Abstract
Protein phosphatase 2A (PP2A) is a heterotrimeric protein serine/threonine phosphatase and is involved in a broad range of cellular processes. PPP2R5D is a regulatory B subunit of PP2A and plays an important role in regulating key neuronal and developmental regulation processes such as PI3K/AKT and GSK3β-mediated cell growth, chromatin remodeling and gene transcriptional regulation. Using WES, we identified four de novo variants in PPP2R5D in a total of seven unrelated individuals with ID and other shared clinical characteristics, including autism spectrum disorder, macrocephaly, hypotonia, seizures and dysmorphic features. Among the four variant, two have been previously reported, and two are novel. All four amino acids are highly conserved among the PP2A subunit family, and all change a negatively charged acidic glutamic acid (E) to a positively charged basic lysine (K), and are predicted to disrupt the PP2A subunits binding and impair the dephosphorylation capacity. Our data provides further support for PPP2R5D as a genetic cause of ID.
Keywords: PPP2R5D, Intellectual Disabilities, Whole Exome Sequencing, de novo mutations, Protein phosphatase, Autism spectrum disorder
Introduction
Intellectual disability (ID) and autism spectrum disorder (ASD) are common neurodevelopmental disorders that occur in ~1% of the general population. Identifying the etiology of ID and ASD remains challenging due to disease heterogeneity. Whole exome sequencing (WES) provides an effective strategy to identify de novo mutations, which account for a significant portion of ID and ASD [1,2].
PP2A is an abundant, multifunctional heterotrimeric serine/threonine-specific phosphatase, which is involved in > 90% of all Ser/Thr phosphatase activities together with protein phosphatase 1 (PP1) [3,4]. Protein phosphorylation is a major mechanism for the regulation of key processes and signaling pathways, and dysregulation of phosphatases has been implicated in ID and other developmental disorders [5,6]. Mutations in IGBP1 (Alpha 4), a regulatory subunit of protein phosphatase 2, 4, and 6, have been identified in patients with agenesis of corpus callosum, ID and other developmental disorders [7,8].
PPP2R5D (HGNC: 9312) encodes B56δ, a regulatory subunit B of PP2A [9], which controls the involvement of PP2A in negative regulation of the PI3K/AKT signaling pathway and the regulation of tau phosphorylation via modulation of cyclin-dependent kinase 5 (CDK5) and GSK3β activities [10], and other key ID-associated cellular processes [11,12]. Mutations in the PP2A regulatory subunit B family genes PPP2R5B, PPP2R5C and PPP2R5D; as well as its scaffolding Aα subunit PPP2R1A, have been recently reported to be associated with developmental disorders, autism, overgrowth, and ID [13-17]. In the present study, we performed clinical WES in 2790 individuals with ID and related neurodevelopmental disorders and identified four independent, de novo, predicted pathogenic variants in PPP2R5D in a total of seven individuals. Our study underscores the importance of the protein phosphatase family in neurodevelopmental processes and provides confirmation in a large series that PPP2R5D is a gene associated neurodevelopmental disorders and ID.
Materials and Methods
Consent
Informed consent was obtained from all participants included in this study, including any identifying information included. This study was approved by the Institutional Review Board of Columbia University.
Whole-exome sequencing
Whole-exome sequencing was performed as described previously [18]. Briefly, Genomic DNA extracted from whole blood was fragmented and exomes were captured using the Agilent SureSelect Human All Exon V4 (50 Mb) kit (Agilent Technologies, Santa Clara, CA). The final isolated DNA products were sequenced using the Illumina HiSeq 2000 or 2500 sequencing system with 100-bp paired-end reads (Illumina, San Diego, CA). DNA sequence was mapped to the human genome reference sequence human assembly hg19/GRCh37 using Burrows-Wheeler Aligner (BWA) with the latest internally validated version at the time of sequencing, progressing from BWA v0.5.8 through BWA-Mem v0.7.8 [19]. Targeted coding exons and splice junctions of known protein-coding RefSeq genes were assessed for average depth of coverage with a minimum depth of 10X required for inclusion in downstream analysis. Local realignment around insertion-deletion sites was performed using the Genome Analysis Toolkit v1.6 [20]. Variant calls were generated simultaneously on all sequenced family members using SAMtools v0.1.18 [19]. All coding exons and surrounding intron/exon boundaries were analyzed. Whole exome sequence data for all sequenced family members was analyzed using GeneDx’s XomeAnalyzer (a variant annotation, filtering, and viewing interface for WES data) and variants were filtered based on inheritance patterns, gene lists of interest, phenotype and population frequencies, as appropriate with resources listed previously [18]. The general assertion criteria for variant classification are publicly available on the GeneDx ClinVar submission page (http://www.ncbi.nlm.nih.gov/clinvar/submitters/26957/). Additional searches were performed using specific gene lists related to the probands’ clinical features. Identified PPP2R5D variants were confirmed in all family members with a new DNA preparation by di-deoxy Sanger sequencing using an ABI3730 (Life Technologies, Carlsbad, CA).
Protein structure analysis
Homology modeling of PPP2R5D was carried out using the program MODELLER [21] based on the structure of PPP2R5C and using an alignment generated by the program HHblits [22]. Modeling of the E420K variant was carried out with the program SCAP [23]. Calculation of the electrostatic potential and its visualization on the protein surface were carried out using the program GRASP2 [24].
Results
Exome sequencing was performed in 2790 probands with developmental delay and/or ID, with or without additional clinical features. Seven individuals (0.25%) were found to have de novo pathogenic variants in PPP2R5D and produced an average of ~11GB of sequence per sample. Mean coverage of captured regions was ~130X per sample, with >97% covered with at least 10x coverage, an average of 90% of base call quality of Q30 or greater, and an overall average mean quality score of >Q35. Common SNPs (>10% frequency present in 1000 Genomes database and subsequently further curated for alleles with <1% frequency) were filtered out and remaining data analyzed for de novo and inherited rare variants. We evaluated 152 genes (169 unique sequence changes) of interest in these seven cases with an average of 24 variants per case.
Seven unrelated probands from seven families were identified with four de novo variants in PPP2R5D. None of the four variants are present in ExAC database, the Database of Single Nucleotide Polymorphisms (dbSNP, http://www.ncbi.nlm.nih.gov/SNP/), 1000 Genomes (http://www.1000genomes.org/), Exome Variant Server (ESP), or in a local database of over 15,000 control exomes. All four variants change a highly conserved negatively charged glutamic acid (E) residue to positively charged lysine (K) and are predicted to have a pathogenic effect by various algorithms (Table 1). Two of the variants we identified, E198K and E200K, have been reported in previous studiess of developmental disorders, overgrowth, autism, and intellectual disability [13,15-17]. The E197K and the recurrent E420K variant have not been previously reported.
Table 1.
Predicted pathogenicity and allele frequencies of PPP2R5D variants
| Mutation | Position | SIFT | PROVEAN | Polyphen (HDIV score) |
Mutation Taster |
MetaSVM | CADD Phred |
ExAC Allele Frequency |
1000 Genomes Allele Frequency |
EVS Allele Frequency |
XomeDx Allele Frequency |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p.E197K | Chr6:42975000G>A | Damaging (0.001) |
Deleterious (−3.77) |
Damaging (0.999) |
Disease causing (1) |
Deleterious (0.3599) |
22.5 | 0 | 0 | 0 | 0 |
| p.E198K | Chr6:42975003G>A | Damaging (0) | Deleterious (−3.82) |
Damaging (1) | Disease causing (1) |
Deleterious (0.4031) |
22.5 | 0 | 0 | 0 | 0 |
| p.E200K | Chr6:42975009G>A | Damaging (0.001) |
Deleterious (−3.82) |
Damaging (0.991) |
Disease causing (1) |
Deleterious (0.1505) |
22.5 | 0 | 0 | 0 | 0 |
| p.E420K | Chr6:42977066G>A | Damaging (0.004) |
Deleterious (−3.94) |
Damaging (0.999) |
Disease causing (1) |
Deleterious (0.6314) |
22.6 | 0 | 0 | 0 | 0 |
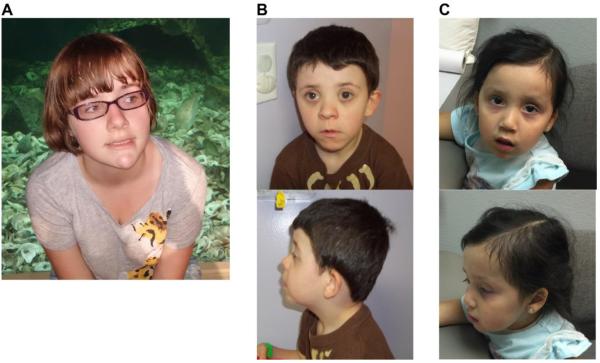
Clinically, these seven individuals with de novo PPP2R5D variants range in age from 22 months to 15 years, and all have some degree of developmental delay and/or ID (Table 2). All individuals demonstrated delayed walking as well as significantly delayed speech, with the majority being non-verbal or speaking only single words. Developmental quotient (DQ)/intelligence quotient (IQ) ranges from ‘mild’ to ‘severe’ (DQ < 50 to IQ of 54). Hypotonia was a consistent feature in all seven individuals, and all but one of the individual also had macrocephaly (occipital frontal circumference >97th percentile for age). Five out of six individuals old enough to be diagnosed were affected with ASD. Four children had unusual habits or behavioral issues including stereotypic behavior, trouble adjusting to new situations, tantrums, aggressiveness, and problems with impulse control. Less commonly observed features were ophthalmologic abnormalities (5/7), brain abnormalities (4/6), ataxia or unsteady gait (2/7), scoliosis (2/7), and seizures (1/7). Mild dysmorphic features were noted in six individuals and included a long face, plagiocephaly, and downslanting palpebral fissures (Figure 1). Some individuals also had birth defects, including one individual with a submucosal cleft palate and two individuals with congenital heart defects (atrial and ventricular septal defect, bicuspid aortic valve). Individual 2 also had a de novo variant in the SOS1 gene, which is associated with Noonan syndrome 4 [25]. Individual 3 also had a de novo variant in the DCAF7 gene, which has not been associated with a phenotype in humans. It has been suggested in the literature that this gene may be involved with left-right asymmetry [26], but this individual does not exhibit any asymmetry. Individual 4 also had a de novo variant in the CACNA1H gene. Evidence has shown that the susceptibility to childhood absence epilepsy-6 [27] and idiopathic generalized epilepsy-6 can be conferred by variation in the CACNA1H gene [28]. These candidate genes were excluded as disease causative in these individuals based on gene functions and lack of phenotypic correlation.
Table 2.
Clinical features of individuals with PPP2R5D variants
| Individual # | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Sex | F | M | M | F | M | F | F |
| Age | 15 yrs | 10 yrs | 6 yrs | 9 yrs | 22 mos | 4 yrs | 4 yrs |
| Variant | c.G592A p.E198K |
c.G592A p.E198K |
c.G598A p.E200K |
c.G1258A p.E420K |
c.G1258A p.E420K |
c.G1258A p.E420K |
c.G589A p.E197K |
| Developmental delay |
+ | + | + | + | + | + | + |
| Age at walking | 3 yrs | 8 yrs | 28 mos | 5 yrs | Not currently walking |
3 yrs 5 mos | 26 mos |
| Speech | First words at 3 yrs; has 100-200 words and can form short sentences |
Non-verbal | Signed at 24 mos, first word at 3 yrs |
Non-verbal | 7 words & 7 signs at 22 mos |
First words 2.5 yrs; single words only (prefers to gesture) |
Says ~75 words and occasional 2 word sentences |
| ID | + IQ unknown but is “low” |
+ | + IQ = 54 |
+ DQ < 50 |
n/a | + | + “Moderate” |
| Macrocephaly | + | + | − | + | + | + | + |
| OFC | 58.1cm (>97%) | 57cm (>97%) | 50.2cm (10%) | 54.5cm at 2yrs (>97%) |
54cm at 19 mos (>97%) |
56.5cm (>97%) | 54.6cm (>97%) |
| Hypotonia | + | + | + | + | + | + | + |
| Autism Spectrum |
+ | + | + | + | n/a | + | |
| Behavioral Abnormalities |
Anxiety in new situations |
- | Water fascination, licks items, excitable |
Aggressive, stereotypies, impulse control issues |
− | Tantrums, perseverative behavior |
− |
| Seizures | − | + (complex partial) | − | − | − | − | − |
| Dysmorphic features | Downslanting palpebral fissures, doligocephaly |
Prominent forehead, large anterior fontanelle, mild midface hypoplasia |
Plagiocephaly, triangular face, long philtrum, short nose, thin upper lip, midface hypoplasia, slightly low set ears |
− | Prominent forehead, slightly low-set ears, high-arched palate, small nose |
Frontal bossing, triangular face, supernumerary nipple, hypoplastic fifth toenails |
Mild facial asymmetry |
| Brain abnormalities | n/a | Bilateral small arachnoid cysts, cavum septum pellucidum |
− | Prominent CSF spaces, mildly enlarged lateral ventricles, cavum septum, & cavum verge |
Moderate dilatation of lateral ventricles |
White matter dysgenesis, dysmorphic corpus callosum |
− |
| Neurological abnormalities |
− | − | Torticollis | Ataxia, broad-based gait, absent deep tendon reflexes |
− | Wide-based unsteady gait, autism |
Chewing difficulties |
| Skeletal abnormalities |
Scoliosis | − | Pectus carinatum, 3rd/5th toes over- riding 4th, camptodactyly of 4th toe |
− | − | − | Congenital scoliosis |
| Ophthalmological abnormalities |
Myopia | − | Rotational nystagmus in infancy; delayed visual maturation |
Alternating esotropia | Strabismus | Astigmatism | − |
| Cardiac abnormalities |
− | − | ASD, VSD, bicuspid aortic valve |
− | VSD, PFO, sinus tachycardia |
− | − |
| Additional clinical features |
− | Failure to thrive, possible short stature |
Submucosal cleft palate, short stature, easy bruising |
− | − | − | − |
|
Other genetic
findings |
− |
SOS1 (de novo, p.I52M) |
DCAF7 (de novo, p.C280Y) |
CACNA1H (de novo, p.P277L) |
− | − | − |
OFC, occipital frontal circumference; ASD, atrial septal defect; VSD, ventricular septal defect; PFO, patent foramen ovale
Figure 1. Facial characteristics of individuals with PPP2R5D variants.
A. Individual 1, B. Individual 3, C. Individual 7
Based on the functional studies of Houge et al., E198K and E200K disrupt the PP2A holoenzyme subunit binding and impair the dephosphorylation of specific substrates [17]. One of the new variants we identified, E197K is also located in the highly-conserved acidic B56δ loop, which is essential for holoenzyme formation. Based on the biochemical data for E198K and E200K, A-C binding could be disturbed by the E197K variant, with the substitution of a positively charged lysine (K) for the negatively charged glutamic acid (E) in the acidic B56δ loop.
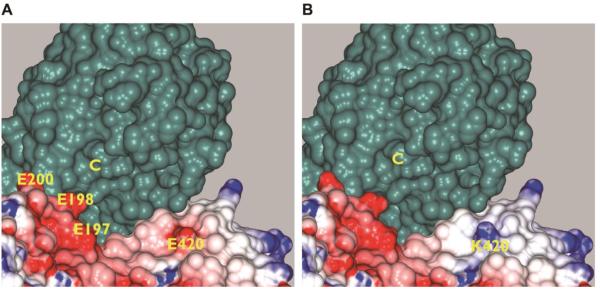
Among the seven cases, three of the patients have a de novo E420K variant, which has not been reported in previous cohorts. E420 is located outside of the A-C binding loop, but is positioned near the active site of the catalytic subunit and is on the surface (Figure 2) implying that mutation to lysine could be accommodated with little disruption of the structure of the regulatory subunit itself, similar to the other variants. However, as shown in the figure, the charge reversal dramatically changes the electrostatic character of the protein-protein surface near the active site of the holoenzyme, suggesting that the functional effects are also similar, either disrupting holoenzyme formation or substrate recognition.
Figure 2. Effects of E420K mutation in PPP2R5D.
Both panels show the molecular surfaces of the regulatory and catalytic subunit of PPP2R5D, calculated from homology models (see Methods). The surface of the catalytic subunit is colored green. The surface of the regulatory subunit is colored according to electrostatic potential with red regions indicating electronegative regions and blue indicating electropositive regions. Panel A shows the surface of the wild-type regulatory subunit, and Panel B shows the surface of the E420K mutant. The active site cavity of the catalytic subunit is labeled with a “C”
Discussion
We have identified PPP2R5D as a gene associated with ID and ASD. We identified four de novo missense variants in PPP2R5D in 7 of 2790 individuals with ID and neurodevelopmental disorders (0.25%). All of the individuals with a PPP2R5D pathogenic variant displayed moderate to severe global developmental delay, ID, and hypotonia. Additionally, ASD was observed in five of the seven individuals. Macrocephaly is a specific feature of this condition observed in 6 of the 7 individuals. Mild dysmorphic features and additional neurological, ophthalmological, and congenital abnormalities were also described in this cohort.
PP2A consists of three subunits: a 65-kDa structural A subunit and a 36-kDa catalytic C subunit, which together form the core enzyme, and the third regulatory B subunit [29], PPP2R5D (NP_006236.1) belongs to the largest PP2A B’ (B56) subunit family, comprised of at least eight members which have 71-88% protein sequence identity over a 400-amino acid conserved region. PPP2R5D (B56δ) codes a 602 amino acid protein. The four variant amino acid residues in our study individuals - E197, E198, E200 and E420 - are all highly conserved across the B56 family [30].
Variants in the PP2A regulatory subunit B family genes including, PPP2R5B, PPP2R5C and PPP2R5D; as well as its scaffolding Aα subunit PPP2R1A, have recently been reported in association with developmental disorders, ASD, human overgrowth and intellectual disability [13-17]. Two variants we identified, E198K and E200K, were also previously identified in individuals with clinical features including overgrowth, ID, hypospadias and Parkinsonism [15]; and severe, undiagnosed developmental disorders [13], which are also observed in some of the individuals in our study (Table 2). One characteristic clinical feature in our series is macrocephaly, suggesting abnormal cell proliferation and overgrowth.
Consistent with the previous report [17], we also observed less severe ID in our patient with the E200K mutation. This “mild” phenotype suggests that within the acidic pocket formed by the glutamic acid (E) residues 197, 198 and 200, E198 is the core for subunits interactions, while E197 and E200, may not cause severe effects on subunit binding. We also note that all three patients who carry the E420K variant show severe ID. As discussed above, the position of the E420 is similar to these other variants with respect to the active site (Figure 2), so it is reasonable to expect that mutation to lysine will have similar effects, but functional studies will be needed to clarify the effect of this mutation on the holoenzyme assembly as well as catalytic capacity on the substrates.
PPP2R5D is highly expressed in the developing human and mouse brain, and is expressed at lower levels in heart and skeletal muscle [9,31]. PPP2R5Ds plays an important role in regulating PI3K/AKT and GSK3β mediated growth control and tau phosphorylation [10,32]. PPP2R5D is also involved in other cellular processes that are associated with ID such as chromatin remodeling and transcriptional regulation. PPP2R5D directly interacts with ADA (alteration/deficiency in activation) 3, which is a conserved component of several transcriptional adaptor and histone acetyltransferase complexes [11]. PPP2R5D binds and stimulates the nuclear translocation of Cacnb4 (Voltage-gated calcium channel, β4 subunit), an important channel in the brain [33]. Disruption of the Cacnb4/Ppp2r5d/PP2A complex by the R482X mutation in β4 is associated with juvenile epilepsy [12]. PPP2R5D was detected to localize with human shugoshin (SGOL1) at centromeres and is required for centromeric cohesion protection during mitosis and meiosis [34]. PPP2R5D plays a key role in regulation of Cdc25C and Cdk1, controlling exit from mitosis [35]. PPP2R5D also regulates the dephosphorylation of DARPP-32 (Dopamine- and cAMP-regulated phosphoprotein, 32-KD; PPP1R1B), thereby regulating the dopaminergic neurotransmission in neurons [36].
In this study, we provide further evidence supporting the role for PPP2R5D in ID and ASD. The four de novo missense variants we identified all alter electrostatic potential and are located at highly conserved amino acids within key functional domains. The de novo variants in PPP2R5D are associated with neurodevelopmental disorders and macrocephaly, most likely through a dominant-negative mechanism [13,17]. Further functional studies are needed to characterize the new mutations we have identified to better understand the disease mechanism.
Acknowledgements
We thank the probands and families for their generous contributions. This work was supported in part by a grant from the Simons Foundation and from the NIH (GM030518).
Footnotes
Conflicts of Interest
Lindsay Henderson, Megan Cho, Leandra Folk, Kyle Retterer, and Kristin Monaghan are employees of GeneDx.
Wendy Chung is a consultant to BioReference Laboratories.
The other authors declare that they have no conflict of interest.
References
- 1.Vissers LE, de Ligt J, Gilissen C, Janssen I, Steehouwer M, de Vries P, van Lier B, Arts P, Wieskamp N, del Rosario M, van Bon BW, Hoischen A, de Vries BB, Brunner HG, Veltman JA. A de novo paradigm for mental retardation. Nature genetics. 2010;42(12):1109–1112. doi: 10.1038/ng.712. doi:10.1038/ng.712. [DOI] [PubMed] [Google Scholar]
- 2.Ku CS, Polychronakos C, Tan EK, Naidoo N, Pawitan Y, Roukos DH, Mort M, Cooper DN. A new paradigm emerges from the study of de novo mutations in the context of neurodevelopmental disease. Molecular psychiatry. 2013;18(2):141–153. doi: 10.1038/mp.2012.58. doi:10.1038/mp.2012.58. [DOI] [PubMed] [Google Scholar]
- 3.Depaoli-Roach AA, Park IK, Cerovsky V, Csortos C, Durbin SD, Kuntz MJ, Sitikov A, Tang PM, Verin A, Zolnierowicz S. Serine/threonine protein phosphatases in the control of cell function. Advances in enzyme regulation. 1994;34:199–224. doi: 10.1016/0065-2571(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 4.Kiely M, Kiely PA. PP2A: The Wolf in Sheep's Clothing? Cancers. 2015;7(2):648–669. doi: 10.3390/cancers7020648. doi:10.3390/cancers7020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gipson TT, Johnston MV. Plasticity and mTOR: towards restoration of impaired synaptic plasticity in mTOR-related neurogenetic disorders. Neural plasticity. 2012;2012:486402. doi: 10.1155/2012/486402. doi:10.1155/2012/486402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim KC, Crino PB. Focal malformations of cortical development: new vistas for molecular pathogenesis. Neuroscience. 2013;252:262–276. doi: 10.1016/j.neuroscience.2013.07.037. doi:10.1016/j.neuroscience.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Peterson RT, Schreiber SL. Alpha 4 associates with protein phosphatases 2A, 4, and 6. Biochemical and biophysical research communications. 1998;247(3):827–832. doi: 10.1006/bbrc.1998.8792. doi:10.1006/bbrc.1998.8792. [DOI] [PubMed] [Google Scholar]
- 8.Graham JM, Jr., Wheeler P, Tackels-Horne D, Lin AE, Hall BD, May M, Short KM, Schwartz CE, Cox TC. A new X-linked syndrome with agenesis of the corpus callosum, mental retardation, coloboma, micrognathia, and a mutation in the Alpha 4 gene at Xq13. American journal of medical genetics Part A 123A. 2003;(1):37–44. doi: 10.1002/ajmg.a.20504. doi:10.1002/ajmg.a.20504. [DOI] [PubMed] [Google Scholar]
- 9.McCright B, Brothman AR, Virshup DM. Assignment of human protein phosphatase 2A regulatory subunit genes b56alpha, b56beta, b56gamma, b56delta, and b56epsilon (PPP2R5A-PPP2R5E), highly expressed in muscle and brain, to chromosome regions 1q41, 11q12, 3p21, 6p21.1, and 7p11.2 --> p12. Genomics. 1996;36(1):168–170. doi: 10.1006/geno.1996.0438. [DOI] [PubMed] [Google Scholar]
- 10.Louis JV, Martens E, Borghgraef P, Lambrecht C, Sents W, Longin S, Zwaenepoel K, Pijnenborg R, Landrieu I, Lippens G, Ledermann B, Gotz J, Van Leuven F, Goris J, Janssens V. Mice lacking phosphatase PP2A subunit PR61/B'delta (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3beta. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(17):6957–6962. doi: 10.1073/pnas.1018777108. doi:10.1073/pnas.1018777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zencir S, Sike A, Dobson MJ, Ayaydin F, Boros I, Topcu Z. Identification of transcriptional and phosphatase regulators as interaction partners of human ADA3, a component of histone acetyltransferase complexes. The Biochemical journal. 2013;450(2):311–320. doi: 10.1042/BJ20120452. doi:10.1042/BJ20120452. [DOI] [PubMed] [Google Scholar]
- 12.Tadmouri A, Kiyonaka S, Barbado M, Rousset M, Fablet K, Sawamura S, Bahembera E, Pernet-Gallay K, Arnoult C, Miki T, Sadoul K, Gory-Faure S, Lambrecht C, Lesage F, Akiyama S, Khochbin S, Baulande S, Janssens V, Andrieux A, Dolmetsch R, Ronjat M, Mori Y, De Waard M. Cacnb4 directly couples electrical activity to gene expression, a process defective in juvenile epilepsy. The EMBO journal. 2012;31(18):3730–3744. doi: 10.1038/emboj.2012.226. doi:10.1038/emboj.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deciphering Developmental Disorders S Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519(7542):223–228. doi: 10.1038/nature14135. doi:10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, del Rosario M, Hoischen A, Scheffer H, de Vries BB, Brunner HG, Veltman JA, Vissers LE. Diagnostic exome sequencing in persons with severe intellectual disability. The New England journal of medicine. 2012;367(20):1921–1929. doi: 10.1056/NEJMoa1206524. doi:10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 15.Loveday C, Tatton-Brown K, Clarke M, Westwood I, Renwick A, Ramsay E, Nemeth A, Campbell J, Joss S, Gardner M, Zachariou A, Elliott A, Ruark E, van Montfort R, Childhood Overgrowth C, Rahman N. Mutations in the PP2A regulatory subunit B family genes PPP2R5B, PPP2R5C and PPP2R5D cause human overgrowth. Human molecular genetics. 2015 doi: 10.1093/hmg/ddv182. doi:10.1093/hmg/ddv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee YH, Grabowska E, Dalkic E, Wang Z, Marks S, Andrews P, Leotta A, Kendall J, Hakker I, Rosenbaum J, Ma B, Rodgers L, Troge J, Narzisi G, Yoon S, Schatz MC, Ye K, McCombie WR, Shendure J, Eichler EE, State MW, Wigler M. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–221. doi: 10.1038/nature13908. doi:10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houge G, Haesen D, Vissers LE, Mehta S, Parker MJ, Wright M, Vogt J, McKee S, Tolmie JL, Cordeiro N, Kleefstra T, Willemsen MH, Reijnders MR, Berland S, Hayman E, Lahat E, Brilstra EH, van Gassen KL, Zonneveld-Huijssoon E, de Bie CI, Hoischen A, Eichler EE, Holdhus R, Steen VM, Doskeland SO, Hurles ME, FitzPatrick DR, Janssens V. B56delta-related protein phosphatase 2A dysfunction identified in patients with intellectual disability. The Journal of clinical investigation. 2015;125(8):3051–3062. doi: 10.1172/JCI79860. doi:10.1172/JCI79860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang L, Cho MT, Retterer K, Folk L, Humberson J, Rohena L, Sidhu A, Saliganan S, Iglesias A, Vitazka P, Juusola J, O'Donnell-Luria AH, Shen Y, Chung WK. Mutations in ARID2 are associated with intellectual disabilities. Neurogenetics. 2015;16(4):307–314. doi: 10.1007/s10048-015-0454-0. doi:10.1007/s10048-015-0454-0. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. doi:10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature genetics. 2011;43(5):491–498. doi: 10.1038/ng.806. doi:10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eswar N, John B, Mirkovic N, Fiser A, Ilyin VA, Pieper U, Stuart AC, Marti-Renom MA, Madhusudhan MS, Yerkovich B, Sali A. Tools for comparative protein structure modeling and analysis. Nucleic acids research. 2003;31(13):3375–3380. doi: 10.1093/nar/gkg543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remmert M, Biegert A, Hauser A, Soding J. HHblits: lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nature methods. 2012;9(2):173–175. doi: 10.1038/nmeth.1818. doi:10.1038/nmeth.1818. [DOI] [PubMed] [Google Scholar]
- 23.Xiang Z, Honig B. Extending the accuracy limits of prediction for side-chain conformations. Journal of molecular biology. 2001;311(2):421–430. doi: 10.1006/jmbi.2001.4865. doi:10.1006/jmbi.2001.4865. [DOI] [PubMed] [Google Scholar]
- 24.Petrey D, Honig B. GRASP2: visualization, surface properties, and electrostatics of macromolecular structures and sequences. Methods in enzymology. 2003;374:492–509. doi: 10.1016/S0076-6879(03)74021-X. doi:10.1016/S0076-6879(03)74021-X. [DOI] [PubMed] [Google Scholar]
- 25.Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, Sarkozy A, Pandit B, Oishi K, Martinelli S, Schackwitz W, Ustaszewska A, Martin J, Bristow J, Carta C, Lepri F, Neri C, Vasta I, Gibson K, Curry CJ, Siguero JP, Digilio MC, Zampino G, Dallapiccola B, Bar-Sagi D, Gelb BD. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nature genetics. 2007;39(1):75–79. doi: 10.1038/ng1939. doi:10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Doan D, Roman Petersen Y, Alvarado E, Alvarado G, Bhandari A, Mohanty A, Mohanty S, Nissen RM. Wdr68 requires nuclear access for craniofacial development. PloS one. 2013;8(1):e54363. doi: 10.1371/journal.pone.0054363. doi:10.1371/journal.pone.0054363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, Ding K, Lo WH, Qiang B, Chan P, Shen Y, Wu X. Association between genetic variation of CACNA1H and childhood absence epilepsy. Annals of neurology. 2003;54(2):239–243. doi: 10.1002/ana.10607. doi:10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- 28.Heron SE, Phillips HA, Mulley JC, Mazarib A, Neufeld MY, Berkovic SF, Scheffer IE. Genetic variation of CACNA1H in idiopathic generalized epilepsy. Annals of neurology. 2004;55(4):595–596. doi: 10.1002/ana.20028. doi:10.1002/ana.20028. [DOI] [PubMed] [Google Scholar]
- 29.McCright B, Virshup DM. Identification of a new family of protein phosphatase 2A regulatory subunits. The Journal of biological chemistry. 1995;270(44):26123–26128. doi: 10.1074/jbc.270.44.26123. [DOI] [PubMed] [Google Scholar]
- 30.McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. The Journal of biological chemistry. 1996;271(36):22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- 31.Martens E, Stevens I, Janssens V, Vermeesch J, Gotz J, Goris J, Van Hoof C. Genomic organisation, chromosomal localisation tissue distribution and developmental regulation of the PR61/B' regulatory subunits of protein phosphatase 2A in mice. Journal of molecular biology. 2004;336(4):971–986. doi: 10.1016/j.jmb.2003.12.047. doi:10.1016/j.jmb.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Yu UY, Yoo BC, Ahn JH. Regulatory B Subunits of Protein Phosphatase 2A Are Involved in Site-specific Regulation of Tau Protein Phosphorylation. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2014;18(2):155–161. doi: 10.4196/kjpp.2014.18.2.155. doi:10.4196/kjpp.2014.18.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronjat M, Kiyonaka S, Barbado M, De Waard M, Mori Y. Nuclear life of the voltage-gated Cacnb4 subunit and its role in gene transcription regulation. Channels. 2013;7(2):119–125. doi: 10.4161/chan.23895. doi:10.4161/chan.23895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441(7089):46–52. doi: 10.1038/nature04663. doi:10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- 35.Forester CM, Maddox J, Louis JV, Goris J, Virshup DM. Control of mitotic exit by PP2A regulation of Cdc25C and Cdk1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):19867–19872. doi: 10.1073/pnas.0709879104. doi:10.1073/pnas.0709879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(8):2979–2984. doi: 10.1073/pnas.0611532104. doi:10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]