Abstract
Background
Congenital cytomegalovirus (CMV) is reported to affect up to 1% of all live births in the United States. T-cell immunity may be important for controlling CMV replication in congenital CMV-infected infants. We describe the natural history of CMV-specific T-cell evolution and CMV replication in infants with congenital CMV infection.
Methods
Cytomegalovirus viral load, CMV urine culture, and CMV-specific CD4 and CD8 T-cell responses were assessed in a prospective longitudinal cohort of 51 infants with congenital CMV infection who were observed from birth to 3 years of age.
Results
We found a kinetic pattern of decreasing urinary CMV replication and increasing CMV-specific CD4 and CD8 T-cell responses during the first 3 years of life. We also found higher CMV-specific CD8 T-cell responses were associated with subsequent reduction of urine CMV viral load.
Conclusion
For infants with congenital CMV infection, our data suggest an age-related maturation of both CMV-specific CD4 and CD8 T-cell immunity that is associated with an age-related decline in urinary CMV replication.
Keywords: CMV-specific T cells; congenital CMV, kinetics, PCR; T cells
Congenital cytomegalovirus (CMV) is reported to affect up to 1% of all live births in the United States. An estimated 40% to 58% of infected newborns with symptomatic presentations and 13.5% of infected newborns with asymptomatic presentations will suffer long-term sequelae, most prominently sensorineural-hearing loss (SNHL) [1]. Delayed presentation and worsening of sequelae can also occur [2, 3]. The pathogenesis of sequelae from congenital CMV infection is not well defined. The leading theory is that SNHL is due to persistent chronic viral infection [4–6], but the immunologic mechanisms responsible for controlling CMV replication after congenital infection are still not completely understood.
In immunocompromised hosts, T-cell responses to CMV seem to be important for controlling viral replication and limiting disease [7, 8]. Limited prior studies of congenital CMV infection suggest that T-cell immunity is also important for controlling CMV replication. Compared with responses to other antigens, CD4 T-cell responses to CMV were impaired in infants with congenital CMV infection [9, 10], with impairment sometimes lasting for years [11]. The majority of these children continued to shed CMV, and in 1 cross-sectional study, higher CMV-specific cell-mediated immunity in older children (≥37 months) was associated with a lower rate of CMV viruria [11]. However, in infants with congenital CMV infection, the longitudinal kinetics of T-cell immunity to CMV and its relationship to CMV replication have not been well characterized.
We describe the natural history of circulating CMV-specific T-cell immunity and CMV shedding in infants with congenital CMV infection. These congenitally infected infants were identified at birth and observed for the first 3 years of life.
METHODS
Patient Population and Study Design
Infants with congenital CMV infection, identified as part of a large newborn screening study conducted at 3 hospitals in the Northern California region, were enrolled into a prospective study to determine the T-cell and virologic responses to congenital CMV infection; 4 additional infants were referred to us with a congenital CMV infection diagnosis. The study was conducted with approval from each hospital's Institutional Review Board, and written informed consent was obtained from a parent. Of 59 total infants, 51 were confirmed to have congenital CMV infection with a positive urine shell vial culture within the first 3 weeks of life. Four infants were CMV positive by saliva testing, detecting CMV early antigen via a fluorescent foci method [12]. However, urine culture confirmations came late at days 27, 31, and 45 days and 16 months. Infants had scheduled visits at 4, 8, 12, 24, and 36 months of age. Urine collection was attempted at each visit. Blood collection was attempted at 4, 12, and 36 months of age. Missed collections of blood or urine samples were mainly due to missed visits; also, late enrolled infants were observed for less than 3 years because of the finite funding period. For each infant, the average number of urine samples obtained was 3.5 (range, 1–6) and average number of blood samples obtained was 1.8 (range, 0–3). Four symptomatic neonates who received antiviral treatment [13] were excluded because ganciclovir would affect the viral load and potentially the immune response to CMV. In total, we analyzed 178 urine and 93 blood samples from 51 patients, 5 of which were symptomatic. Viral load and CMV-specific CD4 and CD8 T-cell responses in the 4 excluded patients who received ganciclovir are shown in the Supplementary Material.
Flow Cytometric Assays for Cytomegalovirus-Specific T Cells
Aliquots of heparinized fresh blood were incubated with whole human CMV antigen (lysates of AD169-infected fibroblasts), mock antigen (uninfected fibroblast lysates), or staphylococcus enterotoxin B (positive control) at a 1:20 (vol/vol) ratio. We followed our previous approach for T-cell stimulation, peripheral blood mononuclear cell surface staining, and intracellular cytokine staining [14]. The assay protocol is provided in the Supplementary Material section. Cells were considered to have a positive response if they coexpressed CD69 and interferon-gamma (IFN-γ) [15, 16]. We defined a positive CMV-specific T-cell response as ≥0.1% [17]. Staphylococcus enterotoxin B responses served as the positive control, and frequencies were found to be higher than CMV-specific CD4 T-cell responses in 97% of the samples (90 of 93) and higher than CMV-specific CD8 T-cell responses in 98% of the samples (91 of 93).
Cytomegalovirus Urine Polymerase Chain Reaction Assay
Samples were stored at −80°C before extraction. DNA extraction was carried out per manufacturer's instructions (Epicentre Biotechnologies). Quantitative polymerase chain reaction (qPCR) was performed in triplicate using CMV IE-1 gene (forward primer: 5′-TCGTTGCAATCCTCGGTCA-3′ and reverse primer: 5′- GGCCGAAGAATCCCTCAAAA-3′) (Operon Biotechnologies) and ABI Prism 7900 Sequence Detection System and SYBR Green chemistry with AmpliTaq Gold DNA polymerase (Applied Biosystems, Carlsbad, CA). Thermocycling parameters were 10 minutes at 95°C for 1 cycle, 30 seconds at 95°C, 1 minute at 55–60°C, 1.5 minutes at 72°C for 40 cycles, and 3 minutes at 72°C for 1 cycle. Known amounts of an IE-1 plasmid were included in reactions to generate a standard curve. Negative controls included no template (distilled water) and urine DNA extracted from a CMV-seronegative adult donor. We used genomic equivalents/mL units for reporting viral load. This assay was run before the development of the World Health Organization International Standard for CMV [18].
Cytomegalovirus Urine Culture
Initial urine cultures were done using the standard shell vial culture method. After parallel testing with shell viral cultures, urine cultures were done with a modified approach. We adapted the shell vial culture to a 24-well plate with a monolayer of human fetal lung tissue (MRC-5; American Type Culture Collection). The method change decreased costs and increased efficiency. Assay protocol is provided in the Supplementary Material. Each well was examined with an inverted fluorescence microscope at 10 × power [Zeiss Axiovert 100]. One or more fluorescent nuclei were considered a positive culture result for CMV detection.
Statistical Methods
Our analysis approach was to build a model that optimized statistical power, and we accomplished this with a shared random effect regression model. The 2 CMV virus-specific outcomes, CMV qPCR value and CMV shell vial culture results, were fit simultaneously within 1 regression model by linking them within each patient using a shared random effect [19]. Because shared random effect models are a type of mixed model, fitted means represent an estimate of the response of the average child rather than the average response across children. We use the phrase, “average child,” when describing results from the mixed model.
The rationale for using the shared random effect regression model was 2-fold. First, it provided improved statistical power because standard errors on regression coefficient estimates for the binary outcome of shell vial culture were reduced through linkage to the continuous outcome of the qPCR value [19]. Second, it reduced bias because, although qPCR has finer resolution, false-negative and false-positive results can occur causing systematic (nonrandom) errors (ie, bias) [20].
The CMV-specific CD4 and CD8 T-cell outcomes were linked within a single shared random effect regression model. The rationale for use of this approach was based on the biologic link of CD4 T cells providing “help” to CD8 T cells and that it optimized statistical power. Before analysis, paired negative control values were subtracted from each value of %CD4 + /CD69 + /IFN-γ + and % CD8 + /CD69 + /IFN-γ +.
In a separate model, we examined the association between CMV-specific T cells and the subsequent CMV urine viral load longitudinally within patients. We modeled the logarithm of CMV viral load on multiple explanatory variables: the first viral load for the patient (baseline viral load), age in weeks, elapsed time between linked visits (details in Supplementary Material), and the CMV-specific CD4 and CD8 T-cell responses. These data were fit to the regression model using generalized least squares.
Analyses were performed in SAS, version 9.3 (SAS Institute, Cary, NC) and R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria). Results of hypothesis testing are statistically significant for levels of P ≤ .05. Code is available upon request. Additional details on the statistical methods are provided in the Supplementary Material.
RESULTS
Kinetics of Cytomegalovirus Urinary Viral Load
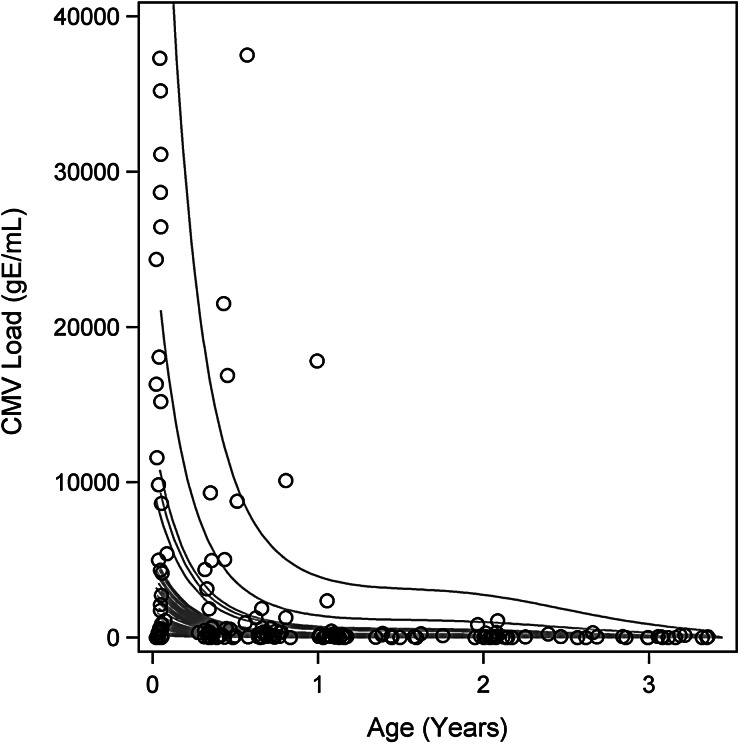
During the first 3 years of life, children with congenital CMV infection showed an overall decrease in urinary CMV shedding, as measured by CMV qPCR and urine culture. Figure 1 illustrates the fitted trajectories of urine CMV viral load for each child. Immediately after birth, neonates’ viral loads varied widely. The highest values were seen in symptomatic infants. Viral load decreased rapidly during infancy and then transitioned to a continued but slower decrease through early childhood.
Figure 1.
Cytomegalovirus (CMV) urine viral load of each individual child in the cohort for the first 3 years of life. Gray lines are fitted trajectories of CMV viral load for individual children from the fit of the shared random effect model and thus include adjustment for bias in quantitative polymerase chain reaction (qPCR) results by the shell vial culture gold standard. Open circles are observed values. The vertical axis is truncated just above the 97.5th sample percentile of the qPCR data to facilitate display of data. The 2 largest observed qPCR values were 405, 691 genomic equivalents/mL (gE/mL), and 1,797,655 gE/mL.
From our regression model, both the decrease in CMV viral load with age and the probability of CMV urinary shedding with age were statistically significant for the average child (P < .0001) (data not shown).
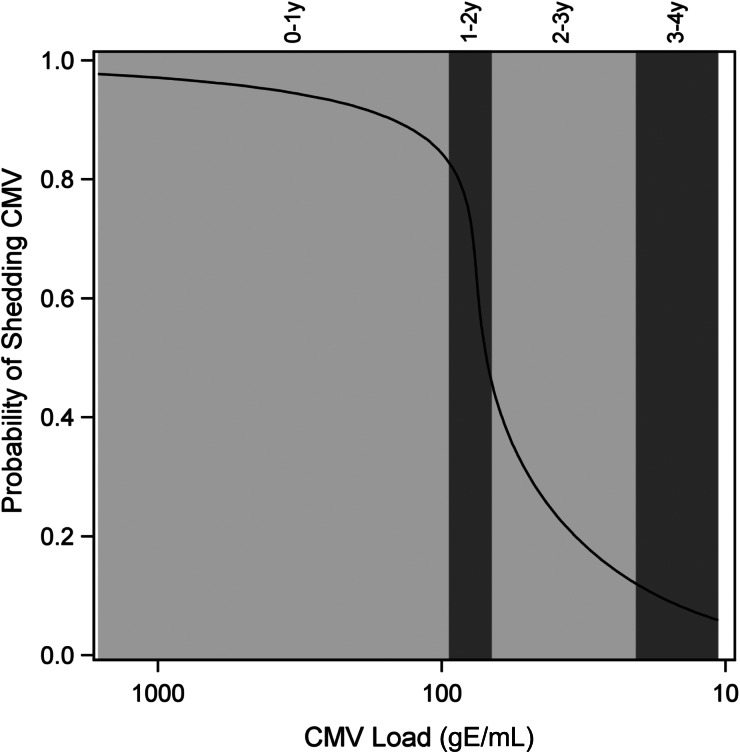
In Figure 2, the magnitude of the decrease in CMV viral load and in the probability of urinary CMV shedding was not proportional. In particular, a decline in the urine CMV viral load coincided with a modest decline in the probability of urinary CMV shedding between 0 and 1 years of age. In contrast, between 1 and 2 years of age, a small decline in urine viral load coincided with a large decline (approximately 85%–45%) in the probability of urinary CMV shedding.
Figure 2.
Probability of cytomegalovirus (CMV) urine shedding versus viral load by age range. Alternating light and dark gray panels denote ages from left to right of infancy, 1–2 years, 2–3 years, and 3–4 years, respectively. The width of each gray panel reflects the range of CMV viral load on a log scale (eg, the range of CMV viral load ranges narrowly approximately 102 for the 1–2 year age range). Solid black line is the developmental trajectory of CMV replication in the urine of the average child.
Kinetics of Cytomegalovirus-Specific CD4 and CD8 T Cells
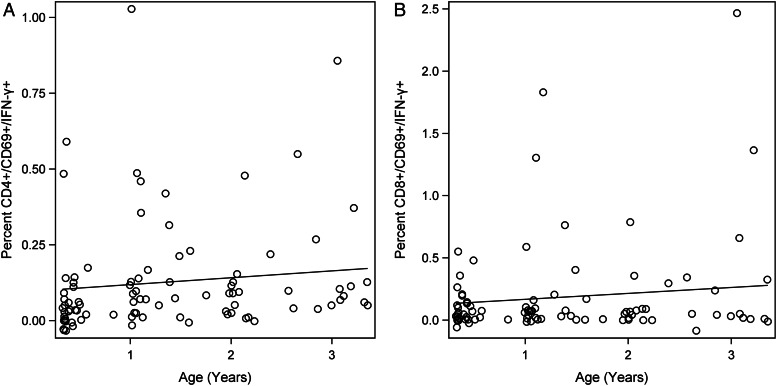
For the first 3 years of life, children with congenital CMV infection showed a gradual and approximately linear increase in their CMV-specific T-cell responses (Figure 3A and B). This increase was statistically significant for both CMV-specific CD4 and CMV-specific CD8 T cells (P < .0001). The slope with age was not statistically different between CMV-specific CD4 and CD8 T-cell responses (P > .05). Mean CMV-specific CD4 and CD8 T-cell frequencies per age group from the fitted regression model are shown in Table 1. The average child's CMV-specific CD8 T-cell response was estimated to be higher than his/her CMV-specific CD4 T-cell response, for each age group, but the overall difference in means across all ages was not statistically significant by a paired t test (P = .1098).
Figure 3.
Kinetics of cytomegalovirus (CMV)-specific CD4 (A) and CD8 (B) T-cell responses for the first 3 years of life. Black line represents trajectory for the average child. Open circles represent observed values. Estimated coefficient of multiple determination (R2) is 0.085 and age trend P < .0001 for both CMV-specific CD4 and CD8 T-cell responses.
Table 1.
Regression Estimates of Mean CMV-Specific CD4 and CD8 T-Cell Responses for the Average Child per Age Rangea
| Age (years) | Mean CMV-Specific CD4 (%) [95% CI] | Mean CMV-Specific CD8 (%) [95% CI] |
|---|---|---|
| Birthb | 0.1 [0.8, 0.11] | 0.12 [0.8, 0.15] |
| 1 | 0.12 [0.11, 0.12] | 0.17 [0.16, 0.17] |
| 2 | 0.14 [0.13, 0.15] | 0.21 [0.2, 0.23] |
| 3 | 0.16 [0.15, 0.18] | 0.26 [0.22, 0.3] |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus.
aTotal sample size is 91 blood samples from 51 patients.
bEstimates at birth are extrapolations from the model because the earliest blood samples were obtained at 17 weeks of age.
From our model, the extrapolated CMV-specific CD4 and CD8 T-cell responses were positive (≥0.1%) at birth [17] for the average child (CMV-specific CD4 0.10% (95% CI: 0.08, 0.11); CMV-specific CD8 0.12% (95% CI: 0.08, 0.15).
Temporal Association of Cytomegalovirus-Specific T-Cell Immunity and Cytomegalovirus Viral Load
Through early childhood, the association between CMV-specific T cells and CMV viral load revealed a statistically significant relationship. Higher levels of CMV-specific CD8 response in blood samples were associated with a reduction of CMV viral load in a subsequent urine sample (obtained at the next visit) (P = .02). The association between CMV-specific CD4 T-cell response and subsequent urine viral load was not statistically significant. The temporal relationship between prior CMV-specific CD8 T cells and subsequent reduction in CMV viral load suggests that CMV-specific CD8 T cells may be contributing to the control of CMV replication.
The temporal relationship between prior CMV-specific CD8 T cells and subsequent reduction in CMV viral load is strengthened by the finding of another expected temporal relationship in our regression model. Higher baseline CMV viral loads were associated with higher subsequent CMV viral loads (P = .0045). Results of the regression model are available in the Supplementary Material.
DISCUSSION
We simultaneously characterized the natural evolution of CMV-specific T-cell immunity and CMV shedding from birth to 3 years of age in infants identified at birth with congenital CMV. A temporal pattern of increasing CMV-specific CD4 and CD8 T-cell responses was associated with decreasing urine CMV viral load and urinary CMV shedding during the first 3 years of life. In addition, higher levels of CMV-specific CD8 T cells, but not CMV-specific CD4 T cells, were associated with decreasing CMV viral load in a subsequent urine sample (obtained at the next visit). The temporal relationship suggests the possibility that CMV-specific CD8 T cells contribute to the control of CMV replication.
Furthermore, from our model, the extrapolated positive CMV-specific T-cell response at birth suggests that T cells were already responding to CMV in utero and contributing to the steep decline in urine CMV viral load that we observed after birth. In addition to the well described prolonged urinary CMV shedding [21], we also found a new 2-phase pattern to the kinetics of CMV replication in the urine. A drop in viral load occurred first followed by cessation of CMV shedding.
To our knowledge, characterization of the association between CMV-specific CD4 and CD8 T-cell immunity and CMV shedding in a prospective longitudinal cohort of infants with congenital CMV infection has not been published previously. Evaluation of CMV-specific T-cell responses in the published literature have predominantly used a cross-sectional study design [9–11] with only 3 studies using a longitudinal design [22–24]. Of these 3 longitudinal studies, only 1 simultaneously assessed CMV-specific immunity and CMV replication [24]. However, this study was limited to 11 patients who were observed for 1 year. This study was published 30 years ago using the lymphocyte proliferation assay, limiting the evaluation to CMV-specific CD4 T-cell with very little information on the CMV-specific CD8 T-cell response.
Our results are supported by reports in the literature. In studies of congenital CMV infection, CMV-specific CD4 T-cell responses appeared to increase with time in 7 infants with more than 1 time point [23], and CMV-specific CD8 responses specific for pp65 and IE-1 epitopes broadened with time in a study of 7 infants <6 months of age [25]. We show a similar pattern of increasing CMV-specific CD4 and CD8 T-cell responses. Cytomegalovirus-specific CD8 T-cell responses have also been detected in 2 newborns [26] and in cord blood from a 28-week-old fetus [27] with congenital CMV infection. These findings suggest that our extrapolations of a positive CMV-specific CD4 and CD8 T-cell response at birth are plausible.
Although no study has definitively proven that CMV-specific T cells directly control CMV replication in children with congenital CMV infection, evidence of this causal relationship is suggested in other populations with CMV infection. Immaturity of the CMV-specific CD4 T-cell response is associated with prolonged CMV replication in young children and transplant patients with CMV infection [28–31]. It is possible that CMV-specific CD4 T-cell activity is necessary for controlling local active CMV replication, whereas CMV-specific CD8 T-cell activity is necessary for controlling CMV dissemination [14].
Our analysis includes some limitations. First, compared with the more frequently collected urine samples, the number of blood samples for each child was limited by design to encourage enrollment. In light of the limited blood samples, we cautiously interpret our results from modeling the association between CMV-specific T-cell responses and viral replication. Our analysis does not address causation. Second, only 5 of 51 (9.8%) of the children in our cohort were symptomatic at birth, so our results essentially reflect on infants with asymptomatic congenital CMV infection. We did not pursue a comparison of symptomatic versus asymptomatic infants due to a very high risk of Type II statistical error. Third, the small blood volume obtained from these young patients limited our choice of immune markers to the most essential in our interrogation of the frequencies and function of CMV-specific CD4 and CD8 T cells.
Our characterization of the natural evolution of both CMV-specific T-cell immunity and CMV replication may be informative for the rational design of future treatment and prevention strategies to reduce the sequelae of congenital CMV [32, 33]. Expansion of T-cell studies in larger cohorts of congenitally infected infants might identify predictive markers for the future risk of sequelae. A similar concept is being pursued in the transplant population [34, 35].
CONCLUSIONS
In summary, for infants with congenital CMV infection, our data suggest an age-related maturation of both CMV-specific CD4 and CD8 T-cell immunity, which is associated with an age-related decline in urinary CMV replication. Our data also suggest the possibility that CMV-specific CD8 T cells contribute to the control of CMV replication in infants with congenital CMV infection. Future studies should continue to refine the characterization of the infant's developing immune response to CMV infection to identify the underlying viral pathogenesis of congenital CMV infection and to determine the immune mechanisms that control CMV replication. In cases in which congenital CMV has been suggested prenatally, future collection of cord blood samples could be very important in validating our estimates of CMV-specific T-cell responses at birth in congenitally infected infants.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We thank our study participants and their parents for enrolling into the study. We also acknowledge efforts by coinvestigators, Drs. Barbara Pahud and Candice Smith; Research Nurse Eileen Cordoba Tongson; Clinical Research Assistants Raquel Fleischmann, Aide Castro, Alicia Gutiérrez, and Alissa Chow; and phlebotomists Irene Prudente and Michele Ugur. We also thank Dr. Edward Mocarski for input on the CMV qPCR assay.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (R01 AI053589) and supported in part by the National Center for Research Resources, National Institutes of Health (M01 RR-00070).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17:355–63. [DOI] [PubMed] [Google Scholar]
- 2.Fowler KB, McCollister FP, Dahle AJ et al. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr 1997; 130:624–30. [DOI] [PubMed] [Google Scholar]
- 3.Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics 1992; 90:862–6. [PubMed] [Google Scholar]
- 4.Boppana SB, Fowler KB, Pass RF et al. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J Pediatr 2005; 146:817–23. [DOI] [PubMed] [Google Scholar]
- 5.Davis LE, Stewart JA, Rarey KE, McLaren LC. Recovery and probable persistence of cytomegalovirus in human inner-ear fluid without cochlear damage. Ann Otol Rhinol Laryngol 1987; 96:380–3. [DOI] [PubMed] [Google Scholar]
- 6.Teissier N, Delezoide AL, Mas AE et al. Inner ear lesions in congenital cytomegalovirus infection of human fetuses. Acta Neuropathol 2011; 122:763–74. [DOI] [PubMed] [Google Scholar]
- 7.Walter EA, Greenberg PD, Gilbert MJ et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med 1995; 333:1038–44. [DOI] [PubMed] [Google Scholar]
- 8.Lacey SF, Diamond DJ, Zaia JA. Assessment of cellular immunity to human cytomegalovirus in recipients of allogeneic stem cell transplants. Biol Blood Marrow Transplant 2004; 10:433–47. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds DW, Dean PH, Pass RF, Alford CA. Specific cell-mediated immunity in children with congenital and neonatal cytomegalovirus infection and their mothers. J Infect Dis 1979; 140:493–9. [DOI] [PubMed] [Google Scholar]
- 10.Starr SE, Tolpin MD, Friedman HM et al. Impaired cellular immunity to cytomegalovirus in congenitally infected children and their mothers. J Infect Dis 1979; 140:500–5. [DOI] [PubMed] [Google Scholar]
- 11.Pass RF, Stagno S, Britt WJ, Alford CA. Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. J Infect Dis 1983; 148:953–61. [DOI] [PubMed] [Google Scholar]
- 12.Boppana SB, Smith RJ, Stagno S, Britt WJ. Evaluation of a microtiter plate fluorescent-antibody assay for rapid detection of human cytomegalovirus infection. J Clin Microbiol 1992; 30:721–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimberlin DW, Lin CY, Sanchez PJ et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003; 143:16–25. [DOI] [PubMed] [Google Scholar]
- 14.Chen SF, Tu WW, Sharp MA et al. Antiviral CD8 T cells in the control of primary human cytomegalovirus infection in early childhood. J Infect Dis 2004; 189:1619–27. [DOI] [PubMed] [Google Scholar]
- 15.Waldrop SL, Davis KA, Maino VC, Picker LJ. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J Immunol 1998; 161:5284–95. [PubMed] [Google Scholar]
- 16.Waldrop SL, Pitcher CJ, Peterson DM et al. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest 1997; 99:1739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn HS, Haney DJ, Ghanekar SA et al. Dynamics of CD4 and CD8 T cell responses to cytomegalovirus in healthy human donors. J Infect Dis 2002; 186:15–22. [DOI] [PubMed] [Google Scholar]
- 18.Kraft CS, Armstrong WS, Caliendo AM. Interpreting quantitative cytomegalovirus DNA testing: understanding the laboratory perspective. Clin Infect Dis 2012; 54:1793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCulloch C. Joint modelling of mixed outcome types using latent variables. Stat Methods Med Res 2008; 17:53–73. [DOI] [PubMed] [Google Scholar]
- 20.van Pelt-Verkuil E, van Belkum A, Hays JP. Principles and Technical Aspects of PCR Amplification. Netherlands: Springer; 2008. [Google Scholar]
- 21.Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011; 21:240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okabe M, Chiba S, Tamura T et al. Longitudinal studies of cytomegalovirus-specific cell-mediated immunity in congenitally infected infants. Infect Immun 1983; 41:128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lidehall AK, Engman ML, Sund F et al. Cytomegalovirus-specific CD4 and CD8 T cell responses in infants and children. Scand J Immunol 2013; 77:135–43. [DOI] [PubMed] [Google Scholar]
- 24.Gehrz RC, Linner KM, Christianson WR et al. Cytomegalovirus infection in infancy: virological and immunological studies. Clin Exp Immunol 1982; 47:27–33. [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson L, Dooley S, Trzmielina S et al. Cytomegalovirus (CMV) IE1- and pp65-specific CD8+ T cell responses broaden over time after primary CMV infection in infants. J Infect Dis 2007; 195:1789–98. [DOI] [PubMed] [Google Scholar]
- 26.Gibson L, Piccinini G, Lilleri D et al. Human cytomegalovirus proteins pp65 and immediate early protein 1 are common targets for CD8+ T cell responses in children with congenital or postnatal human cytomegalovirus infection. J Immunol 2004; 172:2256–64. [DOI] [PubMed] [Google Scholar]
- 27.Marchant A, Appay V, Van Der Sande M et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest 2003; 111:1747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamadia LE, Remmerswaal EB, Weel JF et al. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 2003; 101:2686–92. [DOI] [PubMed] [Google Scholar]
- 29.Hammoud B, Schmueck M, Fischer AM et al. HCMV-specific T-cell therapy: do not forget supply of help. J Immunother 2013; 36:93–101. [DOI] [PubMed] [Google Scholar]
- 30.Schmueck M, Fischer AM, Hammoud B et al. Preferential expansion of human virus-specific multifunctional central memory T cells by partial targeting of the IL-2 receptor signaling pathway: the key role of CD4+ T cells. J Immunol 2012; 188:5189–98. [DOI] [PubMed] [Google Scholar]
- 31.Tu W, Potena L, Stepick-Biek P et al. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation 2006; 114:1608–15. [DOI] [PubMed] [Google Scholar]
- 32.Krause PR, Bialek SR, Boppana SB et al. Priorities for CMV vaccine development. Vaccine 2013; 32:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleiss MR. Could therapeutic vaccination of cytomegalovirus-seropositive persons prevent reinfection and congenital virus transmission? J Infect Dis 2011; 203:1513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avetisyan G, Aschan J, Hagglund H et al. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant 2007; 40:865–9. [DOI] [PubMed] [Google Scholar]
- 35.Gerna G, Lilleri D, Fornara C et al. Monitoring of human cytomegalovirus-specific CD4 and CD8 T-cell immunity in patients receiving solid organ transplantation. Am J Transplant 2006; 6:2356–64. [DOI] [PubMed] [Google Scholar]
- 36.Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed New York: Springer; 2009. [Google Scholar]
- 37.Schlattmann P. Medical Applications of Finite Mixture Models. Berlin: Springer-Verlag, 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.