Abstract
Background
Acetaminophen (APAP)-induced liver toxicity remains the key factor limiting the clinical application of APAP, and herbs are the important sources for isolation of compounds preventing APAP-induced toxicity.
Aims
To investigate the protection mechanism of glycyrrhetinic acid towards APAP-induced liver damage using metabolomics method.
Methods
APAP-induced liver toxicity model was made through intraperitoneal injection (i.p.) of APAP (400 mg/kg). Glycyrrhetinic acid was dissolved in corn oil, and intraperitoneal injection (i.p.) of glycyrrhetinic acid (500 mg/kg body weight) was performed for 20 days before the injection of APAP. UPLC-ESI-QTOF MS was employed to analyze the metabolomic profile of serum samples.
Results
The pre-treatment of glycyrrhetinic acid significantly protected APAP-induced toxicity, indicated by the histology of liver, the activity of ALT and AST. Metabolomics showed that the level of palmtioylcarnitine and oleoylcarnitine significantly increased in serum of APAP-treated mice, and the pre-treatment with GA can prevent this elevation of these two fatty acid-carnitines.
Conclusion
Reversing the metabolism pathway of fatty acid is an important mechanism for the protection of glycyrrhetinic acid towards acetaminophen-induced liver toxicity.
Keywords: Glycyrrhetinic acid (GA), acetaminophen (APAP), metabolomics, fatty acid
Introduction
The human respiratory system is divided into the upper and lower respiratory tract. The upper respiratory tract comprises the nasal cavity, pharynx, and larynx, and the lower respiratory tract is consisted of the trachea, bronchi, and the lungs. The respiratory system diseases contain upper respiratory tract infections, lower respiratory tract infections, asthma, pneumothorax, chronic obstructive pulmonary disease, pulmonary fibrosis, tuberculosis, and even cancers1,2. Searching effective drugs for respiratory system diseases is very important. Glycyrrhetinic acid, also called as enoxolone, is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid which is an important herbal ingredient isolated from herb licorice3. Glycyrrhetinic acid has exerted therapeutic role towards respiratory diseases. For example, glycyrrhetinic acid has been demonstrated to show therapeutic role towards non-small cell lung cancer4.
The adverse effects of acetaminophen (APAP) remain the major reason in limiting the clinical application of APAP which is one of the most important over-the-counter analgesic and antipyretic drugs. The reason for APAP-induced adverse effects is the bioactivation of APAP into the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) which is the key substance basis for APAP-induced toxicity . Metabolomics-based system biology method has been widely used to elucidate the detailed mechanism of APAP-induced liver toxicity. The experiment performed by Chen et al. used metabolomics method to compare the metabolism behavior of APAP between wild type and CYP2E1-null mice, and found several new metabolites of APAP among which APAP dimer has high correlation with the toxicity of APAP5. Serum metabolomics analysis showed that APAP induced mitochondria damage through disrupting oxidative process of fatty acids6. Furthermore, the protective role of PPAR alpha agonist towards APAP-induced toxicity supported the importance of fatty acid metabolism in APAP-induced toxicity7.
Given that the disruption of fatty acids metabolism is an important reason for APAP-induced toxicity, the present study aimed to investigate the influence of glycyrrhetinic acid towards acetaminophen (APAP)-induced fatty acids metabolism.
Materials and methods
Chemicals and reagents
Acetaminophen (APAP), acetonitrile, and formic acid were purchased from Sigma-Aldrich (St. Louis, Mo., USA).
Animal experiment and sample preparation
The animal experiment was performed under the agreement approved by the committee of Yantai Yuhuangding Hospital. 12 Male Sv/129 mice were purchased from Beijing Laboratory Animal Research Center (Beijing, China). The mice were maintained under a standard 12-h light/12-h dark cycle with food and water ad libitum. APAP-induced liver toxicity model was made through intraperitoneal injection (i.p.) of APAP (400 mg/kg). Glycyrrhetinic acid was dissolved in corn oil, and intraperitoneal injection (i.p.) of glycyrrhetinic acid (500 mg/kg body weight) was performed for 20 days before the injection of APAP. Blood was collected at 6 hour after the administration of APAP using BD Microtainer™ serum separator tubes (Franklin Lakes, NJ) by retro-orbital bleeding. After the sacrifice of mice, the livers were excised and used for histology analysis.
Determination of activity of aspartate aminotransferase (AST) and alanine transaminase (ALT)
The mechanism for determination of AST and ALT activity is to the determination of reaction velocity for the catalysis process of oxidation of NADH to NAD+. During this process, 200 µL of ALT or AST assay buffer was added to catalyze the reaction of 1 µL of serum. The whole catalytic process was monitored using the wavelength at 340 nm.
Serum metabolomics analysis
10 µL of serum was firstly mixed with 190 µL of 80% acetonitrile, and the centrifugation at 14,500 rpm was used to deproteinization. 5 µL of the supernatant was added into the vials to undergo UPLC-QTOF-MS analysis. Acquity UPLC BEH C18 column (1.7 µm, 2.1×50 mm, Waters Corp.) was used to seperate the compounds. The elution phase was consisted of water with 0.2% formic acid (A) and acetonitrile with 0.5% formic acid (B). The gradient elution condition was used as followed: 0–3 min, 98%–70% A; 3–8 min, 70%–5% A; 8–9 min, 5% A. Finally, the elution condition was brought back to 98% A. The column temperature was maintained at 40°C. Positive mode was used to monitor all the compounds, and the capillary and cone voltages were set as 4 kV and 30 V. 120 and 360°C were selected as source and desolvation temperature, respectively. The elution rate of nitrogen gas was set as 50 L/h and 600 L/h for cone gas and desolvation gas, respectively. For MS/MS fragmentation, the collision energy ranged from 10 to 40 eV.
Statistical method
The statistical analysis was performed using two-tailed one student's t test.
Results
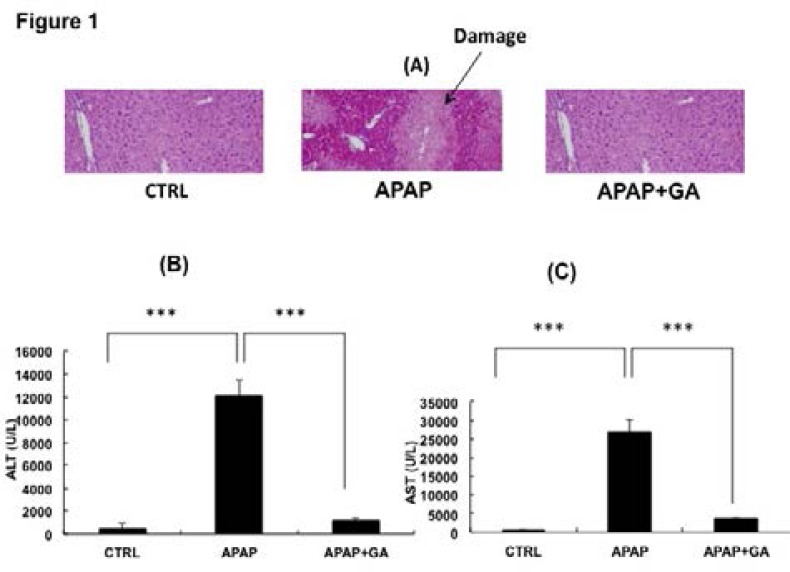
The Figure 1 gave the phenotype results. As shown in Figure 1A, compared with the histology of control mice, the livers from APAP-treated mice had some damaged areas, as indicated using arrow sign. The pretreatment with GA can protect the damage induced by APAP treatment (Figure 1A).
Figure 1.
Pre-treatment with glycyrrhetinic acid (GA) significantly protected acetaminophen (APAP)-induced liver toxicity. (A) H&E staining to analyze the liver histology from control group, APAP-treated group and GA+APAP-treated group. (B) Comparison of ALT activity in control group, APAP-treated group and GA+APAP-treated group. ***, P<0.001. (C) Comparison of AST activity in control group, APAP-treated group and GA+APAP-treated group. ***, P<0.001.
The pre-treatment with GA also prevented the elevation of ALT and AST activity induced by APAP treat ment (Figure 1B &C).
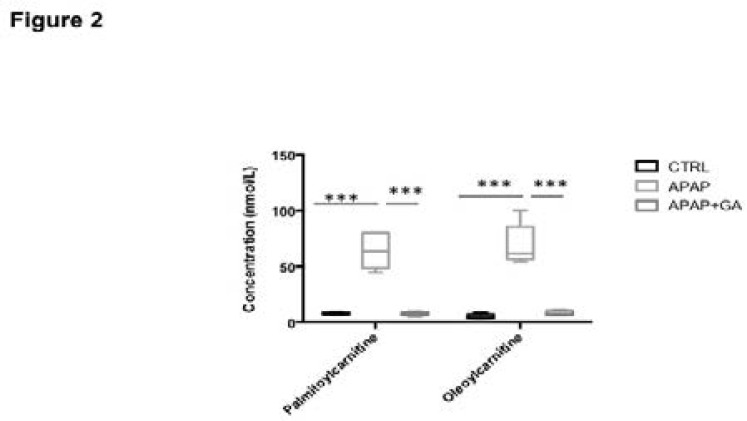
The treatment with APAP significantly increased the level of two ions in serum, and these two ions were identified to be palmtioylcarnitine and oleoylcarnitine (Supplemental Figure 1). The treatment with APAP significantly induced the elevated level of these two compounds (p<0.001, Figure 2), and pre-treatment with GA can significantly decrease the level of these two compounds in serum (p<0.001, Figure 2).
Figure 2.
Comparison of level of palmtioylcarnitine and oleoylcarnitine in control group, APAP-treated group and GA+APAP-treated group. ***, P<0.001.
Discussion
Herbs have become more and more popular in the world, and herbs have exerted therapeutic role towards more and more diseases. The therapeutic behavior of herbs towards APAP-induced liver toxicity has been frequently reported. For example, herb Schisandra Sphenanthera extract has been demonstrated to exhibit therapeutic role towards APAP-induced liver toxicity8, and the compound isolated from this herb Schisandrol B has also exerted its protection role towards APAP-induced liver toxicity9. Herbal ingredient saikosaponin d (SSd), isolated from traditional Chinese herb Bupleurum falcatum, protects against APAP-induced hepato toxicity by inhibiting NF-kB and STAT3 signaling10.
The present study demonstrated the therapeutic role of glycyrrhetinic acid towards APAP-induced liver toxicity through reversing fatty acids metabolic pathway. Given that fatty acids metabolism is a key source for energy production, glycyrrhetinic acid-induced recovery of energy production might be the key mechanism for the protection of glycyrrhetinic acid towards APAP's toxicity. Targeted metabolomics analysis demonstrated that glycyrrhizin protected APAP-induced liver toxicity through reversing fatty acid metabolism11. Therefore, the same protection mechanism of the compounds with such similar structures might exist. It should be noted that glycyrrhetinic acid (GA) can inhibit the activity of CYP2E1 which might also contribute to the protection of GA towards APAP-induced toxcitiy12.
Conclusion
Reversing the metabolism pathway of fatty acid is an important mechanism for the protection of glycyrrhetinic acid towards acetaminophen-induced liver toxicity.
References
- 1.Jenkins G, Goodwin A. Novel approaches to pulmonary fibrosis. Clin Med. 2014;(Suppl 6):s45–s49. doi: 10.7861/clinmedicine.14-6-s45. [DOI] [PubMed] [Google Scholar]
- 2.Froesch P, Martucci F, Gyorik S, Dutly AE, Cafarotti S. Management of non-small cell lung cancer in the elderly. Eur J Intern Med. 2014;25(10):888–894. doi: 10.1016/j.ejim.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 3.AsI MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and itsbioactive compounds. Phyther Res. 2008;22(6):709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.18b-Glycyrrhetinic acid suppresses cell proliferation through inhibiting thromboxane synthase in non-small cell lung cancer. PLoS One. 2014;9(4):e93690. doi: 10.1371/journal.pone.0093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Krausz KW, Idle JR, Gonzalez FJ. Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J Biol Chem. 2008;283(8):4543–4559. doi: 10.1074/jbc.M706299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Krausz KW, Shah YM, Idle JR, Gonzalez FJ. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2009;22(4):699–707. doi: 10.1021/tx800464q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson AD, Shah YM, Matsubara T, Krausz KW, Gonzalez FJ. Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology. 2012;56(1):281–290. doi: 10.1002/hep.25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan X, Jiang Y, Wang Y, Tan H, Zeng H, Wang Y, Chen P, Qu A, Gonzalez FJ, Huang M, Bi H. Wuzhi tablet (Schisandra Sphenanthera extract) protects against acetaminophen-induced hepatotoxicity by inhibition of CYP-mediated bioactivation and regulation of NRF2-ARE and p53/p21 pathways. Drug Metab Dispos. 2014;42(12):1982–1990. doi: 10.1124/dmd.114.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Fan X, Wang Y, Chen P, Zeng H, Tan H, Gonzalez FJ, Huang M, Bi H. Schisandrol B protects against acetaminophen-induced hepatotoxicity by inhibition of CYP-mediated bioactivation and regulation of liver regeneration. Toxicol Sci. 2015;143(1):107–115. doi: 10.1093/toxsci/kfu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu A, Tanaka N, Sun L, Guo B, Kim JH, Krausz KW, Fang Z, Jiang C, Yang J, Gonzalez FJ. Saikosaponin d protects against acetaminophen-induced hepatotoxicity by inhibiting NF-kB and STAT3 signaling. Chem Biol Interact. 223C:80–86. doi: 10.1016/j.cbi.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Jiang YS, Jiang Y, Peng YF, Sun Z, Dai XN, Cao QT, Sun YM, Han JC, Gao YJ. Targeted metabolomics study indicating glycyrrhizin's protection against acetaminophen-induced liver damage through reversing fatty acid. Phytother Res. 2014;28(6):933–936. doi: 10.1002/ptr.5072. [DOI] [PubMed] [Google Scholar]
- 12.Jeong HG, You HJ, Park SJ, Moon AR, Chung YC, Kang SK, Chun HK. Hepatoprotective effects of 18beta-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P450 2E1 expression. Pharmacol Res. 2002;46(3):221–227. doi: 10.1016/s1043-6618(02)00121-4. [DOI] [PubMed] [Google Scholar]