Abstract
Background
There is a paucity of published data regarding upper gastrointestinal diseases in Ugandans with upper gastrointestinal symptoms referred for endoscopy.
Objectives
To study the presenting complaints, pathology and Helicobacter pylori prevalence among patients with upper gastrointestinal symptoms in South-Western Uganda.
Methods
Patients presenting with upper gastrointestinal symptoms underwent upper endoscopy and a urease test for Helicobacter Pylori, all suspicious lesions were biopsied for histopathology review as appropriate.
Results
The most common presenting complaints were epigastric pain (51.6%), dysphagia (13.6%) and odynophagia (7.1%). The most common endoscopy finding was gastritis (40.2%), followed by normal examination (15.2%), oesophageal cancer (13.6%), gastric ulcer (7.6%) and gastric cancer (7.1%). Patients older than 40 years (n=110) had significant findings including gastritis (50.9%), oesophageal cancer (22.7%) and gastric cancer (11.8%). However in younger patients, with the age range of 18–40 years (n=74), most examinations were normal (92.9%). Of the 176 patients able to undergo Helicobacter pylori testing 75.6% were positive. Helicobacter pylori infection was associated with statistically significant increase in gastritis, oesophageal cancer, gastric ulcer, gastric cancer, and duodenal ulcers (p-values< 0.05).
Conclusion
Gastritis, ulcerative disease, and upper gastrointestinal malignancies are common in South-Western Ugandans and are associated with a high prevalence of Helicobacter pylori.
Keywords: Upper gastrointestinal symptoms, Pathology, Urease test, Helicobacter pylori, Endoscopy, Uganda
Introduction
The evaluation of upper gastrointestinal symptoms in a medically resource limited area can be challenging. The lack of medical personnel, including those trained in upper endoscopy and limited facilities, can limit the ability for appropriate evaluation for patients with upper gastrointestinal complaints.
Symptoms such as epigastric pain, dysphagia, odynophagia, heartburn, nausea, bloating and early satiety arising from the upper gastrointestinal tract are common. 1,2 The pathology underlying upper gastrointestinal symptoms is varied and may be associated with significant morbidity and mortality.2
Helicobacter pylori is a gram negative, spiral shaped, flagellated, bacillus; it remains one of the world's most common bacterial infections.3 H. pylori infection is associated with a number of upper gastrointestinal disorders, including chronic active gastritis, peptic ulcer disease and gastric cancer.4 The prevalence of H. pylori infection worldwide varies greatly among countries, the infection is more common in developing countries where the prevalence rate ranges between 70 and 90% as compared to 20–50% in developed countries.5 The overall prevalence rate of H.pylori infection strongly correlates with low socioeconomic status, low living standards, poor personal and environmental hygiene, presence of H.pylori-positive family members and increasing age.6
Data on upper gastrointestinal diseases, H. pylori prevalence based on urease test among patients with upper gastrointestinal symptoms undergoing endoscopy in Uganda are limited. There have been previous reports about endoscopic findings in patients with upper gastrointestinal symptoms.7,8 While this data is informative, its coverage is limited to HIV/AIDS patients and those patients presenting with upper gastrointestinal bleeding. There is thus a need for more data on upper gastrointestinal diseases from Uganda.
Identification of upper gastrointestinal symptoms, associated pathology and H.pylori will enable establishment of preventive measures for these diseases and in addition decrease the need for endoscopy especially in this resource limited setting. Therefore this study was conducted to evaluate the symptoms, pathology, the prevalence of Helicobacter pylori in patients with upper gastrointestinal symptoms presenting in Mbarara, Uganda.
Methods
Study population and data collection
This was a cross-sectional study in which patients were prospectively recruited from those referred for upper gastrointestinal endoscopy at Mbarara University Teaching Hospital, Uganda, a referral center for patients from South-WesternUganda. Data collected on each patient included age, sex, occupation, educational status, referral complaints, HIV status, endoscopic findings, histology, and Helicobacter pylori results.
Endoscopic assessment
All the patients gave informed consent and the procedure was elective. Clinical symptoms were assessed in a systematic manner. The endoscopic evaluation of patients was performed using a sterile Olympus video gastroscope from Olympus Medical. Patients received 2 mls of a 10% lidocaine spray orally while seated on table and waited between 1–5 minutes after the spray for oropharyngeal anesthesia to take effect before the endoscopy procedure. Patients who were referred for endoscopy and turned down procedure after going through with them to obtain informed consent were not included in the study, 6 patients refused the procedure and they were excluded.
All examinations were performed by the same operator (S.O.) who had been trained in endoscopy at Bristol Royal Infirmary Hospital, University of Bristol and Cairo Endoscopy Training Centre, Egypt.
Helicobacter pylori status
Out of 184 patients who were scoped, 176 patients underwent gastric antral mucosal biopsies to determine H. pylori status. These biopsies were tested using a rapid urease test (CLO kit, a container impregnated with rapid urease test manufactured by Cambridge Life Science Ltd 14 St.Thomas' Place, Cambridgeshire Business Park, Ely, Cambs CB74EX UK) which was observed for colour change. Biopsies were placed immediately in the solution in the CLO kit and in the presence of H pylori the solution in the CLO kit which turned from yellow to pink. Where colour change did not occur immediately the solution was observed up to 24 hours later. If no colour change took place at 24 hours, the CLO kit was discarded and the result recorded as negative for H. pylori irrespective of treatment with proton pump inhibitor or other therapy.
Histology
In 8 patients the scope could not be passed beyond the oesophagus to the stomach due oesophageal lumen obliteration by bleedy masses suspicious of malignancies, biopsies were only obtained from the oesophagus for histopathology. In addition to antrum biopsies to determine H.pylori status in 176 patients biopsies were also obtained at the same time from all cases of oesophagitis, gastritis, duodenitis, gastric ulcer, duodenal ulcer, and suspected malignant lesions and the specimens were fixed in 10% buffered formalin and transferred to the histopathology laboratory for histopathology review.
All the biopsy samples were examined by the same histopathologist, Prof. Amnia Diaz of the pathology department Mbarara University Teaching Hospital.
Statistics
For each disease, and for H. pylori infection, prevalence was expressed as the percentage occurrence in the study population. The relationship between H. pylori and endoscopic findings was determined by cross tabulation, calculation of p-values, odd ratios and confidence intervals (a p-value of < 0.05 was considered significant, using Stata program 10.0).
Ethical permission
The study was approved by the Research and Ethics Committee of Mbarara University of Science and Technology. A written informed consent (which was written in both local Runyakore/Rukiga language and English) was obtained from all patients and respect for confidentiality was ensured.
Results
A total of 184 patients who underwent upper gastrointestinal endoscopy were enrolled in this study, their age ranged from 18 to 90 years, with a mean of 53.5 years (SD +/− 17.8).
The majority of patients were men (59.8%). Most of the patients worked in agriculture (65%) followed by civil service positions (18%), students (9%), and other (8%) occupations. Majority of the patients enrolled had primary education (58.7%) followed by tertiary (24.5%) then secondary 16.8%. The HIV status in this patient population was HIV negative 73.9%, HIV positive 8.7%, and the remaining patients (17.4%) declined a HIV test (Table1).
Table 1.
The demographics of patients in the study
| Characteristics | N | (%) | |
| Sex | Males | 110 | 59.8 |
| Females | 74 | 40.2 | |
| Occupation | |||
| Agriculture | 119 | 65 | |
| Civil servant | 34 | 18 | |
| Student | 16 | 9 | |
| Others | 15 | 8 | |
| Education | |||
| Primary | 108 | 58.7 | |
| Secondary | 31 | 16.8 | |
| Tertiary | 45 | 24.5 | |
| HIV status | |||
| Positive | 16 | 8.7 | |
| Negative | 136 | 73.9 | |
| Declined testing | 32 | 17.4 |
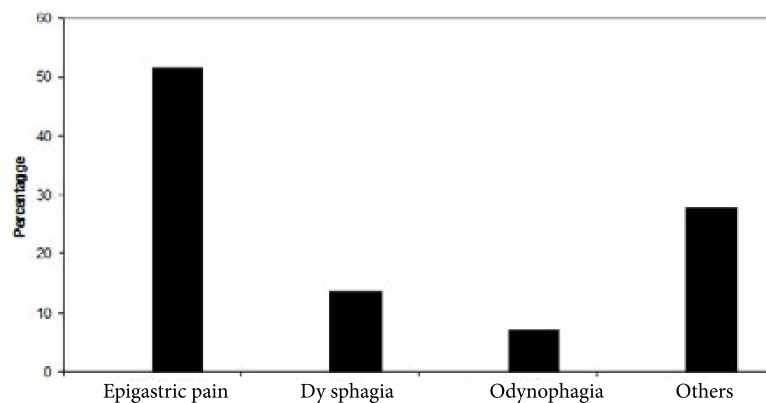
In Fig 1, the main indications for endoscopy were epigastric pain (51.6%), dysphagia (13.6%), and odynophagia (7.1%), other symptoms cited included vomiting, nausea, heartburn, bloating, early satiety and weight loss (27.7%).
Figure 1.
Primary gastrointestinal symptoms at presentation before endoscopy.
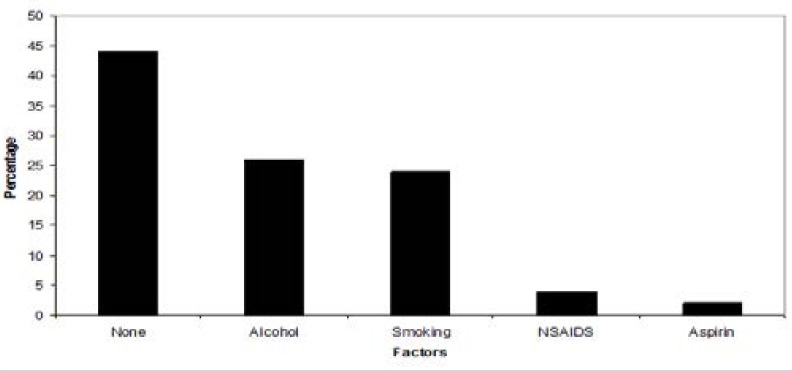
In Fig 2, the majority of patients (44%) did not have obvious sources for their symptoms. Risk factors included alcohol consumption (26%), smoked tobacco (24%), non-steroidal anti-inflammatory drugs (4%) and aspirin use (2%).
Figure 2.
The risk factors for upper gastrointestinal symptoms reported during patient's assessment on history.
Gastritis (40.2%) was the most common finding followed by normal exam (15.2%), oesophageal cancer (13.6%), gastric ulcer (7.6%), and gastric cancer (7.1%). Less common diagnoses included oesophagitis (6.0%) duodenitis (5.4%), duodenal ulcers (3.8%), and oesophageal varices at 1.1% (Table 2).
Table 2.
Histological and endoscopic findings of patients who underwent endoscopy
| Results | No of cases | (%) |
| Gastritis | 74 | 40.2 |
| Normal | 28 | 15.2 |
| Oesophageal cancer | 25 | 13.6 |
| Gastric ulcer | 14 | 7.6 |
| Gastric cancer | 13 | 7.1 |
| Oesophagitis | 11 | 6 |
| Duodenitis | 10 | 5.4 |
| Duodenal ulcer | 7 | 3.8 |
| Oesophageal varices | 2 | 1.1 |
| Total | 184 | 100 |
Gastritis was the most common finding in patients over 40 years of age followed by oesophageal cancer, gastric cancer, gastric ulcer, and oesophagitis. All of the cancers were found in patients older than 40 years. 2 patients over the age of 40 years undergoing endoscopy had a normal examination (Table 3).
Table 3.
The relationship between pathology and age
| Age group | ||||||
| ≤20 | 21 – 30 | 31–40 | >40 | |||
| Findings | Total | (%) | ||||
| Gastritis | 3 | 8 | 7 | 55 | 74 | 40.2 |
| Normal | 7 | 16 | 3 | 2 | 28 | 15.2 |
| Oesophageal canter | 0 | 0 | 0 | 25 | 25 | 13.6 |
| Gastric ulcer | 0 | 4 | 4 | 6 | 14 | 7.6 |
| Gastric cancer | 0 | 0 | 0 | 13 | 13 | 7.1 |
| Oesophagitis | 0 | 2 | 4 | 5 | 11 | 6.0 |
| Duodenitis | 0 | 2 | 5 | 3 | 10 | 5.4 |
| Duodenal ulcer | 1 | 2 | 4 | 0 | 7 | 3.8 |
| Oesophageal varices | 0 | 0 | 2 | 0 | 2 | 1.1 |
| Total | 11 | 34 | 29 | 110 | 184 | 100 |
Patients younger than 40 years of age most commonly had normal findings at endoscopy followed by gastritis, gastric ulcer, both duodenitis and duodenal ulcers. There were no cases of oesophageal or gastric carcinomas found in patients less than 40 years old (Table 3).
There were statistically significantly findings in the setting of Helicobacter pylori infection with p-values<0.05 in patients with gastric lesions which included gastritis (93.2%), oesophageal cancer (82.4%), gastric ulcers (92.9%), gastric cancer (92.3%) and duodenal ulcers at 85.7% (table 4).
Table 4.
The relationship between common gastric lesions and Helicobacter pylori infection
| Common gastric lesions (N) |
H.pylori results N (%) |
OR(95%Cl) | P-value | |
| Positive | Negative | |||
| Gastritis (74) | 69((93.2) | 5(6.8) | 20.7(6.34–67.64) | 0.001 |
| Oesophageal cancer (17) | 14(82.4) | 3(17.6) | 7.2 (1.68–31.04) | 0.003 |
| Gastric ulcer (14) | 13(92.9) | 1(7.1) | 21.6(2.48–188.72) | 0.001 |
| Gastric cancer (13) | 12(92.3) | 1(7.7) | 18.5(2.10–163.47) | 0.001 |
| Duodenal ulcer (7) | 6(85.7) | 1(14.3) | 10.8 ( 1.17–100.44) | 0.021 |
OR: odds ratio; 95%Cl: 95% confidence intervals.
Of the 184 participants of this study we were able to test 176 for Helicobacter pylori. Not all patients could be tested for H. pylori because in 8 patients with oesophageal cancer the scope could not be safely passed into the stomach to obtain the samples. There were 176 individuals in whom it could be safely passed, there were 75.6% H. pylori positive and 24.4% negative.
Discussion
There are multiple reasons patients present with upper gastrointestinal symptoms but epigastric pain is the most common reason for endoscopy. In this study the complaint of epigastric pain (51.6%) was the most common reason for endoscopy similar to studies in Jos and llorin, Nigeria9,10
The most common identifiable lesion at endoscopy in our study was gastritis (40.2%) which is similar to the data from Kenya, and Sudan. These earlier studies in Kenya and Sudan showed a frequency of gastritis at 25.8% and 36.9% respectively.11,12 This disparity may be as a result of the difference in predisposition to risk factors for gastritis among the patients who were studied in these countries.
In this study endoscopy was normal in 15.2% which is similar to data from Kano, Nigeria.13 However there were significant differences in endoscopic findings based on the individual's age in our study. The most common finding on endoscopy was normal tissue in patients younger than 40 years. However, in patients older than 40 years only 2 out of 110 patients had normal endoscopic findings. Thus if a patient presents with upper gastrointestinal symptoms and is older than 40 years there is a very high likelihood of finding significant pathology.
Individuals who present with symptoms and who are over 40 years old have a significant risk of malignancy in our setting. Of the 110 patients over 40 years old 38 (34.5%) were found to have either esophageal or gastric cancer. Our incidence of cancer is higher than what has been reported in earlier studies conducted in Nigeria and Sudan for esophageal cancer at 10.7% and 5.9% respectively and while gastric cancer 3.5% to 6.6 %.9,12,14 It is not completely clear why there is disparity between our study and the Nigerian and Sudanese data but perhaps the differences could be related to the age of patients studied or the increasing incidence of gastrointestinal cancers.
H.pylori was diagnosed in 75.6% among the biopsied patients and this is consistent with results of various studies done in Africa that have shown a high prevalence of H. pylori. In Ghana and Rwanda studies conducted found the H. pylori at a prevalence of 75% using urease test for all biopsies.15,16 Previous studies conducted in various parts of South-Western Nigeria where patients were investigated for H. pylori with the use of (CLO) test showed prevalence rates of 60.5% to 73%.17–19 In addition this study showed that 51.9% of the patients with gastritis had H. pylori infection (P-value<0.001) which is consistent with previous studies in Kenya and Nigeria.17,20,21
As expected almost all patients with gastric and duodenal ulcer were infected with H. pylori. This finding corroborates the results from similar studies conducted in Nigeria and other parts of Africa.21–23 It was also noted that 11 out of 28 patients with normal endoscopy findings were positive for H. pylori.
Limitations
These include the following: first, the fact that it's a single institution hospital based study which may not be a true representation of the burden of upper gastrointestinal diseases of whole population in this region since economic factors influence health-seeking behavior of patients. Secondly we did not specifically exclude patients who had used proton-pump inhibitors in the two weeks or bismuth containing drugs and antibiotics in the 4 weeks preceding the endoscopy and H. pylori testing. Hence, we were unable to assess the association of these variables with H. pylori results. Furthmore we only used a rapid urease test to determine H. pylori status as compared to a combination of two or more methods to improve the accuracy of H.pylori detection.24
Conclusion
Gastritis, peptic ulcer disease and upper gastrointestinal malignancies are common upper gastrointestinal diseases in South-Western Ugandans presenting with upper gastrointestinal symptoms for endoscopy and there is high prevalence of Helicobacter pylori associated with these diseases (p-values< 0.05).
Patients above 40 years old presenting with upper gastrointestinal symptoms are likely to have significant findings at endoscopy including esophageal or gastric malignancies, ulcerations as well as gastritis. Future studies are needed to evaluate if empiric eradication for Helicobacter pylori in the patients who are less than 40 would lead to a decrease in gastritis, ulcers, and malignancy in a Ugandan population. In addition perhaps the healing of these conditions would decrease the need for endoscopy especially in a resource limited setting such as South-Western Uganda.
Acknowledgment
We wish to thank the following: Wyeth Pharmaceuticals in the United Kingdom for sponsoring the CLO kits. Our patients, nursing staff of the endoscopy unit Mbarara University Teaching Hospital. Prof. Amnia Diaz of the pathology department Mbarara University Teaching Hospital for histopathology slide reporting. Patrick Elyanu and Nickolas Matsiko of Mulago-Mbarara Teaching Hospitals Joint AIDS Program (MJAP) for setting up the data base and analysing the data. All staffs of gastroenterology and hepatology units, University of Bristol, for their encouragement in this work. Assistant Professor Jessica Rothman for her editorial assistance during the compilation of this work.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding acknowledgement
This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors.
References
- 1.Olokobo AB, Gashau W, Bwala S, Adamu A, Salawu FK. Helicobacter pylori infection in Nigerians with dyspepsia. Ghana Med J. 2013;47(2):79–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Humayed SM, Mohammed-Elbagir AK, AlWabel AA, Argobi YA. The changing pattern of upper gastrointestinal lesions in Southern Saudi Arabia, an endoscopic study. Saudi J Gastroenterol. 2010;16(1):35–37. doi: 10.4103/1319-3767.58766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. PubMed. [DOI] [PubMed] [Google Scholar]
- 4.Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am Gastroenterol. 1999;94(9):2373–2379. doi: 10.1111/j.1572-0241.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 5.Megraud F, Brassens-Rabbe MP, Denis F, Belbouri A, Hoa DQ. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989;27(8):1870–1873. doi: 10.1128/jcm.27.8.1870-1873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9(2):33–39. [PubMed] [Google Scholar]
- 7.Elshazly MA, Serwadda DM, Freers J. Endoscopic study of African AIDS patients with upper gastrointestinal symptoms. East Afr Med J. 1994;71(8):496–500. [PubMed] [Google Scholar]
- 8.Alema ON, Martin DO, Okello TR. Endoscopic findings in upper gastrointestinal bleeding patients at Lacor hospital, northern Uganda. Afr Health Sci. 2012;4:518–521. doi: 10.4314/ahs.v12i4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashiru O I, Misauno MA. Gastrointestinal endoscopy in Nigeria, a prospective two year audit. Pan Afr Med J. 2013;4:22. doi: 10.11604/pamj.2013.14.22.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olokoba AB, Bojuwoye BJ. Indications for oesophagogastroduodenoscopy in Ilorin Nigeria-a 30 month review. Niger J Clin Pract. 2010;13(3):260–263. [PubMed] [Google Scholar]
- 11.Hudson L, Fasana R, Mutuma GZ, Kabanga JM, Kuria JK, Okoth FA. Patterns of upper gastrointestinal diseases based on endoscopy. Afr J Health Sci. 2005;12(1–2):49–54. doi: 10.4314/ajhs.v12i1.30800. [DOI] [PubMed] [Google Scholar]
- 12.Hussein YA, Doumi EA. Upper Gastrointestinal Endoscopy in El Obeid, Western Sudan. Analysis of the First 1150 Cases. Sudan J Med Sci. 2008;3(2):91–95. [Google Scholar]
- 13.Tijjani BM, Umar AB. Peptic ulcer disease and Helicobacter pylori infection at Kano, Nigeria. The Internet Journal of Gastroenterology. 2009;8(1) [Google Scholar]
- 14.Jemilohum AC, Otegbayo JA, Ola SO, Oluwasola OA, Akere A. Prevalence of Helicobacter pylori among Nigerian patients with dyspepsia in Ibadan. Pan Afr Med J. 2010;6:18. [PMC free article] [PubMed] [Google Scholar]
- 15.Aduful HK, Naaeder SB, Darko R, et al. Upper Gastrointestinal Endoscopy at the Korle Bu Teaching Hospital, Accra, Ghana. Ghana Med J. 2007;41(1):12–16. [PMC free article] [PubMed] [Google Scholar]
- 16.Walker TD, Karemera M, Ngabonziza F, Kyamanywa P. Helicobacter pylori status and associated gastroscopic diagnoses in a tertiary hospital endoscopy population in Rwanda. Trans R Soc Trop Med Hyg. 2014;108(5):305–307. doi: 10.1093/trstmh/tru029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ndububa DA, Agbakwuru AE, Adebayo RA, et al. Upper gastrointestinal findings and incidence of Helicobacter pylori infection among Nigerian patients with dyspepsia. West Afr J Med. 2001;20(2):140–145. [PubMed] [Google Scholar]
- 18.Adesanya AA, Oluwatowoju IO, Oyedeji KS, da Rocha-Afodu JT, Coker AO, Afonja OA. Evaluation of a locally-made urease test for detecting Helicobacter pylori infection. Niger Postgrad Med J. 2002;9(1):43–47. [PubMed] [Google Scholar]
- 19.Ola SO, Yakubu A, Otegbayo JA, et al. The most appropriate site for endoscopic biopsy for the detection of H. pylori among Nigerians in Ibadan. West Afr J Med. 2006;25(4):269–272. [PubMed] [Google Scholar]
- 20.Kalebi A, Rana F, Mwanda W, Lule G, Hale M. Histopathological profile of gastritis in adult patients seen at a referral hospital in Kenya. World J Gastroenterol. 2007;13(30):4117–4121. doi: 10.3748/wjg.v13.i30.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustapha SK, Ajayi NA, Nggada HA, et al. Endoscopic findings and the frequency of Helicobacter pylori among dyspeptic patients in North-Eastern Nigeria. Highland Medical Research Journal. 2007;5(1):78–81. [Google Scholar]
- 22.Ogutu EO, Kang'ethe SK, Nyabola L, Nyong'o A. Endoscopic findings and prevalence of Helicobacter pylori in Kenyan patients with dyspepsia. East Afr Med J. 1998;75(2):85–89. [PubMed] [Google Scholar]
- 23.Baako BN, Darko R. Incidence of Helicobacter pylori infection in Ghanaian patients with dyspeptic symptoms referred for upper gastrointestinal endoscopy. West Afr J Med. 1996;15(4):223–227. [PubMed] [Google Scholar]
- 24.Ramis IB, de Moraes EP, Fernandes MS, et al. Evaluation of diagnostic methods for the detection of Helicobacter pylori in gastric biopsy specimens of dyspeptic patients. Braz J Microbiol. 2012;43(3):903–908. doi: 10.1590/S1517-83822012000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]