Abstract
Introduction
Artemisia annua plant from the family Asteracea is a powerful antimalarial plant introduced to Uganda around 2003. In addition to the artemisinin component, the plant also contains flavonoids which work in synergy to artemisinin against malaria parasites. The plant also contains aromatic oils which repel mosquitoes. In this paper we report the variations in antimalarial components of A. annua samples from the regions cultivating it in Uganda.
Methods
Artemisia annua samples were obtained from three regions that cultivated the plant at the time of this study. The samples were brought to laboratory, authenticated and processed. The levels of artemisinin, total flavonoids and aromatic components were quantified using high performance thin layer chromatography, ultra violet spectrophotometry and gas chromatography respectively.
Results
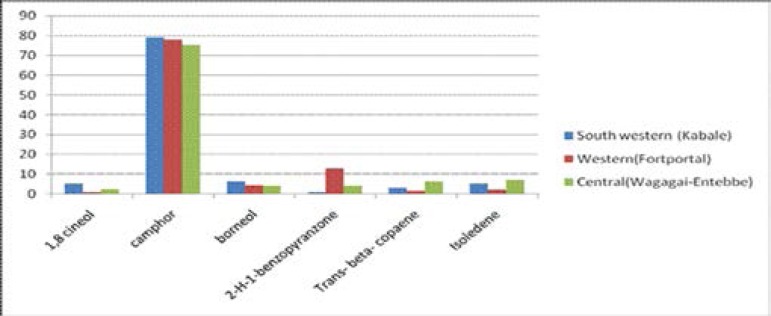
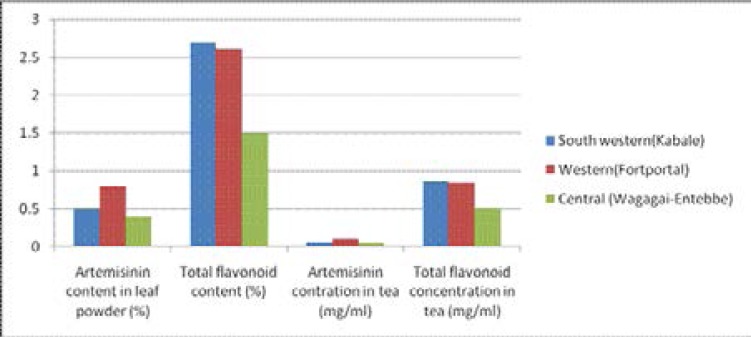
Artemisinin and total flavonoids levels were higher in samples obtained from high land areas (western and south western region) compared to that obtained from lowland regions (central) i.e 0.8% Vs 0.4% and 2.6% Vs 1.5% respectively. The aromatic oils (mosquito repellent components) were similar with camphor component being highest and levels ranging from 75.4% to 79.0%.
Conclusion
Our findings show that the active components in Artemisia annua cultivated and used in the Uganda vary with geographical regions and this calls for standardisation by source.
Keywords: Variations, Antimalarial components, Artemisia annua, Uganda
Introduction
Artemisia annua L. is a medicinal weed that belongs to the Asteracea family of plants. It is native to Asia especially China but also found in Europe and North America. In China, the leaves were used as infusion to cure malaria and also burnt to repel mosquitoes for over 3000 years1. A. annua plant was introduced to Uganda around 2003 as the source of artemisinin needed by the pharmaceutical industries that manufacture Artemisinin combination therapies (ACTs). Ugandan soils are reported to be suitable for cultivation of A.annua2. Artemisinin the most potent antimalarial known or its derivative is not used singly in treatment of malaria but used in combination with other antimalarial drugs. The artemisinin combinations are currently WHO recommended treatments for uncomplicated malaria3. Apart from artemisinin, A. annua also contains other antimalarial compounds that work in synergy with artemisinin offering a natural combination therapy against malaria4,5,6,7.
In Uganda, the cultivation of the plant was initially restricted to farmers in highland areas mainly in western Uganda. However, the drop in the demand for artemisinin from Uganda by the industries that make its derivatives led to uncontrolled and wide spread local cultivation and use of the plant in Uganda. The plant is now cultivated and used by communities in Central, Eastern and Northern Uganda which are low land areas (less than 1200m above sea level) initially not recommended for the plant8,9. A recent study in Uganda reported that although Ugandan soils are suitable for cultivation of artemisinin, artemisinin levels vary with area of cultivation. The variations in levels of other antimalaial components remained unknown until this study. All plant components are known to vary according to geographical locations which in turn may lead to varying clinical outcomes in patients10. The low land areas in Uganda have altitude of about 1200m, receive total rainfall of about 1000mm per annum and temperatures of up to 84°F. The high land areas have altitude of up to 2500m; receive rainfall of up to 1200mm per annum and temperatures of up to 73°F. In this paper, we report variations in the levels of artemisinin, total flavonoids and aromatic oils in samples from the three regions cultivating A.annua in Uganda.
Materials and methods
Material collection
Artemisia annua plant was collected from the Wagagai flower farm garden in Central Uganda in February 2009. The specimen was identified by a taxonomist at Natural Chemotherapeutics Research Institute (NCRI) herbarium, Ministry of Health, Uganda. A voucher specimen, NCJ 257 was deposited at the herbarium of NCRI for use as reference. Dry leaf powder of A. annua samples (1kg) was also obtained from Wagagai health clinic, where it has been used as tea for malaria prophylaxis among the farm workers since 20069. Other dry leaf powder samples (1kg each) were obtained from the two major commercial cultivators in Uganda located in high land areas i.e Kabale district in south western Uganda and Fortportal district in Western Uganda. The samples brought to the laboratory were stored in air tight opaque containers at room temperatures till the time of analysis.
Phytochemical screening of artemisia annua materials
The A.annua dry leaf powders (100g) were extracted with soxhlet apparatus in a sequential manner using petroleum ether, followed by ethanol and then methanol solvent. While A.annua tea was prepared by adding boiling water to dry leaf powder to make 10g/L infusion following procedures previously described9. Phytochemical ingredients in both leaf powders and tea extracts were determined using methods described in the manual of analysis of vegetable drugs11 as briefly described;
Polyuronides
To a test tube containing (10ml of formulation) was added drops of water, leading to formation of a thick precipitate. The precipitate obtained was placed on the filter paper and on staining with hematoxylin formed a blue precipitate for presence of polyuronides.
Reducing compounds
1ml of formulation was diluted with water (2ml) in test tube. Fehling's solutions I (1ml) and Fehling's solution II (1ml) were added and heated in a water bath at 90°C forming a brick-red precipitate.
Saponins
A diluted solution of the formulation (2 ml) was placed in a test tube and shaken for 15 minutes. A soapy like column of about 2cm formed above liquid level.
Tannins
To the formulation (1ml) was added water (2ml) and 3 drops of ferric chloride. A blackish blue color formed.
Alkaloid salts
The formulation (15ml) was evaporated to dryness in an oven at 55°C and residue dissolved in 10% v/v Hydrochloric acid (10 mL). 10 % v/v ammonia solution (10ml) was added to precipitate the alkaloids and then extracted with ether (15ml). The ether portion was evaporated to dryness and hydrochloric acid (1.5ml) added. To 0.5ml of the acidic solution was added 2–3 drops of Mayer's reagents forming opalescence precipitate.
To detect Steroid glycosides, Anthracenosides, coumarins and flavonosides, 25ml of the formulation was mixed in 10% v/v hydrochloric acid (15ml), refluxed for 30minutes, cooled and extracted with diethyl ether (36ml) in portions of 12ml each.
Steroid glycosides
To a residue obtained by evaporating to dryness ether extract (10 ml) was added acetic anhydride (0.50ml) and chloroform (0.50 ml) and transferred into a dry tube. Conc. Sulphuric acid (2 ml) was added by means of a pipette at the bottom of the tube forming reddish-brown ring at the contact zone of the two layers.
Anthracenosides
The ether extract (4 mL) was added to conc. Sulphuric acid (2 mL) and shaken with 25% v/v ammonia solution (2ml) forming cherished-red solution on the top layer.
Coumarin derivatives
To a residue obtained by evaporating ether extract (5 mL) was added hot water (2ml) to dissolve. 10%v/v ammonium solution (0.5 ml) was then added forming a blue fluorescence solution under UV.
Flavonosides
The residue obtained by evaporating ether extract (5ml) was heated in 50% methanol (2 mL). Metallic magnesium (0.5g) and conc. Hydrochloric acid (5drops) was added forming a red solution.
Aromatic oil profile in artemisia annua materials
The aromatic oils were extracted by hydro-distillation of 500g of A. annua dry leaf powder. The distillate was cooled and fractionated with petroleumether (500ml) to extract the volatile oils. The oils in the ether fraction were analysed using Shimadzu GC-MS; model, C70374300170. The peak area of each component of the oil was identified through the in built database and content of each identified component computed based on peak areas as percentages.
Determination of artemisinin content in the artemisia annua samples
Construction of artemisinin calibration curve
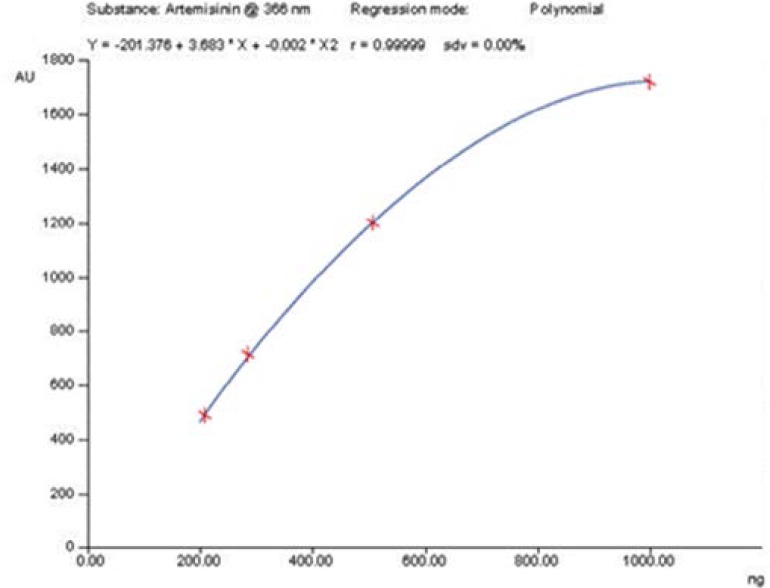
Method previous described for quantification of artemisinin in bulk forms was adopted12. Pure artemisinin (1mg) donated by African Laboratory for Natural Products (ALNAP) in Ethiopia was dissolved in ethanol to make 100ml stock solution. Concentrations of 200ng/ml, 300ng/ml, 500ng/ml and 1000ng/ml of pure artemisinin were applied onto Thin Layer Chromatographic (TLC) plate ( Merck) by the automated High Perfomance Thin Layer Chromatography (HPTLC) applicator, the plate was developed in HPTLC chamber using solvent system consisting of Heptane- diethyl ether in the ratio 1:1. The plate after drying was dipped into Acetic acid- H2SO4- Anisaldehyde (10:0.02:0.1) solvent to derivatise the artemisinin. The retardation factor (Rf) value for artemisinin was 0.5. After drying, the absorbances for each concentration was measured at 366 nm UV-wave length utilising radiation source combining Deuterium (D2) and tungstem lamps. The concentrations and absorbances were used to construct the calibration curve giving line of best fit R2=0.99999 (Figure 1)
Figure 1.
Percentage of Aromatic oil constituents of A. annua by region.
Quantification of artemisinin in dry leaf powder and tea
Artemisia annua dry powder samples (1g) was extracted in 10mls of n-toluene in sonicator for 45 minutes. The extract was filtered through a whatmann filter (No.1). Extract was then applied onto Thin Layer Chromatographic (TLC) plates (Merck) by the automated High Perfomance ThinLayer Chromatography (HPTLC) applicator. The plate was developed in HPTLC chamber using solvent system, heptane- diethyl ether (1:1) and derivatised as described above in 2.4.1. The absorbance was measured at 366 nm UV-wave length and artemisinin content obtained from the calibration curve shown. The concentration of artemisinin in A.annua tea was determined using the same procedures.
Determination of total flavonoids in the Artemisia annua samples
Construction of casticin calibration curve
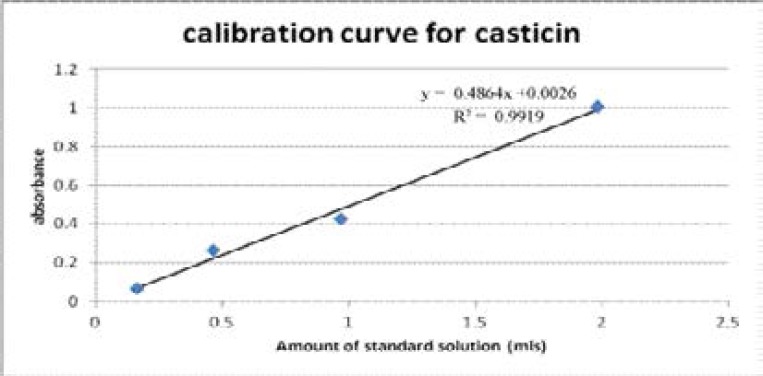
Casticin is one of the antimalarial and immunomodulatory flavonoids present in A.annua that works in synergy to artemisinin. The method previously described by Chen et al for determination of total flavonoids was adopted with slight modification13. Casticin used as a standard was prepared by dissolving 14mg in 25mls of 70% ethanol to give a concentration of 0.56mg/ml. The standard (4 ml) was then pipetted into 10mls volumetric flask and 0.4mls of 5% NaNO2 added, mixed and allowed to react for 6 minutes. A solution of 10% Al(NO3)3 (0.4ml) instead of AlCl3 previously used by Chen et al was added, mixed and allowed to complex for 6 minutes before adding 4 mls of 4% NaOH to neutralise the acidity. Double distilled water was added to top to the 10mls mark giving a yellow green solution of the final concentration 0.224mg/ml. Of the standard prepared, 0.2mls, 0.5mls, 1.0mls, 2mls and 3mls were each placed into cuvetts and topped to 4ml using double distilled. Their absorbance was then measured at 510nm using UV-spectroscopy, model PERKIN ELMER LAMBDA 35 uv/vis computerised spectrophotometer double beam. The respective concentrations gave absorbance of 0.098, 0.2795, 0.4417, 0.9907 and 1.1556. A calibration curve was constructed using absorbance and concentration giving line of best fit in which R2 value= 0.991.
Quantification of total flavonoids in artemisia annua powder
Ten (10 g) of dried A.annua of each sample was weighed into a separate conical glass and 100mls of 80% methanol then added, coked and allowed to stand for 4 h with constant shaking to extract flavonids according method previously described by Edeoga et al14. The extractives were filtered using whatmann filter paper (No.1) to obtain clear filtrates. Filtrate (4ml) was then pipette into 10mls volumetric flask and 0.4mls of 5% NaNO2 added, mixed and allowed to react for 6 minutes. A solution of 10% Al(NO3)3 (0.4ml) instead of AlCl3 was added, mixed and allowed to complex for 6 minutes before adding 4 mls of 4% NaOH to neutralise the acidity. Double distilled water was added to top to the 10mls mark. For each sample, 2mls was placed into cuvette and topped to 4ml using double distilled, its absorbance measured at 510nm and total flavonoid concentration estimated from the calibration curve in reference to casticin. The concentrations were used to calculate the total flavonoids content in dry leaf samples as percentage of dry weight.
Quantification of total flavonoids in artemisia annua tea
The tea of A.annua from Wagagai farm and that prepared using powders from Kabale and Fortportal were filtered through what filter paper (no.1). Filtrate (4ml) was then pipetted into 10mls volumetric flask and 0.4mls of 5% NaNO2 added, mixed and allowed to react for 6 minutes. A solution of 10% Al(NO3)3 (0.4ml) instead of AlCl3 was added, mixed and allowed to complex for 6 minutes before adding 4 mls of 4% NaOH to neutralise the acidity. Double distilled water was added to top to the 10mls mark. For each sample, 2mls was placed into cuvet and topped to 4ml using double distilled water, its absorbance measured at 510nm and total flavonoid concentration estimated from the calibration curve with reference to casticin.
Results and discussion
Major phytochemical groups identified in artemisia annua samples
The qualitative phytochemical test results showed that A. annua from low land and high land areas had similar phytochemical groups including flavonoids and triterpenes (Table 1) the known antimalarial groups. Similarity in phytochemical constituents shows that the A.annua cultivated in the different regions of Uganda are of the same variety.
Table I.
Phytochemical groups identified in Artemisia annua samples
| Phytochemical group |
South western-Kabale | Western-Fort portal |
Central-Wakiso (Entebbe) |
| Tannins | (++) | (++) | (++) |
| Reducing compounds | (++) | (++) | (++) |
| Polyuronides | (++) | (++) | (++) |
| Saponins | (−) | (−) | (−) |
| Alkaloid salts | (++) | (++) | (++) |
| Anthracenosides | (++) | (++) | (++) |
| Coumarin derivatives | (++) | (++) | (++) |
| Steroid glycosides | (++) | (++) | (++) |
| Flavonosides | (++) | (++) | (++) |
| Present (+), Present in abundance (++), absent (−) | |||
Aromatic oil constituents in artemisia annua L.
The major aromatic oil component in all three samples was camphor which constituted more than 75% of the oils. Camphor has been previously reported as the major aromatic oil component in A.annua cultivated in India15 and in Iran16. Unlike other aromatic components, camphor and borneol levels did not vary much between the regions (Figure 1). Camphor oil has been shown have mosquito repellent activity and provided up to 97.6% protection against Anopheles culicifacies17.
Artemisinin and total flavonoid contents in powder and teas
Artemisinin content was highest in the sample from Fort portal in Western Uganda followed by that from Kabale in south western Uganda. The total flavonoids in samples from Wakiso in central was about half that from Fortportal and Kabale (Figure 4). These variations call for quality control and standardization of material for not only of artemisinin levels but also of total flavonoids. This is because flavonoids have been shown to play major role in use of A.annua for malaria treatment and prophylaxis4,8,9.
Figure 4.
Artemisinin and total flavonoids content A.annua powder and teas by region.
The flavonoids content in Kabale and Fort Portal samples were similar to that previously reported in study on Brazillian A.annua cultivar which had 2.6% total flavonoid content4. The flavonoid contents in A.annua have been shown to vary with stage of growth, with highest amounts found during full bloom just like it is for artemisinin18. Although the time of harvesting the A.annua was not considered in this present study, farmers in Uganda generally harvest A.annua just before flowering which is the full bloom period. In lowland areas A.annua flowers much earlier than in highland areas perhaps due to higher temperatures and low rainfall compared to high land areas. The variations observed in this study could therefore be associated with the age of the plant at harvesting time.
Conclusion
This study reveals for the first time that the antimalarial components in A.annua vary with geographical area where the plant is cultivated. Standardization by source of A.annua raw materials used against malaria is vital for consistent clinical outcomes. A study on the variation of the components due to the age of the plant visavis geographical location is recommended to enable commercialization of the plant, and effective use by the communities.
Figure 2.
Artemisinin calibration curve used for estimation of artemisinin content
Figure 3.
Casticin calibration used in estimation of total flavonoid content
Acknowledgements
Authors thank Ms. Marion Borden, the in-charge Wagagai Health Centre for granting permission to study the Artemisia annnua used in their farm and clinic. Authors are also grateful to the staff of Natural Chemotherapeutics Research Institute and African Natural Products Laboratory in Addis Ababa University Ethiopia for the equipment and technical support rendered especially in the quantification of artemisinin and total flavonoid levels in the A. annua ‘tea’ and leaf powder used by Wagagai flower farm community.
Financial support
Authors express their gratitude to the CARNEGIE Corporation through RISE-AFNNET Makerere University node and the Government of Uganda through the Presidential support to scientists under Uganda National Council for Science and Technology for the financial support.
References
- 1.Willcox M, Bodeker G, Bourdy G, Dhingra V, Falquet J, Ferreira JFS, Graz B, Hirt H, Hsu , Magalhães P, Provendier D, Wright C. Traditional Medicinal Plants and Malaria. Boca Raton, London, New York, Washington DC: CRC press; 2004. Artemisia annua as a Traditional Herbal Antimalarial; pp. 43–59. [Google Scholar]
- 2.Byamugisha T, Byamukama R, Ntale M. Tests confirm suitability of Ugandan soils for commercial growing of Artemisia annua Linn. African Journal of Agricultural Research. 2013;8(37):4565–4572. [Google Scholar]
- 3.Lyda O, Iveth G, Piero O, Walter RJT. Artemisininbased combination therapy for Uncomplicated Plasmodium falciparum malaria in Colombia. Malaria Journal. 2007:6. doi: 10.1186/1475-2875-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakuni RS, Jain DC, Sharma RP. Phytochemistry of Artemisia annua and the development of artemisinin-derived antimalarial agents. In: Wright CW, editor. Artemisia. London: Taylor & Francis; 2002. [Google Scholar]
- 5.Bilia A R, Gabriele C, Bergonzi MC, Malgalhaes Melillo DE. Variation in Artemisinin and Flavonoid content in different extracts of Artemisia annua L. Natural Products Communication. 2006;0:1–5. [Google Scholar]
- 6.Jorge FSF, Devanand LL, Tomikazu S, Arne H. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules. 2010;15:3135–3170. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasoanaivo P, Wright CW, Willcox ML, Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar J. 2011;15(10 Suppl 1):S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogwang PE, Ogwal JO, Kasasa S, Olila D, Ejobi F, Kabasa D, Obua C. Artemisia annua L. infusion consumed once a week reduces risk of multiple episodes of malaria: a randomised trial in a Ugandan community. Trop J Pharmaceu Res. 2012;13:445–453. [Google Scholar]
- 9.Ogwang PE, Ogwal-Okeng J, Kasasa S, Ejobi F, Kabasa D, Obua C. Use of Artemisia annua L. Infusion for Malaria Prevention: Mode of Actionand Benefits in a Ugandan Community. British J Pharm Res. 2011;1:124–132. [Google Scholar]
- 10.Wallaart TE. Seasonal variation of artemisinin and its biosynthetic precussors in plants of Artemisia annua of different geographical origin: proof for the existence of chemotypes. Planta Med. 2000;66:57–62. doi: 10.1055/s-2000-11115. [DOI] [PubMed] [Google Scholar]
- 11.Culei I. Methodology for the analysis of vegetable drugs: Practical Manual on the industrial Utilisation of Medicinal and Aromatic Plants. 1st ed. Romania: Center Building; 1982. [Google Scholar]
- 12.Agarwal SP, Ali A, Dua Y, Ahuja S. Determination of artemisinin in bulk and Pharmaceutical dosage forms using HPTLC. Indian J Pharm Sci. 2009;71:98–100. doi: 10.4103/0250-474X.51948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Wang J, Wan D. Determination of total flavonoids in three Sedum crude drugs by UV-Vis spectrophotometry. Pharmacogn Mag. 2010;6(24):259–263. doi: 10.4103/0973-1296.71784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal Plants. African Journal of Biotechnology. 2005;4(7):685–688. [Google Scholar]
- 15.Padalia RC, Verma RS, Chauhan A, Chanotiya CS, Yadav A. Variation in volatile constitutents of Artemisia annua var. CIM-Arogya during plant ontogeny. Nat Prod commun. 2011;6(2):239–242. [PubMed] [Google Scholar]
- 16.Mohammadreza Verdian-Rizi. Variation in essential oil composition of Artemisia annua L. of different growth stage cultivated in Iran. Botany Research Journal. 2008;1(2):33–35. [Google Scholar]
- 17.Ansari MA, Razdan RK. Relative efficacy of various oils in repelling mosquitoes. Indian J Malariol. 1995;32(3):104–111. [PubMed] [Google Scholar]
- 18.Baraldi R, Isacchi B, Predieri S, Marconi G, Vincieri FF, Bilia AR. Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem System Ecol. 2008;36:340–348. [Google Scholar]