Sickle cell disease (SCD) refers to a group of genetic hemolytic anemias in which the erythrocytes have a predominance of sickle hemoglobin (HbS) due to inheritance of a β-globin mutation (βS). The βS mutation is the result of a single amino acid substitution (HbS, HBB Glu6Val) in the β-globin of the hemoglobin heterotetramer, thus forming HbS. Affected individuals typically are homozygous for the sickle mutation (HbSS) or have a compound heterozygous state (eg, HbSC, HbS β-thalassemia). The βS mutation creates a hydrophobic region that, in the deoxygenated state, facilitates a noncovalent polymerization of HbS molecules that damages the erythrocyte membrane and changes the rheology of the erythrocyte in circulation, causing hemolytic anemia, vaso-occlusion, and vascular endothelial dysfunction.
SCD is the most common inherited hemolytic anemia in the United States. Approximately 70,000 to 100,000 individuals in the United States are affected, most commonly those who have ancestry from Africa, the Indian subcontinent, the Arabian Peninsula, or the Mediterranean Basin. Worldwide, millions of persons are affected with SCD, especially in regions with endemic malaria, such as Africa, the Middle East, and India. SCD is characterized by a lifelong hemolytic anemia with an ongoing risk for acute medical complications and inexorable accrual of organ damage in most affected individuals.
There is wide variability in the phenotypic severity of SCD that is not well understood. This variation can be explained partly by differences in the total hemoglobin concentration, the mean corpuscular hemoglobin concentration, erythrocyte rheology, the percentage of adhesive cells, the proportion of dense cells, the presence or absence of α-thalassemia, and the β-globin haplotype [1–5]. The percentage of fetal hemoglobin (HbF), however, is perhaps the most important laboratory parameter influencing clinical severity in SCD [6, 7]. In unaffected individuals, HbF comprises only 5% of the total hemoglobin by age 3 to 6 months and falls to below 1% in adults [8]. In contrast, patients with SCD typically have HbF levels ranging from 1% to 20% [9] and those with genetic mutations leading to hereditary persistence of HbF (HPFH) can have HbF levels that reach 30% to 40% of the total hemoglobin [10].
Based on the observation that infants with SCD have few complications early in life, it was hypothesized that HbF, the predominant hemoglobin in fetal and infant stages of life, might ameliorate the phenotypic expression of SCD [11]. In addition, compound heterozygotes for the sickle mutation and HPFH are relatively protected from severe clinical symptoms [12]. Subsequently, it was shown that increased HbF percentage is associated with decreased clinical severity in SCD, using endpoints, such as the number of vaso-occlusive painful events, transfusions, and hospitalizations [1, 13]. HbF does not, however, seem to protect from some complications [14], perhaps because the HbF levels were inadequate to provide protection [3, 15]. A potential threshold of 20% HbF is suggested, above which patients experience fewer clinical events [16]. The % HbF also has emerged as the most important predictor of early mortality in patients with SCD [6, 17].
Although the genetic and molecular pathophysiology of SCD are well described and understood in considerable detail, there has been disappointing progress toward definitive, curative therapy. Bone marrow transplantation offers a cure but currently requires an HLA-matched sibling donor for best results. This requirement limits the number of patients who can benefit from this approach. Moreover, even using a matched sibling donor, bone marrow transplantation remains associated with considerable morbidity (primarily graft-versus-host disease) and low, but not negligible, mortality.
In lieu of curative therapy, one approach given considerable effort over the past 25 years has been the pharmacologic induction of HbF beyond the fetal and newborn period. Several pharmacologic agents have shown promise, including demethylating agents, such as 5-azacytidine [18] and decitabine [19–21], and short-chain fatty acids, such as butyrate [22–25], but each has limitations in route of administration, safety, or sustained efficacy. Hydroxyurea, in contrast, has a long and growing track record in inducing HbF in patients with SCD. In addition, hydroxyurea has a variety of salutary effects on other aspects of the pathophysiology of SCD, such as increased erythrocyte hydration, improved rheology, and reduced adhesiveness. Hydroxyurea also decreases leukocyte count, and releases nitric oxide. This article reviews the usefulness of hydroxyurea for children with SCD but is not intended to be an exhaustive review of the drug’s biochemistry, its therapeutic rationale, or previously published data. Interested readers may read more thorough reviews of hydroxyurea for the management of SCD [26, 27]. This article is intended as a practical user’s guide for clinicians who wish to know how and why treatment with hydroxyurea should be considered for children with SCD.
An ideal drug for sickle cell disease?
Hydroxyurea may be an ideal therapeutic agent for use in children with SCD. It has excellent bioavailability after oral administration; requires only once-daily dosing, which improves medication adherence; has few if any immediate side effects; has predictable hematologic toxicities that are dose dependent, transient, and reversible; and has potential benefits against multiple pathophysiologic mechanisms of SCD. Although several therapeutic agents currently under development address specific aspects of the pathophysiology of SCD, only hydroxyurea offers a broad range of beneficial effects that collectively can ameliorate the overall clinical severity of disease.
The drug is classified as an antimetabolite and antineoplastic agent. The exact mechanism of its antineoplastic activity is not elucidated fully but believed to be S-phase specific. Hydroxyurea is converted in vivo to a free radical nitroxide that quenches the tyrosyl free radical at the active site of the M2 subunit of ribonucleotide reductase. As a potent ribonucleotide reductase inhibitor, hydroxyurea blocks the conversion of ribonucleotides to deoxyribonucleotides, which interferes with the synthesis of DNA without any effects on RNA or protein synthesis. The drug is used widely in oral doses (ranging from 20 to 80 mg/kg/d) for the long-term treatment of chronic myeloproliferative disorders, such as polycythemia vera and essential thrombocythemia. In combination with reverse transcriptase inhibitors (eg, didanosine), hydroxyurea is finding a role within HIV therapy as a virostatic agent that produces potent and sustained viral suppression [28].
In patients with hemoglobinopathies, the myelosuppressive and cytotoxic effects of hydroxyurea seem to induce erythroid regeneration and the premature commitment of erythroid precursors, with resulting increased production of HbF-containing reticulocytes and total HbF [29]. Additional pharmacologic effects of hydroxyurea that may contribute to its beneficial effects in SCD include increasing erythrocyte HbF through nitric oxide dependent pathways, decreasing the neutrophil count, increasing erythrocyte volume and hydration, increasing deformability of sickle erythrocytes, and altering the adhesion of sickle erythrocytes to the endothelium [29–33]. The release of nitric oxide directly from the hydroxyurea molecule [34, 35] should allow beneficial local effects on the endothelium, thereby ameliorating the vaso-occlusive process and limiting vascular dysfunction.
Clinical experience
Preclinical studies in anemic cynomolgus monkeys showed that hydroxyurea increased HbF levels [33]. Pilot trials in patients with SCD demonstrated that hydroxyurea also increased HbF in humans and caused little short-term toxicity [29–32]. These proof-of-principle experiments were critical first steps toward an important multicenter phase I/II trial involving adults with HbSS, which identified the short-term efficacy and toxicities of hydroxyurea used at maximum tolerated dose (MTD) [32].
Developed on the basis of favorable results from the phase I/II trial, the National Heart, Lung, and Blood Institute (NHLBI) sponsored the pivotal Multicenter Study of Hydroxyurea (MSH), a double-blinded, placebo-controlled, randomized control trial conducted from 1992 to 1995 in 21 centers in the United States. and Canada [36]. Two hundred and ninety-nine adult patients with HbSS were randomized (152 on hydroxyurea and 147 received placebo) but because of the beneficial effects observed, the trial was stopped early and only 134 subjects completed the planned 24 months of treatment. The hydroxyurea-treated subjects had a 44% reduction in painful crises per year (2.5 events per year versus 4.5 events per year) and a 58% reduction in median annual hospitalization rate for painful crisis (1.0 versus 2.4). In addition there were significantly fewer hydroxyurea-treated subjects who developed acute chest syndrome (ACS) (25 versus 51) and who received blood transfusions (48 versus 73); the number of units of blood transfused also was significantly less (336 versus 586). The incidence of death and stroke did not differ between the two treatment arms; there were no deaths related to hydroxyurea treatment and none of the patients who had received hydroxyurea developed cancer during the trial. The study did not address long-term safety or potential reversibility or prevention of chronic organ damage [36].
The results of this study led the Food and Drug Administration in 1998 to add to the indications for hydroxyurea"to reduce the frequency of painful crises and to reduce the need for blood transfusions in adult patients with sickle cell anemia with recurrent moderate to severe painful crises.” This additional labeling refers only to adults severely affected by painful events rather than the broader spectrum of patients with SCD. Now, 10 years later, there is no change in the manufacturer’s drug labeling for hydroxyurea. Therefore, at this time, all children or patients with mild-to-moderate disease severity or those who do not have painful events but who have ACS or end-organ damage require off-label usage.
The initial success of hydroxyurea in adults led to the first pediatric multicenter phase I/II trial, known as HUG-KIDS, from 1994 to 1996 [37]. Eighty-four children ages 5 to 15 years with severe HbSS disease (defined as three or more painful events within the year before entry, three episodes of ACS within 2 years of entry, or three episodes of ACS or pain within 1 year of entry) were enrolled. Sixty-eight reached MTD and 52 were treated at MTD for 12 months. Similar hematologic effects were seen as in the MSH trial with decreased hemolysis (increased Hb and decreased reticulocytosis, decreased lactate dehydrogenase, and decreased total bilirubin), macrocytosis, improved erythrocyte hydration, myelosuppression, and increased HbF and F cells (Table 1). Laboratory toxicities were mild and reversible with temporary interruption of the medication, and no life-threatening clinical adverse events were observed. Subsequent evaluation of this cohort revealed no adverse effect on height or weight gain or pubertal development [38]. Predictors of HbF response were complex, but a higher treatment HbF was associated with higher baseline HbF, Hb, white blood cell count (WBC), and reticulocytes and compliance [39].
Table 1.
Children with homozygous sickle cell anemia have similar laboratory efficacy using hydroxyurea at maximum tolerated dose as adults
| Adults | Children | |
|---|---|---|
| MTD (mg/kg/d) | 21.3 | 25.6 |
| Δ Hb (g/dL) | +1.2 | +1.2 |
| Δ MCV (fL) | +23 | +14 |
| Δ HbF (%) | +11.2 | +9.6 |
| Δ Reticulocytes (109/L) | −158 | −146 |
| Δ WBC (109/L) | −5.0 | −4.2 |
| Δ ANC (109/L) | −2.8 | −2.2 |
| Δ Bilirubin (mg/dL) | −2.0 | −1.0 |
Short-term clinical efficacy in children initially was reported in small open-label studies [40, 41]. In a small, randomized study from Belgium, children with HbSS treated with hydroxyurea had significantly fewer hospitalizations for pain, with shorter lengths of stay, compared with those receiving placebo [42]. Additional European data showed improved laboratory and clinical response without significant toxicity and no growth or pubertal delay [43]. Follow-up studies have revealed continued efficacy in association with long-term hydroxyurea use in children [44], including a sustained HbF response greater than 20% using hydroxyurea at MTD [45].
The role of hydroxyurea in preserving organ function in SCD is not yet determined. From a practical standpoint, these beneficial effects are difficult to assess prospectively because organ damage develops broadly over the whole pediatric age range, beginning with splenic and renal changes in infancy and evolving to pulmonary and neurologic deficits with vasculopathy among older children. In the Hydroxyurea Safety and Organ Toxicity (HUSOFT) study, infants with HbSS tolerated open-label liquid hydroxyurea and had preserved splenic filtrative function compared with historical controls [46]. A follow-up study on this cohort identified preservation of splenic function and apparent gain of function in some cases [47]. In a recent retrospective study, 43 children with HbSS had splenic function measured before and during treatment with hydroxyurea for a median duration of 2.6 years; six patients (14%) completely recovered splenic function and two (5%) had preserved splenic function, suggesting that hydroxyurea might help preserve or recover splenic function [48]. Similar beneficial effects of hydroxyurea are reported anecdotally for children who have proteinuria [49], priapism [50, 51], or hypoxemia [52].
The role of hydroxyurea in the prevention of stroke in SCD is an area of active investigation. In a retrospective study, hydroxyurea therapy was associated with lower transcranial Doppler (TCD) flow velocities [53]. In a recent prospective single institution study, hydroxyurea was shown to decrease elevated TCD velocities significantly, often into the normal range [54], suggesting that hydroxyurea might serve as an alternative to chronic erythrocyte transfusions for primary stroke prophylaxis. Hydroxyurea also is reported as an alternative to chronic transfusions for secondary stroke prophylaxis in children for whom transfusions cannot be continued safely (eg, erythrocyte allosensitization) [55, 56]. Hydroxyurea in combination with serial phlebotomy effectively prevented secondary stroke and led to resolution of transfusional iron overload in 35 children from a single institution [57]. Based on these encouraging preliminary results, the NHLBI-sponsored Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) trial is underway [58]; this study randomizes children with previous stroke to standard therapy (transfusions and chelation) or alternative therapy (hydroxyurea and phlebotomy) for the prevention of secondary stroke and management of iron overload.
There are limited data regarding the prolonged use of hydroxyurea in SCD, particularly with regard to its long-term risks and benefits, but current clinical experience has not identified any clear detrimental effects or safety concerns. The possibility of hydroxyurea having negative effects on growth and development in children has not been realized [45, 47]. Similarly, concerns about DNA damage and leukemogenesis are not validated, with more than 15 years of exposure among adults and more than 12 years of exposure among children; continued vigilance is warranted but current data are encouraging regarding the long-term safety of this therapy. A 9-year observational follow-up study suggests that adults taking hydroxyurea had a significant 40% reduction in overall mortality [59]. During the MSH Patients’ Follow-up study, there was little risk associated with the careful use of hydroxyurea; however, it was stated that hydroxyurea must be taken indefinitely to be effective and concerns remain over long-term safety. The teratogenicity of hydroxyurea for SCD is not elucidated fully. Anecdotes of normal offspring of women taking hydroxyurea during pregnancy [60, 61] are supported by the lack of birth defects observed in the MSH cohort (Abdullah Kutlar, personal communication, December 2007). Recent reports, however, document abnormal spermatogenesis in men taking hydroxyurea [62, 63], so further investigation in this area is needed. A recently opened study at St. Jude Children’s Research Hospital, entitled Long Term Effects of Hydroxyurea Therapy in Children with Sickle Cell Disease [64], should provide important data regarding long-term risks and benefits of hydroxyurea in this young patient population.
Practical considerations
Hydroxyurea therapy cannot be prescribed, monitored, and adjusted properly according to exact and specific written guidelines. Instead, optimal treatment with hydroxyurea (as with many other medications) requires careful attention to the details of each patient’s treatment response; such individualized therapy often involves as much art as science. The following sections represent the distillation of a combined 25 years of experience with hydroxyurea (>300 treated children), but none of the text should be considered dogma. Instead, these recommendations and suggestions represent a workable and historically successful approach that can serve as a good starting point for health care teams.
Beginning hydroxyurea therapy
The decision to initiate hydroxyurea therapy in a child who has SCD should be made deliberately and thoughtfully. The medical history of a patient should be reviewed carefully to document the number and severity of acute vaso-occlusive events plus any evidence of clinical or laboratory evidence of chronic organ damage, such as hypoxemia, proteinuria, or elevated TCD velocities. Indications for hydroxyurea therapy are not universally agreed upon and each health care team must determine their own threshold; a proposed list is in Table 2. In addition to the laboratory and clinical profile, previous compliance with outpatient clinic visits should be reviewed and the neurocognitive status and psychosocial milieu for the child considered. There are many nonpharmacologic reasons that hydroxyurea therapy can fail in children with SCD, and anticipation of problems with development of creative solutions represents the best way to promote adherence and obtain the optimal drug effects for a child receiving treatment.
Table 2.
Potential indications for hydroxyurea therapy in children with homozygous sickle cell anemia
| Acute vaso-occlusive complications | Painful events | |
| Dactylitis | ||
| Acute chest syndrome | ||
| Laboratory markers of severity | Low hemoglobin | |
| Low HbF | ||
| Elevated WBC | ||
| Elevated LDH | ||
| Organ dysfunction | Brain | Elevated TCD velocities |
| Silent MRI or MRA changes | ||
| Stroke prophylaxis | ||
| Lungs | Hypoxemia | |
| Kidney | Proteinuria | |
| Miscellaneous | Sibling on hydroxyurea | |
| Parental request |
Most pediatric hematologists have accepted clinical severity with acute vaso-occlusive complications as an indication for hydroxyurea therapy, but there is little agreement about indications for children with laboratory abnormalities or organ dysfunction. Similarly, the appropriate age for hydroxyurea initiation has not been determined, although clinical trials have demonstrated safety and efficacy for infants, young children, and school-aged children with SCD.
Healthcare providers never should make the decision to start hydroxyurea unilaterally; team members must discuss the recommendation openly with patients and families. Ideally, all of a patient’s caregivers who might dispense the medication should be present during the initial pretreatment discussions, to ensure that all questions are answered and all concerns addressed. Only if all of the parties involved (patient, parents/guardians, extended family members providing care, and the health care team) are in agreement should hydroxyurea therapy be started. Treatment is likely to fail due to medication nonadherence if any key family member (including the child) is not fully supportive of the decision to begin treatment. Families are told that 6 to 12 months of therapy with monthly clinic visits for examination and blood draw are needed to establish an optimal dose and dosing regimen, so they should make a commitment to this duration before commencing treatment. This verbal contract emphasizes the importance of the commitment to therapy, which is being made by all involved parties. Two to three pretreatment visits also are advised, to explain the nuances of therapy and answer questions, because the decision to begin an indefinite treatment with monthly visits should not be made quickly by a single person at a single visit. Occasionally, an apparently motivated family member fails to return for a follow-up informational visit with an additional parent/guardian or other family member. This kind of missed visit may reflect some unspoken reluctance to begin treatment by parent or patient, unforeseen psychosocial obstacles, or unidentified financial or transportation barriers but allows an early appraisal of the likelihood of treatment success.
Explaining the rationale
The recommendation to begin hydroxyurea therapy and a description of the potential risks and benefits of taking the drug should be communicated to patients and family members in a straightforward and honest way, using age-appropriate and culturally sensitive language and vocabulary. Some families have access to the Internet and already have acquired detailed information and formed specific questions, whereas others have little knowledge of the drug beyond what is provided by the health care team. Providing a rationale that includes mechanisms of HbF induction or nitric oxide metabolism generally is not helpful or persuasive in the majority of cases. Instead, a general review of the pathophysiology of sickle cell vaso-occlusion typically is sufficient, indicating where hydroxyurea might be beneficial. Most children recognize that sickled erythrocytes have an elongated shape; hence, comments like “hydroxyurea helps your blood cells stay round” can help motivate even young patients to stay on therapy and serve as easy reminders of the benefits of treatment during subsequent visits. Many families realize that their children were generally healthy during the first few months of life, so the benefits of HbF can be put into this context. The importance of daily medication adherence cannot be overemphasized. To help children understand this principle, hydroxyurea can be likened to a powerful vitamin to be taken daily. Families are reminded that a child will not feel better or worse immediately after each dose, and the beneficial effects occur in the blood cells over time and leading eventually to overall improvement.
Describing risks and benefits
The potential benefits of hydroxyurea therapy are best discussed with patients and families not only in terms of preventing acute clinical complications, such as pain and ACS, but also as helping avoid hospitalizations and transfusions, enhancing growth, and possibly preventing chronic organ damage. Adverse short-term side effects of taking hydroxyurea are described as usually minimal and often none, except for occasional mild gastrointestinal discomfort. The treatment effects of lowering the blood counts to modest neutropenia are described as predictable and actually desired but requiring periodic dose escalation with monthly monitoring to achieve a stable MTD. Potential deleterious effects on hair or skin are mentioned but minimized, except for occasional (< 5%) hyperpigmentation and melanonychia; hepatic and renal drug-related toxicity is described as rare, probably no more than approximately 1 in 1000.
The long-term risks for hydroxyurea therapy are discussed as largely unknown, although accumulating evidence of the drug’s long-term safety and efficacy (currently > 15 years in adults and > 12 years in children) makes this particular point easier to discuss with each passing year. The risks of hydroxyurea for fertility and offspring are discussed; the potential of hydroxyurea as a teratogen in animals provides the strongest rationale for contraception, but the absence of teratogenicity or sterility observed to date among humans, including adult patients from the MSH study, is emphasized. Among the most important discussion points with families are those related to the potential of long-term hydroxyurea exposure to cause cancer in their child. First, it is noted that hydroxyurea initially was developed as an anticancer agent and still is used to treat certain forms of cancer. Next, it is noted that children with SCD, just like other children, can develop leukemia and other pediatric cancers [65]. Third, it is noted that adult patients who have preleukemic conditions, such as myeloproliferative disorders, may have an increased risk for developing cancer after 10 to 20 years of hydroxyurea therapy, but this has not yet been observed in children or adults with SCD. Finally, the theoretic risk of developing cancer in 20 years should be compared with the known natural history of untreated children with SCD and clinical severity, and to the high likelihood of acute and chronic clinical complications, poor quality of life, and increased risk for early death [6, 66]. With this approach, the long-term risks of malignancy are not trivialized but are placed into context. A recent National Institutes of Health Consensus Conference concluded that the risk for cancer associated with hydroxyurea therapy in SCD does not appear to be higher than the baseline rate for this patient population [67].
Dose initiation
Before initiating hydroxyurea therapy, baseline laboratory studies should be obtained (Fig. 1). Based on data from the HUG-KIDS [37],HUSOFT [46], Toddler HUG [68], and other studies [45, 54], the vast majority of children with HbSS tolerate an initial hydroxyurea dose of 20 mg/kg/d given as a single dose. Earlier studies used a lower initial starting dose of 15 mg/kg/d [32, 37], but almost every pediatric patient tolerates hydroxyurea at 20 mg/kg/d unless there is concomitant renal dysfunction. The dose of hydroxyurea does not need to be adjusted for ideal body weight, because obesity is rare among untreated children with SCD. Hydroxyurea capsules are available commercially (200 mg, 300 mg, 400 mg, and 500 mg capsules), allowing fairly precise dosing regimens with accuracy within 2 mg/kg/d. At some centers, dosing is achieved with only 500mgcapsules, using doses such as: one capsule per day (500 mg/d), one capsule alternating with two capsules per day (750 mg/d), two capsules per day (1000 mg/d), and so forth. Adherence is improved, however, when the same dose is administered every day. Giving the entire daily dose at once, as opposed to a twice or three times daily dosing, improves adherence and offers some pharmacokinetic advantages [69].
Fig. 1.
Guideline for initiating, modifying, and monitoring hydroxyurea therapy (see text for further details). Asterisk indicates prehydroxyurea laboratory studies that are performed to help determine the etiology of potential treatment related laboratory changes or toxicities (eg, transaminitis, macrocytosis, and reticulocytopenia).
For young children or those who cannot tolerate swallowing capsules, a liquid hydroxyurea formulation often can be prepared by a local or institutional pharmacy. Hydroxyurea capsule contents or bulk hydroxyurea powder can be dissolved in water with vigorous stirring and sweetener can be added for flavoring palatability; such liquid formulations are stable for weeks to months with refrigeration or at room temperature [70]. The initial hydroxyurea slurry should not be heated to speed up dissolution, however, because structural and functional activity is diminished. Liquid hydroxyurea formulations are easy to dose (usually to 0.2 mL precision), allowing fine tuning of daily doses before and after MTD is achieved.
It is recommended that the hydroxyurea dose be administered at a time of day that is most convenient for patients and families. In many instances, this is in the morning or before school or the workday but can be in the afternoon, early evening, or before bedtime. The exact timing should not be regimented or overly emphasized; the critical feature is reliable dosing once each day. Families may worry about “missing a dose” by several hours but this is not a problem; it should be emphasized, however, that the daily dose just needs to be swallowed at some time during each day. Occasional patients (approximately 5%) mention gastrointestinal symptoms, such as stomachache or nausea, after taking hydroxyurea in the morning; in these instances, changing to evening dosing almost always leads to resolution of symptoms.
Dose escalation to maximum tolerated dose
Beneficial effects of hydroxyurea can begin in the first few weeks after commencing therapy [71], which can lead to some reluctance by medical providers to increase the dose beyond that needed for subjective clinical improvement. Because the salutary laboratory effects of hydroxyurea, especially induction of HbF and diminution of WBC and absolute neutrophil count (ANC), are dose dependent [32,45], however, it seems logical and advisable to increase the daily hydroxyurea dose to achieve the MTD. Based on comparative data documenting superior laboratory effects when hydroxyurea is prescribed at MTD [45], the goal of hydroxyurea should be to achieve modest marrow suppression without undue hematologic toxicity.
After initiating hydroxyurea therapy (approximately 20 mg/kg/d), the child is seen in the outpatient clinic setting approximately every 4 weeks. At each interval visit, medical history is obtained and physical examination performed along with a discussion of dosing issues and emphasis on daily adherence. A complete blood cell count with WBC differential and reticulocyte count should be performed at each interval visit, and the next month’s dose should not be ordered or dispensed until that day’s weight and blood counts are available. The daily dose should be increased by approximately 5 mg/kg/d every 8 weeks if no toxicity occurs. The 4-week interval is too short for most dose adjustments, because hematologic toxicity can accumulate and not manifest fully until 8 weeks after a dose increase. It is critical to examine the trends in peripheral blood counts at each visit—sometimes toxicity is slowly cumulative and can be anticipated based on changes identified over 8 to 16 weeks.
Hydroxyurea is titrated most easily according to the peripheral blood counts and typically is limited by neutropenia, occasionally by reticulocytepenia, and more rarely by thrombocytopenia. The target ANC for MTD should be approximately 2 to 4 × 109/L (2000–4000 per µL), although other hematologic toxicity may limit dose escalation. Based on published data [45, 55, 57], most children with HbSS require a dose of 25 to 30 mg/kg/d to reach this MTD. The maximum daily dose of hydroxyurea should not exceed 35 mg/kg/d or 2000 mg/d; failure to achieve marrow suppression at these doses strongly suggests medication nonadherence. The MTD, measured in mg/kg/d, typically is established within 4 to 8 months of initiating hydroxyurea therapy but should be assigned only after a child tolerates a particular dose for at least 8 weeks. The MTD then usually remains relatively stable unless there is substantial weight gain, development of splenomegaly, or change in renal function. Once a child reaches MTD, the dose in mg/kg/d, should not be modified frequently, because multiple dose changes and blood count checks are unnecessary and may incorrectly suggest a narrow therapeutic window. Periodic increases in absolute daily dose due to weight gain are appropriate. When a stable MTD is reached, it may be appropriate to decrease the frequency of clinic visits to bimonthly, depending on patient response and family reliability. Extension to quarterly visits usually is associated, however, with a decline in adherence, likely because of lack of frequent reminders from medical providers.
Dose modification
Hematologic toxicity is by far the most common reason to modify the hydroxyurea dose, usually before reaching the MTD. Although early studies on children with SCD used conservative thresholds for medication stoppage and subsequent dose modifications (eg, ANC < 2000 in HUG-KIDS) [37], a more liberal approach can be used safely in the majority of children. Practical toxicity definitions and thresholds for erythrocytes, reticulocytes, neutrophils, and platelets are listed in Table 3. Traditionally, hydroxyurea toxicity guidelines also include thresholds for hepatic or renal toxicity (eg, alanine aminotransferase increase > 3–5 × the upper limit of normal or a doubling of creatinine) but such organ toxicity almost never is associated with hydroxyurea treatment. An increase in alanine aminotransferase or creatinine should never be assumed to be drug related, and additional investigations with ultrasonography or other tests should be strongly considered.
Table 3.
Hematologic toxicity thresholds requiring hydroxyurea dose modifications
| Neutrophils | ANC < 1.0 × 109/L (1000 per µL) |
| Hemoglobin | < 7.0 g/dL with low reticulocytes (eg, absolute reticulocyte count < 100 × 109/L [100 K per µL]) |
| Decrease by > 20% from previous value, with low reticulocytes (as previously) |
|
| Reticulocytes | < 80 × 109/L (80 K per µL) unless the hemoglobin concentration is > 8.0 g/dL |
| Platelets | < 80 × 109/L (80 K per µL) |
When a hematologic toxicity occurs on hydroxyurea therapy, the medication should be discontinued to allow the counts to recover. Almost all hematologic toxicities are transient, reversible, and dose dependent and recover within 1 week of drug interruption, although severe toxicities may feature pancytopenia and take 2 to 3 weeks until recovery. If the counts recover in 1 week, then the dose can either be resumed at the previous amount or decreased modestly (eg, reduced by 2.5–5 mg/kg/d). Conversely, if laboratory values suggest that a dose increase would be tolerated after 2 months at a stable dose, the MTD dose can be increased by a small amount (such as 2.5 mg/kg/d). Before increasing a hydroxyurea dose beyond a previously established stable MTD, however, the likelihood of diminished medication adherence should be strongly considered.
Increasing adherence
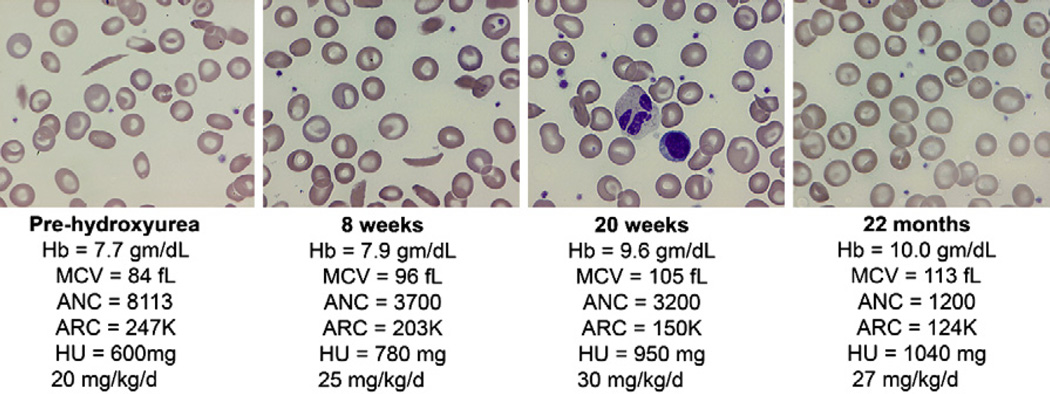
Medication adherence is “perhaps the best documented but least understood health-related behavior” [72]. Children and their family members are much more likely to be adherent to hydroxyurea therapy and the frequent clinic visits if they believe that treatment will be beneficial. At each clinic visit, the importance of daily medication should be emphasized; specific questions should be asked regarding who gives the dose, what time it is administered, how many doses are missed per week, and so forth. Visualization of the peripheral blood smear is an effective way to illustrate the benefits of hydroxyurea therapy. The de-identified peripheral blood smears of several patients, which were obtained pre- and post-treatment MTD, can be shown to children and family members. The authors use a multiheaded microscope before initiating hydroxyurea therapy to demonstrate the obvious changes that occur with good adherence and a good treatment response (Fig. 2), including anisocytosis, macrocytosis, decreased polychromasia, and fewer sickled forms. This viewing and explanation should be performed by an experienced medical provider who can emphasize that adherence also can be monitored by review of the blood counts and the peripheral blood smear.
Fig. 2.
Changes in complete blood cell count parameters and erythrocyte morphology in association with hydroxyurea therapy, from dose initiation through escalation to MTD. The initial panel shows blood counts and the peripheral blood smear at dose initiation, with hemolytic anemia and leukocytosis evident along with sickled forms. The second panel is after 8 weeks of hydroxyurea therapy (at approximately 20 mg/kg/d) with some macrocytes and anisocytosis present, along with reductions in the ANC and ARC; the dose was escalated (to approximately 25 mg/kg/d). The third panel is after 20 weeks of hydroxyurea therapy, with less anemia and sickling, more macrocytosis, and modest myelosuppression; the dose was escalated (to approximately 30 mg/kg/d). The fourth panel is after 22 months of hydroxyurea therapy (at MTD of 27 mg/kg/d); there is improved Hb with pronounced macrocytosis and no sickled forms, along with modest neutropenia and reticulocytopenia. ARC, absolute reticulocyte count; Hb, hemoglobin; HU, hydroxyurea.
When explanations of risks and benefits of hydroxyurea therapy are given to patients and family members, it is emphasized that a parent must be in charge of ensuring that the medication actually is swallowed each day. It is imperative to anticipate that occasionally children miss a dose of hydroxyurea without any ill effect, and therefore be tempted to miss several days, wondering if they somehow have been cured. Explaining that blood cells are produced every day, hence the medication must be taken every day, is logical even for young patients. Parents must be reminded at each interval visit that they must be sure to give the medication; teenagers are especially notorious for embellishing adherence. In some instances, patients can be remarkably adherent and even remind parents about dosing.
The use of a “medication score card” can be helpful for improving hydroxyurea adherence. Serial listing of monthly blood counts according to various blood count parameters can be used to show beneficial changes, such as increased hemoglobin concentration, mean corpuscular volume (MCV), and HbF; concomitant decreases in WBC and ANC also easily can be seen. Additional strategies to improving hydroxyurea adherence include providing a calendar to mark off days after medicine has been swallowed, preloading a weekly/biweekly/monthly pill container with prescribed capsules, keeping the pill bottle in plain sight (eg, the kitchen table) to minimize forgotten doses, and counting leftover or unused pills. Whatever the mnemonic devices used, among the best strategies for successful treatment are a thorough understanding of the rationale for treatment, a limited number of health care providers for continuity to patients and family, and regular clinic visits on a 1 to 2 month basis, to engender trust and loyalty with emphasis on the beneficial treatment effects and the need for daily adherence.
Summary
Hydroxyurea is a powerful therapeutic agent with proved laboratory and clinical efficacy for children with SCD. Although there are important questions regarding its long-term efficacy and safety, hydroxyurea has the potential to ameliorate many of the signs and symptoms of the disease. Ongoing clinical trials will help answer questions about the proper clinical indications for its use and, in particular, its ability to prevent organ damage and preserve organ function and long-term safety.
Acknowledgments
The authors thank Nicole A. Mortier, MHS PA-C, and William H. Schultz, MHS PA-C, for years of experience and dedication to treating children with SCD. We appreciate their insights and advice regarding the optimal use of hydroxyurea in this patient population.
Dr. Heeney is supported by NIH K12 HL087164 and U54 HL070819. Dr. Ware is supported by U54 HL070590, U01 HL078787, N01 HB 07155, and American Syrian Lebanese Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg MH, Rosenstock W, Coleman MB, et al. Effects of thalassemia and microcytosis on the hematologic and vasoocclusive severity of sickle cell anemia. Blood. 1984;63(6):1353–1360. [PubMed] [Google Scholar]
- 3.Baum KF, Dunn DT, Maude GH, et al. The painful crisis of homozygous sickle cell disease. A study of the risk factors. Arch Intern Med. 1987;147(7):1231–1234. [PubMed] [Google Scholar]
- 4.Phillips G, Jr, Coffey B, Tran-Son-Tay R, et al. Relationship of clinical severity to packed cell rheology in sickle cell anemia. Blood. 1991;78(10):2735–2739. [PubMed] [Google Scholar]
- 5.Powars DR. Sickle cell anemia: beta s-gene-cluster haplotypes as prognostic indicators of vital organ failure. Semin Hematol. 1991;28(3):202–208. [PubMed] [Google Scholar]
- 6.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 7.Charache S. Fetal hemoglobin, sickling, and sickle cell disease. Adv Pediatr. 1990;37:1–31. [PubMed] [Google Scholar]
- 8.Wood WG. Increased HbF in adult life. Baillieres Clin Haematol. 1993;6(1):177–213. doi: 10.1016/s0950-3536(05)80070-8. [DOI] [PubMed] [Google Scholar]
- 9.Serjeant GR. Fetal haemoglobin in homozygous sickle cell disease. Clin Haematol. 1975;4(1):109–122. [PubMed] [Google Scholar]
- 10.Wood WG, Stamatoyannopoulos G, Lim G, et al. F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F. Blood. 1975;46(5):671–682. [PubMed] [Google Scholar]
- 11.Watson J, Stahman AW, Bilello FP. Significance of paucity of sickle cells in newborn negro infants. Am J Med Sci. 1948;215:419–423. doi: 10.1097/00000441-194804000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg MH. Compound heterozygous and other hemoglobinopathies. In: Steinberg MH, Forget BG, Higgs DR, editors. Disorders of hemoglobin: genetics, pathophysiology, and clinical management. Cambridge (UK): Cambridge University Press; 2001. pp. 786–810. [Google Scholar]
- 13.Odenheimer DJ, Sarnaik SA, Whitten CF, et al. The relationship between fetal hemoglobin and disease severity in children with sickle cell anemia. Am JMed Genet. 1987;27(3):525–535. doi: 10.1002/ajmg.1320270305. [DOI] [PubMed] [Google Scholar]
- 14.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 15.Powars DR, Schroeder WA, Weiss JN, et al. Lack of influence of fetal hemoglobin levels or erythrocyte indices on the severity of sickle cell anemia. J Clin Invest. 1980;65(3):732–740. doi: 10.1172/JCI109720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powars DR, Weiss JN, Chan LS, et al. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984;63(4):921–926. [PubMed] [Google Scholar]
- 17.Leikin SL, Gallagher D, Kinney TR, et al. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics. 1989;84(3):500–508. [PubMed] [Google Scholar]
- 18.Ley TJ, DeSimone J, Noguchi CT, et al. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983;62(2):370–380. [PubMed] [Google Scholar]
- 19.DeSimone J, Koshy M, Dorn L, et al. Maintenance of elevated fetal hemoglobin levels by decitabine during dose interval treatment of sickle cell anemia. Blood. 2002;99(11):3905–3908. doi: 10.1182/blood.v99.11.3905. [DOI] [PubMed] [Google Scholar]
- 20.Koshy M, Dorn L, Bressler L, et al. 2-Deoxy 5-azacytidine and fetal hemoglobin induction in sickle cell anemia. Blood. 2000;96(7):2379–2384. [PubMed] [Google Scholar]
- 21.Saunthararajah Y, Hillery CA, Lavelle D, et al. Effects of 5-aza-2′-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood. 2003;102(12):3865–3870. doi: 10.1182/blood-2003-05-1738. [DOI] [PubMed] [Google Scholar]
- 22.Atweh GF, Sutton M, Nassif I, et al. Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood. 1999;93(6):1790–1797. [PMC free article] [PubMed] [Google Scholar]
- 23.Dover GJ, Brusilow S, Charache S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood. 1994;84(1):339–343. [PubMed] [Google Scholar]
- 24.Resar LM, Segal JB, Fitzpatric LK, et al. Induction of fetal hemoglobin synthesis in children with sickle cell anemia on low-dose oral sodium phenylbutyrate therapy. J Pediatr Hematol Oncol. 2002;24(9):737–741. doi: 10.1097/00043426-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Sher GD, Ginder GD, Little J, et al. Extended therapy with intravenous arginine butyrate in patients with beta-hemoglobinopathies. N Engl J Med. 1995;332(24):1606–1610. doi: 10.1056/NEJM199506153322404. [DOI] [PubMed] [Google Scholar]
- 26.Davies SC, Gilmore A. The role of hydroxyurea in the management of sickle cell disease. Blood Rev. 2003;17(2):99–109. doi: 10.1016/s0268-960x(02)00074-7. [DOI] [PubMed] [Google Scholar]
- 27.Halsey C, Roberts IA. The role of hydroxyurea in sickle cell disease. Br J Haematol. 2003;120(2):177–186. doi: 10.1046/j.1365-2141.2003.03849.x. [DOI] [PubMed] [Google Scholar]
- 28.Lori F, Foli A, Groff A, et al. Optimal suppression of HIV replication by low-dose hydroxyurea through the combination of antiviral and cytostatic (‘virostatic’) mechanisms. AIDS. 2005;19(11):1173–1181. doi: 10.1097/01.aids.0000176217.02743.d1. [DOI] [PubMed] [Google Scholar]
- 29.Dover GJ, Humphries RK, Moore JG, et al. Hydroxyurea induction of hemoglobin F production in sickle cell disease: relationship between cytotoxicity and F cell production. Blood. 1986;67(3):735–738. [PubMed] [Google Scholar]
- 30.Platt OS, Orkin SH, Dover G, et al. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984;74(2):652–656. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charache S, Dover GJ, Moyer MA, et al. Hydroxyurea-induced augmentation of fetal hemoglobin production in patients with sickle cell anemia. Blood. 1987;69(1):109–116. [PubMed] [Google Scholar]
- 32.Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79(10):2555–2565. [PubMed] [Google Scholar]
- 33.Letvin NL, Linch DC, Beardsley GP, et al. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984;310(14):869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- 34.Gladwin MT, Shelhamer JH, Ognibene FP, et al. Nitric oxide donor properties of hydroxyurea in patients with sickle cell disease. Br J Haematol. 2002;116(2):436–444. doi: 10.1046/j.1365-2141.2002.03274.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Yakubu M, Kim-Shapiro DB, et al. Rat liver-mediated metabolism of hydroxyurea to nitric oxide. Free Radic Biol Med. 2006;40(9):1675–1681. doi: 10.1016/j.freeradbiomed.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 37.Kinney TR, Helms RW, O’Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94(5):1550–1554. [PubMed] [Google Scholar]
- 38.Wang WC, Helms RW, Lynn HS, et al. Effect of hydroxyurea on growth in children with sickle cell anemia: results of the HUG-KIDS Study. J Pediatr. 2002;140(2):225–229. doi: 10.1067/mpd.2002.121383. [DOI] [PubMed] [Google Scholar]
- 39.Ware RE, Eggleston B, Redding-Lallinger R, et al. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood. 2002;99(1):10–14. doi: 10.1182/blood.v99.1.10. [DOI] [PubMed] [Google Scholar]
- 40.Scott JP, Hillery CA, Brown ER, et al. Hydroxyurea therapy in children severely affected with sickle cell disease. J Pediatr. 1996;128(6):820–828. doi: 10.1016/s0022-3476(96)70335-9. [DOI] [PubMed] [Google Scholar]
- 41.Jayabose S, Tugal O, Sandoval C, et al. Clinical and hematologic effects of hydroxyurea in children with sickle cell anemia. J Pediatr. 1996;129(4):559–565. doi: 10.1016/s0022-3476(96)70121-x. [DOI] [PubMed] [Google Scholar]
- 42.Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88(6):1960–1964. [PubMed] [Google Scholar]
- 43.de Montalembert M, Belloy M, Bernaudin F, et al. Three-year follow-up of hydroxyurea treatment in severely ill children with sickle cell disease. The French Study Group on Sickle Cell Disease. J Pediatr Hematol Oncol. 1997;19(4):313–318. doi: 10.1097/00043426-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Ferster A, Tahriri P, Vermylen C, et al. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97(11):3628–3632. doi: 10.1182/blood.v97.11.3628. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 46.Wang WC, Wynn LW, Rogers ZR, et al. A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. J Pediatr. 2001;139(6):790–796. doi: 10.1067/mpd.2001.119590. [DOI] [PubMed] [Google Scholar]
- 47.Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood. 2005;106(7):2269–2275. doi: 10.1182/blood-2004-12-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hankins JS, Helton KJ, McCarville MB, et al. Preservation of spleen and brain function in children with sickle cell anemia treated with hydroxyurea. Pediatr Blood Cancer. 2008;50(2):293–297. doi: 10.1002/pbc.21271. [DOI] [PubMed] [Google Scholar]
- 49.Fitzhugh CD, Wigfall DR, Ware RE. Enalapril and hydroxyurea therapy for children with sickle nephropathy. Pediatr Blood Cancer. 2005;45(7):982–985. doi: 10.1002/pbc.20296. [DOI] [PubMed] [Google Scholar]
- 50.Saad ST, Lajolo C, Gilli S, et al. Follow-up of sickle cell disease patients with priapism treated by hydroxyurea. Am J Hematol. 2004;77(1):45–49. doi: 10.1002/ajh.20142. [DOI] [PubMed] [Google Scholar]
- 51.Maples BL, Hagemann TM. Treatment of priapism in pediatric patients with sickle cell disease. Am J Health Syst Pharm. 2004;61(4):355–363. doi: 10.1093/ajhp/61.4.355. [DOI] [PubMed] [Google Scholar]
- 52.Singh S, Koumbourlis A, Aygun B. Resolution of chronic hypoxemia in pediatric sickle cell patients after treatment with hydroxyurea. Pediatr Blood Cancer. doi: 10.1002/pbc.21480. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Kratovil T, Bulas D, Driscoll MC, et al. Hydroxyurea therapy lowers TCD velocities in children with sickle cell disease. Pediatr Blood Cancer. 2006;47(7):894–900. doi: 10.1002/pbc.20819. [DOI] [PubMed] [Google Scholar]
- 54.Zimmerman SA, Schultz WH, Burgett S, et al. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007;110(3):1043–1047. doi: 10.1182/blood-2006-11-057893. [DOI] [PubMed] [Google Scholar]
- 55.Ware RE, Zimmerman SA, Schultz WH. Hydroxyurea as an alternative to blood transfusions for the prevention of recurrent stroke in children with sickle cell disease. Blood. 1999;94(9):3022–3026. [PubMed] [Google Scholar]
- 56.Sumoza A, de Bisotti R, Sumoza D, et al. Hydroxyurea (HU) for prevention of recurrent stroke in sickle cell anemia (SCA) Am J Hematol. 2002;71(3):161–165. doi: 10.1002/ajh.10205. [DOI] [PubMed] [Google Scholar]
- 57.Ware RE, Zimmerman SA, Sylvestre PB, et al. Prevention of secondary stroke and resolution of transfusional iron overload in children with sickle cell anemia using hydroxyurea and phlebotomy. J Pediatr. 2004;145(3):346–352. doi: 10.1016/j.jpeds.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 58.ClinicalTrials.gov. [Accessed March 1, 2008];Stroke with transfusions changing to hydroxyurea (SWiTCH) Available at: http://clinicaltrials.gov/ct2/show/NCT00122980.
- 59.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 60.Pata O, Tok CE, Yazici G, et al. Polycythemia vera and pregnancy: a case report with the use of hydroxyurea in the first trimester. Am J Perinatol. 2004;21(3):135–137. doi: 10.1055/s-2004-823773. [DOI] [PubMed] [Google Scholar]
- 61.Byrd DC, Pitts SR, Alexander CK. Hydroxyurea in two pregnant women with sickle cell anemia. Pharmacotherapy. 1999;19(12):1459–1462. doi: 10.1592/phco.19.18.1459.30901. [DOI] [PubMed] [Google Scholar]
- 62.Grigg A. Effect of hydroxyurea on sperm count, motility and morphology in adult men with sickle cell or myeloproliferative disease. Intern Med J. 2007;37(3):190–192. doi: 10.1111/j.1445-5994.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 63.Masood J, Hafeez A, Hughes A, et al. Hydroxyurea therapy: a rare cause of reversible azoospermia. Int Urol Nephrol. 2007;39(3):905–907. doi: 10.1007/s11255-006-9107-4. [DOI] [PubMed] [Google Scholar]
- 64.ClinicalTrials.gov. [Accessed March 1, 2008];Longtermeffects of hydroxyurea therapy in children with sickle cell disease. Available at: http://clinicaltrials.gov/ct2/show/NCT00305175.
- 65.Schultz WH, Ware RE. Malignancy in patients with sickle cell disease. Am J Hematol. 2003;74(4):249–253. doi: 10.1002/ajh.10427. [DOI] [PubMed] [Google Scholar]
- 66.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342(2):83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 67.NIH. [Accessed March 1, 2008];National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. 2008 doi: 10.7326/0003-4819-148-12-200806170-00220. Available at: http://consensus.nih.gov/2008/Sickle%20Cell%20Draft%20Statement%2002-27-08.pdf. [DOI] [PubMed]
- 68.Thornburg CD, Dixon N, Burgett S, et al. Efficacy of hydroxyurea to prevent organ damage in young children with sickle cell anemia. Blood. 2007;110(11):3386. doi: 10.1002/pbc.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan JH, Ataga K, Kaul S, et al. The influence of renal function on hydroxyurea pharmacokinetics in adults with sickle cell disease. J Clin Pharmacol. 2005;45(4):434–445. doi: 10.1177/0091270004273526. [DOI] [PubMed] [Google Scholar]
- 70.Heeney MM, Whorton MR, Howard TA, et al. Chemical and functional analysis of hydroxyurea oral solutions. J Pediatr Hematol Oncol. 2004;26(3):179–184. doi: 10.1097/00043426-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Bridges KR, Barabino GD, Brugnara C, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood. 1996;88(12):4701–4710. [PubMed] [Google Scholar]
- 72.Becker MH, Maiman LA. Sociobehavioral determinants of compliance with health and medical care recommendations. Med Care. 1975;13(1):10–24. doi: 10.1097/00005650-197501000-00002. [DOI] [PubMed] [Google Scholar]