Abstract
Background
Neonatal enterovirus sepsis has high mortality. Antiviral therapy is not available.
Methods
Neonates with suspected enterovirus sepsis (hepatitis, coagulopathy, and/or myocarditis) with onset at ≤15 days of life were randomized 2:1 to receive oral pleconaril or placebo for 7 days. Serial virologic (oropharynx, rectum, urine, serum), clinical, pharmacokinetic, and safety evaluations were performed.
Results
Sixty-one subjects were enrolled (43 treatment, 18 placebo), of whom 43 were confirmed enterovirus infected (31 treatment, 12 placebo). There was no difference in day 5 oropharyngeal culture positivity (primary endpoint; 0% in both groups). However, enterovirus-infected subjects in the treatment group became culture negative from all anatomic sites combined faster than placebo group subjects (median 4.0 versus 7.0 days, P = .08), and fewer subjects in the treatment group remained polymerase chain reaction (PCR)–positive from the oropharynx when last sampled (23% versus 58%, P = .02; median, 14.0 days). By intent to treat, 10/43 (23%) subjects in the treatment group and 8/18 (44%) in the placebo group died (P = .02 for 2-month survival difference); among enterovirus-confirmed subjects, 7/31 (23%) in the treatment group died versus 5/12 (42%) in the placebo group (P = .26). All pleconaril recipients attained concentrations greater than the IC90 after the first study day, but 38% were less than the IC90 during the first day of treatment. One subject in the treatment group and three in the placebo group had treatment-related adverse events.
Conclusions
Shorter times to culture and PCR negativity and greater survival among pleconaril recipients support potential efficacy and warrant further evaluation.
Keywords: enterovirus, hepatitis, neonatal, pleconaril, sepsis
(See the Editorial Commentary by Modlin on pages 63–4.)
Nonpoliovirus enteroviruses (EVs) are an important cause of neonatal illness, with an incidence approximating or exceeding that of illness caused by Streptococcus agalactiae, herpes simplex virus, or symptomatic cytomegalovirus infections [1, 2]. A subset of affected newborns, particularly those with disease onset in the first few days of life, develops severe disease manifesting as hepatitis, coagulopathy, myocarditis, pneumonitis, and/or meningoencephalitis. Hepatitis with coagulopathy and myocarditis are associated with mortality as high as 24%–83% and 30%–50%, respectively. Long-term morbidity including persistent hepatic and cardiac dysfunction and neurodevelopmental deficits may occur in survivors [1–5].
Antiviral therapy for neonatal EV infections is not commercially available. The mainstay of treatment for severe disease is supportive care, including cardiorespiratory and blood product support. Small studies suggest potential antiviral or immunomodulatory benefit of intravenous immune globulin (IVIG), but efficacy remains unproven [6]. Pleconaril is an oral viral capsid inhibitor with activity against picornaviruses, including most EVs and many rhinoviruses. In studies of EV meningitis, EV infections in immunocompromised hosts, and picornavirus upper respiratory tract infections [7–13] and in a phase 1 study in neonates and uncontrolled case reports and case series in newborns [14, 15], pleconaril was generally well tolerated, with suggestion of benefit in some studies. The NIH-sponsored Collaborative Antiviral Study Group (CASG) performed a randomized, double-blind, placebo-controlled study of the virologic and clinical efficacy, pharmacokinetics, and safety of pleconaril for the treatment of severe neonatal EV sepsis (www.clinicaltrials.gov NCT00031512).
METHODS
Study Population
Neonates with suspected EV sepsis were enrolled at 19 centers from June 1999 to December 2010. Inclusion criteria included illness onset at age ≤15 days, birthweight ≥1500 g and gestational age ≥32 weeks, and ≥1 of (1) serum alanine aminotransferase >3 times the upper limit of normal (ULN); (2) platelet count <100,000/mm3, prothrombin time >1.5 times the ULN, and positive fibrin split products; or (3) cardiac shortening fraction <25% or ejection fraction <50% by echocardiography. Study drug was to be initiated ≤10 days after onset of illness. Subjects with known bacterial or non-EV viral infection, imminent demise, cyanotic congenital heart lesion, alimentary tract abnormalities that might interfere with medication absorption, or known maternal human immunodeficiency virus infection were excluded. The study was approved by the institutional review board of each participating institution and informed consent was obtained in accordance with guidelines of the U.S. Department of Health and Human Services.
Study Design
A 2:1 study drug:placebo randomization with randomly permuted block sizes was used. Randomization codes were accessible only to biostatistical personnel at the coordinating center. Subjects initially received 5.0 mg/kg per dose of a 40 mg/mL liquid formulation of pleconaril (ViroPharma, Inc., Exton, PA) or matching placebo identical in composition except lacking active study drug. The liquid formulation expired on May 31, 2002, after which the formulation was changed by the manufacturer to a 40 mg/mL suspension. Subjects received 8.5 mg/kg per dose of pleconaril suspension (calculated to provide similar drug exposure as 5.0 mg/kg per dose of pleconaril liquid based on previous comparative pharmacokinetic studies) or matching placebo. Both pleconaril formulations and matching placebos were administered every 8 hours for 7 days in 1.0 mL of breast milk or formula by mouth or orogastric tube and were given to subjects whether or not they were receiving anything else by mouth. Subjects were permitted to receive antibiotics, acyclovir (inactive against enteroviruses), cardiotropic medications, and, with protocol version 3.0 (May 2000), IVIG. Study drug/placebo was discontinued for subjects in whom an etiology for illness other than EV infection was documented after enrollment.
Laboratory and Clinical Assessments
Specimens for EV culture and polymerase chain reaction (PCR) were obtained from the oropharynx, rectum, urine, and serum on days 1–5, 7, 10, and 14. Plasma for pharmacokinetic analysis was obtained on day 1 (2, 4, and 8 hours after study drug administration) and days 3 and 7 (before and 2 and 4 hours after administration). Hematology and chemistry tests were obtained on study days 1, 3, 5, 7, 10, and 14 and urinalyses on days 1 and 7. Follow-up visits occurred at 2, 6, 12, 18, and 24 months. Adverse events were recorded at each evaluation, and relationship to study treatment was determined by site investigators. Abnormal laboratory values were graded using the Division of AIDS Toxicity Table for Grading Severity of Pediatric Adverse Experiences (April 1994).
The primary endpoint was the percentage of patients with a positive viral culture from the oropharynx 5 days after initiating study drug. Secondary endpoints included duration of culture positivity from oropharynx, rectum, urine, and serum; changes in laboratory values; duration of hospitalization; survival; and pleconaril pharmacokinetics. Duration of PCR positivity was added post hoc as a secondary endpoint. Tertiary endpoints included number of days of blood product (fresh frozen plasma, packed red blood cells, platelets, other products) and inotropic support.
Virologic Methods
Specimens for viral culture and PCR were frozen (–70°C) and batched shipped on dry ice to the CASG Central Laboratory at University of Alabama at Birmingham. Virus cultures were performed by methods reported previously [7]. For reverse transcriptase PCR (RT-PCR), nucleic acid was extracted from 200 µL of each specimen utilizing bioMérieux NucliSENS easyMAG, according to the manufacturer's suggested protocol (bioMérieux, Inc., Durham, NC). Ten microliters of purified nucleic acid were amplified using Qiagen OneStep RT-PCR mastermix (Qiagen, Valencia, CA) and EV-specific primers, followed by detection of the 114-bp amplified fragment by gel electrophoresis as previously described [7, 16].
Pharmacokinetic Methods
Pleconaril concentrations were determined by liquid chromatography/mass spectrometry assays at MDS Pharma Services (Montreal, Quebec, Canada; 60 samples from 9 subjects) [17] and the University of Alabama (178 samples from 23 subjects). The latter assay consisted of a reversed-phase high-performance liquid chromatographic assay coupled to a triple quadrupole mass spectrometer validated for the determination of pleconaril concentrations. Sample preparation involved addition of a labeled internal standard (Pleconaril-d4 [IS]) from Medical Isotopes, Inc. (Pelham, NH) and acetonitrile precipitation followed by supernatant dilution with 50% acetonitrile. Reversed-phase chromatographic separation of pleconaril and IS was performed on an XBridge™ C18 analytical column (2.1 × 50 mm, 3.5 μm) under isocratic conditions. A binary mobile phase was used consisting of 0.2% formic acid and acetonitrile (20/80). Detection and quantitation were achieved by multiple reaction monitoring. The protonated molecular ions [M + H]+ were monitored at m/z of 382.4 > 54.1 for pleconaril and 386.4 > 128.0 for IS. Calibration curves were linear over the range of 5–5000 ng/mL using a 20-μL aliquot of human plasma.
A 1-compartment model with an absorption lag phase was fit to the pharmacokinetic data using the maximum likelihood estimation maximization algorithm in ADAPT 5.0 [18]. Steady-state area-under-the-curve (AUC), time to maximum concentration (Tmax,ss), and maximum concentration (Cmax,ss) were calculated based on modeled parameters. Individual concentrations attained were compared with a concentration of 70 ng/mL reported to inhibit >90% of non-poliovirus EVs in vitro [15].
Sample Size and Statistical Analysis
A target sample size of 45 subjects with confirmed EV disease, with an expectation that approximately 67% of enrolled subjects would be confirmed to be EV infected, was chosen to provide sufficient power to detect a reduction in viral shedding from the oropharynx from 96% to 70% on day 5. EV-confirmed subjects (culture- or PCR-positive) were included in virologic and clinical efficacy analyses, all enrolled subjects were included in safety analyses, and pleconaril recipients for whom pharmacokinetic data were obtained (whether or not EV infected) were included in pharmacokinetic analyses. Survival analyses were conducted on the total enrolled cohort (intent to treat) and on EV-confirmed subjects.
Fisher exact test was used for comparisons of categorical factors and the Kruskal–Wallis and Mann–Whitney tests for continuous factors, with medians and ranges presented. Kaplan–Meier curves and log-rank statistics were used to assess differences in duration of culture and PCR positivity and survival between study groups. Cox proportional hazards models were used to adjust for covariates. In analyses of time to culture and PCR negativity, subjects who died or were lost to follow-up had their data censored at the time of their last culture or PCR. Changes in toxicity grades from baseline and adverse event rates were compared by χ2 tests.
RESULTS
Study Population
Sixty-one subjects were enrolled; 43 randomized to the treatment group and 18 to the placebo group (Table 1; Supplementary Figure 1; see online supplementary material for this figure). Forty-three of 61 (70%) were ultimately confirmed to be EV positive: 31 in the treatment group and 12 in the placebo group. The study was terminated before subject accrual was complete due to expiration of the study drug. Four of 43 (9%) subjects in the treatment group and 5/18 (28%) in the placebo group did not receive a full 7-day course of study treatment/placebo (P = .11). Eleven of 31 (36%) and 4 of 12 (33%) of EV-confirmed subjects in the treatment and placebo groups, respectively, received IVIG.
Table 1.
Maternal and Neonatal Characteristics
| Characteristic | Total Enrolled |
Enterovirus Confirmed |
||||
|---|---|---|---|---|---|---|
| Pleconaril | Placebo | P Value | Pleconaril | Placebo | P Value | |
| (N = 43) | (N = 18) | (N = 31) | (N = 12) | |||
| Maternal fever (%)a | 28 (65) | 7 (44)b | 0.15 | 22 (71) | 5 (46)c | 0.16 |
| Cesarean section delivery (%) | 25 (58) | 9 (50) | 1.0 | 19 (61) | 7 (58) | 0.50 |
| Race/ethnicity (%) | 0.98 | 0.68 | ||||
| White | 29 (67) | 13 (72) | 23 (74) | 9 (75) | ||
| African American | 4 (9) | 1 (6) | 1 (3) | 1 (8) | ||
| Hispanic | 7 (16) | 3 (17) | 5 (16) | 1 (8) | ||
| Asian/Pacific Islander | 1 (2) | 1 (6) | 1 (3) | 1 (8) | ||
| Other | 2 (5) | 0 (0) | 1 (3) | 0 (0) | ||
| Male (%) | 26 (61) | 11 (61) | 1.0 | 20 (65) | 7 (58) | 0.74 |
| Median (range) Apgar scores | ||||||
| 1 min | 8 (1–9) | 8 (1–9) | 0.61 | 8 (4–9) | 8 (1–8) | 0.36 |
| 5 min | 9 (5–10) | 9 (4–9) | 0.90 | 9 (7–10) | 9 (4–9) | 0.24 |
| Median (range) gestational age at delivery, wks | 37 (33–42) | 37 (32–40) | 0.40 | 37 (34–41) | 37 (34–40) | 0.81 |
| <37 Weeks gestation at delivery (%) | 12 (28) | 7 (39) | 0.40 | 10 (32) | 4 (33) | 1.0 |
| Breastfed (%) | 22 (51) | 12 (67) | 0.40 | 16 (52) | 9 (75) | 0.19 |
| Median (range) age at illness onset, d | 4.0 (1–15) | 5.0 (1–10) | 0.70 | 4.5 (1–15) | 5.0 (1–10) | 0.91 |
| Median (range) duration of illness prior to enrollment, d | 6.0 (1–15) | 7.0 (2–14) | 0.09 | 6.0 (2–15) | 7.0 (4–14) | 0.04 |
| Duration of illness ≤5 days prior to enrollment (%) | 20 (50)d | 3 (17) | 0.02 | 14 (47)c | 2 (17) | 0.09 |
| Median (range) postnatal age at enrollment, d | 8 (1–17) | 10 (6–16) | 0.12 | 8 (6–17) | 10 (7–16) | 0.40 |
| Inclusion criterion for study entry, % | ||||||
| • Alanine aminotransferase >3 times ULN | 58 | 61 | 1.0 | 61 | 55 | 0.74 |
| • Platelet count <100,000/mm3, prothrombin time >1.5 times ULN, positive fibrin split products | 34 | 28 | 0.77 | 41 | 18 | 0.27 |
| • Cardiac shortening fraction <25% or ejection fraction <50% | 52 | 56 | 1.0 | 50 | 55 | 1.0 |
| Median (range) weight at enrollment, g | 3070 | 3300 | 0.93 | 2983 | 3000 | 0.86 |
| (1980–5905) | (2200–4290) | (1980–5905) | (2200–4290) | |||
Abbreviation: ULN, upper limit of normal.
aIn the 2 weeks prior to delivery to onset of neonatal disease.
bInformation missing for 2 subjects.
cInformation missing for 1 subject.
dInformation missing for 3 subjects.
Maternal and Neonatal Characteristics
Maternal and neonatal characteristics were similar between the treatment and placebo groups (Table 1). There were high rates of maternal illness preceding neonatal disease among EV-confirmed subjects, and 60% mothers of EV-positive subjects had caesarean sections. Fourteen (33%) EV-confirmed subjects were born at <37 weeks gestation. The median (range) age at onset of neonatal illness and the median (range) duration of illness prior to enrollment were 5.0 (1–15) days and 6.0 (2–15) days, respectively, for EV-positive subjects, with 1 day longer duration in the placebo group compared with the treatment group and more subjects in the treatment group with duration of illness less than or equal to 5 days prior to enrollment. A majority of enrolled subjects fulfilled hepatitis and myocarditis inclusion criteria and one third met the coagulopathy criterion; distributions were similar in the treatment and placebo groups. Most EV-positive subjects assessed had cerebrospinal fluid pleocytosis (>25 white blood cells/mm3 in 9/15, 60%) and abnormal chest radiographs (37/43, 86%), with no differences between treatment and placebo groups.
Virologic Efficacy
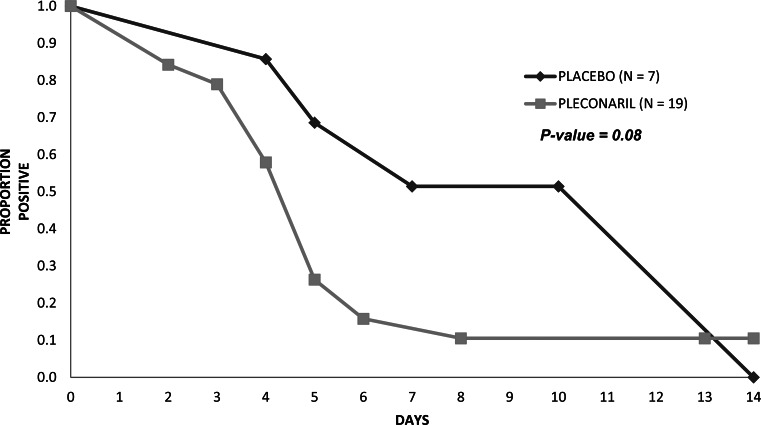
Nineteen subjects in the treatment group (44%) and 7 in the placebo group (39%) were culture positive from any site at entry or during the treatment period, and 31 subjects in the treatment group (72%) and 12 in the placebo group (67%) were PCR positive from any site at entry or during the treatment period. All culture-positive subjects were also PCR positive. Six of 24 (25%) EV-confirmed subjects in the treatment group and 3/10 (30%) in the placebo group were culture positive from the oropharynx on day 1, and no subject shed cultivable virus from the oropharynx on day 5 (Table 2). The median time to sustained oropharyngeal culture negativity was 3.0 days in both groups. Among subjects who were culture positive from any site, those in the treatment group became culture negative from all sites faster than those in the placebo group, with a median time to culture negativity in the treatment group of 4.0 days versus 7.0 days in the placebo group (P = .08; Figure 1). A similar pattern was observed for urine (Table 2), but not other individual sites.
Table 2.
Viral Culture Results Among Enterovirus-Confirmed Subjects
| Source | No. of Subjects |
No. of Subjects |
No. of Subjects |
Median (Range) Days to |
||||
|---|---|---|---|---|---|---|---|---|
| Positive During |
Positive on Study |
Positive on Study |
Sustained Negativitya |
|||||
| Treatment Period (%) |
Day 1/No. Tested (%) |
Day 5/No. Tested (%) |
Pleconaril | Placebo | ||||
| Pleconaril | Placebo | Pleconaril | Placebo | Pleconaril | Placebo | (N = 31) | (N = 12) | |
| Oropharynx | 10/31 (32) | 4/11 (36)b | 6/24 (25) | 3/10 (30) | 0/29 (0) | 0/9 (0) | 3.0 (2–4) | 3.0 (2–3)c |
| Rectum | 11/30 (37)b,d | 0/11 (0)b,d | 7/24 (29) | 0/10 (0) | 1/27 (4) | 0/10 (0) | 3.0 (2–9)e | — |
| Urine | 15/30 (50)b | 7/12 (58) | 9/21 (43) | 5/9 (56) | 1/24 (4) | 3/10 (30) | 4.0 (2–8)f | 7.0 (4–14)f |
| Serum | 9/31 (29) | 5/11 (45)b | 5/16 (31) | 3/7 (43) | 0/28 (0) | 1/8 (13) | 3.0 (2–5) | 4.0 (2–10)g |
aSustained negativity defined as the day on which viral cultures became negative and remained negative at all subsequent timepoints among subjects who were culture positive from the anatomic site and became consistently culture negative at that site.
bSpecimens were missing for 1 subject.
cExcludes 1 placebo subject who was positive when last sampled at 10 days.
dP = 0.02 for the difference in proportion of subjects positive during the treatment period.
eExcludes 1 pleconaril subject who was positive when last sampled at 14 days.
fExcludes 1 pleconaril subject who was positive when last sampled at 13 days and 2 placebo subjects who were positive when last sampled at 4 and 10 days; P = 0.05 for the difference in number of days to sustained negativity.
gExcludes 1 placebo subject who was positive when last sampled at 1 day.
Figure 1.
Time to culture negativity for all anatomic sites (oropharynx, rectum, urine, and serum) combined among the 19 subjects in the treatment group and 7 in the placebo group who were culture positive from any site. A subject was considered culture negative on a given study day only when culture assays from all sampled sites on that day were negative.
Rates of PCR positivity at entry or during the treatment period among EV-confirmed subjects exceeded 80% from each anatomical site, with 75%–100% positivity at each site on day 1 in the treatment and placebo groups and 43%–97% positivity on day 5 (Table 3). Seven of 31 subjects (23%) in the treatment group and 7/12 (58%) in the placebo group continued to be PCR positive from the oropharynx when last sampled at a median of 14.0 days (P = .02). For other sites, 40%–77% of subjects remained PCR positive at last sampling (medians = 9–15 days), and differences between groups in positivity at last sampling were not significant. Sustained PCR negativity of serum occurred earlier in the treatment group compared with the placebo group among the 25% of subjects who became PCR negative (P = .06). For all anatomic sites combined, there was no difference in the time to PCR negativity between EV-infected subjects in the treatment and placebo groups.
Table 3.
Polymerase Chain Reaction Results Among Enterovirus-Confirmed Subjects
| Source | No. of Subjects |
No. of Subjects |
No. of Subjects |
No. of Subjects Positive |
||||
|---|---|---|---|---|---|---|---|---|
| Positive During |
Positive on Study |
Positive on Study |
at Last Sampling (%) |
|||||
| Treatment Period (%) |
Day 1/No. Tested (%) |
Day 5/No. Tested (%) |
Pleconaril | Placebo | ||||
| Pleconaril | Placebo | Pleconaril | Placebo | Pleconaril | Placebo | (N = 31) | (N = 12) | |
| Oropharynx | 28/31 (90) | 10/12 (83) | 20/24 (83) | 8/8 (100) | 13/30 (43) | 7/11 (64) | 7 (23)a | 7 (58)a |
| Rectum | 27/30 (90)b | 12/12 (100) | 19/24 (79) | 8/8 (100) | 21/28 (75) | 7/11 (64) | 15 (50)c | 9 (75)c |
| Urine | 26/30 (87)b | 11/12 (92) | 15/20 (75) | 8/8 (100) | 11/25 (44) | 8/10 (80) | 12 (40)d | 6 (50)d |
| Serum | 30/31 (97) | 10/11 (91)b | 19/21 (90) | 8/8 (100) | 28/29 (97) | 9/10 (90) | 24 (77)e | 8 (73)e |
aPleconaril subjects positive at last testing were last sampled at a median of 14.0 (range 7–15) days and placebo subjects positive at last testing were last sampled at a median of 14.0 (range 4–15) days; P = 0.02 for the difference in proportion of subjects who were positive when last sampled. Among subjects who attained sustained negativity, median (range) days to sustained negativity = 14.0 (4–16) for pleconaril subjects and 5.0 (5–14) for placebo subjects.
bSpecimens were missing for 1 subject.
cPleconaril subjects positive at last testing were last sampled at a median of 15.0 (range 7–15) days and placebo subjects positive at last testing were last sampled at a median of 14.0 (range 4–15) days. Among subjects who attained sustained negativity, median (range) days to sustained negativity = 13.5 (2–16) for pleconaril subjects and 14.0 (10–14) for placebo subjects.
dPleconaril subjects positive at last testing were last sampled at a median of 14.0 (range 7–15) days and placebo subjects positive at last testing were last sampled at a median of 9.0 (range 4–14) days. Among subjects who attained sustained negativity, median (range) days to sustained negativity = 9.0 (2–15) for pleconaril subjects and 14.0 (8–15) for placebo subjects.
ePleconaril subjects positive at last testing were last sampled at a median of 14.0 (range 3–16) days and placebo subjects positive at last testing were last sampled at a median of 10.5 (range 4–15) days. Among subjects who attained sustained negativity, median (range) days to sustained negativity = 8.0 (6–14) for pleconaril subjects and 14.5 (14–15) for placebo subjects; P = .06 for the difference in number of days to sustained negativity.
Analyses of viral culture and PCR according to receipt of pleconaril versus placebo and IVIG versus no IVIG were limited by small numbers within subgroups, but did not reveal trends that differed from overall comparisons of the pleconaril versus placebo groups. There were not significant trends by year over the course of the study in rates of conversion from culture positivity to culture negativity for all anatomic sites combined or in rates of conversion from PCR positivity to PCR negativity for all anatomic sites combined.
Clinical Efficacy
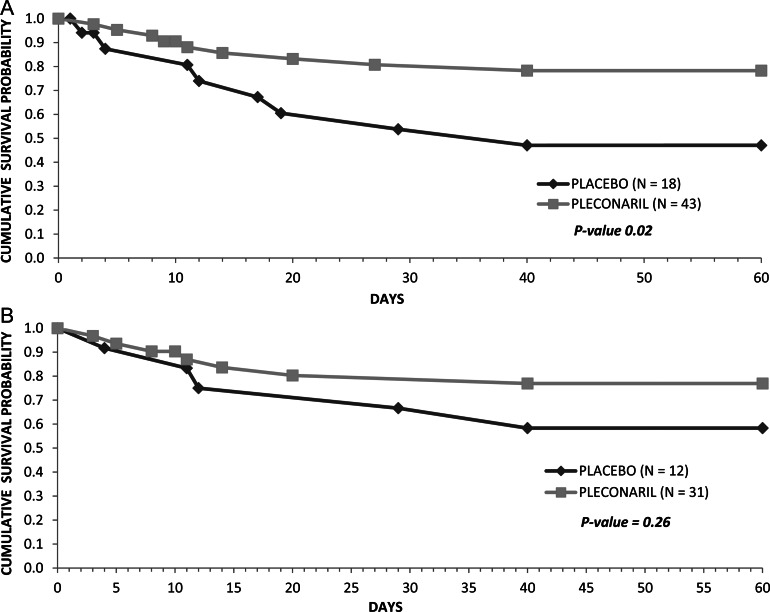
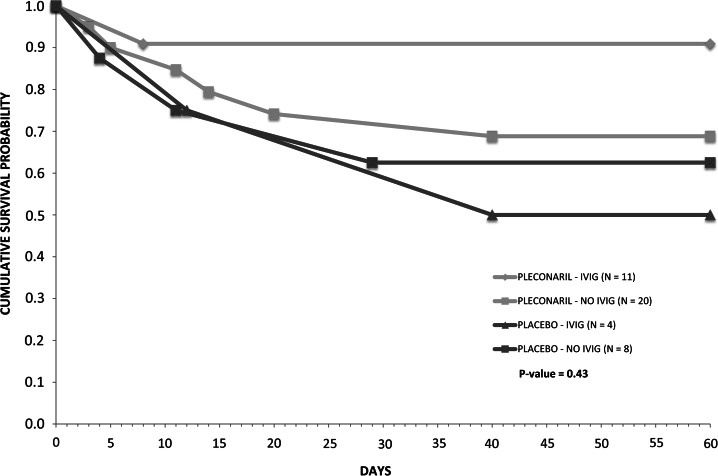
There were no differences in durations of intensive care, blood product support, inotropic support, or hospitalization, or in total hospital charges between the 2 groups (Supplementary Table 1; see online supplementary material for this table). Among all enrolled subjects, 10/43 (23%) in the treatment group and 8/18 (44%) in the placebo group died during the study period. Cumulative survival probability over 2 months was greater in the treatment group (P = .02, Figure 2; P = .057 after adjustment for duration of symptoms prior to enrollment). Among EV-confirmed subjects, 7/31 (23%) in the treatment group and 5/12 (42%) in the placebo group died, with cumulative survival probability favoring the treatment group (P = .26, Figure 2; P = .29 after adjustment for duration of symptoms prior to enrollment). Cumulative survival probability in the treatment group continued to exceed that in the placebo group over 18 months (P = .07 among all subjects and P = .23 among EV-confirmed subjects). Survival among EV-confirmed subjects according to treatment group and receipt of IVIG was greater among pleconaril recipients than placebo recipients regardless of IVIG administration, but differences among subgroups were not significant (Figure 3). There was not a significant trend in survival by year over the course of the study.
Figure 2.
Survival over 2 months among all enrolled subjects (A) and among enterovirus-confirmed subjects (B).
Figure 3.
Survival over 2 months among enterovirus-confirmed subjects according to treatment group and receipt of intravenous immune globulin (IVIG).
Pharmacokinetic Analyses
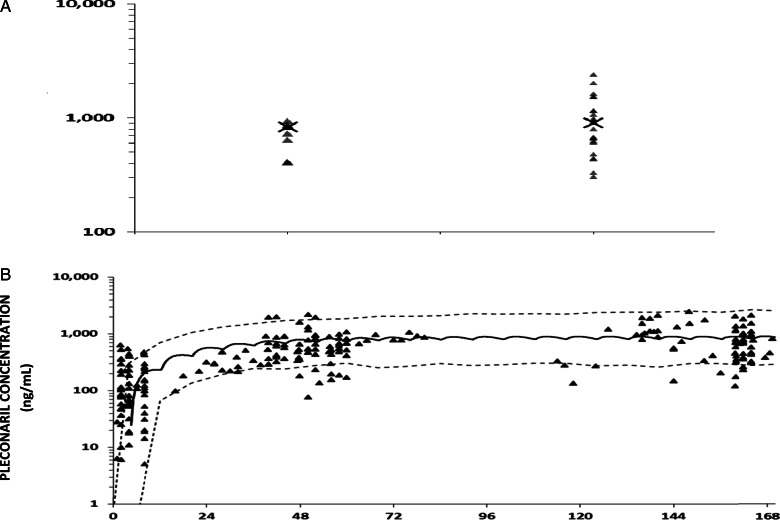
Concentration–time data were available for 32 pleconaril recipients (9 liquid formulation and 23 suspension; 238 concentration–time points); specimens/data were missing for 11 pleconaril recipients. The liquid and suspension formulations as dosed produced similar drug exposure, as indicated by simulated Cmax,ss (median = 834 ng/mL versus 903 ng/mL, respectively, P = .30; Figure 4A) and modeled AUC (median AUC = 5812 ng × h/mL versus 7829 ng × h/mL, respectively, P = .16). For both formulations combined, the median Cmax,ss; AUC; Tmax,ss; and elimination half-life were 836 (range, 305–2408) ng/mL, 6824 (range, 2228–24,446) ng × h/mL, 3.1 hours, and 22.8 hours, respectively. Subjects with hepatitis had lower exposures than those without hepatitis (median Cmax,ss = 782 ng/mL versus 986 ng/mL, P = .06, and AUC = 5894 ng × h/mL versus 8964 ng × h/mL, P = .09) and had lower distribution volumes (median 88.5 L versus 158.9 L, P = .20).
Figure 4.
Pleconaril concentration as a function of formulation (A) and time (B). In (A), the X denotes the median simulated steady-state maximum concentration (Cmax,ss) for each formulation. In (B), subjects dosed with the 2 formulations are combined. The solid line represents simulated median concentration–time data and dashed lines are the 5th and 95th percentile confidence intervals.
Despite considerable variability in the pleconaril concentration–time data, all concentrations beyond the first day exceeded 70 ng/mL. However, 13/32 (41%) pleconaril recipients did not attain values ≥70 ng/mL during the first 24 hours, and steady state was not reached until approximately day 4 (Figure 4). Cumulative survival analysis suggested greater survival among EV-confirmed pleconaril recipients who achieved pleconaril levels ≥70 ng/mL during the first 24 hours of treatment versus those with levels <70 ng/mL in the first 24 hours and placebo recipients (Supplementary Figure 2; see online supplementary material for this figure).
Safety
Eighty-six percent of subjects in the treatment group and 94% in the placebo group experienced adverse events, of whom only 3% and 18%, respectively, had events judged possibly/probably treatment related (Table 4). The number of events/subject and severity distribution were similar between groups. One subject (placebo group) had a serious adverse event considered possibly/probably treatment related. No subjects had treatment withdrawn due to adverse events other than death (5).
Table 4.
Safety Endpoints Among All Enrolled Subjects
| Safety Endpoint | Pleconaril | Placebo | P Value |
|---|---|---|---|
| (N = 43) | (N = 18) | ||
| Subjects with ≥1 adverse eventa (%) | 37 (86) | 17 (94) | 0.66 |
| Median (range) no. events/subjectb | 10 (1–35) | 10 (1–26) | 0.80 |
| Maximum adverse event severity experienced by each subjectb | |||
| Mild, % | 11 | 6 | 0.91 |
| Moderate, % | 31 | 29 | |
| Severe, % | 57 | 65 | |
| Subjects with ≥1 adverse event possibly or probably related to treatment (%b) | 1 (3)c | 3 (18)c | 0.09 |
| Percent of subjects with grade 3 or 4 laboratory toxicity on study days 3–7 and < grade 3 toxicity at entryd | |||
| Hemoglobin | 19 | 0 | 0.08 |
| Platelet count | 16 | 13 | 1.0 |
| Absolute neutrophil count | 5 | 23 | 0.05 |
| Creatinine | 5 | 13 | 0.49 |
| Alanine aminotransferase | 9 | 7 | 0.65 |
| Aspartate aminotransferase | 13 | 17 | 0.72 |
| Total bilirubin | 24 | 7 | 0.37 |
aAny time during the study period, after study enrollment.
bAmong subjects who experienced at least 1 event after study enrollment.
cOne subject in the pleconaril group with diaper rash and thrush, each graded as moderate; 1 subject in the placebo group with elevated liver function tests graded as mild; 1 subject in the placebo group with emesis graded as mild; and 1 subject in the placebo group with elevated liver function tests graded as severe. The latter event was considered a life-threatening serious adverse event that subsequently resolved without sequelae.
dAbnormal laboratory values were graded using the Division of AIDS Toxicity Table for Grading Severity of Pediatric Adverse Experiences (April 1994).
DISCUSSION
Neonates in this study had unexpectedly low culture yields and brief shedding, particularly from the oropharynx and rectum. Accordingly, the primary endpoint (reduction in oropharyngeal culture positivity on day 5) was not met, nor were differences observed in duration of shedding of cultivable virus from the oropharynx and rectum. Nevertheless, indicators of potential virologic efficacy were observed. Time to culture negativity of all anatomic sites combined was shorter in the treatment group. PCR of oropharyngeal, rectal, urine, and serum specimens had very high yields, consistent with published data [19], and persistence of PCR positivity from the oropharynx was lower and time to PCR negativity of serum was shorter in the treatment group. Clinical efficacy was suggested by differences among all enrolled subjects in cumulative survival favoring the treatment group and a similar trend among EV-confirmed subjects.
Adverse events were frequent in this study, reflecting the severity of illness in the population studied. However, adverse event profiles were similar in the treatment and placebo groups, and few adverse events and no deaths were attributed to study treatment. These findings support the safety of pleconaril in this cohort of very ill newborns.
Two pleconaril formulations were used in this study; however, pharmacokinetic data indicated comparable exposures with these formulations as dosed, allowing their data to be combined. Cmax values were in the range of concentrations reported in adult (800–3400 ng/mL) and pediatric (200–1500 ng/mL) studies, including the mean 2-hour postdose concentration of 500 ng/mL observed in an earlier CASG study in infants with EV meningitis [7] and the mean peak concentration of 690 ng/mL measured in a single-dose study in neonates [15]. Furthermore, they were well above the concentration of pleconaril of 70 ng/mL reported to inhibit >90% of nonpoliovirus EVs in vitro [15]. The median AUC was comparable to AUCs reported in adult studies (3582–19,365 ng × h/mL), the single-dose neonatal study (mean 5152 ng × h/mL), and a single-dose study in children (mean 8131 ng × h/mL) [15, 20]. However, more than a third of subjects had concentrations <70 ng/mL during the first 24 hours, suggesting inadequate initial dosing. Additionally, Cmax values and AUCs were lower among subjects with hepatic involvement, despite an expectation that concentrations of this hepatically metabolized drug might be higher in subjects with liver disease.
The major limitation of this study is the small size of the study population. Anticipating that sample size would be a limiting factor in proving clinical efficacy, it was hoped that viral culture would provide a feasible surrogate marker. However, the yield of cultures was unexpectedly low and the duration of shedding brief, particularly from the oropharynx and rectum; furthermore, not all specimens were obtained at each timepoint for all subjects. Despite these limitations, it is notable that time to culture negativity of all anatomic sites combined was shorter in the treatment group and survival differences favoring pleconaril recipients were observed. Reasons for the unexpectedly low yield of viral cultures are unclear, but could include inadequate sample collection, loss of virus during storage and/or shipping, or suboptimal culture technique. Other study limitations include delays from illness onset to initiation of therapy and use of an oral agent in a very ill population of neonates in whom gastrointestinal absorption could be predicted to be impaired. Although pharmacokinetics appeared favorable overall, suboptimal concentrations in the first 24 hours may reflect impaired and/or delayed absorption during severe illness. Delays in initiation of treatment and achieving satisfactory drug concentrations early in the course may have been limiting factors for demonstrating efficacy. An intravenous formulation of the study drug is not available; an oral loading dose may be a potential strategy to overcome the latter challenge. There was an imbalance between study groups in duration of illness prior to enrollment; however, adjustment for this imbalance only modestly affected survival analyses. Determination of viral type, which likely varied among subjects over the study period, was not performed and neutralizing antibody titers in IVIG administered to some subjects were not measured, precluding matching based on these parameters or analysis of their potential confounding effects.
In summary, shorter times to achieving negative viral cultures and PCR for multiple anatomic sites and suggestion of greater survival among EV-infected subjects who received pleconaril lend credence to its potential efficacy for neonatal EV infections. No safety concerns emerged, and drug concentrations that were achieved exceeded concentrations that inhibit most nonpoliovirus EVs in vitro. As no effective antiviral therapy for neonatal EV infections exists and these infections are associated with unacceptably high mortality and morbidity, signals of efficacy and absence of safety concerns should compel further development and evaluation of pleconaril for neonates (and other populations) with potentially devastating EV infections.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
The authors gratefully thank the research staff at study sites and the families that participated in this study. Drs. Harley Rotbart, John Modlin, Janet Kinney, John Bradley, Leonard Weiner, and Mark McKinlay contributed to the design of this study. Drs. Charles Grose (Chair), Jeffrey Cohen, Mark Van Raden, Gerald Fisher, and Shirley Jankelevich served on the data safety monitoring board. Sharon Blount, University of Alabama at Birmingham, performed the virology laboratory studies; Linda Austin, University of Alabama at Birmingham, provided programming and data management support; and Dunia Ritchey, University of Alabama at Birmingham, assisted with preparation of the figures.
Additional personnel who contributed to the performance of this study were Kathryn Edwards, MD, and Natasha Halasa, MD, Vanderbilt University; Elizabeth Esterl, RN, Sharolene Goodman, RT, and Leslie Smitely, RN, Children's Hospital Colorado; and Anna Winborn, BA, CCRC, Dewan Perry, RN, MS, Fiker Zeray, RN, CPNP, and Shanda Johnson, RRT, MHA, CCRP, University of Texas Southwestern.
Financial support. This work was supported under contract with the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (contract numbers N01-AI-30025, N01-AI -65306, N01-AI -15113, N01-AI-62554). Study drug and matching placebo were supplied by ViroPharma, Inc. and Schering-Plough Corp.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group, Harley Rotbart, John Modlin, Janet Kinney, John Bradley, Leonard Weiner, Mark McKinlay, Charles Grose, Jeffrey Cohen, Mark Van Raden, Gerald Fisher, Shirley Jankelevich, Sharon Blount, Linda Austin, Dunia Ritchey, Kathryn Edwards, Natasha Halasa, Elizabeth Esterl, Sharolene Goodman, Leslie Smitely, Anna Winborn, Dewan Perry, Fiker Zeray, and Shanda Johnson
References
- 1.Abzug MJ. Presentation, diagnosis, and management of enterovirus infection in neonates. Paediatr Drugs 2004; 6:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Abzug MJ. The enteroviruses: problems in need of treatments. J Infect 2014; 68:S108–14. [DOI] [PubMed] [Google Scholar]
- 3.Abzug MJ. Prognosis for neonates with enterovirus hepatitis and coagulopathy. Pediatr Infect Dis J 2001; 20:758–63. [DOI] [PubMed] [Google Scholar]
- 4.Freund MW, Kleinveld G, Krediet TG, van Loon AM, Verboon-Maciolek MA. Prognosis for neonates with enterovirus myocarditis. Arch Dis Child Fetal Neonatal Ed 2010; 95:F206–12. [DOI] [PubMed] [Google Scholar]
- 5.Tebruegge M, Curtis N. Enterovirus infections in neonates. Semin Fetal Neonatal Med 2009; 14:222–7. [DOI] [PubMed] [Google Scholar]
- 6.Abzug MJ, Keyserling HL, Lee ML, Levin MJ, Rotbart HA. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin Infect Dis 1995; 20:1201–6. [DOI] [PubMed] [Google Scholar]
- 7.Abzug MJ, Cloud G, Bradley J, et al. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr Infect Dis J 2003; 22:335–41. [DOI] [PubMed] [Google Scholar]
- 8.Desmond RA, Accortt NA, Talley L, Villano SA, Soong SJ, Whitley RJ. Enteroviral meningitis: natural history and outcome of pleconaril therapy. Antimicrob Agents Chemother 2006; 50:2409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden FG, Herrington DT, Coats TL, et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis 2003; 36:1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotbart HA. Antiviral therapy for enteroviral infections. Pediatr Infect Dis J 1999; 18:632–3. [DOI] [PubMed] [Google Scholar]
- 11.Rotbart HA, Webster AD. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis 2001; 32:228–35. [DOI] [PubMed] [Google Scholar]
- 12.Pevear DC, Hayden FG, Demenczuk TM, Barone LR, McKinlay MA, Collett MS. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob Agents Chemother 2005; 49:4492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawyer MH. Enterovirus infections: diagnosis and treatment. Pediatr Infect Dis J 1999; 18:1033–40. [DOI] [PubMed] [Google Scholar]
- 14.Aradottir E, Alonso EM, Shulman ST. Severe neonatal enteroviral hepatitis treated with pleconaril. Pediatr Infect Dis J 2001; 20:457–9. [DOI] [PubMed] [Google Scholar]
- 15.Kearns GL, Bradley JS, Jacobs RF, et al. Single dose pharmacokinetics of pleconaril in neonates. Pediatr Infect Dis J 2000; 19:833–9. [DOI] [PubMed] [Google Scholar]
- 16.Yang CF, De L, Yang SJ, et al. Genotype-specific in vitro amplification of sequences of the wild type 3 polioviruses from Mexico and Guatemala. Virus Res 1992; 24:277–96. [DOI] [PubMed] [Google Scholar]
- 17.Ma JD, Nafziger AN, Rhodes G, Liu S, Bertino JS. Duration of pleconaril effect on cytochrome P450 3A activity in healthy adults using the oral biomarker midazolam. Drug Metab Dispos 2006; 34:783–5. [DOI] [PubMed] [Google Scholar]
- 18.D'Argenio D, Schumitzky A, Wang X. ADAPT 5 User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software . Los Angeles: Biomedical Simulations Resource; 2009: 1–316. [Google Scholar]
- 19.Abzug MJ, Loeffelholz M, Rotbart HA. Diagnosis of neonatal enterovirus infection by polymerase chain reaction. J Pediatr 1995; 126:447–50. [DOI] [PubMed] [Google Scholar]
- 20.Kearns GL, Abdel-Rahman SM, James LP, et al. Single-dose pharmacokinetics of a pleconaril (VP63843) oral solution in children and adolescents. Antimicrob Agents Chemother 1999; 43:634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.