Abstract
Background
Rhinovirus is the most common cause of viral respiratory tract infections in children. Virologic predictors of lower respiratory tract infection (LRTI), such as viral load and the presence of another respiratory virus (coinfection), are not well characterized in pediatric outpatients.
Methods
Mid-nasal turbinate samples were collected from children presenting for care to the Seattle Children's Hospital emergency department (ED) or urgent care with a symptomatic respiratory infection between December 2011 and May 2013. A subset of samples was tested for rhinovirus viral load by real-time polymerase chain reaction. Clinical data were collected by chart reviews. Multivariate logistic regression was used to evaluate the relationship between viral load and coinfection and the risk for LRTI.
Results
Rhinovirus was the most frequent respiratory virus detected in children younger than 3 years. Of 445 patients with rhinovirus infection, 262 (58.9%) had LRTIs, 231 (51.9%) required hospital admission and 52 (22.5%) were hospitalized for 3 days or longer. Children with no comorbidities accounted for 142 (54%) of 262 rhinovirus LRTIs. Higher viral load was significantly associated with LRTI among illness episodes with rhinovirus alone (OR, 2.11; 95% confidence interval [CI], 1.24–3.58). Coinfection increased the risk of LRTI (OR, 1.83; 95% CI, 1.01–3.32).
Conclusions
Rhinovirus was the most common pathogen detected among symptomatic young children in a pediatric ED who had respiratory viral testing performed, with the majority requiring hospitalization. Higher rhinovirus viral load and coinfection increased disease severity. Virologic data may assist clinical decision making for children with rhinovirus infections in the pediatric ED.
Keywords: coinfection, disease severity, emergency department, rhinovirus, viral load
Human rhinovirus (HRV) is the most common etiology of viral upper respiratory tract infections (URTIs) in children worldwide and causes primarily mild self-limited infections with rhinorrhea, cough, sore throat, and nasal congestion [1, 2]. HRV also causes lower respiratory tract infections (LRTIs), particularly in young infants, the elderly, and patients with immunocompromising conditions such as asthma, malignancy, cystic fibrosis, or chronic obstructive pulmonary disease [3, 4]. Among outpatients presenting for evaluation in the clinic setting, HRV is associated with a burden of disease similar to that of influenza [5]. However, frequent detection of HRV among asymptomatic children or in the presence of respiratory viral coinfections, such as respiratory syncytial virus (RSV), makes it difficult to define its true etiologic role [4, 6, 7].
Recent improvements in molecular techniques, including real-time quantitative polymerase chain reaction (qPCR) assays, have permitted increased identification of HRV as the cause of respiratory tract infections [8]. An understanding of how to interpret virologic data is increasingly relevant to outpatient clinical care, because rapid multiplex PCR assays are used for the point-of-care diagnosis of respiratory viruses in emergency department (ED) and urgent care (UC) settings [9, 10]. Rapid tests can provide results, including a semiquantitative measure of viral load (VL), in less than 1 hour [11]. Rapid diagnosis of influenza in a pediatric ED has been shown to decrease antiviral use, diagnostic test ordering, antibiotic use, and hospital length of stay [12]. Rapid testing for RSV in a pediatric ED increased cohorting and decreased hospital overcrowding during respiratory viral infection season [10]. To our knowledge, no previous studies have described the effects of rapid HRV testing in the ED setting on hospital admissions, lengths of stay, and use of antibiotics in children.
HRV infections have typically been evaluated in hospitalized children among whom HRV disease severity was similar to that of RSV or influenza [13]. Studies of respiratory viral coinfections often do not evaluate HRV specifically, which makes it difficult to interpret the impact of coinfection on the severity of HRV illness episodes. We hypothesize that among children presenting to an acute care setting, higher HRV VL and coinfection with other respiratory viruses are associated with more severe disease.
PATIENTS AND METHODS
Mid-nasal turbinate swabs were collected from symptomatic patients during their initial presentation to Seattle Children's Hospital ED or UC at the discretion of the clinician. Children were tested for respiratory viruses in the ED/UC if the treating physicians felt that they were ill enough to search for an etiology for their symptoms and that the result might affect their medical care (ie, a positive test may prevent additional testing for bacterial causes, and a negative test may lead to additional testing). Samples were tested using the FilmArray respiratory virus panel for 15 respiratory viruses, including RSV A/B, influenza A/B, parainfluenza viruses (PIVs) 1 to 4, coronaviruses (CoVs) HKU1, 229E, OC43, and NL63, adenovirus (AdV), human metapneumovirus (hMPV), and HRV/enteroviruses (EntVs), in an automated system that provides qualitative results and does not differentiate between HRV and EntV (Idaho Technology, Salt Lake City, UT) [9, 14]. Results were available to the clinician within 1.6 hours [9].
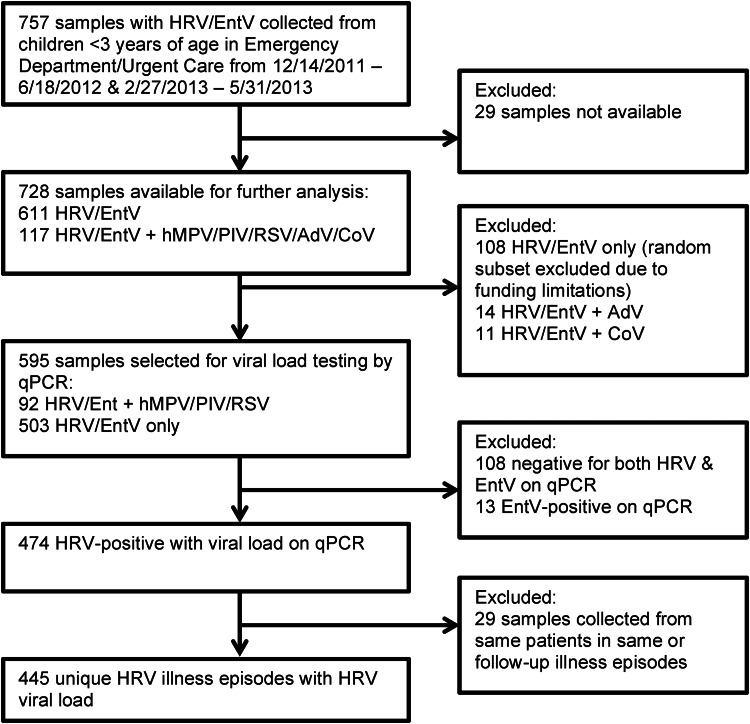
Residual samples from patients younger than 3 years with HRV/EntV detected were subsequently tested for HRV by qPCR assay to differentiate HRV from EntV infections and to obtain a cycle threshold (CT) value, which is a semiquantitative estimate of the HRV VL (Figure 1) [15]. Samples selected for qPCR testing were a random subset with HRV/EntV only and all samples with HRV/EntV in combination with RSV, hMPV, or PIV, which we defined as viral coinfection. Samples with HRV/EntV in combination with AdV/CoV only were not included. Samples with HRV/EntV in combination with influenza A/B were also not included, because they were not detected in any of our subjects with HRV/EntV. We stratified VL as high or low, defined as a CT value of ≤30 (high) or >30 (low). An illness episode was defined as a respiratory illness in the same patient within a 2-week period. Only a patient's first illness episode was included in the analysis. The VL of the first sample collected during an illness episode was used.
Figure 1.
Flow diagram showing sample selection for VL testing and chart review. Of a total of 757 samples collected in patients younger than 3 years during the time period of the study, analysis was performed using clinical and virologic data from 445 HRV illness episodes.
Chart review was performed to collect sociodemographic, clinical, radiologic, and laboratory data using Project Redcap [16]. Laboratory results were included if they were obtained on the day of initial diagnosis, and chest radiology results within 48 hours of diagnosis. Abnormal chest imaging results were defined as a finding of consolidation or infiltrate consistent with pneumonia. LRTI was defined as the presence of abnormal chest imaging results, auscultation of crackles or wheezing, hypoxia (oxygen saturation, <92%), physician diagnosis of severe respiratory distress, respiratory failure, or bronchiolitis, or the need for hospitalization as a result of respiratory illness. URTI was defined as the presence of rhinorrhea, sore throat, nasal congestion, or secondary bacterial infection without any of the criteria listed above. Comorbidity was defined as the presence of malignancy, solid organ transplant, primary immunodeficiency, and/or underlying pulmonary, cardiac, hematologic, genetic/metabolic, neurologic, or gastrointestinal conditions.
Continuous and categorical variables were compared using t tests and χ2 tests, respectively. Unadjusted and adjusted logistic regression models were used to estimate the association between high VL and LRTI, coinfection and LRTI, and coinfection and high VL. Covariates used in the adjusted model included age (<12 months vs ≥12 months), gender, race, preexisting comorbidities (yes vs no), and premature birth (birth at <37 weeks gestation). The association between high VL and LRTI was estimated for HRV-only illness episodes and all illness episodes. The unadjusted and adjusted associations between LRTI and a 5-unit CT decrease were also estimated as a sensitivity analysis for HRV-only illness episodes and all illness episodes. With exploratory analyses, we repeated the primary analyses for children <12 months versus ≥12 months old and for children with or without comorbidities. In other exploratory models, we estimated the associations between hospitalization and higher VL and coinfection with other respiratory viruses and between LRTI and specific coinfections (RSV/HRV vs HRV alone, hMPV/PIV and HRV vs HRV alone). Adjusted linear regression was used to investigate the association between length of hospital stay (in days) and coinfection and higher VL, as well as between length of hospital stay and specific coinfections (RSV/HRV vs HRV alone, hMPV/PIV and HRV vs HRV alone). All statistical tests were 2-sided with an alpha value of .05. Analyses were performed using Stata 12.0 (Stata Corp, College Station, TX). Institutional review board approval was obtained from Seattle Children's Hospital.
RESULTS
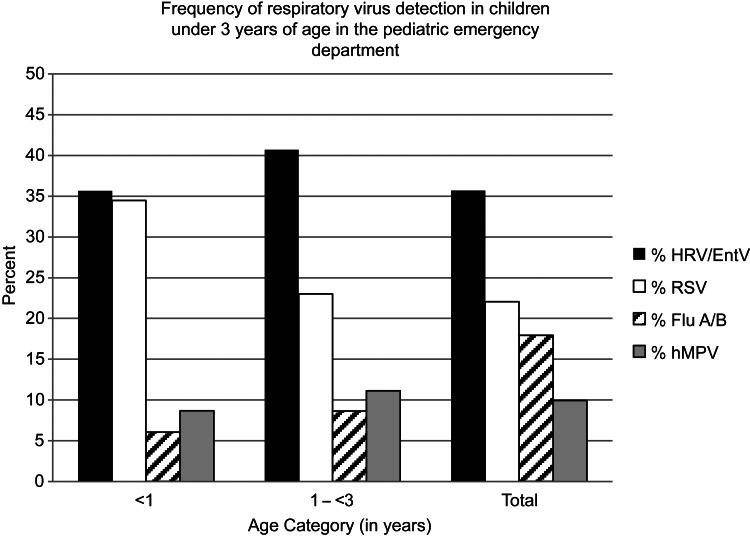
HRV/EntV was the most frequently detected respiratory virus in nasal samples collected from children younger than 3 years from December 14, 2011, to June 18, 2012, and February 27, 2013, to May 31, 2013, accounting for 366 (35.6%) of 1026 respiratory viruses in children younger than 1 year and 391 (40.6%) of 961 respiratory viruses in children aged 1 to 3 years (Figure 2). Samples collected between June 19, 2012, and February 26, 2013, were not available for analysis.
Figure 2.
Frequencies of HRV/EntV, RSV, influenza A/B (Flu A/B), and hMPV detection in samples collected from children younger than 3 years presenting to the Seattle Children's Hospital ED or UC between December 14, 2011 to June 18, 2012, and February 27, 2013 to May 31, 2013.
Overall, 445 unique patients had a symptomatic HRV illness episode, of which 262 (58.9%) were LRTIs (Table 1). Children with no comorbidities (n = 142 [54.1%]) accounted for the majority of HRV LRTIs. A total of 126 (48%) children with HRV LRTIs were older than 12 months. Children with underlying pulmonary conditions had a higher frequency of LRTI (n = 78 [29.8%]) than URTI (n = 15 [8.2%]). Most patients with HRV illness episodes (n = 410 [92.1%]) were seen initially in the ED, and 231 (51.9%) were admitted to the hospital; of these children, 52 (22.5%) were hospitalized for 3 days or longer.
Table 1.
Clinical and Sociodemographic Characteristics of Children with HRV URTI and LRTI
| Characteristic | URTIs (N = 183) |
LRTIs (N = 262) |
Total (N = 445) |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Gender, male | 102 | 56 | 172 | 66 | 274 | 62 |
| Age | ||||||
| <1 mo | 8 | 4 | 17 | 7 | 25 | 6 |
| 1 to 6 mo | 64 | 35 | 71 | 27 | 135 | 30 |
| 7 to 12 mo | 30 | 16 | 48 | 18 | 78 | 18 |
| 13 to <36 mo | 81 | 44 | 126 | 48 | 207 | 47 |
| Race | ||||||
| White | 78 | 43 | 125 | 48 | 203 | 46 |
| Black | 17 | 9 | 21 | 8 | 38 | 9 |
| Asian | 11 | 6 | 17 | 7 | 28 | 6 |
| Other* | 77 | 43 | 99 | 37 | 176 | 40 |
| Ethnicity | ||||||
| Not Hispanic | 126 | 69 | 180 | 69 | 306 | 69 |
| Hispanic | 46 | 25 | 62 | 24 | 108 | 24 |
| No information | 11 | 6 | 20 | 8 | 31 | 7 |
| Premature (<37 wk gestation) | 26 | 14 | 45 | 17 | 71 | 16 |
| Underlying disease | ||||||
| None | 136 | 74 | 142 | 54 | 278 | 63 |
| Malignancy | 2 | 1 | 5 | 2 | 7 | 2 |
| Solid organ transplant | 2 | 1 | 2 | 1 | 4 | 1 |
| Primary immunodeficiency | 2 | 1 | 1 | 0 | 3 | 1 |
| Underlying pulmonary disease | 15 | 8 | 78 | 30 | 93 | 21 |
| Underlying cardiac disease | 8 | 4 | 22 | 8 | 30 | 7 |
| Hematologic | 2 | 1 | 2 | 1 | 4 | 1 |
| Genetic/metabolic | 2 | 1 | 5 | 2 | 7 | 2 |
| Neurologic | 12 | 7 | 14 | 5 | 26 | 6 |
| Gastrointestinal | 3 | 2 | 3 | 1 | 6 | 1 |
| Other | 28 | 15 | 58 | 22 | 86 | 19 |
*Other includes Native American/Pacific Islander (n = 10 [2%]), American Indian/Alaska native (n = 4 [1%]), more than 1 race (n = 25 [6%]), other race (n = 105 [24%]), and no information (n = 32 [7%]).
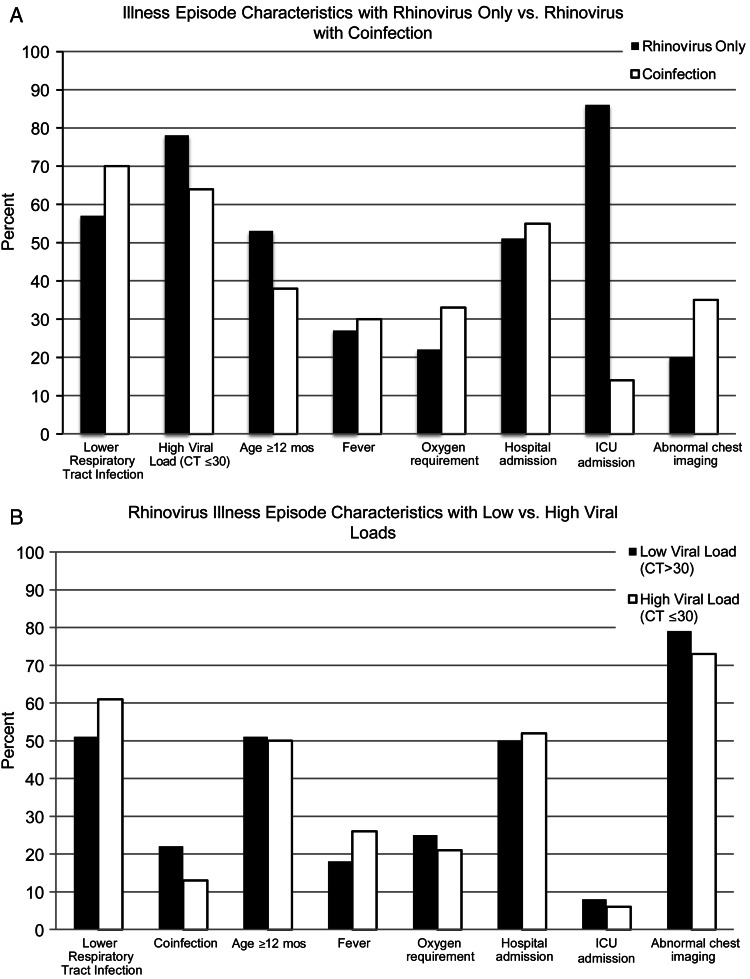
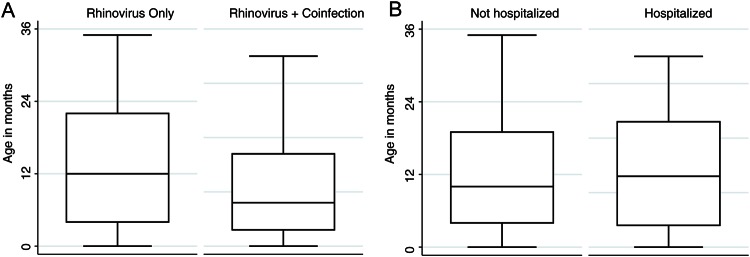
The characteristics of HRV illness episodes with or without coinfection and illness episodes with low or high HRV VLs are shown in Figures 3A and B, respectively. The most common coinfections were RSV in 42 (64.6%) and hMPV in 19 (28.7%) illness episodes. Influenza A and B were not detected in any HRV illness episodes. The mean age of the children with HRV-only illness episodes was 14 months and of the children with illness episodes with coinfection was 11 months (P = 0.027; Figure 4A). The mean HRV PCR CT value was 27 (range, 13–40).
Figure 3.
Percentages of patients with clinical and virologic characteristics in HRV only versus coinfection illness episodes (A) and low versus high VL illness episodes (B).
Figure 4.
Box plots showing the ages of children with HRV illness episodes with or without respiratory viral coinfection (A) (P = 0.027) and of children who were or were not hospitalized (B) (P = 0.002).
Hospital Course
The mean ages of the children who were and were not hospitalized were 15 and 12 months, respectively (P = 0.002; Figure 4B). The administration of supplemental oxygen (n = 50 [11.2%]), antibiotics (n = 82 [18.4%]), corticosteroids (n = 74 [16.6%]), albuterol (n = 95 [21.3%]), and/or intravenous fluids (n = 139 [33.4%]) was frequent (Table 2). The mean white blood cell count was 12.1 × 103/μL (range, 0.2–34.0 x 103/μL). No relationship was found between antibiotic use and white blood cell count (P = 0.94) or abnormal chest x-ray results (P = 0.89). Fever was associated with antibiotic use (56% in patients with fever vs 31% in patients without fever; P = 0.002). The median length of hospital stay was 1 day (range, 0–163 days). Of 14 (3.1%) patients admitted to the intensive care unit (ICU), the median age was 18 months (range, 0–35 months). Among the children admitted to the ICU, 2 (14.3%) had other respiratory viruses, including RSV (n = 1) and hMPV (n = 1). Four (0.8%) patients in our study died, but none was attributed to HRV-associated respiratory failure. The patients who died were aged 5, 11, 14, and 16 months and had underlying conditions including malignancy (n = 1), primary immunodeficiency (n = 1), underlying respiratory disease (n = 2), underlying cardiac disease (n = 1), and neurologic disease (n = 1); 2 were hospitalized, 1 of whom was admitted to the ICU.
Table 2.
Clinical and Virologic Characteristics of HRV URTI and LRTI Episodes
| Characteristic | URTI (N = 183) |
LRTI (N = 262) |
Total (N = 445) |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Location of initial care | ||||||
| ED | 158 | 86 | 252 | 96 | 410 | 92 |
| UC | 25 | 14 | 10 | 4 | 35 | 8 |
| Fever (temperature > 38.0°C) | 59 | 32 | 47 | 18 | 106 | 24 |
| Admitted to hospital | 31 | 17 | 200 | 76 | 231 | 52 |
| Hospitalized for ≥3 days | 6 | 3 | 46 | 18 | 52 | 12 |
| Coinfection present | 20 | 11 | 46 | 18 | 66 | 15 |
| Viruses in sample | ||||||
| HRV only | 163 | 89 | 216 | 82 | 379 | 85 |
| HRV and RSV | 9 | 5 | 30 | 12 | 39 | 9 |
| HRV and parainfluenza | 4 | 2 | 2 | 1 | 6 | 1 |
| HRV and hMPV | 6 | 3 | 11 | 4 | 17 | 4 |
| HRV and multiple viruses* | 1 | 1 | 3 | 0 | 4 | 0 |
| Supplemental oxygen administered | 1 | 1 | 49 | 19 | 50 | 11 |
| Antibiotics administered | 13 | 7 | 69 | 26 | 82 | 18 |
| Steroids administered | 2 | 1 | 72 | 28 | 74 | 17 |
| Albuterol during hospitalization | 0 | 0 | 95 | 36 | 95 | 21 |
| Intravenous fluids | 24 | 13 | 115 | 44 | 139 | 31 |
| Admitted to the intensive care unit | 2 | 1 | 12 | 5 | 14 | 3 |
| Intensive care unit stay for ≥3 days | 0 | 0 | 8 | 3 | 8 | 2 |
| Patient died | 2 | 1 | 2 | 1 | 4 | 1 |
*Includes HRV, RSV, and hMPV (n = 1 [0%]), HRV, RSV, and CoV (n = 2 [0%]), and HRV, hMPV, and CoV (n = 1 [0%]).
Multivariate Analysis
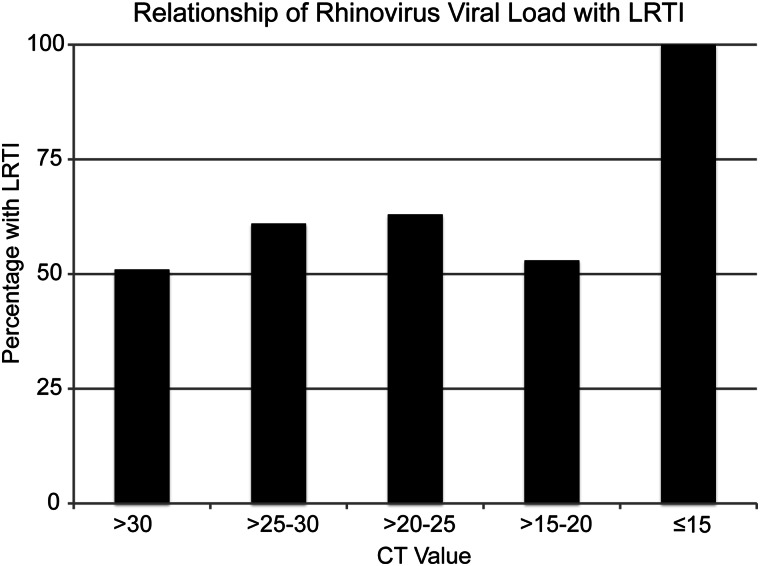
Higher HRV VLs (CT ≤30) were associated with LRTI among HRV-only illness episodes on multivariate analysis (OR, 2.11; 95% confidence interval [CI], 1.24–3.58; Table 3). The relationship between the HRV VL and LRTI when including all HRV illness episodes (OR, 1.57; 95% CI, 0.99–2.51) did not reach statistical significance. A sensitivity analysis revealed an increased risk of LRTI with each 5-unit decrease in CT value for HRV-only illness episodes (OR, 1.28; 95% CI, 1.01–1.62; Figure 5). Coinfected patients were less likely to have a high HRV VL (OR, 0.38; 95% CI, 0.21–0.69).
Table 3.
Univariate and Multivariate Logistic Regression Analysis Evaluating the Relationship of VL and Coinfection to LRTI
| Relationship of VL and Coinfection to LRTI | OR (95% CI) |
|---|---|
| Association of LRTI with higher VL | |
| All HRV illness episodes | |
| Univariate analysis | 1.50 (0.97–2.32) |
| Multivariate analysis* | 1.57 (0.99–2.51) |
| HRV-only illness episodes | |
| Univariate analysis | 1.90 (1.17–3.09) |
| Multivariate analysis* | 2.11 (1.24–3.58) |
| Sensitivity analysis (effect of decreasing CT value by 5 units) | |
| All HRV illness episodes | |
| Univariate analysis | 1.14 (0.94–1.39) |
| Multivariate analysis* | 1.15 (0.94–1.42) |
| HRV-only illness episodes | |
| Univariate analysis | 1.26 (1.01–1.57) |
| Multivariate analysis* | 1.28 (1.01–1.62) |
| Association of coinfection with higher VL | |
| Univariate analysis | 0.51 (0.29–0.88) |
| Multivariate analysis* | 0.38 (0.21–0.69) |
| Association of coinfection with LRTI | |
| Univariate analysis | 1.74 (0.99–3.05) |
| Multivariate analysis* | 1.83 (1.01–3.32) |
*Adjusted for age (<12 months or ≥12 months), gender, race (white, black, Asian, Native Hawaiian/Pacific Islander, American Indian/Alaska native, more than race 1, or other race), underlying disease (yes or no), and premature birth.
Figure 5.
Bar chart showing the percentages of LRTIs in children with HRV illness episodes with VLs indicated by CT values of >30, >25 to 30, >20 to 25, >15 to 20, or ≤15.
Illness episodes with a coinfection increased the risk of LRTI over that of illness episodes with HRV alone (OR, 1.83; 95% CI, 1.01–3.32). Specifically, RSV coinfections were associated with an increased risk of LRTI (OR, 2.80; 95% CI, 1.25–6.30) over that of illness episodes with HRV alone. Coinfection with other respiratory viruses (PIV or hMPV) did not increase the risk of LRTI (OR, 0.89; 95% CI, 0.37–2.16).
We found that HRV-only illness episodes with higher VLs were significantly associated with LRTI among children ≥12 months of age (OR, 2.57; 95% CI, 1.21–5.49) but not in children <12 months of age (OR, 1.81; 95% CI, 0.83–3.95). Coinfection was not statistically significantly associated with increased risk of LRTI in children ≥12 months of age (OR, 1.55; 95% CI, 0.59–4.10) or children <12 months of age (OR, 2.07; 95% CI, 0.97–4.41). Hospitalization was not associated with high VL (OR, 1.10; 95% CI, 0.70–1.74) or coinfection (OR, 1.03; 95% CI, 0.60–1.79). Among those who were hospitalized, the mean length of stay was 2.1 days longer (95% CI, 2.5–6.7) in HRV illness episodes with a high VL than in those with a low VL.
Repeat-Illness Episodes
Eighteen children had repeat illness episodes during the study, with a median of 33 days (range, 16–359 days) between the first and second illness episodes. Fifteen of 18 first illness episodes and 12 of 17 (1 unknown) second illness episodes were LRTIs. The median CT values of the first and second illness episodes were similar at 24 (range, 15–34) and 25 (range, 21–33). Repeat-illness episodes were uniformly caused by a different HRV subtype [17].
DISCUSSION
HRV was the most common pathogen detected in children younger than 3 years presenting for care in a pediatric ED who had respiratory viral testing performed. The majority of children with HRV required hospital admission. Of those hospitalized, more than 20% stayed for 3 days or longer, suggesting that HRV-associated illness was severe enough to require prolonged inpatient care. Factors associated with more severe disease in these children included higher HRV VL and coinfection with RSV. The majority of children with HRV LRTI had no comorbidities and were older than 1 year. This demonstrates a substantial burden of HRV disease in healthy older children.
Our results confirm those of a small study that showed that children younger than 2 years with HRV-associated illnesses have hospitalization rates greater than 50%, although this study included inpatients and patients seen in the ED or outpatient clinic [18]. Our results are also consistent with those of a large surveillance study of children and adults seeking care in outpatient clinics for influenza-like illness that showed that HRV was as commonly detected as influenza at a rate of 21%, although that study excluded children younger than 2 years [6]. It should be noted that our study results contrast with the findings of asymptomatic HRV infection in pediatric patients, which was well described by Peltola et al [19], because we required all patients in our study to be symptomatic before respiratory specimens were obtained.
The contribution of VL and coinfection to HRV disease severity in hospitalized children has been debated. Some studies found no effect of HRV VL on duration of hospital stay, supplemental oxygen use, presence of fever, or use of antibiotics or corticosteroids [20, 21]. Other inpatient studies have shown that mean HRV VL was associated with disease severity in hospitalized adults and children without underlying medical conditions and in children older than 11 months [22–24]. A higher median HRV VL has also been associated with viremia [25]. A Spanish study found that children with multiple respiratory viruses were more likely to be admitted [26], whereas a US study found that coinfection was associated with lower oxygen requirements, decreased risk of hospital or ICU admission, and shorter hospitalization duration [27].
The relationship between disease severity, VL, and HRV coinfection is not well defined in pediatric outpatients. Childcare attendees coinfected with other respiratory viruses have lower HRV VLs and incidence of fever but prolonged duration of symptoms [28]. In another study, VL did not seem to differ in older children with or without asthma, although the role of VL in disease severity was not studied [29]. A study of HRV transmission within families did not find a relationship between VL and symptom duration [19]. A recent study of a combined inpatient and outpatient population in Brazil found that RSV coinfection was the predominant cause of severe HRV disease, but it did not examine the effect of VL [30].
Our study included 56% of patients with URTI symptoms only and 48% who did not require hospitalization. In this patient population, we found a clear association between higher VL and increased risk of LRTI. In a sensitivity analysis, progressive increases in VL were associated with increased risk of LRTI. Hospitalized and younger children may have higher VLs as a result of impaired immune status or lack of previous exposure, which makes it difficult to differentiate the role of virus versus that of the immune system in disease severity. When we evaluated the effect of VL separately for children <1 year versus those ≥1 year of age, VL was more strongly associated with LRTI in older children.
In this study, we originally considered a sample with RSV, PIV, influenza, or hMPV a coinfection. It is interesting to note that no patients in our study had influenza detected as a coinfection. We did not evaluate coinfections of HRV solely with CoV or AdV, because both of them have been frequently detected in asymptomatic children [28, 31]. RSV was the most common coinfection and was associated with increased risk of LRTI, whereas hMPV and PIV coinfections were not. These results indicate that RSV is the most important viral coinfection in HRV illness episodes and are in agreement with the results from a recent study from Brazil [30]. Because data from children with RSV infection alone were not captured in our study, we are not able to determine whether the primary cause of severe disease in cases of HRV/RSV coinfections is RSV alone or whether HRV is a significant contributor as well.
A limitation of our study is our estimation of HRV VL based on CT values, which may not be a true indicator of quantitative VL because of the genetic diversity of the HRV serotypes being amplified. However, no standard method of quantifying HRV VL has been developed [32]. In practice, CT values are used by clinicians for decision making, and a sensitivity analysis found an effect of incrementally lower CT values on increased risk of LRTI. To our knowledge, no other studies have used a CT value of less than 30 as a definition for high VL; previous studies that compared HRV VL and disease severity used the mean or median VL, which is specific to the population being studied [23, 25]. We chose the CT criteria because we believe that this definition may be extrapolated to other study populations. Another limitation includes the gap in sample collection between July and January, including the peak incidence of HRV in the United States in the early fall [33]. Rhinovirus circulates year-round in the Pacific Northwest region, and there are increased rates of detection between September and June (http://depts.washington.edu/rspvirus/respiratory.htm). Circulating viral strains have been shown to vary according to season and are potentially associated with disease severity [34]. An additional limitation of our study is the exclusion of all samples with HRV/EntV in combination with only AdV/CoV and of a random subset of samples with HRV only, introducing a sampling bias. Because of these exclusion criteria, we were not able to determine whether HRV illness with AdV/CoV coinfections differed in clinical characteristics from those with HRV alone. We were also were not able to determine the incidence of coinfection in our study. This sample-selection process was a result of funding limitations. We also did not capture information regarding bacterial coinfection in our study, and it is possible that antibiotic use, hospital admission, and ICU admission is related to bacterial coinfection rather than rhinovirus infection alone.
In addition, our study was performed using retrospective chart review and samples that were collected at the clinicians' discretion at the time of illness. Patients selected for testing were likely more ill-appearing than those who were not. Hospitalization rates are subsequently higher than those of a study population that would include all children with respiratory virus symptoms. An additional limitation is the lack of an asymptomatic control group in our study. Rhinovirus is often detected in asymptomatic children, making its clinical significance unclear when detected on PCR testing [35]. However, this study was designed to evaluate rhinovirus infections in symptomatic children only. Finally, an important caveat is that this study was performed in a tertiary care institution in a major metropolitan center, and its population of ED attendees may not be generally representative of other institutions. In particular, 21% of our patients had underlying pulmonary disease, including asthma. A genetic basis has been found for childhood asthma and HRV-associated wheezing illness episodes [36]. Notably, because we excluded patients with enterovirus from analysis, none of these patients had enterovirus D68.
In conclusion, HRV infections represent a substantial disease burden in the pediatric ED. Higher VLs and simultaneous detection of RSV were associated with HRV LRTI. Knowledge of how to interpret virologic data may assist clinical decision making in a general pediatric population.
Acknowledgments
We acknowledge Emma Roberts and Jessie Gritton for their assistance with data entry.
Financial support. All phases of this study were supported by the Seattle Children's Hospital Translational Research Ignition Projects Program Pilot Project Fund (to J.A.E. and E.J.K.) and National Institutes of Health grant K23-AI103105 (to H.Y.C.). Statistical support was provided by the National Center For Advancing Translational Sciences of the National Institutes of Health (grant UL1 TR000423). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Project Redcap was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR000002.
Potential conflicts of interest. J.A.E. has received research support from Gilead, Chimerix, GlaxoSmithKline, and Roche, has received payment for lectures from Abbvie, and serves as a consultant for GlaxoSmithKline and Gilead. K.L. has received research support from Chimerix and Gilead. All other authors: no reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev 2013; 26:135–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998; 36:539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg SB. Respiratory consequences of rhinovirus infection. Arch Intern Med 2003; 163:278–84. [DOI] [PubMed] [Google Scholar]
- 4.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010; 23:74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowlkes A, Giorgi A, Erdman D, et al. Viruses associated with acute respiratory infections and influenza-like illness among outpatients from the Influenza Incidence Surveillance Project, 2010–2011. J Infect Dis 2014; 209:1715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol 2011; 49:2631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singleton RJ, Bulkow LR, Miernyk K, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol 2010; 82:1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuypers J, Campbell AP, Cent A, et al. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis 2009; 11:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu M, Qin X, Astion ML, et al. Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care. Am J Clin Pathol 2013; 139:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills JM, Harper J, Broomfield D, Templeton KE. Rapid testing for respiratory syncytial virus in a paediatric emergency department: benefits for infection control and bed management. J Hosp Infect 2011; 77:248–51. [DOI] [PubMed] [Google Scholar]
- 11.Woodberry MW, Shankar R, Cent A, et al. Comparison of the Simplexa FluA/B and RSV direct assay and laboratory-developed real-time PCR assays for detection of respiratory virus. J Clin Microbiol 2013; 51:3883–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonner AB, Monroe KW, Talley LI, et al. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 2003; 112:363–7. [DOI] [PubMed] [Google Scholar]
- 13.Iwane MK, Prill MM, Lu X, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis 2011; 204:1702–10. [DOI] [PubMed] [Google Scholar]
- 14.Renaud C, Crowley J, Jerome KR, Kuypers J. Comparison of FilmArray Respiratory Panel and laboratory-developed real-time reverse transcription-polymerase chain reaction assays for respiratory virus detection. Diagn Microbiol Infect Dis 2012; 74:379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 2006; 44:2382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin EK, Chu HY, Klein EJ, et al. Rhinovirus and Lower Respiratory Tract Disease in the Pediatric Emergency Department, Pediatric Academic Society Annual Meeting, May 3–7, 2014, Vancouver, WA.
- 18.Piotrowska Z, Vazquez M, Shapiro ED, et al. Rhinoviruses are a major cause of wheezing and hospitalization in children less than 2 years of age. Pediatr Infect Dis J 2009; 28:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peltola V, Waris M, Osterback R, et al. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis 2008; 197:382–9. [DOI] [PubMed] [Google Scholar]
- 20.Franz A, Adams O, Willems R, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol 2010; 48:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Leeuwen JC, Goossens LK, Hendrix RM, et al. Equal virulence of rhinovirus and respiratory syncytial virus in infants hospitalized for lower respiratory tract infection. Pediatr Infect Dis J 2012; 31:84–6. [DOI] [PubMed] [Google Scholar]
- 22.Gerna G, Piralla A, Rovida F, et al. Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J Med Virol 2009; 81:1498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeyama A, Hashimoto K, Sato M, et al. Rhinovirus load and disease severity in children with lower respiratory tract infections. J Med Virol 2012; 84:1135–42. [DOI] [PubMed] [Google Scholar]
- 24.Piralla A, Lilleri D, Sarasini A, et al. Human rhinovirus and human respiratory enterovirus (EV68 and EV104) infections in hospitalized patients in Italy, 2008–2009. Diagn Microbiol Infect Dis 2012; 73:162–7. [DOI] [PubMed] [Google Scholar]
- 25.Esposito S, Daleno C, Scala A, et al. Impact of rhinovirus nasopharyngeal viral load and viremia on severity of respiratory infections in children. Eur J Clin Microbiol Infect Dis 2013; 33:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cilla G, Onate E, Perez-Yarza EG, Montes M, Vicente D, Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol 2008; 80:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses 2012; 6:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin ET, Fairchok MP, Stednick ZJ, et al. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis 2013; 207:982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy JL, Shaker M, McMeen V, et al. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am J Respir Crit Care Med 2014; 189:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa LF, Queiroz DA, Lopes da Silveira H, et al. Human rhinovirus and disease severity in children. Pediatrics 2014; 133:e312–21. [DOI] [PubMed] [Google Scholar]
- 31.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet 2011; 377:1264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schibler M, Yerly S, Vieille G, et al. Critical analysis of rhinovirus RNA load quantification by real-time reverse transcription-PCR. J Clin Microbiol 2012; 50:2868–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol 2006; 78:644–50. [DOI] [PubMed] [Google Scholar]
- 34.Miller EK, Williams JV, Gebretsadik T, et al. Host and viral factors associated with severity of human rhinovirus-associated infant respiratory tract illness. J Allergy Clin Immunol 2011; 127:883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhedin S, Lindstrand A, Rotzen-Ostlund M, et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics 2014; 133:e538–45. [DOI] [PubMed] [Google Scholar]
- 36.Caliskan M, Bochkov YA, Kreiner-Moller E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med 2013; 368:1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]