Abstract
Osteoporosis is one of the most prevalent skeletal disorders and has enormous public health consequences due to the morbidity and mortality of the resulting fractures. This article discusses the developmental origins of osteoporosis and outlines some of the modifiable and non-modifiable risk factors in both intrauterine and postnatal life that contribute to the later onset of osteoporosis. Evidence for the effects of birth size and early growth in both preterm and term born infants are discussed and the role of epigenetics within the programming hypothesis is highlighted. This review provides compelling evidence for the developmental origins of osteoporosis and highlights the importance of osteoporosis prevention at all stages of the life course.
Keywords: Bone mineral density, Life course, Programming, Osteoporosis.
INTRODUCTION
Osteoporosis is characterized by the depletion of bone mineral mass, combined with bone micro-architecture deterioration, greater bone fragility and a resultant increased fracture risk [1]; a 10% loss of vertebral bone mass can double the risk of a vertebral fracture [2]. Osteoporosis is one of the most prevalent skeletal disorders and has a similar lifetime risk to coronary heart disease [3]. It affects approximately 3 million people in the UK and worldwide, an osteoporotic fracture occurs every 3 seconds [4]. Osteoporosis has enormous public health consequences due to the morbidity and mortality of the resulting fractures and the associated healthcare expenditure, particularly as aging populations increase in many parts of the world. As there is no cure, it is important to identify early life influences on later bone mineral density, which may aid the development of interventions to optimize bone health and reduce osteoporosis risk. This article discusses the developmental origins of osteoporosis and outlines some of the modifiable and non-modifiable risk factors in both intrauterine and postnatal life that contribute to the later onset of osteoporosis.
NORMAL SKELETAL DEVELOPMENT AND THE DEVELOPMENTAL ORIGINS OF HEALTH AND DISEASE
Bone mineral content (BMC) and bone mineral density (BMD) in adulthood depends predominantly on growth and mineralization of the skeleton and the resultant peak bone mass achieved and then, to a lesser extent, on the subsequent loss. Bone mass shows strong tracking during childhood and adolescent growth and into adulthood. Reduced peak bone mass in childhood is associated with increased fracture riskand has been proposed as one of the most accurate predictors of later life fracture risk [5]. Genetic predisposition accounts for up to 50% of the variance in bone mass and gender also influences bone composition with males attaining greater bone mass than females [6]. Environmental influences during both childhood and adulthood, such as smoking [7], corticosteroid use [8] and exercise [9] are also important. However, a significant portion of variance in bone mass remains unexplained [10]. It is likely that much of this remaining variation results from the programming of systems controlling skeletal growth trajectory and so ultimately influencing peak bone mass during critical growth periods [11, 12]. The Developmental Origins of Health and Disease (DoHaD) hypothesis suggests that nutritional imbalance during critical windows in early life can permanently influence or ‘programme’ long-term development and disease in later life [13], (see Table 1). Much of the original work was by Barker and colleagues who reported the relationship with low birth weight (used as a proxy for fetal growth) with coronary heart disease [14, 15]. Further studies suggested, however, that these mechanisms and effects were not restricted to fetal life and that nutrition and growth in infancy (and perhaps in later childhood) were also crucial, leading to the incorporation of elements of evolutionary biology and the adoption of the term DOHaD.
Table 1.
Developmental risk factors for osteoporosis.
| MATERNAL |
| Vitamin D status Calcium intake Social class and pre-pregnancy dietary factors Maternal fat stores and nourishment during pregnancy |
| FETAL |
| In utero growth effects on birthweight and birth length Length of gestation (prematurity) Genetic predisposition including maternal and paternal birthweights, gene-environment interactions, vitamin D polymorphisms In-utero activity |
| INFANT |
| Slow growth throughout infancy Lack of breast feeding and dietary factors Vitamin D intakes Socio-demographic factors e.g. exposure to smoking |
| CHILDHOOD |
| Lifestyle and socio-demographic factors Nutrient intakes Physical activity and bone stress Co- morbidities and drug treatments e.g. steroids |
Factors that affect early life bone development [16] have not been fully elucidated and the lack of prospective research in this area has been highlighted [17].
Some of the greatest insights into programming of bone come from large epidemiological studies, either those with detailed early exposure information and prolonged follow-up, or mother-offspring cohorts. Examples of large longitudinal studies include the Avon Longitudinal Study of Parents and Children (ALSPAC) [18, 19], which consists of a cohort of approximately 14000 women anticipated to give birth in 1991 or 1992. Utilising frequent questionnaires and clinic assessments of the mothers and their offspring, detailed information is available on early-life exposures and subsequent skeletal development. Other studies include the Hertfordshire cohort study [20], consisting of 3000 men and women aged 60-75 years old living in Hertfordshire, who were recruited to a study to determine influences of birth weight and infant growth on adult disease; and the Finnish cohort of over 7000 people born in Helsinki university hospital between 1924 and 1933 and still residing in Finland in 1971 [21]. The Finnish cohort is unique in that early life data is linked to later hip fracture rather than relying on proxy markers of fracture risk such as bone mineral density, as assessed by non-invasive DXA methods. The Southampton Women’s survey is a mother-offspring cohort and is the only study in Europe where the mothers were interviewed before conception; up to 2000 offspring have now been followed up at 10 years of age [22].
The potential for confounding in observational studies can make establishing causation difficult. For example, poor nutrition is an inevitable consequence in the sickest neonate who in turn will be more likely to have a poorer metabolic outcome. Similarly, socioeconomic status may be an important confounder when investigating the effects of programming, as socioeconomic status in itself is known to have a important effect on BMD [23], therefore it is vital that such potential confounders are adjusted for. Another challenge in longitudinal cohort studies, especially involving children, is that of attritional losses over time introducing a risk of bias. A 2011 meta-analysis stated that research from a variety of populations may help clarify inconsistencies concerning the relationship between early life events and subsequent bone health [10]. Further evidence for the role of programming comes from twin studies, where statistically significant differences in the relationship between birth weight and bone mineral content were found between monozygous twin pairs [24]. In this study, associations were largely accounted for by environmental factors independent of maternal factors (gestation, smoking, nutrition etc.) and were largely mediated by skeletal size and especially adult height.
It can be technically challenging to account for bone size at different stages of growth when interpreting DXA bone mineral density data, particularly in longitudinal studies. Bone strength is based on size as well as mineral content and as a child grows, their bones will change in shape and size, and the body will also change in size and composition. This can make it difficult when comparing results to previous scans. Some scanners automatically produce T scores based on the BMD with reference to a healthy adult- clearly this is completely meaningless in a young child. DXA measures the total amount of bone mineral content contained within the skeletal region scanned and the two- dimensional projected bone area in order to calculate the areal BMD in grams/metre squared, rather than by calculating the mineral content within the volume. Therefore if the bone is larger, the areal BMD will appear greater although the true BMD would be the same. This is particularly important in children with chronic diseases who are often small as their BMD may be underestimated by DEXA. This problem is further compounded by pubertal delay, which is often seen in chronic disease. Therefore it is vital to consider height, bone age (rather than chronological age) and puberty when interpreting results and the conclusions of studies and there are several different methods available to help adjust for bone size and growth [25].
EFFECTS OF BIRTH SIZE AND EARLY GROWTH ON LATER BONE MASS
Bone mineral accrual is greatest during the last trimester of pregnancy, when growth velocity is rapid. Birth weight and birth length are a reflection of intra-uterine growth and therefore are also affected by environmental influences during pregnancy. A study by Harvey et al. using the Southampton cohort showed that intrauterine growth was strongly associated with childhood bone size and density at 4 years of age. Change in femur length between 19 and 34 weeks gestation was associated with childhood skeletal size at 4 years of age, while changes in fetal abdominal circumference predicted bone density [26].
There are however, conflicting data regarding the influence of birth weight on later BMD. Baird et al. performed a systematic review and meta-analysis to determine whether birth weight predicted bone mass in adulthood [10]. The study identified 14 studies that met the inclusion criteria. Most showed a consistent association between higher birth weight and greater adult bone mineral content at both the lumbar spine and the hip but found that birth weight was not a strong predictor of later lumbar spine or hip BMD. The Hertfordshire cohort study (which formed the basis for several of the studies of Barker et al.) showed that birth weight was independently associated with BMD in men, but not women, at 63 years of age.
Baird suggests that in most of the studies, the weak association between birth weight and BMD was likely to be a result of other postnatal factors such as childhood physical activity and pubertal timing as well as genetic variation playing a more influential role. Studies in the ALSPAC cohort demonstrated independent effects of birth weight and weight at one year on bone size and strength during the sixth and seventh decades after adjustment for confounding lifestyle factors [20]. This provides strong evidence for both programming and tracking of bone mass throughout the lifecourse. Although another study found no association with preterm birth itself and peak bone mass [27], an effect of being small for gestational age was apparent, suggesting that a proportion of later bone mass is determined by fetal growth. Some studies suggests that very low birth weight infants, whether preterm or not, attain a sub-optimal peak bone mass in part due to their small size and subnormal skeletal mineralization [28].
Endocrine mechanisms may be responsible for the programming, for example via the growth hormone (GH)-Insulin-growth factor 1 (IGF-1) axis, which regulates both growth, and bone remodeling [29]. In support of this, Javaid et al. [30] showed that the concentration of IGF-1 in the umbilical cord correlates strongly with birth weight (after adjustment for gestational age) and bone mineral content. Similarly, there is evidence that birth weight and infant weight predict GH and cortisol levels during adulthood, which in turn are determinants of later bone loss [31, 32].
Multiple studies have emphasized the importance of early growth for programming later bone health. For example in a study by Cooper et al., those who were lightest at 1 year of age had the lowest BMC [33]. In a further study, weight gain during the first two years of life predicted BMD at age 9-14 years in children who were born preterm [34]. The Finnish cohort showed that low rates of childhood growth were a major determinant of later hip fracture risk [21]. Studies as part of the Hertfordshire Cohort Study found that birth weight and weight at 1 year of age were strongly associated with measures of bone strength at both the radius and tibia [20], but that low weight in infancy was also associated with reduced femoral neck width, independently of bone mineral content (BMC) [35]. This supports the theory that intrauterine and postnatal growth influences later fracture risk not only by affecting bone mass, but also by effects on bone geometry.
THE EFFECTS OF BIRTH WEIGHT AND INFANT GROWTH ON ADULT BMD IN THOSE BORN PRETERM
Eighty percent of fetal bone mineral accumulation occurs during the last trimester of pregnancy, with a surge in placental transfer of calcium, magnesium and phosphorus to the fetus [36]. A preterm infant who spends this period without the placenta and the associated endocrine and physical maternally controlled environments is therefore more susceptible to a lower BMD and BMC than an infant born at term. There is, however, conflicting data regarding the long-term consequences of preterm birth on the skeleton and the potential for peak BMD compared to their term counterparts. Premature infants are known to have a lower bone mass [37], BMD [16] and BMC [38] at the corrected age of term, as well as a lower weight and ponderal index [16]. A study of 7-year-old boys showed greater measures of cortical thickness, whole body BMC and hip BMD in term compared to preterm boys after adjustment for weight, height and age. These differences remained after adjustment for birth weight, length of neonatal hospital stay and current activity level [39].
A study by Fewtrell et al. [40] showed that former preterm infants who were followed up at around 10 years of age were shorter, lighter and had lower BMC than controls. These differences continue through childhood and appear to persist until puberty [38, 39], although results are difficult to interpret due to the confounding effects of the endocrine changes during puberty, and the interaction with bone size and later BMD. In a study by Backstrom et al., individuals who were born preterm were assessed with computerized tomography as young adults. Lower bone strength was demonstrated at the distal tibia and radius compared to age and sex matched controls [17]. This effect was more pronounced in males and remained after adjustment for potential confounders. Several studies have failed to demonstrate an association between preterm birth and later bone strength, although all of these [28, 39, 41] were undertaken in relatively small cohorts. A possible explanation for the variation in study results may be in the timing of follow-up as catch up in bone mineralization may occur primarily in late childhood and adolescence. Other studies have found that although preterm born individuals were smaller, their BMD was appropriate for size. Adults who were born preterm may also be shorter than their term born counterparts. As some studies may not have made appropriate adjustments for current size it is difficult to determine whether BMD is appropriate for current size or not [39].
Several other studies in infants have shown the influence of early growth on later bone health in those born preterm. In a study by Cooper et al., those who were lightest at 1 year of age had the lowest BMC [33]. In a further study, weight gain during the first two years of life predicted BMD at age 9-14 [34]. Fewtrell et al. suggested that preterm infants with the most substantial increase in height (length) between birth and 8-12 years of age showed the greatest bone mass at follow-up [40] , (see Table 2). They also demonstrated that birth length alone was a strong predictor of later bone mass, suggesting that optimising linear growth in early life may be beneficial to later bone health. However, although conducted with a large cohort (n=201), few measurements were taken after discharge and dual-energy x-ray absorptiometry (DXA) was only performed at 8-12 years. As a result, the impact of changes in growth and corresponding bone mass at potentially critical epochs of infancy could not be assessed. There are scarce data looking at the effects of birth length and ponderal index on later bone health; further work in this area would add additional insight into the effects of growth.
Table 2.
Key findings from relevant studies.
| Study | Population and Gender (M/F) | Key Exposures & Bone Outcomes Explored | Key Findings | Gender Differences Described | Comments |
|---|---|---|---|---|---|
| Boot et al. (1997) [41] | (n=500) Children and adolescents 4-20 years M=205 F=295 |
Puberty, dietary and lifestyle and current bone density (DXA) | Pubertal development in girls and current weight in boys, are main factors in current BMD Low birthweight and prematurity not significantly associated with BMD |
Key factors: Tanner stage in girls versus weight in boys | Puberty and later childhood growth are key determinants of skeletal development Pubertal factors may be sex specific Large study, but limited numbers of preterm born children |
| Cooper et al. (2001) | (n=7086) Born in 1924-33 and residing in Finland in 1971 M=3639 F=3447 |
Growth measured at birth and during childhood and linked to risk of hip fracture | Children born to tall mothers and those with slow childhood growth rates have increased hip fracture risk | Fracture more likely in taller women Differing growth patterns predict risk |
Measures actual fracture outcome rather than predictive markers of risk Cohort were largely working class; dietary factors and activity level may no longer be as comparable with modern patterns |
| Dennison et al. (2001) [50] | (n=291) Adults 61-73 years M=165 F=126 |
Vitamin D receptor genotype, birthweight and adult bone mass (DXA) | Significant interaction between birthweight and VDR genotype Association between lumbar BMD and VDR genotype varies according to birthweight |
Women had a greater rate of bone loss over the follow-up period | Large study with later life outcomes Supports role of interactions between genetic factors and ‘programming’ of osteoporosis |
| Godfrey et al. (2001) [53] | (n=145) Term infants M= 81 F= 64 |
Maternal and paternal demographic and lifestyle factors, and neonatal bone mass (DXA) | Parental birthweight and paternal height positively correlated with neonatal total BMC Smoking during pregnancy, increased maternal exercise and decreased triceps skinfold thickness correlated with lower BMC and BMD |
Gender differences in neonatal bone mineral measurements were not significant | Detailed parental exposures and good study size Suggests interaction of genetic and environmental factors on skeletal development in-utero |
| Javaid et al. (2004) [30] | (n=119) Term infants M=68 F=51 |
Umbilical cord IGF-1 and neonatal bone mass (DXA) | IGF- 1 concentration correlates with birth weight and BMC after adjustment for gestational age | Females had a greater IGF-1 level and fat mass at birth | Unable to determine interaction of growth factors and other previously measured attributes of maternal smoking, body habitus and exercise |
| Oliver et al. (2007) [20] | (n=631) Adults aged 65-73 years M=313 F=318 |
Early infant growth and adult bone strength (CT) | Strong association between birthweight or infant weight with bone length and strength, but not volumetric density in adults | Adult male BMI strongly associated with BMD Not significant in women |
Large study Supports role of intrauterine and early life exposures on late adult life skeletal characteristics |
| Hovi et al. (2009) [28] | Adults born preterm/ VLBW (n=144) Term born controls (n=139) M=115 F=168 |
Low birthweight and adult bone density (DXA) at 18 - 27 years | Reduced lumbar spine and femoral neck BMD in VLBW infants 2-fold increased risk for low lumbar spine BMD after adjusting for height in VLBW infants |
Gender differences not discussed | Measured around age of peak bone mass acquisition No later life follow-up of osteoporotic fractures Lower BMD compared to controls identifies birthweight as a possible risk factor |
| Fewtrell et al. (2009) [43] | (n=202) Adults born preterm M=87 F=115 |
Neonatal diet and early adult bone density (DXA) | No nutrient effect on peak bone mass between diets Positive association between proportion of human milk and later BMC No significant difference in childhood fractures |
No evidence for relationship between early diet and gender on bone outcomes | Dietary intervention was brief (4 weeks) but long follow up period Maternal recall of supplemental breastfeeding may be inaccurate Potential for residual confounding of breast milk provision by socio-demographic factors |
| Harvey et al. (2010) [26] | (n=380) Children (age 4 years) born at term M=197 F=183 |
Fetal growth velocity and childhood bone density (DXA) at 4 years | Higher velocity of femur growth between 19-34 weeks positively associated with skeletal size at 4 years but not volumetric density Higher velocity of fetal abdominal growth associated with greater childhood volumetric density but not skeletal size |
Gender differences not discussed | Large study with detailed measures Different mechanisms may exist for programming skeletal size and volumetric density |
| Steer et al. (2011) [19] | (n=6876) Children from the ALSPAC study age 9.9 years |
Maternal vitamin D status and dietary factors, birthweight, and childhood bone measurements (DXA) | Association of birthweight with bone mass explained after adjusting for body size Inverse association of birthweight on bone mineral content Maternal vitamin D and folate have lasting effects on development |
No difference described in intrauterine programming between genders | Large cohort Used a proxy measure of vitamin D (UVB exposure) Possible links between intrauterine environment and bone development |
Promoting adequate growth during NICU remains a challenge and the initial dramatic fall off in growth centiles, followed by a period of rapid growth acceleration, represents a pattern that is very different to that seen following normal pregnancies. Whether this type of growth trajectory represents an independent risk for later adverse metabolic outcome requires further study, but highlights that growth rather than absolute size is the key variable determining longer-term health.
Optimising early growth through nutritional interventions generates positive and lasting effects on bone mineralization [39] and it is hypothesized that this may partially counteract preterm bone deficits. A systematic review by Kuschel and Harding in 2009 showed that fortifying the nutrition of preterm babies improves growth and bone mineral accretion [42]. There is conflicting evidence as to whether breastfeeding has a protective role in the primary prevention of osteoporosis. In some studies, such as that of Fewtrell, breast milk consumption was found to result in higher adult BMD [43] despite the milk being unfortified and having a lower mineral content than formula. This suggests a possible beneficial role for non-nutrient components such as growth factors. In another study, bone mass at follow-up age of approximately 10 years was positively associated with the duration of breastfeeding [44], yet other studies have shown no benefits at a similar age [45, 46]. Other studies have not demonstrated an ongoing relationship into adulthood between breastfeeding and bone mass [33]. Given the known benefits of breastfeeding and the lack of proven negative association, It seems prudent to strongly encourage breastfeeding, despite the slower infant growth trajectories that may be seen compared to preterm infants fed using artificial milk formula.
THE PROGRAMMING EFFECT OF AN ADVERSE IN-UTERO ENVIRONMENT AND THE ROLE OF EPIGENETICS
It is clear that there is a strong genetic predisposition to osteoporosis and although the genes that regulate bone mass have not been completely established, responsible gene variants include vitamin D receptor polymorphisms [47], collagen-1 receptor [48] and oestrogen receptor variants [49]. There is also some evidence that gene-environment interactions may play an important role. Using vitamin D receptor polymorphisms as an example, Dennison et al. showed that the relationship between lumbar spine BMD and VDR genotype varied according to birth weight and remained after adjusting for adult body weight, and that a significant statistical interaction occurred between birth weight and VDR genotype [50]. This suggests that in-utero programming may modify genetic influences on osteoporosis risk. Many of the long-term effects on bone health may be modulated by epigenetic mechanisms - mitotically heritable alterations in gene expression that are not caused by changes in DNA sequence. The classic examples are DNA methylation and histone acetylation [51, 52] and result in differences in gene expression and transcription, but may also involve post-transcriptional effects on other processes such as protein translation. Early life growth and nutritional exposures appear to affect the ‘cellular memory’ and result in variation in later life phenotypes. Much of this work is preliminary but initial data suggest that epigenetic mechanisms may underlie the process of developmental plasticity and its effect on the risk of osteoporosis.
One of the models that has been postulated is the role of maternal vitamin D status and postnatal calcium transfer. Early work on methylation and vitamin D receptors and placental calcium transporters suggests that epigenetic regulation might explain how maternal vitamin D levels affect bone mineralization in the neonate [51]. Much of the current research is in animal models, but if the changes can be replicated in humans, epigenetic or other biomarkers may provide risk assessment tools to enable targeted intervention to those at greatest risk of osteoporosis, (see Fig. 1).
Fig. (1).
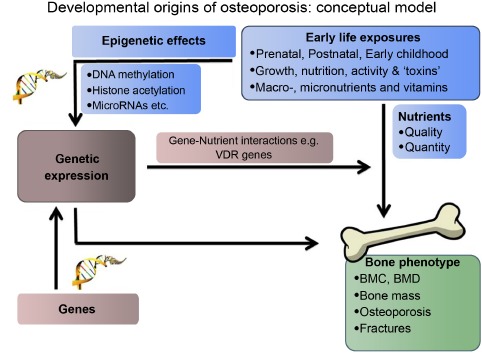
Developmental origins of osteoporosis: conceptual model.
Mother-offspring cohorts enable the observation of environmental influences and characteristics of pregnant women in relation to the bone mass of their offspring. Adverse environmental conditions such as smoking during pregnancy [53], poor diet [54, 55], low fat stores [56, 57] and low maternal vitamin D levels [5, 33] are all associated with suboptimal bone mineral density in later life. Studies using the ALSPAC cohort show that bone development of the child is clearly related to the in utero environment, but that some of the associations can be explained in part by the shared associations with bone and body size, although maternal vitamin D status exerts persisting effects on bone mass development [19]. Findings from the Southampton Women’ survey also corroborate the role of low maternal vitamin D levels on offspring bone mass and suggests that the mechanism is related to umbilical venous calcium concentrations [56, 58]. Further work from that group also suggests that maternal vitamin D stores can influence fetal femoral development at as early as 19 weeks gestation [59].
There is strong animal evidence to support the programming hypothesis. Maternal protein restriction in rats results in a reduction in bone area and bone mineral content, probably by programming the skeletal growth trajectory through modification of the responsiveness of epiphyseal cells [60, 61]. Supplemental calcium in adolescent rats does not rescue the reduction in BMC associated with placental restriction of rats in utero [62], suggesting that early life environment is critical for bone programming, and dietary phosphate restriction in neonatal pigs results in reduced growth and bone mineral content [63]. Perinatal dietary deficiency of essential fatty acids, and accompanied by reduced leptin and IGF-1 levels also influenced bone density of adult rats [64]. The critical role of leptin in regulating bone metabolism is now also acknowledged in humans and there are clinical trials currently investigating the role of leptin treatment on bone mineral density, both in hypoleptinaemic and post-menopausal women [65].
CONCLUSION
This review provides compelling evidence for the developmental origins of osteoporosis. It highlights the importance of osteoporosis prevention at all stages of the life course, including optimising the in utero environment and maternal nutrition, and the importance of infant nutrition as preventative strategies for future osteoporosis. It is important to continue to determine the mechanisms behind skeletal programming to further aid the development of preventative strategies.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
DISCLOSURE
“Part of this article has been previously published in International Journal of Endocrinology Volume 2013 (2013), Article ID 902513, 7 pages”
REFERENCES
- 1.Consensus development conference Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993;94(6):646–650. doi: 10.1016/0002-9343(93)90218-E. [DOI] [PubMed] [Google Scholar]
- 2.Klotzbuecher C.M., Ross P.D., Landsman P.B., Abbott T.A., III, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J. Bone Miner. Res. 2000;15(4):721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 3.WHO. In Summary Meeting Report. Brussels, Belgium: World Health Organization; 2004. May 5-7, WHO scientific group on the assessment of osteoporosis at primary health care level. [Google Scholar]
- 4.Johnell O., Kanis J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 5.Rigo J., Pieltain C., Salle B., Senterre J. Enteral calcium, phosphate and vitamin D requirements and bone mineralization in preterm infants. Acta Paediatr. 2007;96(7):969–974. doi: 10.1111/j.1651-2227.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 6.Orwoll E.S., Belknap J.K., Klein R.F. Gender specificity in the genetic determinants of peak bone mass. J. Bone Miner. Res. 2001;16(11):1962–1971. doi: 10.1359/jbmr.2001.16.11.1962. [DOI] [PubMed] [Google Scholar]
- 7.Cornuz J., Feskanich D., Willett W.C., Colditz G.A. Smoking, smoking cessation, and risk of hip fracture in women. Am. J. Med. 1999;106(3):311–314. doi: 10.1016/S0002-9343(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 8.Van Staa T.P., Leufkens H.G., Abenhaim L., Zhang B., Cooper C. Use of oral corticosteroids and risk of fractures. J. Bone Miner. Res. 2000;15(6):993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 9.Heinonen A., Kannus P., Sievänen H., Oja P., Pasanen M., Rinne M., Uusi-Rasi K., Vuori I. Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet. 1996;348(9038):1343–1347. doi: 10.1016/S0140-6736(96)04214-6. [DOI] [PubMed] [Google Scholar]
- 10.Baird J., Kurshid M.A., Kim M., Harvey N., Dennison E., Cooper C. Does birthweight predict bone mass in adulthood? A systematic review and meta-analysis. Osteoporos. Int. 2011;22(5):1323–1334. doi: 10.1007/s00198-010-1344-9. [DOI] [PubMed] [Google Scholar]
- 11.Gale C.R., Martyn C.N., Kellingray S., Eastell R., Cooper C. Intrauterine programming of adult body composition. J. Clin. Endocrinol. Metab. 2001;86(1):267–272. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- 12.Dennison E.M., Cooper C., Cole Z.A. Early development and osteoporosis and bone health. J. Dev. Orig. Health Dis. 2010;1(3):142–149. doi: 10.1017/S2040174409990146. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Barker Theory. New insights into ending chronic disease. http://www.thebarkertheory.org/index.php . [Accessed 01/07/2012].
- 15.Barker D.J., Winter P.D., Osmond C., Margetts B., Simmonds S.J. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad I., Nemet D., Eliakim A., Koeppel R., Grochow D., Coussens M., Gallitto S., Rich J., Pontello A., Leu S.Y., Cooper D.M., Waffarn F. Body composition and its components in preterm and term newborns: A cross-sectional, multimodal investigation. Am. J. Hum. Biol. 2010;22(1):69–75. doi: 10.1002/ajhb.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backström M.C., Kuusela A.L., Koivisto A.M., Sievänen H. Bone structure and volumetric density in young adults born prematurely: a peripheral quantitative computed tomography study. Bone. 2005;36(4):688–693. doi: 10.1016/j.bone.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Fraser A., Macdonald-Wallis C., Tilling K., Boyd A., Golding J., Davey Smith G., Henderson J., Macleod J., Molloy L., Ness A., Ring S., Nelson S.M., Lawlor D.A. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steer C.D., Tobias J.H. Insights into the programming of bone development from the Avon Longitudinal Study of Parents and Children (ALSPAC). Am. J. Clin. Nutr. 2011;94(6) Suppl.:1861S–1864S. doi: 10.3945/ajcn.110.001495. [DOI] [PubMed] [Google Scholar]
- 20.Oliver H., Jameson K.A., Sayer A.A., Cooper C., Dennison E.M., Hertfordshire Cohort Study Group Growth in early life predicts bone strength in late adulthood: the Hertfordshire Cohort Study. Bone. 2007;41(3):400–405. doi: 10.1016/j.bone.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper C., Eriksson J.G., Forsén T., Osmond C., Tuomilehto J., Barker D.J. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos. Int. 2001;12(8):623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 22.Inskip H.M., Godfrey K.M., Robinson S.M., Law C.M., Barker D.J., Cooper C., SWS Study Group Cohort profile: The Southampton Women’s Survey. Int. J. Epidemiol. 2006;35(1):42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark E.M., Ness A., Tobias J.H. ALSPAC Study Team. Social position affects bone mass in childhood through opposing actions on height and weight. J. Bone Miner. Res. 2005;20(12):2082–2089. doi: 10.1359/JBMR.050808. [DOI] [PubMed] [Google Scholar]
- 24.Antoniades L., MacGregor A.J., Andrew T., Spector T.D. Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology (Oxford) 2003;42(6):791–796. doi: 10.1093/rheumatology/keg227. [DOI] [PubMed] [Google Scholar]
- 25.Crabtree N.J., Arabi A., Bachrach L.K., Fewtrell M., El-Hajj Fuleihan G., Kecskemethy H.H., Jaworski M., Gordon C.M. International Society for Clinical Densitometry. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014;17(2):225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Harvey N.C., Mahon P.A., Robinson S.M., Nisbet C.E., Javaid M.K., Crozier S.R., Inskip H.M., Godfrey K.M., Arden N.K., Dennison E.M., Cooper C. SWS Study Group. Different indices of fetal growth predict bone size and volumetric density at 4 years of age. J. Bone Miner. Res. 2010;25(4):920–927. doi: 10.1359/jbmr.091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalziel S.R., Fenwick S., Cundy T., Parag V., Beck T.J., Rodgers A., Harding J.E. Peak bone mass after exposure to antenatal betamethasone and prematurity: follow-up of a randomized controlled trial. J. Bone Miner. Res. 2006;21(8):1175–1186. doi: 10.1359/jbmr.060516. [DOI] [PubMed] [Google Scholar]
- 28.Hovi P., Andersson S., Järvenpää A-L., Eriksson J.G., Strang-Karlsson S., Kajantie E., Mäkitie O. Decreased bone mineral density in adults born with very low birth weight: a cohort study. PLoS Med. 2009;6(8):e1000135. doi: 10.1371/journal.pmed.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen R.B., Vielwerth S., Frystyk J., Veldhuis J., Larsen T., Mølgaard C., Greisen G., Juul A. Fetal growth velocity, size in early life and adolescence, and prediction of bone mass: association to the GH-IGF axis. J. Bone Miner. Res. 2008;23(3):439–446. doi: 10.1359/jbmr.071034. [DOI] [PubMed] [Google Scholar]
- 30.Javaid M.K., Godfrey K.M., Taylor P., Shore S.R., Breier B., Arden N.K., Cooper C. Umbilical venous IGF-1 concentration, neonatal bone mass, and body composition. J. Bone Miner. Res. 2004;19(1):56–63. doi: 10.1359/jbmr.0301211. [DOI] [PubMed] [Google Scholar]
- 31.Fall C., Hindmarsh P., Dennison E., Kellingray S., Barker D., Cooper C. Programming of growth hormone secretion and bone mineral density in elderly men: a hypothesis. J. Clin. Endocrinol. Metab. 1998;83(1):135–139. doi: 10.1210/jcem.83.1.4487. [DOI] [PubMed] [Google Scholar]
- 32.Dennison E., Hindmarsh P., Fall C., Kellingray S., Barker D., Phillips D., Cooper C. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J. Clin. Endocrinol. Metab. 1999;84(9):3058–3063. doi: 10.1210/jcem.84.9.5964. [DOI] [PubMed] [Google Scholar]
- 33.Cooper C., Westlake S., Harvey N., Javaid K., Dennison E., Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos. Int. 2006;17(3):337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 34.Bhopal S., Mann K., Embleton N., Korada M., Cheetham T., Pearce M. The Influence of Early Growth on Bone Mineral Density at Age 9-14 Years in Children Born Preterm.; 7th World Congress on Developmental Origins of Health and Disease; Portland, Oregon, USA: Cambridge University Press; 2011. [Google Scholar]
- 35.Javaid M.K., Lekamwasam S., Clark J., Dennison E.M., Syddall H.E., Loveridge N., Reeve J., Beck T.J., Cooper C. Hertfordshire Cohort Study Group. Infant growth influences proximal femoral geometry in adulthood. J. Bone Miner. Res. 2006;21(4):508–512. doi: 10.1359/jbmr.051214. [DOI] [PubMed] [Google Scholar]
- 36.Cooper C., Westlake S., Harvey N., Javaid K., Dennison E., Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos. Int. 2006;17(3):337–347. doi: 10.1007/s00198-005-2039-5. [DOI] [PubMed] [Google Scholar]
- 37.De Schepper J., Cools F., Vandenplas Y., Louis O. Whole body bone mineral content is similar at discharge from the hospital in premature infants receiving fortified breast milk or preterm formula. J. Pediatr. Gastroenterol. Nutr. 2005;41(2):230–234. doi: 10.1097/01.mpg.0000172883.93042.8f. [DOI] [PubMed] [Google Scholar]
- 38.Bowden L.S., Jones C.J., Ryan S.W. Bone mineralisation in ex-preterm infants aged 8 years. Eur. J. Pediatr. 1999;158(8):658–661. doi: 10.1007/s004310051171. [DOI] [PubMed] [Google Scholar]
- 39.Abou Samra H., Stevens D., Binkley T., Specker B. Determinants of bone mass and size in 7-year-old former term, late-preterm, and preterm boys. Osteoporos. Int. 2009;20(11):1903–1910. doi: 10.1007/s00198-009-0896-z. [DOI] [PubMed] [Google Scholar]
- 40.Fewtrell M., Prentice A., Cole T.J., Lucas A. Effects of growth during infancy and childhood on bone mineralization and turnover in preterm children aged 8-12 years. Acta Paediatr. 2000;89(2):148–153. doi: 10.1111/j.1651-2227.2000.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 41.Boot A.M., de Ridder M.A., Pols H.A., Krenning E.P., de Muinck Keizer-Schrama S.M. Bone mineral density in children and adolescents: relation to puberty, calcium intake, and physical activity. J. Clin. Endocrinol. Metab. 1997;82(1):57–62. doi: 10.1210/jcem.82.1.3665. [DOI] [PubMed] [Google Scholar]
- 42.Kuschel C.A., Harding J.E. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Database Syst. Rev. 2000;1(2):CD000433. doi: 10.1002/14651858.CD000433. [Review]. [DOI] [PubMed] [Google Scholar]
- 43.Fewtrell M.S., Williams J.E., Singhal A., Murgatroyd P.R., Fuller N., Lucas A. Early diet and peak bone mass: 20 year follow-up of a randomized trial of early diet in infants born preterm. Bone. 2009;45(1):142–149. doi: 10.1016/j.bone.2009.03.657. [DOI] [PubMed] [Google Scholar]
- 44.Jones G., Riley M., Dwyer T. Breastfeeding in early life and bone mass in prepubertal children: a longitudinal study. Osteoporos. Int. 2000;11(2):146–152. doi: 10.1007/PL00004176. [DOI] [PubMed] [Google Scholar]
- 45.Harvey N.C., Robinson S.M., Crozier S.R., Marriott L.D., Gale C.R., Cole Z.A., Inskip H.M., Godfrey K.M., Cooper C. Southampton Women’s Survey Study Group. Breast-feeding and adherence to infant feeding guidelines do not influence bone mass at age 4 years. Br. J. Nutr. 2009;102(6):915–920. doi: 10.1017/S0007114509317420. [DOI] [PubMed] [Google Scholar]
- 46.Young R.J., Antonson D.L., Ferguson P.W., Murray N.D., Merkel K., Moore T.E. Neonatal and infant feeding: effect on bone density at 4 years. J. Pediatr. Gastroenterol. Nutr. 2005;41(1):88–93. doi: 10.1097/01.MPG.0000162481.81900.E6. [DOI] [PubMed] [Google Scholar]
- 47.Cooper G.S., Umbach D.M. Are vitamin D receptor polymorphisms associated with bone mineral density? A meta-analysis. J. Bone Miner. Res. 1996;11(12):1841–1849. doi: 10.1002/jbmr.5650111203. [DOI] [PubMed] [Google Scholar]
- 48.Uitterlinden A.G., Burger H., Huang Q., Yue F., McGuigan F.E., Grant S.F., Hofman A., van Leeuwen J.P., Pols H.A., Ralston S.H. Relation of alleles of the collagen type Ialpha1 gene to bone density and the risk of osteoporotic fractures in postmenopausal women. N. Engl. J. Med. 1998;338(15):1016–1021. doi: 10.1056/NEJM199804093381502. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi S., Inoue S., Hosoi T., Ouchi Y., Shiraki M., Orimo H. Association of bone mineral density with polymorphism of the estrogen receptor gene. J. Bone Miner. Res. 1996;11(3):306–311. doi: 10.1002/jbmr.5650110304. [DOI] [PubMed] [Google Scholar]
- 50.Dennison E.M., Arden N.K., Keen R.W., Syddall H., Day I.N., Spector T.D., Cooper C. Birthweight, vitamin D receptor genotype and the programming of osteoporosis. Paediatr. Perinat. Epidemiol. 2001;15(3):211–219. doi: 10.1046/j.1365-3016.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 51.Earl S., Harvey N., Cooper C. The Epigenetic Regulation of Bone Mass. IBMS boneKEy. 2010;7(2):54–62. doi: 10.1138/20100428. [DOI] [Google Scholar]
- 52.Groom A., Elliott H.R., Embleton N.D., Relton C.L. Epigenetics and child health: basic principles. Arch. Dis. Child. 2011;96(9):863–869. doi: 10.1136/adc.2009.165712. [DOI] [PubMed] [Google Scholar]
- 53.Godfrey K., Walker-Bone K., Robinson S., Taylor P., Shore S., Wheeler T., Cooper C. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J. Bone Miner. Res. 2001;16(9):1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 54.Cole Z.A., Gale C.R., Javaid M.K., Robinson S.M., Law C., Boucher B.J., Crozier S.R., Godfrey K.M., Dennison E.M., Cooper C. Maternal dietary patterns during pregnancy and childhood bone mass: a longitudinal study. J. Bone Miner. Res. 2009;24(4):663–668. doi: 10.1359/jbmr.081212. [DOI] [PubMed] [Google Scholar]
- 55.Yin J., Dwyer T., Riley M., Cochrane J., Jones G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur. J. Clin. Nutr. 2010;64(2):131–137. doi: 10.1038/ejcn.2009.117. [DOI] [PubMed] [Google Scholar]
- 56.Harvey N.C., Javaid M.K., Arden N.K., Poole J.R., Crozier S.R., Robinson S.M., Inskip H.M., Godfrey K.M., Dennison E.M., Cooper C. SWS Study Team. Maternal predictors of neonatal bone size and geometry: the Southampton Women’s Survey. J. Dev. Orig. Health Dis. 2010;1(1):35–41. doi: 10.1017/S2040174409990055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yajnik C.S., Deshmukh U.S. Maternal nutrition, intrauterine programming and consequential risks in the offspring. Rev. Endocr. Metab. Disord. 2008;9(3):203–211. doi: 10.1007/s11154-008-9087-z. [DOI] [PubMed] [Google Scholar]
- 58.Javaid M.K., Crozier S.R., Harvey N.C., Gale C.R., Dennison E.M., Boucher B.J., Arden N.K., Godfrey K.M., Cooper C. Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 59.Mahon P., Harvey N., Crozier S., Inskip H., Robinson S., Arden N., Swaminathan R., Cooper C., Godfrey K. SWS Study Group. Low maternal vitamin D status and fetal bone development: cohort study. J. Bone Miner. Res. 2010;25(1):14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehta G., Roach H.I., Langley-Evans S., Taylor P., Reading I., Oreffo R.O., Aihie-Sayer A., Clarke N.M., Cooper C. Intrauterine exposure to a maternal low protein diet reduces adult bone mass and alters growth plate morphology in rats. Calcif. Tissue Int. 2002;71(6):493–498. doi: 10.1007/s00223-001-2104-9. [DOI] [PubMed] [Google Scholar]
- 61.Lanham S.A., Roberts C., Perry M.J., Cooper C., Oreffo R.O. Intrauterine programming of bone. Part 2: alteration of skeletal structure. Osteoporos. Int. 2008;19(2):157–167. doi: 10.1007/s00198-007-0448-3. [DOI] [PubMed] [Google Scholar]
- 62.Romano T., Wark J.D., Wlodek M.E. Calcium supplementation does not rescue the programmed adult bone deficits associated with perinatal growth restriction. Bone. 2010;47(6):1054–1063. doi: 10.1016/j.bone.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 63.Alexander L.S., Mahajan A., Odle J., Flann K.L., Rhoads R.P., Stahl C.H. Dietary phosphate restriction decreases stem cell proliferation and subsequent growth potential in neonatal pigs. J. Nutr. 2010;140(3):477–482. doi: 10.3945/jn.109.117390. [DOI] [PubMed] [Google Scholar]
- 64.Korotkova M., Ohlsson C., Gabrielsson B., Hanson L.A., Strandvik B. Perinatal essential fatty acid deficiency influences body weight and bone parameters in adult male rats. Biochim. Biophys. Acta. 2005;1686(3):248–254. doi: 10.1016/j.bbalip.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Upadhyay J., Farr O.M., Mantzoros C.S. The role of leptin in regulating bone metabolism. Metabolism. 2015;64(1):105–113. doi: 10.1016/j.metabol.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


