I, Hatzakis E, Gonzalez FJ, Perdew GH, Patterson AD. Persistent Organic Pollutants Modify Gut Microbiota-Host Metabolic Homeostasis in mice through aryl hydrocarbon receptor activation. Environ Health Perspect. 2015 doi: 10.1289/ehp.1409055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Chard LS, Maniati E, Wang P, Zhang Z, Gao D, Wang J, Cao F, Ahmed J, El Khouri M, Hughes J, Wang S, Li X, Denes B, Fodor I, Hagemann T, Lemoine NR, Wang Y. A vaccinia virus armed with interleukin-10 is a promising therapeutic agent for treatment of murine pancreatic cancer. Clin Cancer Res. 2015;21(2):405–416. doi: 10.1158/1078-0432.CCR-14-0464. [DOI] [PubMed] [Google Scholar]
- 8.Hsia TC, Chang WS, Liang SJ, Chen WC, Tu CY, Chen HJ, Yang MD, Tsai CW, Hsu CM, Tsai CH, Bau DT. Interleukin-10 (IL-10) promoter genotypes are associated with lung cancer risk in Taiwan males and smokers. Anticancer rES. 2014;34(12):7039–7044. [PubMed] [Google Scholar]
- 9.Jenabian MA, El-Far M, Vyboh K, Kema I, Costiniuk CT, Thomas R, Baril JG, LeBlanc R, Kanagaratham C, Radzioch D, Allam O, Ahmad A, Lebouché B, Tremblay C, Ancuta P, Routy JP, for the Montreal Primary infection and Slow Progressor Study Groups Immunosuppressive Tryptophan Catabolism and Gut Mucosal Dysfunction Following Early HIV Infection. J Infect Dis. 2015 doi: 10.1093/infdis/jiv037. pii: jiv037. [DOI] [PubMed] [Google Scholar]