Abstract
Wnt ligands regulate heart morphogenesis but the underlying mechanisms remain unclear. Two Formin-related proteins, DAAM1 and 2, were previously found to bind the Wnt effector Disheveled. Here, since DAAM1 and 2 nucleate actin and mediate Wnt-induced cytoskeletal changes, a floxed-allele of Daam1 was used to disrupt its function specifically in the myocardium and investigate Wnt-associated pathways. Homozygous Daam1 conditional knockout (CKO) mice were viable but had misshapen hearts and poor cardiac function. The defects in Daam1 CKO mice were observed by mid-gestation and were associated with a loss of protrusions from cardiomyocytes invading the outflow tract. Further, these mice exhibited noncompaction cardiomyopathy (NCM) and deranged cardiomyocyte polarity. Interestingly, Daam1 CKO mice that were also homozygous for an insertion disrupting Daam2 (DKO) had stronger NCM, severely reduced cardiac function, disrupted sarcomere structure, and increased myocardial proliferation, suggesting that DAAM1 and DAAM2 have redundant functions. While RhoA was unaffected in the hearts of Daam1/2 DKO mice, AKT activity was lower than in controls, raising the issue of whether DAAM1/2 are only mediating Wnt signaling. Daam1-floxed mice were thus bred to Wnt5a null mice to identify genetic interactions. The hearts of Daam1 CKO mice that were also heterozygous for the null allele of Wnt5a had stronger NCM and more severe loss of cardiac function than Daam1 CKO mice, consistent with DAAM1 and Wnt5a acting in a common pathway. However, deleting Daam1 further disrupted Wnt5a homozygousnull hearts, suggesting that DAAM1 also has Wnt5a-independent roles in cardiac development.
Introduction
In noncompaction cardiomyopathy (NCM), the trabecular and compact layers of the embryonic myocardium fail to integrate into the single-layered myocardium of the adult heart (Carrilho-Ferreira et al., 2014; Oechslin and Jenni, 2011; Towbin et al., 2015). NCM confers an increased risk of heart failure, arrhythmias, embolic events, and sudden death (Carrilho-Ferreira et al., 2014; Oechslin and Jenni, 2011; Towbin et al., 2015). Understanding the mechanisms behind myocardial growth and maturation will aid the detection of NCM and amelioration of its consequences. While the intercellular signals that guide the specification, maintenance, and differentiation of cardiac progenitor cells have been described (Francou et al., 2013; Rochais et al., 2009; Tzahor, 2007; Zaffran and Kelly, 2012), those guiding their cytoskeletal rearrangements and morphogenesis into adult myocardium remain unclear.
Wnt proteins are ligands that regulate developmental processes, including cell fate decisions, differentiation, cytoskeletal remodeling, adhesion, proliferation and survival (Cadigan and Nusse, 1997; Niehrs and Acebron, 2012; Teo and Kahn, 2010). Wnt proteins control gene expression via a canonical signaling pathway that stabilizes β-catenin and allows it to enter the nucleus, where it activates TCF/Lef1 family transcription factors (Angers and Moon, 2009; Eastman and Grosschedl, 1999). Wnt proteins also signal through several non-canonical pathways (De, 2011; Kuhl, 2002; Kuhl et al., 2000; Strutt, 2003; Tada et al., 2002). The best characterized of these resembles the Drosophila planar cell polarity (PCP) pathway, which orients actin-based hairs on the wings of flies by activating RhoA (Gao, 2012; Maung and Jenny, 2011; Sebbagh and Borg, 2014). In vertebrates, Wnt/PCP signaling regulates diverse processes, including neural tube closure and the polarization of cochlear stereocilia (Ezan and Montcouquiol, 2013; Gao, 2012; Sebbagh and Borg, 2014; Wu et al., 2011). Moreover, mutations in the non-canonical Wnt genes, Wnt5a and Wnt11, as well as the PCP pathway component, Vangl2, disrupt cardiac development (Cohen et al., 2012; Etheridge et al., 2008; Henderson et al., 2006; Nagy et al., 2010; Phillips et al., 2008; Phillips et al., 2005; Schleiffarth et al., 2007; Sinha et al., 2015).
A screen for factors bound to the Wnt effector Disheveled (DVL) identified two Diaphanous family Formin-homology (FH) proteins called Disheveled associated activator of morphogenesis 1 and 2 (DAAM1 and 2) (Habas et al., 2001). DAAM1 and 2 have proline-rich FH1 domains, which recruit the actin capping protein Profilin, and FH2 domains, which catalyze the assembly of unbranched actin filaments (Aspenstrom, 2010; Kuhn and Geyer, 2014; Schonichen and Geyer, 2010). Unlike Diaphanous, which is activated by RhoA binding, DAAM1 has low affinity for RhoA under basal conditions (Habas et al., 2001; Kuhn and Geyer, 2014; Liu et al., 2008). However Wnt/PCP signaling causes DVL to bind DAAM1, which increases its affinity for RhoA and promotes its polymerization of actin (Habas et al., 2001; Liu et al., 2008; Young and Copeland, 2010). DAAM1 thus appears to directly link non-canonical Wnt ligands at the cell surface to the actin cytoskeleton. Interestingly, mice homozygous for a β-geo insertion into the Daam1 locus (Daam1β-geo) exhibit NCM (Li et al., 2011), suggesting that DAAM1-mediated Wnt/PCP signaling may orient the cytoskeletons of trabecular cardiomyocytes and guide their integration into the compact myocardium of the adult heart.
Yet despite these findings, a number of questions remain about the roles played by DAAM1 (and 2) in cardiac development. First, because Daam1β-geo introduces a null allele globally (Li et al., 2011), it is unclear whether the heart phenotype reflects autonomous roles for DAAM1 in myocardial cells. Second, these heart phenotypes are mild (Li et al., 2011), suggesting that additional roles for DAAM1 may be masked by redundancy with DAAM2. Third, while the ability of DAAM1 to bind RhoA and DVL suggests that it functions in Wnt/PCP signaling (Habas et al., 2001), mutations in the single Drosophila homolog DAAM do not disrupt PCP (Matusek et al., 2006), raising the question of whether DAAM1 is truly involved in this pathway.
Here, a novel floxed allele of Daam1 (Daam1floxed) was used to create a homozygous conditional knockout (CKO) to examine the autonomous roles for DAAM1 in the myocardial lineage. Further, Daam1floxed mice were bred to mice carrying a lacZ insertion into the Daam2 locus (Daam2lacZ) to determine if DAAM1 and 2 are redundant within the myocardium. Finally, Daam1floxed mice were bred to Wnt5a mutant mice to identify genetic interactions. The resulting data suggest that the morphological abnormalities and NCM exhibited by Daam1 mutants reflect an autonomous role for DAAM1 in the polarized protrusive activity in cardiomyocytes, demonstrate that both DAAM1 and 2 are required for cardiomyocyte maturation, and implicate DAAM1 in both Wnt-dependent and -independent functions.
Methods
Generation of Daam1floxed and Daam2lacZ mice
The targeting vectors used to disrupt Daam1 and Daam2 were generated from BACs covering the Daam1 and Daam2 loci from male CJ7/129/Sv mice (Research Genetics, CITB-CJ7-B, Cat. 96021). The construct used to generate the Daam1floxed allele (Figure S1A) was made from a 9.6 kb ClaI to BamHI digestion fragment spanning exons 5 to 12 of the Daam1 gene. LoxP sites were inserted into SalI and BglII sites within the introns flanking exon 6. A PGK-tk cassette was added upstream of the 5’ homologous arm and an FRT-flanked PGK-neo cassette was placed downstream of the 3’ loxP site. The 5’ homologous arm of the construct used to generate the Daam2lacZ allele (Figure S2A) consisted of a 6.2 kb EcoRV to PstI fragment containing exons 6–9 as well as part of exon 10 of the Daam2 gene. An IRES-lacZ cassette and loxP-flanked PGKneo cassette were inserted into a PstI site in exon 10 followed by a 1.1 kb PCR fragment from intron 14–15 that served as the 3’ homologous arm and a PKG-tk cassette that was used to negatively select against random insertions. The Daam1 and Daam2 targeting vectors were linearized with Not1 and electroporated into the CJ7 embryonic stem cell line (Tessarollo, 2001). Transfected cells were then cultured in the presence of G418 and Gancyclovir to select for homologous recombination events.
Targeted clones of Daam1floxed ES cells were identified by probing Southern blots of BglII digested genomic DNA with digoxigenin (DIG) labeled fragments of regions upstream of the ClaI site in intron 4–5 (SA Probe in Figure S1A,C) and downstream of BamHI site in intron 12–14 (LA probe in Figure S1A,B). Clones of Daam2lacZ ES cells were identified by probing Southern blots of BglII digested genomic DNA with a DIG labeled fragment of intron 6–7 (LAI Probe in Figure S2A,B). Southern blots of genomic DNA digested with HindIII were probed with a DIG labeled fragment from a region upstream of the EcoRV site in intron 5–6 (Probe LAO in Figure S2A,C) to confirm integration of the 5’ end of the homologous arm into the locus. Southern blots of genomic DNA digested with BglII and EcoRI were probed with a DIG labeled fragment of intron 14–15 located downstream of the 3’ homologous arm (Probe SAO in Figure S2A,D) to confirm proper targeting. Probes were generated using the PCR DIG Probe Synthesis Kit (Roche Diagnostics, 11636090910) according to the manufacturer’s protocol. Correctly targeted ES cells were injected int°C57BL6 blastocysts with the aid of the Gene Targeting Facility at the National Cancer Institute Division of Basic Science. Chimeric males were mated with C57BL6 females to produce Daam1floxed and Daam2lacZ progeny, which were identified by PCR with the primer pairs listed in Table S1. Proper targeting was confirmed by Southern blotting as described. Daam1floxed and Daam2lacZ mice were maintained on a mixed CD1 background.
Breeding and genetics
Daam1floxed mice were bred to Nkx2.5cre mice (Moses et al., 2001) to generate homozygous Daam1 conditional knockout (CKO) and Daam1floxed/+; Nkx2.5cre/+ (Daam1 HET) mice. For fate mapping, Daam1floxed and Nkx2.5cre mice were bred with R26Rβ-geo mice (Jackson Laboratories, #008616), which express a nuclear localized GFP-lacZ fusion protein after Cre-mediated excision (Stoller et al., 2008), to generate Daam1 CKO; R26Rβ-geo/+ and Daam1 HET; R26Rβgeo/+ embryos. Daam1floxed and Nkx2.5cre mice were mated to Daam2lacZ mice to produce Daam1/2 DKO and Daam1floxed/+; Daam2lacZ/+; Nkx2.5cre/+ (Daam1/2 DHET) mice. Daam1floxed and Nkx2.5cre mice were bred to mice carrying a null allele of Wnt5a (Yamaguchi et al., 1999) (Jackson Laboratories, #004758) to obtain Daam1floxed/floxed; Wnt5anull/null; Nkx2.5cre/+ (Daam1 CKO; Wnt5a KO), Daam1floxed/floxed; Wnt5anull/+; Nkx2.5cre/+ (Daam1 CKO; Wnt5a HET), and control mice. The Daam1floxed, Daam2lacZ, Wnt5anull, Nkx2.5cre, and R26Rβ-geo alleles were detected by PCR with the primer pairs listed (Table S1). For timed breeding, females were checked for vaginal plugs, which mark pregnancies on E0.5. Mice were euthanized by CO2 asphyxiation and cervical dislocation. Procedures were approved by the Institutional Animal Care and Use Committees at the University of Rochester, University of Pennsylvania, and National Laboratory for Cancer Research.
Isolation and culture of embryonic and neonatal ventricular cardiac myocytes
Embryonic and neonatal ventricular cardiac myocytes (EVCMs and NVCMs, respectively) were isolated with a kit designed for isolating cardiomyocytes from neonatal rat hearts (Worthington Biochemical Corp., LK003300), using a modified version of manufacturer’s protocol. The ventricles of 40–60 hearts were isolated from E12.5-E14.5 CD1 embryos or P0-P1 CD1 neonates under aseptic conditions and pre-digested in Trypsin in HBSS overnight at 4°C. The Trypsin was quenched with the addition of Soybean Trypsin-inhibitor before the tissue was digested with Type II Collagenase for 45 minutes at 37°C with gentle agitation. The dissociated cells were passed through a cell strainer to remove undigested tissue, centrifuged and suspended in 3:1 mixture of DMEM and M-199 (Life Technologies Inc., 11995-073 and 11150-059, respectively) supplemented with 10% horse serum (Life Technologies Inc., 16050122) and 5% FBS (Hyclone™, SH30910.03). NVCMs were pre-plated for 1 hour on gelatin-coated dishes to remove fibroblasts. EVCMs and NVCMs were plated on gelatin-coated chamber slides and incubated at 37°C, 5% CO2 in media containing either 1 µM Akt Inhibitor VIII (Millipore Inc., 124018) or DMSO. After 48 hours, cells were fixed and stained with Alexa Fluor 595 Phalloidin and Hoechst 33342 (Life Technologies Inc., A12381 and H3570, respectively) according to the manufacturer’s protocol. EVCMs were stained with an antibody against NKX2.5 (Santa Cruz Biotechnology Inc., sc-8697) to distinguish EVCMs from non-myocardial cells.
Quantitative PCR (Q-PCR), Western blotting, and Elisa for GTP-bound RhoA
To examine the effects of DAAM1 and DAAM2 loss-of-function on transcription, RNA was isolated from the hearts of at least three embryos of each genotype using TRIzol reagent (Life Technologies Inc., 15596-026). RNA was reverse transcribed using the iScript Reverse Transcription Supermix (Bio-Rad Inc., 170-8840). Q-PCR with the primer pairs listed in Table S1 was performed on a Bio-Rad CFX386 thermocycler with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Inc., 172-5270) as described (Bisson et al., 2015; Cohen et al., 2012; Cohen et al., 2007). Relative gene expression was calculated by the ΔCT method using Gapdh as an endogenous control. The ΔCT values of all samples were normalized to the mean ΔCT of reference samples so that the standard deviation could be calculated from the ΔCT values of controls. Graphs represent mean fold changes in gene expression. Error bars represent standard deviation. P<0.05 by Student’s t-test indicated statistical significance.
To examine the effects of DAAM1 and DAAM2 loss-of-function on protein levels, the hearts of E17.5 embryos were lysed in RIPA buffer. Lysates were subjected to SDS-PAGE and transferred to PVDF membranes, which were then blotted with antibodies recognizing the carboxy-termini of DAAM1 and amino-termini of DAAM2 (Abcam Inc., 71499 and 177919, respectively), CyclinD1, c-MYC, phospho-Rb (Ser780), phospho-Rb (Ser807/Ser811), Rb, phopho-AKT (Ser473), AKT (pan), phospho-c-Jun (Ser73), c-Jun, phospho-PRK1/2 (Thr774/Thr816), phospho-ERK1/2 (Thr202/Try204), phospho-p38 MAPK (Thr180/Try182), phospho-GSK3α/β (Ser21/Ser9), GSK3α/β (Cell Signaling Technologies Inc., 2978, 5605, 8180, 4060, 9309, 8516, 4691, 9164, 9165, 2611, 4370, 9215, 9331 and 5676, respectively), phospho-MYPT1 (Thr696), MYPT1 (Millipore Inc., ABS45 and 07-672, respectively) and β-catenin (BD Transduction Laboratories Inc., 610154) as described (Bisson et al., 2015; Cohen et al., 2012). Antibodies against β-Tubulin (Cell Signaling Technologies Inc., 2128) and GAPDH (Thermo Scientific, PA1987) were used to assess loading. HRP-conjugated anti-mouse and anti-rabbit 2° antibodies (Vector Laboratories, PI-2000 and PI-1000, respectively), Super Signal West Pico ECL substrates (Thermo Scientific Inc., PI-34078) and Hyperfilm ECL (GE Healthcare Inc., 28-9068-39) were used for detection.
The levels of GTP-bound RhoA in embryonic hearts were examined with the RhoA G-LISA Activation Assays Kit (Cytoskeleton Inc., BK121) as described in the manufacturer’s protocol. The hearts of 6 Daam1/2 DKO and 6 Daam1/2 DHET embryos were examined individually. Graphs represent mean GTP-bound RhoA levels relative to the mean value in control samples. Error bars represent standard deviation. P<0.05 by Student’s t-test indicated statistical significance.
Histology, immunological staining, and in situ hybridization
Adult mice were euthanized at 2 months or 8 months of age before their hearts were perfused with 100 mM KCl buffer and 4% paraformaldehyde. Pregnant dams were euthanized and the embryos collected and fixed as described (Bisson et al., 2015; Cohen et al., 2012; Cohen et al., 2007; Nagy, 2003). Tissue was dehydrated with a series of washes in increasing ethanol concentration, embedded in paraffin and sectioned as described (Bisson et al., 2015; Cohen et al., 2012; Cohen et al., 2007; Nagy, 2003). Templates for RNA probes were amplified from Daam1 and Daam2 cDNA (MGD Clone ID 4941186 and 4237922, respectively) by PCR with reverse primers that had T7 promoter sequences added to their 5’ ends (Table S1). In situ hybridization (ISH) was performed as described (Cohen et al., 2012; Cohen et al., 2007; Nagy, 2003). Immunohistochemistry (IHC) with antibodies for DAAM1, ACTC1, Desmin (Abcam Inc., 71499, 199258 and 32362, respectively), β-catenin (BD Transduction Laboratories Inc., 610154), N-cadherin (Sigma-Aldrich Inc., 3865), and Ki67 (Dako Inc., M7249) were performed as described (Cohen et al., 2012; Cohen et al., 2007; Nagy, 2003). DAAM1 staining was detected with biotinylated anti-rabbit 2° antibody, the Vectastain Elite ABC kit, and the DAB Peroxidase Substrate Kit (Vector Laboratories, BA-1000, PK-6100 and SK-4100, respectively) as described (Bisson et al., 2015; Cohen et al., 2012; Cohen et al., 2007; Nagy, 2003). Fluorescent staining was performed with Alexa Fluor 488 and 594 conjugated 2° antibodies as described (Bisson et al., 2015; Cohen et al., 2012; Cohen et al., 2007; Nagy, 2003). Whole mount X-Gal staining was performed as described (Cohen et al., 2012; Cohen et al., 2007; Nagy, 2003). X-Gal stained embryos were sectioned and counterstained with Eosin as described (Cohen et al., 2012; Cohen et al., 2007; Nagy, 2003). Portions of the RV wall were also isolated from the hearts of 2-month-old Daam1/2 DKO and control mice, briefly treated with 100 mM KCl and fixed in 2% glyceraldehyde for transmission electron microscopy, which was performed with the assistance of the Electron Microscopy Resource Laboratory at the University of Pennsylvania School of Medicine as described (Tian et al., 2010).
To calculate the mean widths of the trabecular myocardia, the ruler tool of Photoshop CS6 (Adobe Systems Inc.) was used to measure the widths of the trabecular myocardia at 5 equidistant points along the bases of the RV and LV walls in micrographs of H&E stained sections. For each embryo, 4 sections spaced 50 µm apart were examined. The results were used to calculate the typical widths of the trabecular zones in that embryo. The typical widths of the RV and LV trabecular zones for three independent embryos of each genotype were then averaged to calculate the mean widths of the RV and LV trabecular zones in Daam1 CKO, Daam1/2 DKO and control embryos. Graphs represent mean the mean widths of the RV and LV trabecular zones for each genotype. Error bars represent standard deviation of the typical widths for each genotype. P<0.05 by Student’s t-test indicated statistical significance.
Results
Daam1 and Daam2 are co-expressed within the embryonic myocardium
In situ hybridization (ISH) was performed on sections of wild-type mouse embryos to detect Daam1 and 2 mRNA within the developing heart. At E9.5, Daam1 was detected throughout the myocardial layer of the heart tube (arrows, Figure S3A). At E10.5, Daam1 was expressed in the atrial and ventricular myocardia as well as the inter-ventricular septum (IVS) (arrowhead, Figure S3B). Daam1 continued to localize to the atrial and ventricular myocardia at E12.5 as well as the ventricular trabeculae (arrows, Figure S3C). Daam2 was not expressed in the myocardium at E9.5 but was present in epicardial cells (arrows, Figure S3D) and the pro-epicardial organ (arrowhead, Figure S3D). At E10.5, Daam2 was expressed in the mesenchyme surrounding the ventral foregut and OFT (arrowheads, Figure S3E), areas enriched in cardiac progenitors (Cohen et al., 2007), as well as the epicardium and lining of the pericardial cavity. By E12.5, Daam2 was found throughout the myocardium and ventricular trabeculae (arrows, Figure S3F).
Myocardial-specific deletion of Daam1 results in progressive loss of cardiac function
To determine if Daam1 is autonomously required in myocardial cells, LoxP sites were inserted into the introns surrounding exon 6 of Daam1 (Figure S1A) to generate the Daam1floxed allele. Mice carrying Daam1floxed were bred to Nkx2.5cre mice, which express Cre in cardiac progenitors and their descendants (Moses et al., 2001). Western blotting for DAAM1 revealed a 120 kD band in the hearts of E17.5 Daam1 HET embryos that was reduced in Daam1 CKO hearts (Figure S1D). Immunohistochemistry (IHC) showed that DAAM1 was reduced in the myocardia of E14.5 Daam1 CKO embryos relative to controls (Figure S1E,F) but unaffected in epicardial or endocardial cells (arrowheads and arrows, respectively, Figure S1F).
While Daam1 CKO mice were viable, the free wall of the right ventricle (RV) did not extend as close to the apex in Daam1 CKO mice as in controls (dashed brackets, Figure S4A–D). To determine if DAAM1 deficiency affected cardiac function, echocardiography was performed on 2-month-old Daam1 CKO and Daam1 HET mice. Indicators of systolic function were similar in Daam1 CKO and control mice at this age (Table S2). However the mean E/A ratio, the velocity of flow across the mitral valve early in diastole divided by the velocity of flow across the mitral valve late in diastole (Mottram and Marwick, 2005), was < 1 in Daam1 CKO mice. In healthy hearts, most of the flow across the mitral valve occurs as the left ventricle (LV) relaxes prior to atrial contraction and the E/A ratio is >1, as it was in controls. Conditions that increase the pressure needed to fill the LV cause the majority of filling to occur as the atria contract late in diastole. The reduced E/A ratios in Daam1 CKO mice are thus indicative of diastolic dysfunction.
Echocardiography was performed on 8-month-old Daam1 CKO and control mice to determine if the early diastolic dysfunction in Daam1 CKO mice would lead to a later loss of systolic function. Consistent with the results of our histological analysis, the mean diameter of the RV during diastole was larger in Daam1 CKO mice than in controls (Figure S4E–G, Table S3). The acceleration of pulmonary artery (PA) flow, which is proportional to the force of RV contraction, and RV fractional shortening, an indicator of RV systolic function, were also lower in Daam1 CKO than in control mice (Figure S4H,I, respectively), indicating that RV systolic function was disrupted in older Daam1 CKO mice.
Myocardial-specific Daam1 deletion disrupts heart morphogenesis and ventricular compaction
Sections of E16.5 control (Figure S5A) and Daam1 CKO (Figure S5B) embryos were examined to determine if the aberrant RV morphology in Daam1 CKO mice resulted from alterations in cardiac development. The PA and aortas were unaffected in Daam1 CKO embryos (data not shown). However, while the bases of the ventricles were aligned with one another in controls (dashed lines, Figure S5A), the base of the RV was closer to the tricuspid valve in Daam1 CKO embryos (dashed lines, Figure S5B), consistent with the shortened RVs in Daam1 CKO adults. Examining sections through the RVs (Figure S5C,D) and LVs (Figure S5E,F) also revealed that the trabecular zones were thicker in Daam1 CKO embryos than in controls (Figure S5C–H), consistent with the non-compaction defects found in Daam1β-geo mice (Li et al., 2011).
To understand the etiology of the heart phenotypes in Daam1 CKO mice, Daam1floxed mice were interbred with R26Rβ-geo mice, which carry Cre-inducible β-galactosidase (β-gal) (Stoller et al., 2008). Cardiomyocytes were oriented perpendicular to the walls of the conus in controls (arrows in Figure S5I) but small and rounded in Daam1 CKO embryos (Figure S5J). Cardiomyocytes were also tightly associated with one another in controls but loosely connected by thin cellular projections in Daam1 CKO hearts (arrows in Figure S5J). While cardiomyocytes invading nonmyocardial regions of the OFT extended thick protrusions from their leading edges in controls (arrows, Figure S5K), these structures were absent in Daam1 CKO embryos (arrows, Figure S5L). Cardiomyocytes within the RV formed columns with blood vessels at their bases and a single layer of endocardial cells covering their luminal surfaces in controls (dashed lines, Figure S5M) but were randomly distributed and mixed with endocardial cells in Daam1 CKO embryos (Figure S5N). Together, these data suggest that the abnormal RV morphology and NCM in Daam1 CKO mice reflect a role for DAAM1 in regulating the cardiomyocyte cytoskeleton.
Daam2 deficiency enhances the effects of myocardial-specific Daam1 deletion on cardiac morphogenesis and myocardial function
The overlapping expression of Daam1 and 2 suggest that they have redundant roles during cardiac development. To test this hypothesis, Daam1floxed mice were mated to Daam2lacZ mice, which have an IRES-LacZ insertion in exon 10 of Daam2 (Figure S2A). Western blotting with an antibody for the amino-terminal end of DAAM2 revealed two bands at the expected sizes of ~90 and 70 kD in E17.5 Daam2lacZ/+ hearts (Figure S2E) that were both reduced in Daam2lacZ/lacZ hearts. The amino-terminal FH1 and FH2 domains of DAAM1 have dominant-negative activity when overexpressed in frog embryos and cultured cells (Habas et al., 2001). Since IRES-LacZ is inserted after the exons encoding the FH1 and FH2 domains, Daam2lacZ mice may make a truncated DAAM2 that could block DAAM1 function. However, western blotting did not detect a band at ~45 kD, the predicted size of truncated DAAM2 (Figure S2E), and Daam2lacZ/lacZ mice were viable with normal hearts (data not shown). The mutant protein produced in Daam2lacZ/lacZ mice is thus insufficient to cause heart phenotypes like those of Daam1 CKO mice.
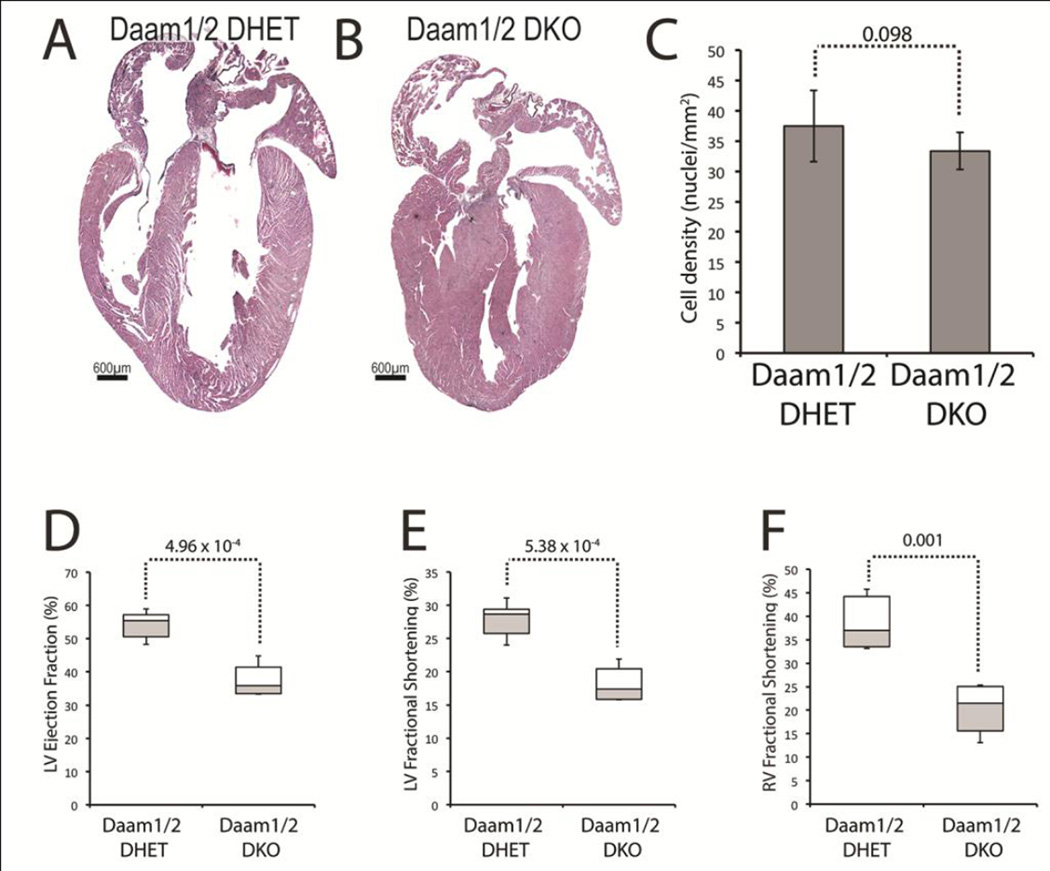
In contrast to those of Daam2lacZ/lacZ mice, the hearts of Daam1/2 double knockout (DKO) mice (Figure 1B) were smaller than controls (Figure 1A) but had thicker ventricular walls and septa. The density of nuclei was unaffected (Figure 1C), suggesting that the thick walls of Daam1/2 DKO hearts were not caused by hypertrophic growth. To determine if the loss of DAAM2 exacerbated cardiac dysfunction under DAAM1 deficiency, echocardiography was performed on 2-month-old Daam1/2 DKO and Daam1/2 DHET control mice (Table S4). Interestingly, the ejection fraction (Figure 1D) and fractional shortening (Figure 1E) of the LV were lower in Daam1/2 DKO mice than controls. The acceleration of blood flow into the PA from the RV was unaffected, but the fractional shortening of the RV was reduced, in Daam1/2 DKO mice relative to controls (Figure 1F). Since LV systolic function was unaffected in Daam1 CKO mice and the effects of Daam1 loss-of-function on RV systolic function were not observed until 8 months of age, these data indicate that the combined deficiency of DAAM1 and 2 exacerbates cardiac dysfunction compared to DAAM1 loss alone and suggest that DAAM1 and 2 have overlapping functions in myocardial cells.
Figure 1.
Loss of DAAM1 and 2 disrupts cardiac morphology and function. A,B H&E stained sections of hearts from 2-month-old Daam1/2 double knockout (DKO, B) and Daam1/2 double heterozygous (DHET, A) mice. Hearts of Daam1/2 DKO mice are smaller and have thicker ventricular walls and IVS. C Mean numbers of nuclei/mm2 in the walls of Daam1/2 DKO and DHET hearts. D–F LV ejection fraction (D), LV fractional shortening (E), and RV fractional shortening (F) in 2-month-old Daam1/2 DKO mice and DHET mice. Center lines of boxplots in D–F represent median values; white upper and gray lower boxes represent the limits of the 2nd and 3rd quartiles, respectively; and the top and bottom whiskers represent the 1st and 4th quartiles, respectively. Student’s t-test results are shown above the dotted lines.
Combined DAAM1 and DAAM2 deficiency disrupts the cytoskeletal architecture and adhesion of cardiomyocytes
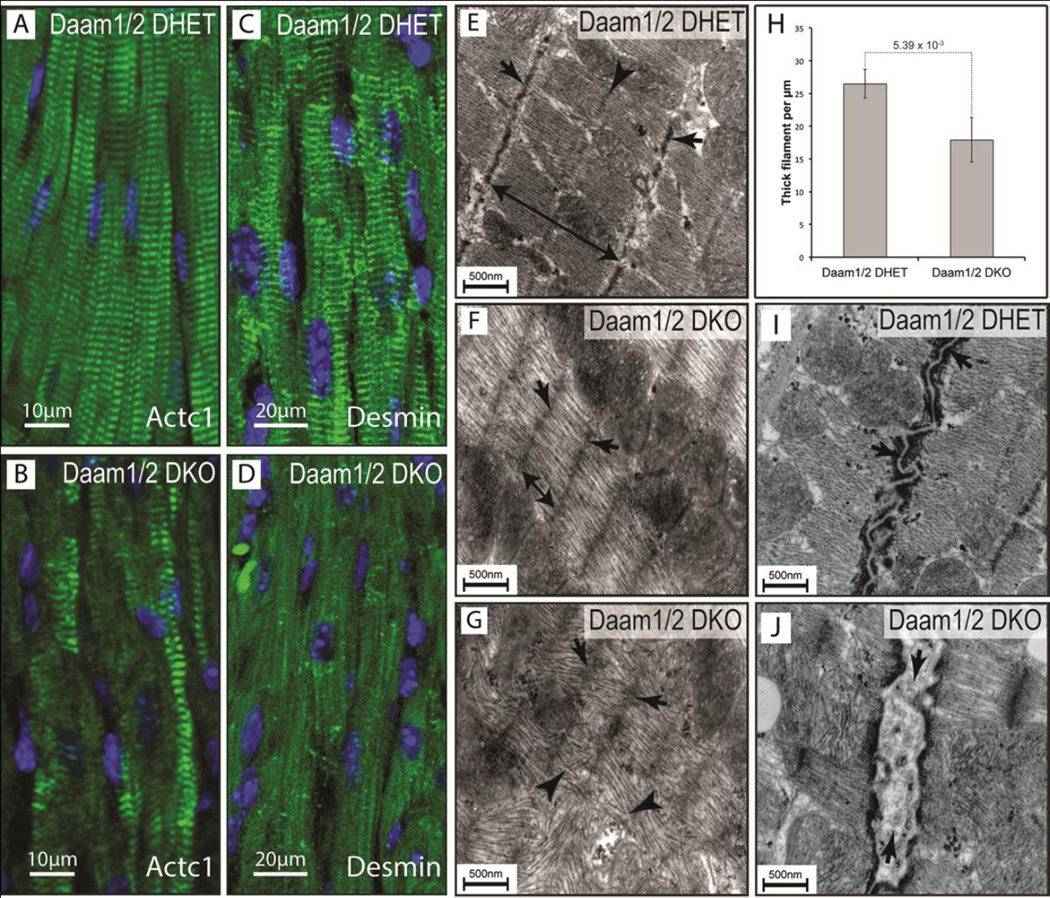
The expedited loss of systolic function in Daam1/2 DKO mice suggested that DAAM1 and 2 could be co-required for sarcomere assembly and/or maintenance. Therefore, sections of hearts from 2-month-old Daam1/2 DHET (Figure 2A,C) and Daam1/2 DKO (Figure 2B,D) mice were stained with antibodies for the A-band marker ACTC1 (Frank et al., 2006; Luther, 2009; Stromer, 1998) and the intermediate filament protein Desmin, which localizes to Z-bands and intercalated discs (Goldfarb et al., 2004; Thornell et al., 1997; Wang et al., 2002). In controls, ACTC1 (Figure 2A) and Desmin (Figure 2C) localized to repeating bands oriented perpendicular to the long axis of cardiomyocytes. In contrast, the striated pattern of ACTC1 staining was reduced in Daam1/2 DKO hearts, with some bands of ACTC1 staining that were diffuse and irregularly spaced (Figure 2B) relative to controls. Similarly, the striated pattern of Desmin staining was nearly absent in Daam1/2 DKO hearts, but residual staining was present in scattered puncta and faint lines running parallel to the long axis of cardiomyocytes (Figure 2D).
Figure 2.
Loss of DAAM1 and 2 disrupts sarcomere architecture. A–D Sections of hearts from 2-month-old Daam1/2 DKO (B,D) and DHET (A,C) mice stained for ACTC1 (green, A,B), Desmin (green, C,D) and DAPI (blue, A–D) showing disrupted protein localization in Daam1/2 DKO hearts. E–G Transmission electron micrographs (TEM) of myocardium from 2-month-old Daam1/2 DKO (F,G) and DHET (E) mice reveals more diffuse (arrows) and closely-spaced Zbands (double-headed arrows) in Daam1/2 DKO mice. The M-line, clearly visible in Daam1 DHET mice (arrowhead, E), is absent from Daam1/2 DKO mice. Thick filaments within the A-band are parallel to one another in controls, but disorganized and intersecting in severely affected areas of Daam1/2 DKO hearts (arrowheads, G). H Mean density of thick filaments in Daam1/2 DKO and control hearts. Result of Student’s t-test is shown above the dotted line. I–J TEM revealing that electron-dense adherence junctions of intercalated discs appear on either side of the closely-opposed membranes of cardiomyocytes in 2-month-old Daam1/2 DHET mice (arrow, I), but are separated by lighter material in Daam1/2 DKO mice (arrow, J).
Transmission electron microscopy was used to directly examine the effects of combined DAAM1/2 deficiency on cytoskeletal architecture. Sarcomeres were well defined in the hearts of 2-month-old Daam1/2 DHET mice (Figure 2E). The Z-bands, where actin filaments are anchored at the ends of individual contractile units (Frank et al., 2006; Luther, 2009; Stromer, 1998), were clearly visible and had well-defined edges (arrows, Figure 2E). The lighter I-bands, in which actin filaments emanating from the Z-bands are found without myosin, were apparent on either side of the Z-discs. The centrally located A-bands (double headed arrow, Figure 2E), in which actin and myosin overlap (Frank et al., 2006; Luther, 2009; Stromer, 1998), H-bands, where the myosin filaments are not associated actin, and M-lines (arrowhead, Figure 2E), where myosin filaments are anchored (Frank et al., 2006; Luther, 2009; Stromer, 1998), were all clearly visible. In contrast, the Z-bands were faint or absent (arrows, Figure 2F,G) and the Abands were shorter (double-headed arrow, Figure 2F) in Daam1/2 DKO hearts. The actinmyosin filaments were also less dense in Daam1/2 DKO mice than in controls (Figure 4H) and the I-bands, H-bands and M-lines were not apparent. In severely affected areas of Daam1/2 DKO heart, the thick filaments were disorganized and intersected one another (arrowheads in Figure 4G). These data are consistent with loss of ACT1C and Desmin staining in Daam1/2 DKO hearts and suggest that Daam1 and 2 are critical for sarcomere assembly and/or maintenance.
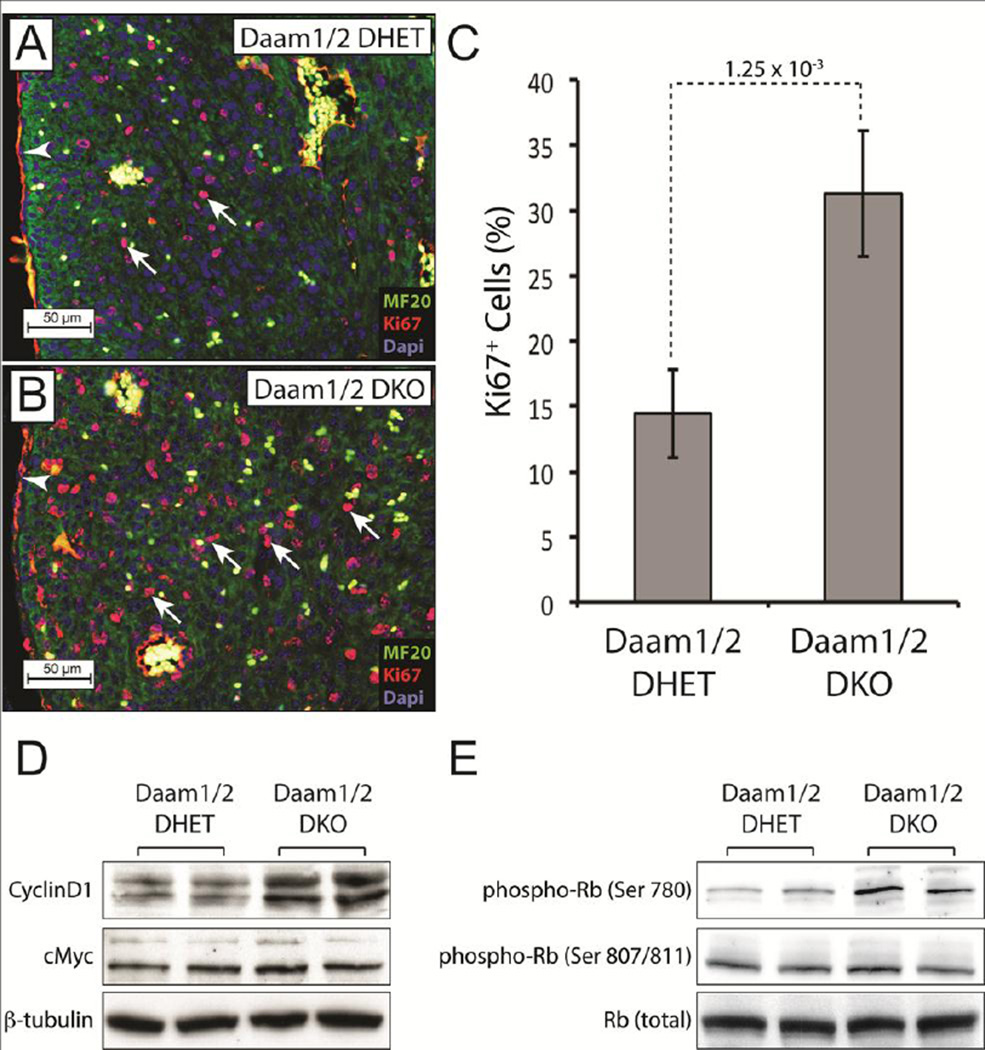
Figure 4.
Loss of DAAM1 and 2 increases cardiomyocyte proliferation. A,B Staining E17.5 Daam1/2 DKO and DHET hearts for the S-phase marker Ki67 (red), the muscle marker MF20 (green) and DAPI (blue) revealed more MF20+/Ki67+ cells (arrows) in Daam1/2 DKO hearts (B) relative to controls (A). Blood cells autofluoresce in the red and green channels and thus appear yellow. Red staining in epicardial and endothelial cells (arrowheads) is non-specific background. C Mean percentages of MF20+/Ki67+ cells in E17.5 Daam1 DKO and control hearts. Result of Student’s t-test is shown above the dotted line. D,E Western blots showing increased CyclinD1 (D) and phosphorylation of Retinoblastoma (Rb) at serine 780 (E) in E17.5 Daam1/2 DKO hearts relative to controls. The levels of cMYC, β-tubulin, total Rb and the phosphorylation of Rb at serine 807/811 were similar in Daam1/2 DHET and Daam1/2 DKO embryos.
Intercalated discs were also abnormal in Daam1/2 DKO mice. In controls, the membranes of adjoining myocytes were closely opposed and formed a discrete line (arrows, Figure 2I) flanked by electron-dense areas containing the adherens junctions, desmosomes, and gap junctions of these specialized adhesion sites (Delmar and McKenna, 2010; Mezzano et al., 2014; Vite and Radice, 2014). In contrast, lower-density material was frequently observed between the membranes of adjacent cardiomyocytes in the hearts of Daam1/2 DKO mice (arrows, Figure 2J).
The combined loss of DAAM1 and DAAM2 increases cardiomyocyte proliferation
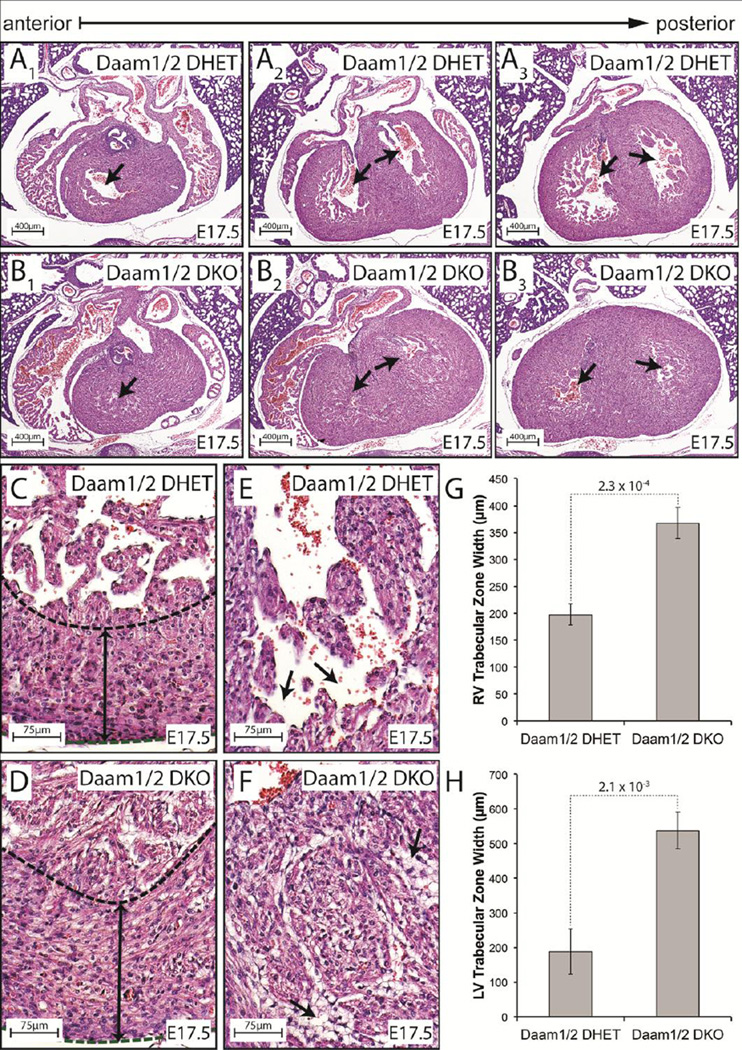
Daam1/2 DKO and control embryos were sectioned to better understand the origins of NCM in Daam1/2 DKO mice. By E14.5, Daam1/2 DKO embryos exhibited NCM phenotypes like those seen in Daam1 CKO embryos (Figure FS6A–F). By E17.5, the trabeculae began to assimilate into the compact zones of control embryos, clearing the ventricular lumen (arrows, Figure 3A1–A3). In contrast, the ventricular lumens were occluded in Daam1/2 DKO hearts (arrows, Figure 3B1–B3). While the width of the compact zones were unaffected in Daam1/2 DKO embryos (dashed lines and double headed arrows, Figure 3C,D), the trabecular layers were wider in Daam1/2 DKO embryos than in controls (Figure 3G,H). Individual trabeculae were also thicker in Daam1/2 DKO embryos than in control hearts (Figure 3E,F). Moreover, the intertrabecular spaces, which were clearly visible in controls (arrows, Figure 3E), were filled with detached endothelial cells in Daam1/2 DKO embryos (arrows, Figure 3F), making it more difficult to distinguish the compact and trabecular myocardia.
Figure 3.
Loss of DAAM1 and 2 enhances noncompaction in Daam1 CKO mice. A,B H&E-stained sections of E17.5 Daam1/2 DHET (A) and DKO (B) hearts reveal thickened myocardia and occluded lumen in Daam1/2 DKO embryos relative to controls. C,D Enlarged images of the base of the LV in Daam1/2 DHET (C) and Daam1/2 DKO (D) hearts. The thickness of the compact myocardium (dashed lines) is similar in Daam1/2 DKO and control hearts at this stage (double headed arrows, C,D). E,F Images of the luminal side of LV wall shows the thick trabeculae and reduced intertrabecular space (arrows, E,F) in Daam1/2 DKO hearts (F) relative to controls (E). G,H Mean widths of the trabecular zones at the base of the RV (G) and LV (H) in E17.5 Daam1/2 DHET and Daam1/2 DKO embryos. Values represent measurements from 3 embryos of each genotype taken as described in supplemental methods. Student’s t-test results are shown above the dotted lines.
Staining sections of E17.5 Daam1/2 DKO and control embryos for the S-phase marker Ki67 and muscle marker MF20 revealed that the percentage of double-labeled cells was higher in Daam1/2 DKO hearts (Figure 4A–C), suggesting that the thick myocardia in these mice resulted from increased cardiomyocyte proliferation. Western blotting demonstrated that the levels of CyclinD1, which promotes entry into S-phase by activating Cyclin-dependent kinases (Bendris et al., 2015; Lim and Kaldis, 2013), were elevated in Daam1/2 DKO hearts relative to controls (Figure 4D). The Cyclin-dependent phosphorylation of the tumor suppressor Retinoblastoma on Serine 780, which relieves its inhibition of cell-cycle entry (Giacinti and Giordano, 2006), was also enriched in Daam1/2 DKO hearts (Figure 4E). In contrast, levels of the mitogenic transcription factor c-MYC (Bretones et al., 2015) and phosphorylation of Retinoblastoma at Serine 807/811 were unaffected.
DAAM1 and DAAM2 are required for the activity of AKT but not RhoA within the developing heart
While early studies indicated that DAAM1 was necessary and sufficient for Wnt ligands to activate RhoA (Habas et al., 2001), RhoA signaling was unaffected in the hearts of Daam1β-geo/βgeo mice (Li et al., 2011). This discrepancy may be explained if functional redundancy with DAAM2 had masked a role for these factors in Wnt-dependent RhoA activation. RhoA activity was therefore assessed in the hearts of E17.5 Daam1/2 DKO and control embryos with an ELISA that detects the binding of GTP-bound RhoA to the immobilized GBD of its effector Rhotekin. However, no changes were observed in the levels or activity of RhoA, phospho- MYPT1 or PRK1/2 (Figure S7A,B,D). Since non-canonical Wnt ligands activate Jun-N-terminal kinase (JNK) (Nomachi et al., 2008; Oishi et al., 2003; Wang et al., 2013), the phosphorylation of c-Jun by JNK was examined in E17.5 Daam1/2 DKO and control hearts. However, the phosphorylation of not only c-Jun, but also P38 and ERK1/2 were unaffected (Figure S7C,D). Finally, the effects of non-canonical Wnt ligands are often mediated by the inhibition of β-catenin (Bisson et al., 2015; Cohen et al., 2012; Kwack et al., 2013; Mikels and Nusse, 2006; Yuan et al., 2011). Yet neither the levels of β-catenin protein, nor expression of the β-catenin targets were affected in the hearts of Daam1/2 DKO embryos (Figure S7D–F). Together, these data suggest that DAAM1 and 2 are dispensable for RhoA activity as well as MAPK and canonical Wnt signaling within the developing heart.
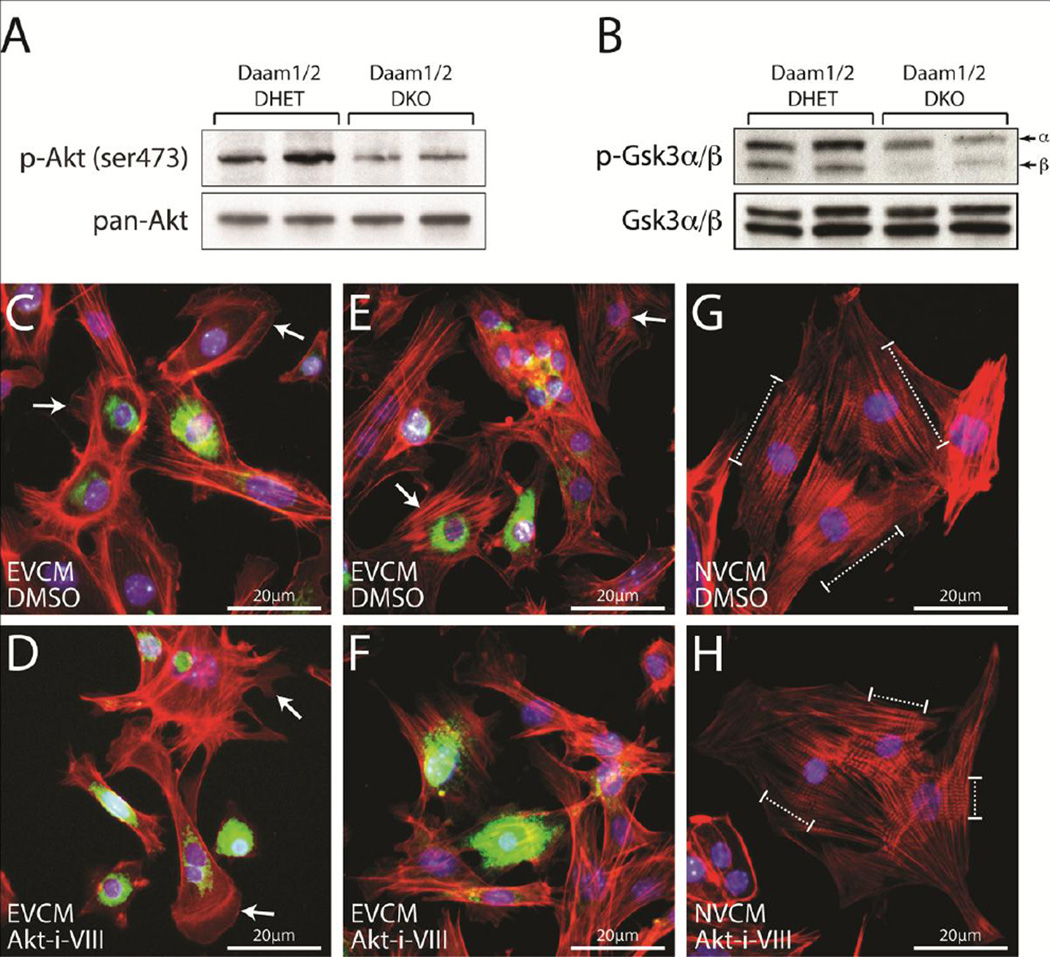
Surprisingly, the phosphorylation of AKT on serine 473 (Figure 5A), which is associated with its activity (Jacinto et al., 2006; Sarbassov et al., 2005), and the AKT-dependent phosphorylation of GSK3α/β (Figure 5B), which inhibits the activities of these kinases (Cross et al., 1995) were reduced in Daam1/2 DKO hearts relative to controls. In contrast, total AKT and GSK3α/β levels were unaffected. To determine if AKT activity was necessary for the formation of cardiac sarcomeres, embryonic ventricular cardiomyocytes (EVCMs) were isolated from wild-type embryos collected at E12.5–E14.5 and plated in media containing DMSO (Figure 5C,E) or 1 µM AKT-Inhibitor-VIII (Figure 5D,F), an isozyme-selective inhibitor of AKT1 and 2 (Lindsley et al., 2005). Staining with phalloidin and DAPI to label filamentous actin (F-actin) and nuclei, respectively, revealed that control EVCMs had “ribbed” lamellipodia, in which filopodia radiate out of the F-actin meshwork, extending from their periphery (arrows, Figure 5C). In contrast, EVCMs treated with 1 µM AKT-Inhibitor-VIII had lamellipodia but not the associated filopodia found in controls (arrows, Figure 5D). Moreover, striated myofibrils observed in control EVCMs (arrows, Figure 5E) were absent in cultures of AKT-Inhibitor-VIII treated EVCMs (Figure 5F). To further explore the effects of AKT inhibition on myofibril formation, neonatal ventricular cardiomyocytes (NVCMs) were isolated from P0–P1 neonates and plated in media containing DMSO (Figure 5G) or 1 µM AKT-Inhibitor-VIII (Figure 5H). Staining with phalloidin and Hoechst revealed that control NVCMs formed long myofibrils (dashed brackets, Figure 5G), but AKTInhibitor- VIII-treated NVCMs formed myofibrils that were much shorter (dashed brackets, Figure 5H), reminiscent of the disrupted sarcomeres in Daam1/2 DKO hearts.
Figure 5.
Loss of DAAM1 and 2 disrupts AKT/GSK3α/β signaling. A,B Western blots show reduced AKT (A) and GSK3α/β (B) phosphorylation in E17.5 Daam1/2 DKO embryos. C–F Embryonic ventricular cardiac myocytes (EVCMs) were isolated from CD1 embryos between E12.5 and E14.5 and plated in media containing DMSO (C and E) or 1 µM AKT-inhibitor-VIII (Akt-i-VIII, D,F). After 24 hours, cells were fixed and stained with phalloidin to label F-actin (red), Hoechst to label nuclei (blue), and an antibody for NKX2.5 (green) to identify EVCMs. DMSO-treated cells have “ribbed” lamellipodia (arrows, C) with filopodia radiating out from the F-actin meshwork. In contrast, AKT-i-VIII-treated cells have lamellipodia without filopodia (arrows, D). Striated myofibrils are observed in control-treated EVCMs (arrows, E) but not AKT-i-VIII-treated EVCMs (F). G,H Neonatal ventricular cardiac myocytes (NVCMs) isolated from P0-P2 neonates and plated in media containing either DMSO (G) or 1 µM AKT-i-VIII (H). AKT-i-VIII-treated cells form fewer myofibrils than controls. Myofibrils in AKT-i-VIII-treated cells are also shorter and less developed than those of controls (dashed brackets).
Heterozygous loss of Wnt5a enhances the effects of myocardial-specific Daam1 deletion on the morphology and function of the adult heart
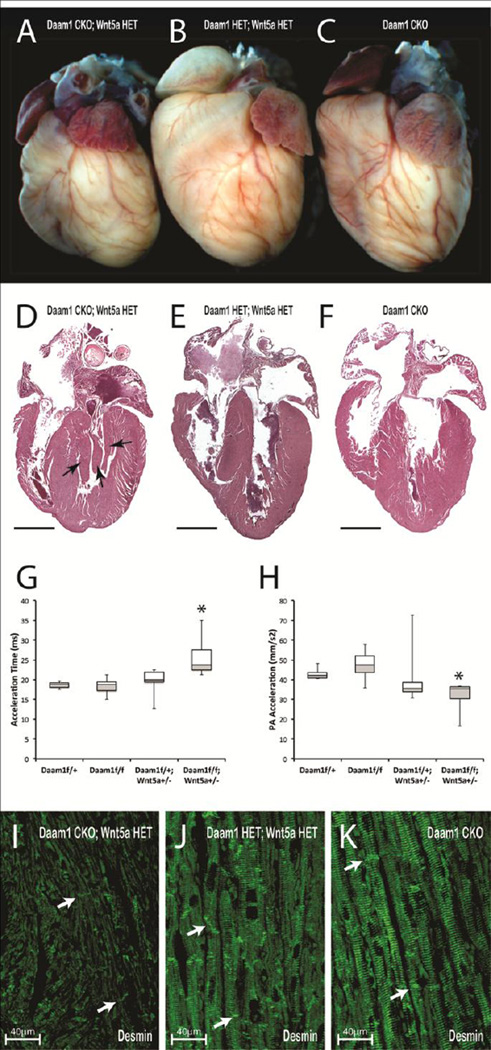
DAAM1, identified by its ability to bind to the Wnt effector DVL, is required for Wnt-dependent RhoA activation in gastrulating Xenopus embryos and cultured human epithelial cells (Habas et al., 2001), suggesting that DAAM1 is an essential component of the Wnt/PCP pathway. To determine if reducing the levels of Wnt5a, a non-canonical Wnt ligand required for cardiac morphogenesis (Schleiffarth et al., 2007; Sinha et al., 2014), would enhance the defects in Daam1 CKO hearts, Daam1floxed mice were bred with mice carrying a null allele of Wnt5a (Wnt5anull) (Yamaguchi et al., 1999) and Nkx2.5cre mice (Moses et al., 2001) to generate Daam1 CKO mice that were heterozygous for Wnt5anull. While the hearts of 2-month-old Daam1 HET; Wnt5a HET (Figure 6B) and Daam1 CKO (Figure 6C) mice were similar in size, the hearts of Daam1 CKO; Wnt5a HET mice (Figure 6A) were small and rounded. H&E-stained sections revealed excess myocardial tissue near the mitral valves of Daam1 CKO; Wnt5a HET mice (arrows, Figure 6D) that was absent in Daam1 CKO (Figure 6F) and Daam1 HET; Wnt5a HET hearts (Figure 6E). Moreover, while the length of the IVS was equivalent in the hearts of Daam1 HET; Wnt5a HET, Daam1 CKO, and Daam1 CKO; Wnt5a HET mice, the apex of the LV did not extend past the base of the IVS in Daam1 CKO; Wnt5a HET mice as it did in controls.
Figure 6.
Heterozygous loss of Wnt5a enhances the heart defects of Daam1 CKO mice. A–F Whole (A–C) and sectioned (D–F) hearts from 2-month-old Daam1floxed/+; Wnt5anull/+ (Daam1 HET; Wnt5a HET) control mice (B,E), Daam1 CKO mice (C,F) and Daam1 CKO; Wnt5a HET mice (A,D). G,H Acceleration times (G) and PA acceleration (H) of 2-month-old Daam1 HET, Daam1 CKO, Daam1 HET; Wnt5a HET and Daam1 CKO; Wnt5a HET mice. Boxplots are described in the legend for Figure 1. Asterisks indicate P-values < 0.05 versus double heterozygous controls. I–K Desmin (green) localizes to Z-bands and intercalated discs (arrows) in Daam1 CKO (K) and Daam1 HET; Wnt5a HET control (J) hearts but is lost from the Z-bands in Daam1 CKO; Wnt5a HET (I) hearts while intercalated discs remain marked.
Echocardiography on 2-month-old Daam1 CKO; Wnt5a HET, Daam1 CKO, and Daam1 HET; Wnt5a HET mice revealed that the E/A ratio was similarly reduced in Daam1 CKO; Wnt5a HET and Daam1 CKO mice (Table S2), suggesting that the heterozygous loss of Wnt5a does not enhance the loss of diastolic function in Daam1 CKO mice. RV systolic function was unaffected in Daam1 CKO mice at this age, but blood flowing into the PA took longer to reach peak velocity in Daam1 CKO; Wnt5a HET mice than in Daam1 CKO and Daam1 HET; Wnt5a HET mice (Figure 6G). The peak velocity PA flow was unaffected, therefore the acceleration of PA flow was reduced in Daam1 CKO; Wnt5a HET mice (Figure 6H). Thus, the heterozygous loss of Wnt5a enhances the loss of RV systolic function in Daam1 CKO mice.
Similarly, sarcomere architecture was examined in sections of hearts from 2-month-old Daam1 CKO; Wnt5a HET (Figure 6I), Daam1 CKO (Figure 6K), and Daam1 HET; Wnt5a HET control (Figure 6J) mice with staining for Desmin. Desmin was nearly absent in the hearts of Daam1 CKO; Wnt5 HET mice, with residual staining localized to what appeared to be intercalated discs (arrows, Figure 6I) and “streaks” running the length of the myocytes like those in the hearts of Daam1/2 DKO mice, indicating an exacerbation of the Daam1 CKO phenotype in Wnt5a null heterozygotes.
DAAM1 insufficiency in the myocardium enhances cardiac defects in Wnt5a null embryos
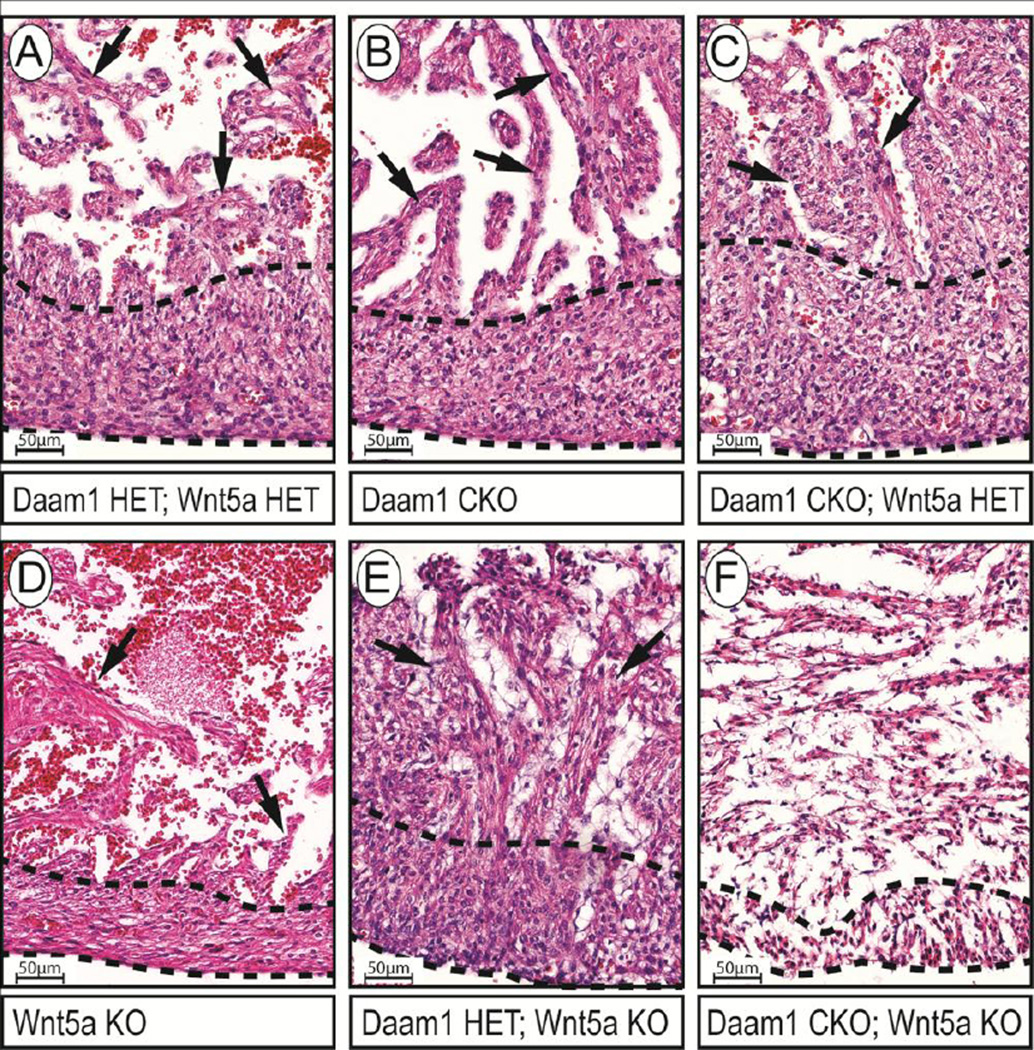
The trabeculae of E16.5 Daam1 CKO (arrows, Figure S8B) and Daam1 CKO; Wnt5a HET (arrows, Figure S8C) embryos were larger and extended further into the lumen than those of double-heterozygous controls (Figure S8A). At the base of the RV in controls (boxed area in Figure S8A, Figure 7A), the trabeculae were folded such that they were oriented parallel to the free wall of the RV (arrows, Figure 7A) and thus closer to the compact zones. In Daam1 CKO embryos (boxed area in Figures S8B, Figure 7B), the trabeculae remained straight such that they were oriented perpendicular to the compact zones (arrows, Figure 7B) and thus extended further into the lumen than in controls. The compact zones were also thinner in Daam1 CKO hearts (dashed lines, Figure 7B) than in Daam1 HET; Wnt5a HET controls (dashed lines, Figure 7A). In contrast, the RV of Daam1 CKO; Wnt5a HET embryos (boxed area in Figure S8C, Figure 7C) had thicker compact zones (dashed lines, Figure 7C) than those of Daam1 CKO and Daam1 HET; Wnt5a HET embryos, and while the trabeculae were straight and perpendicular to the RV wall, they were thicker (arrows, Figure 7C) than those of Daam1 CKO or control embryos and resembled those in Daam1/2 DKO embryos.
Figure 7.
Reducing DAAM1 function enhances the heart defects in Wnt5a mutant embryos. A–F Enlargements of boxed areas in H&E-stained sections of E16.5 embryos shown in Figure S8. The compact zone (dashed lines) is thinner in Daam1 CKO embryos (B) than in Daam1 HET; Wnt5a HET controls (A) but thicker in Daam1 CKO; Wnt5a HET embryos (C) than in either Daam1 CKO or Daam1 CKO; Wnt5a HET embryos. The trabeculae of Daam1 CKO; Wnt5a HET embryos (arrows, C) are perpendicular to the wall but thicker than those of Daam1 CKO embryos. The compact and trabecular myocardia are thinner in Wnt5a KO (D) embryos. In contrast, the compact zone is thicker in Daam1 HET; Wnt5a KO (E) than in Wnt5a KO embryos. There are also long trabeculae in Daam1 HET; Wnt5a KO hearts with detached endothelial cells filling the intertrabecular spaces. Cells in Daam1 CKO; Wnt5a KO hearts (F) form rudimentary compact and trabecular layers but they are disorganized and unlikely to be functional.
Since Wnt5a knockout (Wnt5a KO) mice die at birth (Yamaguchi et al., 1999), the hearts of adult Daam1 CKO; Wnt5a KO mice could not be examined; we therefore assessed hearts of E16.5 Daam1 CKO; Wnt5a KO embryos. Consistent with previous findings, the hearts of Wnt5a KO embryos had OFT and septation defects (Figure S8D). At the base of the RV (boxed area in Figure S8D, Figure 7D), the compact zones (dashed lines, Figure 7D) were thinner in Wnt5a KO embryos than in controls. Interestingly, while the thinning of the compact zone in the hearts of Daam1 CKO embryos was associated with larger trabeculae, the trabeculae were smaller in Wnt5a KO embryos than in controls (arrows, Figure 7D). Surprisingly, deleting one copy of Daam1 from the myocardia of Wnt5a KO embryos caused strong cardiac defects unlike those of Daam1 CKO or Wnt5a KO embryos (Figure S8E). Notably, the walls of the RV and right atrium (RA) were discontinuous in Daam1 HET; Wnt5a KO embryos (arrows, Figure S8E), creating openings where blood had leaked into the pericardial space. At the base of the RV (boxed area in Figure S8E, Figure 7E) the compact zones (dashed lines, Figure 7E) were thicker than those of Daam1 CKO or Wnt5a KO embryos and had long trabeculae separated by loosely attached endothelial cells (arrows, Figure 7E). Daam1 CKO; Wnt5a KO embryos were rarely recovered at E16.5, and those that were found were necrotic (Figure S8F). At the base of the LV (boxed area in Figure S8F, Figure 7F) the cells formed rudimentary compact zones (dashed lines, Figure 7F) and trabeculae.
To better understand the early embryonic lethality caused by deleting Daam1 from the hearts of Wnt5a KO mice, Daam1 CKO; Wnt5a KO embryos were examined at E12.5. While not fully formed by this stage, the IVS extended from the base of the heart to the endocardial cushion in the anterior AV canal of wild type control hearts (arrows in Figure S9A). Congruent with prior reports of septation defects in Wnt5a mutants, the IVS was shorter and did not extend to the cushion in Wnt5a KO or Daam1 CKO; Wnt5a KO embryos (arrows in Figures S9B and C, respectively). However, the morphologies of Wnt5a KO and Daam1 HET; Wnt5a KO hearts were otherwise similar to that of control hearts. In contrast, Daam1 CKO; Wnt5a KO embryos had dysmorphic hearts in which the RV was shifted anterior to the LV (Figure S9D–F). More severely affected Daam1 CKO; Wnt5a KO embryos were necrotic and had AV cushions that sat atop a single ventricle to which both atria were connected (Figure S9G–I). Moreover, closer inspections of the base of the RV revealed that the luminal cardiomyocytes of Daam1 CKO; Wnt5a KO embryos had a mesenchyme-like organization and did not form distinct trabeculae like those in control, Wnt5a KO or Daam1 HET; Wnt5a KO embryos (Figure S9J–M). These data suggest that simultaneously deleting Daam1 and Wnt5a causes early defects in cytoskeleton dynamics and myocardial structure that interfere with the looping morphogenetic movements that position the heart’s chambers.
Discussion
A previous study using a global knockout approach suggested a role for DAAM1 in myocardial growth and differentiation (Li et al., 2011). Here, the cell-autonomous contribution of DAAM1 was investigated using a myocardial-specific knockout of Daam1 driven by Nkx2.5cre. Although Nkx2.5cre can activate some Cre-dependent reporters in non-myocardial cells (Ma et al., 2008; Zhou et al., 2008), IHC indicated that DAAM1 was lost from the myocardia but not the epicardia and endocardia of Daam1 CKO mice. Daam1 CKO mice were viable but exhibited abnormal cardiac morphology in which the RV did not extend as far toward the apex of the heart as it did in controls. Further, cardiac function was also altered in Daam1 CKO mice; early diastolic dysfunction progressed to RV systolic dysfunction as they aged. Similar to Daam1β-geo mice, Daam1 CKO mice had excess trabecular myocardia and thin compact zones as embryos, suggesting the presence of NCM. Daam1 CKO mice did not have OFT and septation defects like those in Daam1β-geo mice. However, the cytoskeletal projections at the leading edges of the myocytes invading the OFT were absent in the hearts of Daam1 CKO embryos, suggesting that DAAM1 plays a role in the migration of these cells despite the lack of overt OFT defects in Daam1 CKO mice. Such phenotypes may require Daam1 to be lost from both myocardial and non-myocardial cells. Alternatively, the prior study was performed on a C57BL/6J background (Li et al., 2011). Since inbred strains exhibit greater sensitivity to genetic insults than the mixed CD1 background used in the present study, the absence of more severe morphological defects in Daam1 CKO mice may reflect the robustness of outcrossed strains.
Interestingly, while cardiomyocytes in the distal RV, which later becomes the conotruncus, were elongated in the axis perpendicular to the plane of the myocardium in the hearts of controls, these cells were smaller and more rounded in the hearts of Daam1 CKO embryos. These cells were also closely associated with one another along their short axes and organized into a “ladder-like” pattern in control hearts but randomly distributed and loosely attached to one another via thin projections in the hearts of Daam1 CKO embryos. Together, these data indicate that DAAM1 is critical for cardiomyocyte polarity and adhesion and are consistent with the proposed role for DAAM1 in PCP signaling. Moreover, while cardiomyocytes in more proximal regions the ventricular walls separated into columns with a single layer of epicardial cells covering their luminal surfaces in control hearts, these cells were disorganized and intermixed with endocardial cells in Daam1 CKO hearts. Since this columnar organization of ventricular cardiomyocytes is thought to pattern the trabeculae, which grow into the ventricular lumen from these sites (Sedmera et al., 2000), the NCM in Daam1 CKO embryos may result from the loss of polarity and adhesion in these cells. Alternatively, the loss of polarized protrusive activity in these cells may disrupt the morphogenetic movements required for the trabecular myocardium to integrate into the compacted walls of the adult heart.
The absence of severe defects in the hearts of Daam1 CKO mice suggested that DAAM2 might compensate for the loss of DAAM1 during myocardial development. In support of this idea, Daam1 and 2 were co-expressed in the myocardium and Daam1/2 DKO mice had a more severe loss of cardiac function and stronger NCM than Daam1 CKO mice. Moreover, while the sarcomeres and intercalated discs of Daam1 CKO mice were grossly normal, these structures were disrupted in Daam1/2 DKO mice and Daam1/2 embryos. The trabeculae of Daam1/2 DKO embryos also grew thicker than those of Daam1 CKO embryos as development proceeded due to elevated myocardial proliferation. Consistent with these data, the levels of CyclinD1 protein were higher in the hearts of Daam1/2 DKO embryos than in controls. Although, canonical Wnt/β-catenin signaling activates CyclinD1 transcription (Tetsu and McCormick, 1999), the levels of CyclinD1 mRNA as well as several other β-catenin target genes were unaffected in Daam1/2 DKO hearts. Therefore, the increased proliferation in Daam1/2 DKO hearts is unlikely to be caused by elevated canonical Wnt signaling. Alternatively, the proliferation of embryonic cardiomyocytes is coupled to the transient breakdown of their sarcomeric cytoskeletons (Ahuja et al., 2004; Porrello et al., 2011), which are thought to be too rigid to allow mitotic division to occur and treatments that disrupt sarcomere integrity can cause normally quiescent adult cardiomyocytes to reenter the cell-cycle (Engel et al., 2005). It is thus possible that increased myocardial proliferation in Daam1/2 DKO mice may result from the loss of cytoskeletal integrity.
Consistent with the findings of the previous study of Daam1β-geo mice, neither the activity of RhoA nor its effectors were reduced in the hearts of Daam1/2 DKO mice. Although studies performed in frog embryos and cultured cells indicated that DAAM1 is required for Wnt ligands to activate RhoA signaling (Habas et al., 2001), genetic interaction studies of the Drosophila Daam1 homolog suggest that DAAM proteins acts downstream of RhoA in a manner consistent with other FH proteins (Matusek et al., 2006). Interestingly, AKT signaling was reduced in the hearts of Daam1/2 DKO embryos relative to controls. DAAM1 was shown to interact with SRCfamily tyrosine kinases (Aspenstrom et al., 2006; Matusek et al., 2006), and SRC promotes the activation of AKT signaling by growth factors (Kassenbrock et al., 2002; Scaltriti and Baselga, 2006). Thus, the loss of AKT activity in Daam1/2 DKO hearts may indicate that DAAM1/2 are needed for SRC to activate AKT. Activating AKT was recently shown to promote sarcomere assembly in skeletal myocytes (Takano et al., 2011) and our data indicate that AKT inhibition impedes myofibril formation in cultured cardiomyocytes. Therefore, the loss of AKT signaling may, at least partially, underlie the cardiac defects observed in Daam1/2 DKO mice.
While Daam1 and 2 were identified as a DVL binding partners (Habas et al., 2001), their Drosophila homolog is not required for PCP (Matusek et al., 2006), raising the question of whether or not these genes are truly involved in Wnt/PCP signaling. Since Daam1 and 2 were redundantly required for sarcomere architecture, we reasoned that reducing the levels of upstream ligand might further degrade signaling and reveal defects in Daam1 CKO mice that mimicked those of Daam1/2 DKO mice. We thus made Daam1 CKO mice that were heterozygous for a null-allele of Wnt5a. The hearts of Daam1 CKO; Wnt5a HET mice had more severe morphological defects, stronger NCM and an earlier onset of RV systolic dysfunction than Daam1 CKO mice, consistent Daam1 and Wnt5a acting in a common pathway. However, if Daam1 and 2 only mediated Wnt5a signaling, deleting Daam1 would not enhance the defects in Wnt5a KO hearts since the ligand activating its signaling would already be absent. Yet deleting one or both copies of Daam1 from the myocardia dramatically enhanced the heart defects in Wnt5a KO embryos and produced strong synthetic phenotypes unlike those of Daam1/2 DKO or Wnt5a KO embryos. Daam1 therefore has Wnt5a-independent functions. While these data may reflect the loss of signaling by other non-canonical Wnt ligands, deleting one copy of Wnt11 did not exacerbate the defects in Daam1 CKO or Wnt5a KO mice (data not shown). Alternatively, Daam1/2 may act downstream of other classes of ligands such as growth factors, as suggested by the loss of AKT signaling in Daam1/2 DKO hearts.
NCM, found in 3.7% of heart failure patients (Almeida and Pinto, 2013; Kovacevic-Preradovic et al., 2009; Oechslin and Jenni, 2011), may have a significant impact on human health. The findings reported here indicate that DAAM1 is cell-autonomously required in the myocardial lineage to prevent NCM in mice. Daam1 CKO mice may thus provide a model for studying the causes of NCM. The NCM in Daam1 CKO mice was preceded by the loss of cardiomyocyte protrusive activity and polarity early in post-progenitor development, suggesting that NCM results from disrupting the cellular rearrangements that pattern the trabeculae. Interestingly, NCM has been linked to mutations in sarcomere components (Almeida and Pinto, 2013; Klaassen et al., 2008). Consistent with these data, Daam1 and 2 were co-expressed in the trabecular myocardium at mid-gestation and redundantly required for sarcomere assembly. These data suggest that the sarcomeric cytoskeleton may play a role in the migration and polarization of embryonic cardiomyocytes. The enhanced cardiac defects in Daam1 CKO mice lacking one copy of Wnt5a were consistent with DAAM1 and 2 mediating Wnt5a signaling and suggest that NCM may be caused by the loss of Wnt/PCP signaling. However, reducing Daam1 gene dosage exacerbated the defects in Wnt5a KO hearts, consistent with Daam1 and 2 playing other Wnt-independent roles in myocardial maturation.
Supplementary Material
Generation of Daam1floxed mice. A Schematic of the targeting construct used to generate Daam1floxed mice. B,C Southern blots of BglII digested genomic DNA with digoxigenin (DIG) labeled fragments of regions downstream of the BamHI site in intron 12–14 (LA probe, A,B) and upstream of the ClaI site in intron 4–5 (SA Probe, A,C). D Western blot for DAAM1 protein performed on hearts of E17.5 Daam1floxed/floxed; Nkx2.5cre (Daam1 CKO) and Daam1floxed/+; Nkx2.5cre (Daam1 HET) embryos. E,F Images of E14.5 Daam1 CKO (F) and control embryos (E) stained for DAAM1 (brown). DAAM1 is reduced in the myocardium of Daam1 CKO embryos relative to controls but not affected in the epicardium (arrowheads) and endocardium (arrows) cells.
Generation of Daam2lacZ mice. A Schematic of the IRES-lacZ insertion in Daam2lacZ mice. B Southern blots of BglII digested genomic DNA with a fragment of intron 6–7 (LAI Probe, A,B). C Southern blots of genomic DNA digested with HindIII probed with a fragment of a region upstream of the EcoRV site in intron 5–6 (LAO Probe, A,C). D Southern blots of genomic DNA digested with BglII and EcoRI probed with a fragment of intron 14–15 located downstream of the 3’ homologous arm (SAO Probe, A,D) to confirm proper targeting. E Western blot for DAAM2 performed on hearts of E17.5 Daam2lacZ/lacZ and Daam2lacZ/+ embryos.
Daam1 and 2 are co-expressed in the trabecular myocardium. In situ hybridizations for Daam1 (red, A–C) and Daam2 (red, D–F) performed on sections of E9.5 (A,D), E10.5 (B,E), and E12.5 (C,F) wild-type mouse embryos. Sections were counterstained with DAPI (blue) to label nuclei. A At E9.5, Daam1 is expressed throughout the myocardium (arrows). B At E10.5, Daam1 persists in the myocardium and interventricular septum (IVS, arrowhead). C At E12.5, Daam1 is found throughout the ventricular walls and trabeculae (arrows). D Daam2 was absent from the myocardium at E9.5 but present in the pro-epicardial organ (arrowhead) and epicardial cells (arrows). E At E10.5, Daam2 was expressed in regions of the mesenchyme and outflow tract (arrowheads) that contain cardiac progenitors. F At E12.5, Daam2 was expressed throughout the ventricular walls and trabeculae (arrows).
DAAM1 is autonomously required for the systolic function of the right ventricle. A–D Hearts of 8-month-old Daam1floxed/floxed; Nkx2.5cre/+ (Daam1 CKO, B,D) and Daam1floxed/+; Nkx2.5cre/+ (Daam1 HET, A,C) mice perfused with KCl buffer. The wall of the right ventricle (RV) is shorter and extends further from the interventricular septum (IVS) in Daam1 CKO than in Daam1 HET (brackets) mice. E,F Echocardiographs of short axis views of 8-month-old Daam1 CKO (F) and Daam1 HET (E) mice with narrower RVs (dotted lines) that extend further from the IVS in Daam1 CKO mice. G Diameters of the RV in 8-month-old Daam1 CKO and control mice, as measured by echocardiography. H,I Acceleration of PA flow (H) and fractional shortening of the RV (I) in 8-month-old Daam1 CKO and control mice, as measured by echocardiography. Center lines of boxplots in G–I represent median values; white upper and gray lower boxes represent the limits of the 2nd and 3rd quartiles, respectively; and the top and bottom whiskers represent the 1st and 4th quartiles, respectively. Student’s t-test results are listed above the dotted lines.
DAAM1 is required for ventricular compaction, cytoskeletal protrusions, and cardiomyocyte polarity. A,B H&E-stained sections of E16.5 Daam1 HET (A) and Daam1 CKO (B) embryos. The proximal-distal axis of the RV is shorter in Daam1 CKO embryos, causing misalignment of the bases of the ventricles (dashed lines, A,B). C–F Enlarged images of the RV (C,D) and LV (E,F) of Daam1 CKO (D,F) and control (C,E) embryos. The compact zones (dashed lines, C–F) were thinner near the apexes of the RV and left ventricle (LV) in Daam1 CKO embryos than in controls (double-headed arrows). The trabeculae in the RVs of Daam1 CKO embryos were also not as regularly spaced or consistent in size as those in the RVs of controls, while those in the LVs of Daam1 CKO embryos were disorganized and occupied much of the luminal space. G,H Graphs show the widths of the trabecular zones in the RVs (G) and LVs (H) of Daam1 HET and Daam1 CKO embryos. Values are the means of measurements taken from 4 embryos of each genotype as described in supplemental methods. Error bars represent standard deviation. Student’s t-test results are listed above the dotted lines. I–N Hearts of E13.5 Daam1 CKO; R26Rβ-Geo (J,L,N) and Daam1 HET; R26Rβ-Geo (I,K,M)) embryos stained with X-gal (blue) and eosin (pink). Cardiomyocytes are tightly associated with one another and oriented perpendicular to the long-axis of the myocardium in the conotruncal regions of control hearts (arrows, I) but randomly distributed and loosely attached to each other in the coni of Daam1 CKO embryos (arrows, J). Thick protrusions on cardiomyocytes invading the OFT in controls (arrows, K) are absent in Daam1 CKO hearts (arrows, L). Cardiomyocytes in walls of the RV form columns in controls (dashed lines, M) but are disorganized and mixed with non-myocardial cells in Daam1 CKO embryos (N).
Simultaneous loss of Daam1 and 2 causes noncompaction. A, B H&E stained sections of E14.5 Daam1/2 DKO (B) and Daam1/2 DHET (A) hearts reveal more trabeculae in Daam1/2 DKO hearts than in controls. C–F Enlarged images of the RV (C,D) and LV (E,F) of Daam1/2 DHET (C,E) and Daam1/2 DKO (D,F) hearts. The compact myocardia (dashed lines) are thinner in the RV of Daam1/2 DKO hearts than controls (double-headed arrows, C,D). Trabeculae are more numerous and thicker in both the RV and LV of Daam1/2 DKO hearts than in controls (C–F). G,H Graphs show the widths of the trabecular zones in the RVs (G) and LVs (H) of Daam1/2 DHET and Daam1/2 DKO embryos. Values are the means of measurements from 4 embryos of each genotype, which were taken as described in supplemental methods. Error bars represent standard deviation. Student’s t-test results are listed above the dotted lines.
DAAM1 loss-of-function does not affect MAPK and Wnt/β-catenin signaling. A Mean relative levels of GTP-bound RhoA in E17.5 Daam1/2 DKO and control hearts. Result of Student’s t-tests is shown above the dotted line. B,C Western blots for the phosphorylation of MYPT1 by RhoA-associated Kinase (B) and phosphorylation of cJun by Jun-N-terminal kinase (C). D Western blots shows the levels of phosphorylated PRK1/2, P38, ERK1/2, β-catenin, and β-tubulin in E17.5 Daam1/2 DKO and control embryos. E Graph shows the relative expression of the β-catenin target genes Axin2, Lef1, Nkd2, CyclinD1, and Myc in the hearts of E17.5 Daam1/2 DKO and control embryos. F Relative expression of Axin2 and another β-catenin target, Tcf4, in the hearts of E14.5 Daam1/2 DKO and control embryos. Values are the means from 3 independent embryos of each genotype and error bars represent standard deviation.
Reducing DAAM1 function enhances the heart defects in Wnt5a mutant embryos. AC H&E-stained sections of E16.5 Daam1 HET; Wnt5a HET (A), Daam1 CKO (B) and Daam1 CKO; Wnt5a HET (C) embryos reveal excess trabeculae in Daam1 CKO and Daam1 CKO; Wnt5a HET embryos (arrows, B,C, respectively). D–F H&E-stained sections of E16.5 Wnt5a knockout (Wnt5a KO, D), Daam1 HET; Wnt5a KO (E), and Daam1 CKO; Wnt5a KO embryos (F). Wnt5a KO embryos have RVs with thin, compact, and trabecular myocardia while the LV walls are thickened. The hearts of Daam1 HET; Wnt5a KO embryos have a disrupted morphology in which the myocardia are discontinuous, allowing blood to escape into the pericardial cavity (arrows, E). Daam1 CKO; Wnt5a KO embryos were necrotic (F). Enlarged images of the boxed areas are shown in Figure 7.
Simultaneously deleting Daam1 and Wnt5a disrupts looping morphogenesis. A–C H&E-stained sections of E12.5 wild type (A), Wnt5a KO (B) and Daam1 HET; Wnt5a KO (C) embryos. Arrows mark the IVS. D-F H&E-stained sections of a moderately affected E12.5 Daam1 CKO; Wnt5a KO embryo arranged from anterior (D) to posterior (F). G-I H&E-stained sections of a severely affected E12.5 Daam1 CKO; Wnt5a KO embryo arranged from anterior (G) to posterior (I). J-M Enlarged images of the base of the RV in E12.5 wild type (J), Wnt5a KO (K), Daam1 HET; Wnt5a KO (L) and Daam1 CKO; Wnt5a KO (M) embryos.
Highlights.
Daam1 is autonomously required for cardiomyocyte polarity and adhesion
Daam1 and 2 are co-required for sarcomere assembly and myocardial maturation
Reducing Wnt5a levels exacerbates the heart defects in Daam1 mutants
Deleting Daam1 in Wnt5a mutants causes severe heart defects and early lethality
Daam1 and 2 may have Wnt-dependent and Wnt-independent roles in heart development
Acknowledgements
We would like to thank Dr. Vikas V. Patel and the Penn CVI Mouse Cardiovascular Physiology and Microsurgery Core as well as Min Min Lu and the Histology and Gene Expression Core at the University of Pennsylvania Cardiovascular Research Institute.
Sources of Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, as well as the Progenitor Cell Biology Consortium Grant (HL100405) and American Heart Association (10SDG2610019 and 15GRNT23020024).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- Ahuja P, Perriard E, Perriard JC, Ehler E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. Journal of cell science. 2004;117:3295–3306. doi: 10.1242/jcs.01159. [DOI] [PubMed] [Google Scholar]
- Almeida AG, Pinto FJ. Non-compaction cardiomyopathy. Heart. 2013;99:1535–1542. doi: 10.1136/heartjnl-2012-302048. [DOI] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nature reviews. Molecular cell biology. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P. Formin-binding proteins: modulators of formin-dependent actin polymerization. Biochimica et biophysica acta. 2010;1803:174–182. doi: 10.1016/j.bbamcr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Experimental cell research. 2006;312:2180–2194. doi: 10.1016/j.yexcr.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Bendris N, Lemmers B, Blanchard JM. Cell cycle, cytoskeleton dynamics and beyond: the many functions of cyclins and CDK inhibitors. Cell cycle. 2015;0 doi: 10.1080/15384101.2014.998085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson JA, Mills B, Paul Helt JC, Zwaka TP, Cohen ED. Wnt5a and Wnt11 inhibit the canonical Wnt pathway and promote cardiac progenitor development via the Caspase-dependent degradation of AKT. Developmental biology. 2015;398:80–96. doi: 10.1016/j.ydbio.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Bretones G, Delgado MD, Leon J. Myc and cell cycle control. Biochimica et biophysica acta. 2015;1849:506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes & development. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Carrilho-Ferreira P, Almeida AG, Pinto FJ. Non-compaction cardiomyopathy: prevalence, prognosis, pathoetiology, genetics, and risk of cardioembolism. Current heart failure reports. 2014;11:393–403. doi: 10.1007/s11897-014-0227-3. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development. 2012;139:1931–1940. doi: 10.1242/dev.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta biochimica et biophysica Sinica. 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circulation research. 2010;107:700–714. doi: 10.1161/CIRCRESAHA.110.223412. [DOI] [PubMed] [Google Scholar]
- Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Current opinion in cell biology. 1999;11:233–240. doi: 10.1016/s0955-0674(99)80031-3. [DOI] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes & development. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS genetics. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezan J, Montcouquiol M. Revisiting planar cell polarity in the inner ear. Seminars in cell & developmental biology. 2013;24:499–506. doi: 10.1016/j.semcdb.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Francou A, Saint-Michel E, Mesbah K, Theveniau-Ruissy M, Rana MS, Christoffels VM, Kelly RG. Second heart field cardiac progenitor cells in the early mouse embryo. Biochimica et biophysica acta. 2013;1833:795–798. doi: 10.1016/j.bbamcr.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. Journal of molecular medicine. 2006;84:446–468. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Gao B. Wnt regulation of planar cell polarity (PCP) Current topics in developmental biology. 2012;101:263–295. doi: 10.1016/B978-0-12-394592-1.00008-9. [DOI] [PubMed] [Google Scholar]
- Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Vicart P, Goebel HH, Dalakas MC. Desmin myopathy. Brain : a journal of neurology. 2004;127:723–734. doi: 10.1093/brain/awh033. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Phillips HM, Chaudhry B. Vang-like 2 and noncanonical Wnt signaling in outflow tract development. Trends in cardiovascular medicine. 2006;16:38–45. doi: 10.1016/j.tcm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Kassenbrock CK, Hunter S, Garl P, Johnson GL, Anderson SM. Inhibition of Src family kinases blocks epidermal growth factor (EGF)-induced activation of Akt, phosphorylation of c-Cbl, and ubiquitination of the EGF receptor. The Journal of biological chemistry. 2002;277:24967–24975. doi: 10.1074/jbc.M201026200. [DOI] [PubMed] [Google Scholar]
- Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, Schuler P, Greutmann M, Hurlimann D, Yegitbasi M, Pons L, Gramlich M, Drenckhahn JD, Heuser A, Berger F, Jenni R, Thierfelder L. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation. 2008;117:2893–2901. doi: 10.1161/CIRCULATIONAHA.107.746164. [DOI] [PubMed] [Google Scholar]
- Kovacevic-Preradovic T, Jenni R, Oechslin EN, Noll G, Seifert B, Attenhofer Jost CH. Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience. Cardiology. 2009;112:158–164. doi: 10.1159/000147899. [DOI] [PubMed] [Google Scholar]
- Kuhl M. Non-canonical Wnt signaling in Xenopus: regulation of axis formation and gastrulation. Seminars in cell & developmental biology. 2002;13:243–249. doi: 10.1016/s1084-9521(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends in genetics : TIG. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Geyer M. Formins as effector proteins of Rho GTPases. Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwack MH, Kim MK, Kim JC, Sung YK. Wnt5a attenuates Wnt/beta-catenin signalling in human dermal papilla cells. Experimental dermatology. 2013;22:229–231. doi: 10.1111/exd.12101. [DOI] [PubMed] [Google Scholar]
- Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, Chen H, Haneline LS, Chan RJ, Schwartz RJ, Field LJ, Atkinson SJ, Shou W. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138:303–315. doi: 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorganic & medicinal chemistry letters. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther PK. The vertebrate muscle Z-disc: sarcomere anchor for structure and signalling. Journal of muscle research and cell motility. 2009;30:171–185. doi: 10.1007/s10974-009-9189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Developmental biology. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133:957–966. doi: 10.1242/dev.02266. [DOI] [PubMed] [Google Scholar]
- Maung SM, Jenny A. Planar cell polarity in Drosophila. Organogenesis. 2011;7:165–179. doi: 10.4161/org.7.3.18143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzano V, Pellman J, Sheikh F. Cell junctions in the specialized conduction system of the heart. Cell communication & adhesion. 2014;21:149–159. doi: 10.3109/15419061.2014.905928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS biology. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. doi: 10.1002/gene.10022. [DOI] [PubMed] [Google Scholar]
- Mottram PM, Marwick TH. Assessment of diastolic function: what the general cardiologist needs to know. Heart. 2005;91:681–695. doi: 10.1136/hrt.2003.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy II, Railo A, Rapila R, Hast T, Sormunen R, Tavi P, Rasanen J, Vainio SJ. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovascular research. 2010;85:100–109. doi: 10.1093/cvr/cvp254. [DOI] [PubMed] [Google Scholar]
- Nagy A. Manipulating the mouse embryo : a laboratory manual. 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- Niehrs C, Acebron SP. Mitotic and mitogenic Wnt signalling. The EMBO journal. 2012;31:2705–2713. doi: 10.1038/emboj.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. The Journal of biological chemistry. 2008;283:27973–27981. doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? European heart journal. 2011;32:1446–1456. doi: 10.1093/eurheartj/ehq508. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Phillips HM, Hildreth V, Peat JD, Murdoch JN, Kobayashi K, Chaudhry B, Henderson DJ. Non-cell-autonomous roles for the planar cell polarity gene Vangl2 in development of the coronary circulation. Circulation research. 2008;102:615–623. doi: 10.1161/CIRCRESAHA.107.160861. [DOI] [PubMed] [Google Scholar]
- Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circulation research. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochais F, Mesbah K, Kelly RG. Signaling pathways controlling second heart field development. Circulation research. 2009;104:933–942. doi: 10.1161/CIRCRESAHA.109.194464. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- Schleiffarth JR, Person AD, Martinsen BJ, Sukovich DJ, Neumann A, Baker CV, Lohr JL, Cornfield DN, Ekker SC, Petryk A. Wnt5a is required for cardiac outflow tract septation in mice. Pediatric research. 2007;61:386–391. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- Schonichen A, Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochimica et biophysica acta. 2010;1803:152–163. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Borg JP. Insight into planar cell polarity. Experimental cell research. 2014;328:284–295. doi: 10.1016/j.yexcr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sinha T, Li D, Theveniau-Ruissy M, Hutson MR, Kelly RG, Wang J. Loss of Wnt5a disrupts second heart field cell deployment and may contribute to OFT malformations in DiGeorge syndrome. Human molecular genetics. 2014 doi: 10.1093/hmg/ddu584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha T, Li D, Theveniau-Ruissy M, Hutson MR, Kelly RG, Wang J. Loss of Wnt5a disrupts second heart field cell deployment and may contribute to OFT malformations in DiGeorge syndrome. Human molecular genetics. 2015;24:1704–1716. doi: 10.1093/hmg/ddu584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller JZ, Degenhardt KR, Huang L, Zhou DD, Lu MM, Epstein JA. Cre reporter mouse expressing a nuclear localized fusion of GFP and beta-galactosidase reveals new derivatives of Pax3-expressing precursors. Genesis. 2008;46:200–204. doi: 10.1002/dvg.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromer MH. The cytoskeleton in skeletal, cardiac and smooth muscle cells. Histology and histopathology. 1998;13:283–291. doi: 10.14670/HH-13.283. [DOI] [PubMed] [Google Scholar]
- Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Seminars in cell & developmental biology. 2002;13:251–260. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Takano J, Mihira N, Fujioka R, Hosoki E, Chishti AH, Saido TC. Vital role of the calpain-calpastatin system for placental-integrity-dependent embryonic survival. Molecular and cellular biology. 2011;31:4097–4106. doi: 10.1128/MCB.05189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JL, Kahn M. The Wnt signaling pathway in cellular proliferation and differentiation: A tale of two coactivators. Advanced drug delivery reviews. 2010;62:1149–1155. doi: 10.1016/j.addr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Tessarollo L. Manipulating mouse embryonic stem cells. Methods Mol Biol. 2001;158:47–63. doi: 10.1385/1-59259-220-1:47. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Thornell L, Carlsson L, Li Z, Mericskay M, Paulin D. Null mutation in the desmin gene gives rise to a cardiomyopathy. Journal of molecular and cellular cardiology. 1997;29:2107–2124. doi: 10.1006/jmcc.1997.0446. [DOI] [PubMed] [Google Scholar]
- Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Developmental cell. 2010;18:275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin JA, Lorts A, Jefferies JL. Left ventricular non-compaction cardiomyopathy. Lancet. 2015 doi: 10.1016/S0140-6736(14)61282-4. [DOI] [PubMed] [Google Scholar]
- Tzahor E. Wnt/beta-catenin signaling and cardiogenesis: timing does matter. Developmental cell. 2007;13:10–13. doi: 10.1016/j.devcel.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Vite A, Radice GL. N-cadherin/catenin complex as a master regulator of intercalated disc function. Cell communication & adhesion. 2014;21:169–179. doi: 10.3109/15419061.2014.908853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhao Y, Su Y, Li R, Lin Y, Zhou X, Ye L. C-Jun N-terminal kinase (JNK) mediates Wnt5a-induced cell motility dependent or independent of RhoA pathway in human dental papilla cells. PloS one. 2013;8:e69440. doi: 10.1371/journal.pone.0069440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Osinska H, Gerdes AM, Robbins J. Desmin filaments and cardiac disease: establishing causality. Journal of cardiac failure. 2002;8:S287–S292. doi: 10.1054/jcaf.2002.129279. [DOI] [PubMed] [Google Scholar]
- Wu G, Huang X, Hua Y, Mu D. Roles of planar cell polarity pathways in the development of neural [correction of neutral] tube defects. Journal of biomedical science. 2011;18:66. doi: 10.1186/1423-0127-18-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Young KG, Copeland JW. Formins in cell signaling. Biochimica et biophysica acta. 2010;1803:183–190. doi: 10.1016/j.bbamcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Niu CC, Deng G, Li ZQ, Pan J, Zhao C, Yang ZL, Si WK. The Wnt5a/Ror2 noncanonical signaling pathway inhibits canonical Wnt signaling in K562 cells. International journal of molecular medicine. 2011;27:63–69. doi: 10.3892/ijmm.2010.560. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Kelly RG. New developments in the second heart field. Differentiation; research in biological diversity. 2012;84:17–24. doi: 10.1016/j.diff.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Zhou B, von Gise A, Ma Q, Rivera-Feliciano J, Pu WT. Nkx2-5-and Isl1-expressing cardiac progenitors contribute to proepicardium. Biochemical and biophysical research communications. 2008;375:450–453. doi: 10.1016/j.bbrc.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of Daam1floxed mice. A Schematic of the targeting construct used to generate Daam1floxed mice. B,C Southern blots of BglII digested genomic DNA with digoxigenin (DIG) labeled fragments of regions downstream of the BamHI site in intron 12–14 (LA probe, A,B) and upstream of the ClaI site in intron 4–5 (SA Probe, A,C). D Western blot for DAAM1 protein performed on hearts of E17.5 Daam1floxed/floxed; Nkx2.5cre (Daam1 CKO) and Daam1floxed/+; Nkx2.5cre (Daam1 HET) embryos. E,F Images of E14.5 Daam1 CKO (F) and control embryos (E) stained for DAAM1 (brown). DAAM1 is reduced in the myocardium of Daam1 CKO embryos relative to controls but not affected in the epicardium (arrowheads) and endocardium (arrows) cells.
Generation of Daam2lacZ mice. A Schematic of the IRES-lacZ insertion in Daam2lacZ mice. B Southern blots of BglII digested genomic DNA with a fragment of intron 6–7 (LAI Probe, A,B). C Southern blots of genomic DNA digested with HindIII probed with a fragment of a region upstream of the EcoRV site in intron 5–6 (LAO Probe, A,C). D Southern blots of genomic DNA digested with BglII and EcoRI probed with a fragment of intron 14–15 located downstream of the 3’ homologous arm (SAO Probe, A,D) to confirm proper targeting. E Western blot for DAAM2 performed on hearts of E17.5 Daam2lacZ/lacZ and Daam2lacZ/+ embryos.
Daam1 and 2 are co-expressed in the trabecular myocardium. In situ hybridizations for Daam1 (red, A–C) and Daam2 (red, D–F) performed on sections of E9.5 (A,D), E10.5 (B,E), and E12.5 (C,F) wild-type mouse embryos. Sections were counterstained with DAPI (blue) to label nuclei. A At E9.5, Daam1 is expressed throughout the myocardium (arrows). B At E10.5, Daam1 persists in the myocardium and interventricular septum (IVS, arrowhead). C At E12.5, Daam1 is found throughout the ventricular walls and trabeculae (arrows). D Daam2 was absent from the myocardium at E9.5 but present in the pro-epicardial organ (arrowhead) and epicardial cells (arrows). E At E10.5, Daam2 was expressed in regions of the mesenchyme and outflow tract (arrowheads) that contain cardiac progenitors. F At E12.5, Daam2 was expressed throughout the ventricular walls and trabeculae (arrows).
DAAM1 is autonomously required for the systolic function of the right ventricle. A–D Hearts of 8-month-old Daam1floxed/floxed; Nkx2.5cre/+ (Daam1 CKO, B,D) and Daam1floxed/+; Nkx2.5cre/+ (Daam1 HET, A,C) mice perfused with KCl buffer. The wall of the right ventricle (RV) is shorter and extends further from the interventricular septum (IVS) in Daam1 CKO than in Daam1 HET (brackets) mice. E,F Echocardiographs of short axis views of 8-month-old Daam1 CKO (F) and Daam1 HET (E) mice with narrower RVs (dotted lines) that extend further from the IVS in Daam1 CKO mice. G Diameters of the RV in 8-month-old Daam1 CKO and control mice, as measured by echocardiography. H,I Acceleration of PA flow (H) and fractional shortening of the RV (I) in 8-month-old Daam1 CKO and control mice, as measured by echocardiography. Center lines of boxplots in G–I represent median values; white upper and gray lower boxes represent the limits of the 2nd and 3rd quartiles, respectively; and the top and bottom whiskers represent the 1st and 4th quartiles, respectively. Student’s t-test results are listed above the dotted lines.
DAAM1 is required for ventricular compaction, cytoskeletal protrusions, and cardiomyocyte polarity. A,B H&E-stained sections of E16.5 Daam1 HET (A) and Daam1 CKO (B) embryos. The proximal-distal axis of the RV is shorter in Daam1 CKO embryos, causing misalignment of the bases of the ventricles (dashed lines, A,B). C–F Enlarged images of the RV (C,D) and LV (E,F) of Daam1 CKO (D,F) and control (C,E) embryos. The compact zones (dashed lines, C–F) were thinner near the apexes of the RV and left ventricle (LV) in Daam1 CKO embryos than in controls (double-headed arrows). The trabeculae in the RVs of Daam1 CKO embryos were also not as regularly spaced or consistent in size as those in the RVs of controls, while those in the LVs of Daam1 CKO embryos were disorganized and occupied much of the luminal space. G,H Graphs show the widths of the trabecular zones in the RVs (G) and LVs (H) of Daam1 HET and Daam1 CKO embryos. Values are the means of measurements taken from 4 embryos of each genotype as described in supplemental methods. Error bars represent standard deviation. Student’s t-test results are listed above the dotted lines. I–N Hearts of E13.5 Daam1 CKO; R26Rβ-Geo (J,L,N) and Daam1 HET; R26Rβ-Geo (I,K,M)) embryos stained with X-gal (blue) and eosin (pink). Cardiomyocytes are tightly associated with one another and oriented perpendicular to the long-axis of the myocardium in the conotruncal regions of control hearts (arrows, I) but randomly distributed and loosely attached to each other in the coni of Daam1 CKO embryos (arrows, J). Thick protrusions on cardiomyocytes invading the OFT in controls (arrows, K) are absent in Daam1 CKO hearts (arrows, L). Cardiomyocytes in walls of the RV form columns in controls (dashed lines, M) but are disorganized and mixed with non-myocardial cells in Daam1 CKO embryos (N).
Simultaneous loss of Daam1 and 2 causes noncompaction. A, B H&E stained sections of E14.5 Daam1/2 DKO (B) and Daam1/2 DHET (A) hearts reveal more trabeculae in Daam1/2 DKO hearts than in controls. C–F Enlarged images of the RV (C,D) and LV (E,F) of Daam1/2 DHET (C,E) and Daam1/2 DKO (D,F) hearts. The compact myocardia (dashed lines) are thinner in the RV of Daam1/2 DKO hearts than controls (double-headed arrows, C,D). Trabeculae are more numerous and thicker in both the RV and LV of Daam1/2 DKO hearts than in controls (C–F). G,H Graphs show the widths of the trabecular zones in the RVs (G) and LVs (H) of Daam1/2 DHET and Daam1/2 DKO embryos. Values are the means of measurements from 4 embryos of each genotype, which were taken as described in supplemental methods. Error bars represent standard deviation. Student’s t-test results are listed above the dotted lines.
DAAM1 loss-of-function does not affect MAPK and Wnt/β-catenin signaling. A Mean relative levels of GTP-bound RhoA in E17.5 Daam1/2 DKO and control hearts. Result of Student’s t-tests is shown above the dotted line. B,C Western blots for the phosphorylation of MYPT1 by RhoA-associated Kinase (B) and phosphorylation of cJun by Jun-N-terminal kinase (C). D Western blots shows the levels of phosphorylated PRK1/2, P38, ERK1/2, β-catenin, and β-tubulin in E17.5 Daam1/2 DKO and control embryos. E Graph shows the relative expression of the β-catenin target genes Axin2, Lef1, Nkd2, CyclinD1, and Myc in the hearts of E17.5 Daam1/2 DKO and control embryos. F Relative expression of Axin2 and another β-catenin target, Tcf4, in the hearts of E14.5 Daam1/2 DKO and control embryos. Values are the means from 3 independent embryos of each genotype and error bars represent standard deviation.
Reducing DAAM1 function enhances the heart defects in Wnt5a mutant embryos. AC H&E-stained sections of E16.5 Daam1 HET; Wnt5a HET (A), Daam1 CKO (B) and Daam1 CKO; Wnt5a HET (C) embryos reveal excess trabeculae in Daam1 CKO and Daam1 CKO; Wnt5a HET embryos (arrows, B,C, respectively). D–F H&E-stained sections of E16.5 Wnt5a knockout (Wnt5a KO, D), Daam1 HET; Wnt5a KO (E), and Daam1 CKO; Wnt5a KO embryos (F). Wnt5a KO embryos have RVs with thin, compact, and trabecular myocardia while the LV walls are thickened. The hearts of Daam1 HET; Wnt5a KO embryos have a disrupted morphology in which the myocardia are discontinuous, allowing blood to escape into the pericardial cavity (arrows, E). Daam1 CKO; Wnt5a KO embryos were necrotic (F). Enlarged images of the boxed areas are shown in Figure 7.
Simultaneously deleting Daam1 and Wnt5a disrupts looping morphogenesis. A–C H&E-stained sections of E12.5 wild type (A), Wnt5a KO (B) and Daam1 HET; Wnt5a KO (C) embryos. Arrows mark the IVS. D-F H&E-stained sections of a moderately affected E12.5 Daam1 CKO; Wnt5a KO embryo arranged from anterior (D) to posterior (F). G-I H&E-stained sections of a severely affected E12.5 Daam1 CKO; Wnt5a KO embryo arranged from anterior (G) to posterior (I). J-M Enlarged images of the base of the RV in E12.5 wild type (J), Wnt5a KO (K), Daam1 HET; Wnt5a KO (L) and Daam1 CKO; Wnt5a KO (M) embryos.