Abstract
Background and Aims The identification of stoichiometric homeostasis is crucial for understanding plant adaptive strategies under a changing environment. However, current knowledge of plant stoichiometric homeostasis has mainly been obtained from mature leaves, with little from other organs across different developmental stages.
Methods We conducted a greenhouse nitrogen (N) and phosphorus (P) addition experiment to evaluate the strength of stoichiometric homeostasis across different organs and developmental stages of Arabidopsis thaliana.
Key Results Homeostatic regulation coefficients (H) for N (HN), P (HP) and N : P ratio (HNP) were highest in reproductive tissue, followed by stem and leaf at the same stage. All H parameters in the same organ decreased significantly over the developmental stages. Leaf HN, HP and HNP were highest at stage 1, followed by stages 2 and 3. Both stem and silique at stage 2 relative to stage 3 had higher HN, HP and HNP. These results suggested that reproductive tissue relative to other organs and young tissue relative to old tissue showed more constrained elemental composition in response to nutrient availabilities, and such trends were also evidenced by stoichiometric scaling relationships.
Conclusions Our findings highlight that stoichiometric homeostasis is tightly related to the ontogenesis of plant tissue. These results could have a strong implication for diagnosing relative availabilities of N and P in ecosystems, suggesting that the N and P stoichiometry of old tissues might be stronger indicators of nutrient status for plants, but further study is needed to test the generality across species with more distinguishable functional traits.
Keywords: Arabidopsis thaliana, developmental stage, N and P concentration, N : P ratio, organ, scaling relationship, stoichiometric homeostasis.
INTRODUCTION
Stoichiometric homeostasis is the ability of an organism to maintain constant elemental compositions in response to variations of ambient nutrient availabilities (Sterner and Elser, 2002). Plant growth depends largely on homeostatic regulation because nutrient contents below a minimum requirement induce the senescence of tissues, whereas excess uptake of nutrients may cause toxic disturbance (Sterner and Elser, 2002; Güsewell, 2004). Previous studies have revealed that stoichiometric homeostasis in leaves during the growing season was affected by species, light density, elemental type and nutrient supply level (Güsewell, 2004; Elser et al., 2010; Yu et al., 2011; Dijkstra et al., 2012). By contrast, homeostatic regulation capacities of other plant organs and how developmental stages affect them remain little known (Kerkhoff et al., 2006; Persson et al., 2010).
Different plant organs may have variations in stoichiometric homeostasis related to them performing different functions (Elser et al., 2010; Persson et al., 2010). Due to the pivotal role of nitrogen (N) and phosphorus (P) in carbon gain (Lambers et al., 2008), leaves may constrain their elemental composition to the greatest extent possible, accompanied by concurrent nutrient resorption strategies or nutrient remobilization from other organs (Aerts and Chapin, 2000; Lambers et al., 2008). Stems and roots could act as nutrient reservoirs that support the optimal N and P stoichiometry in leaves, and store excessive N and P taken up from soils (Cernusak et al., 2010; Pallardy, 2010). In contrast to stem and root, leaves thus should have higher stoichiometric homeostasis with less sensitivity to soil nutrient availability. This speculation has been recently supported by greenhouse studies on tree seedlings and field studies across woody species (Garrish et al., 2010; Minden and Kleyer, 2014; Schreeg et al., 2014). For example, stem and root N : P ratios of tree seedlings in a tropical forest are more sensitive indicators of soil nutrient availability than are those of newly expanded leaves (Schreeg et al., 2014). The generality of this pattern across other species, however, requires further study.
Stoichiometric homeostasis in reproductive tissues remains largely unexplored but could be very important for plants (Ågren, 2008). Nutrients in seeds stored as phytic acid and proteins (Chapin, 1980) determine several processes in the plant reproductive phase, including seedling establishment and seed dispersal (Lee and Fenner, 1989; Naegle et al., 2005; Obeso, 2012; Fujita et al., 2014). In this case, the reproductive allocations of N and P occupy a large fraction of a plant’s nutrients (Fenner, 1986). During reproductive growth, nutrients are remobilized from vegetative parts to seeds and fruits (Lambers et al., 2008). Reproductive tissues thus should maintain higher nutrient concentrations and constrained elemental compositions to facilitate seedling establishment and propagation (Fenner, 1986).
Plant N and P concentrations generally decrease but N : P ratio increases with ontogenetic development of individual plants, probably resulting from translocation of nutrients out of older tissues during senescence or the dilution effect caused by increased structural materials during plant growth (Chapin et al., 1980; Olsen and Bell, 1990; Santa Regina et al., 1997; Ågren, 2008; Zhang et al., 2013). Accordingly, stoichiometric homeostasis may depend on the developmental stage due to different metabolic activity and nutrient requirements (Güsewell, 2004; Ågren, 2008). Recent studies have revealed that newly expanded leaves (or mature green-leaves) showed lower variances of N and P stoichiometry than old leaves (or senesced-leaves) (Han et al., 2013; Schreeg et al., 2014; González-Zurdo et al., 2015). Old leaves remain metabolically active without further growth, which greatly reduces nutrient requirements and shuttles nutrients to young leaves (Usuda, 1995), causing more fluctuation of their elemental composition. Young leaves that grow and assimilate concurrently may require nutrients based on the stoichiometry of essential metabolic processes, constraining their elemental compositions (Güsewell, 2004). However, it is unknown to what extent results from leaves describing stage-dependent patterns in stoichiometric homeostasis can be extrapolated to other organs.
In this study, we address the hypothesis that reproductive tissue and young tissue should maintain more constrained elemental compositions under the changing soil nutrient availability. To test this hypothesis, we conducted a greenhouse N and P addition experiment using Arabidopsis thaliana, a species in the family Brassicaceae with an annual life-history strategy and a model organism for plant molecular biological studies (Meinke et al., 1998). The N and P concentrations of leaf, stem and silique (i.e. reproductive tissues) across different developmental stages were collected and measured to examine whether silique showed higher stoichiometric regulation coefficients (H) (i.e. more constrained nutrient concentrations) than other organs at the same stage, and whether each organ at earlier developmental stages showed higher H for N and P and N : P ratio. We further tested these expectations by evaluating the stoichiometric scaling relationships across pairwise organ combinations at the same stage and across pairwise stage combinations within each organ.
MATERIALS AND METHODS
Plant materials and experimental treatments
The experiment was conducted in a phytotron with long days (16-h light/8-h dark photoperiod) at approx. 20 °C and 60–70 % relative humidity. Light was produced by one yellow sodium fluorescent lamp and five white fluorescent lamps. Seeds of A. thaliana from ecotype ‘Columbia’ were used to perform the N and P addition experiment. Before sowing, seeds were surface sterilized using 70 % (v/v) ethanol/0·5 % (v/v) Tween 20 and then stratified in a 0.1 % (w/v) agar dish at 4 °C in the dark for 4 d. These seeds were then sown in 27 pots (3 replicates × 9 treatments), each having 24 small divisions to grow 24 individuals. The pots were filled with sterilized vermiculite (medium particle size) and immersed in respective nutrient solutions containing different N and P concentrations. To minimize micro-environmental effects, we randomly rearranged the pots every 2 d.
There were five N-level treatments (1, 2, 4, 8 and 12 mmol N L−1, added as NH4NO3) at the intermediate P supply level (0·25 mmol P L−1) and five P-level treatments (0·0625, 0·125, 0·25, 0·5 and 1·0 mmol P L−1, added as KH2PO4 and NaH2PO4) at the intermediate N supply level (4 mmol N L−1), respectively and a total of nine treatments (Table 1). With the exceptions of N and P, all pots were supplied with the same concentrations of macro- and microelements (0·75 mm K2SO4, 0·65 mm MgSO4, 1 µm MnSO4, 0·1 µm CuSO4, 1 µm ZnSO4, 0·035 µm Na2MoO4, 0·1 mm Fe-EDTA, 0·01 mm H3BO3 and 2 mm CaCl2). The pH of the nutrient solution was adjusted to 5·8. The composition and concentration of these solutions were based on Hoagland’s formula (Hoagland and Arnon, 1950) and then modified according to our previous experiments (Yan et al., 2015).
Table 1.
N and P concentrations and N : P molar ratio in nutrient solutions used in nine treatments [five N levels (N1–N5) under the intermediate P level (P3), and five P levels (P1–P5) under the intermediate N level (N3)]
| Treatment | N (mmol L−1) | P (mmol L−1) | N : P ratio (mol:mol) | Nutrient status |
|---|---|---|---|---|
| N1P3 | 1 | 0·25 | 4·0 | N limited |
| N2P3 | 2 | 0·25 | 8·0 | N limited |
| N3P3 | 4 | 0·25 | 16·0 | Nutrient balanced |
| N4P3 | 8 | 0·25 | 32·0 | P limited |
| N5P3 | 12 | 0·25 | 48·0 | P limited |
| N3P1 | 4 | 0·0625 | 64·0 | P limited |
| N3P2 | 4 | 0·125 | 32·0 | P limited |
| N3P4 | 4 | 0·5 | 8·0 | N limited |
| N3P5 | 4 | 1 | 4·0 | N limited |
Sampling and measurement
Samples were harvested at three different growth stages: stage 1, the initiation of bolting (designated as the ‘young leaf stage’); stage 2, 2 weeks after stage 1 with the four largest rosette leaves fully expanded (designated as the ‘mature green-leaf’ stage) (Weaver et al., 1998); and stage 3, the end of main stem inflorescences (designated as the ‘silique maturity stage’). Harvesting dates were adjusted to match the individual developmental stage considering the variations in individual growth rate among the treatments. Leaves were harvested across the three stages, whereas stems and siliques were only harvested at stages 2 and 3 given that they did not occur at stage 1. Leaves shifted from young rosette leaves without full expansion at stage 1 to mature green-leaves at stage 2, and finally to senesced-leaves at stage 3. Stems and siliques shifted from young tissues with active meristems at stage 2 to old tissues with increased structural materials at stage 3. Five or six individuals with closely similar growth per plot were sampled and pooled to represent a replicate.
After harvest, samples were oven-dried at 65 °C to constant weight and then powdered using a mortar and pestle before measuring N and P concentrations. Total N concentration was determined by the Dumas combustion method using an elemental analyser (Elementar vario EL III, Elementar, Hanau, Germany; Jones, 2001), and total P concentration was measured by inductively coupled plasma optical emission spectroscopy (Thermo 6300; Thermo Scientific, West Palm Beach, FL, USA) after HNO3–HF–HClO4 digestion (Jones, 2001).
Data analyses
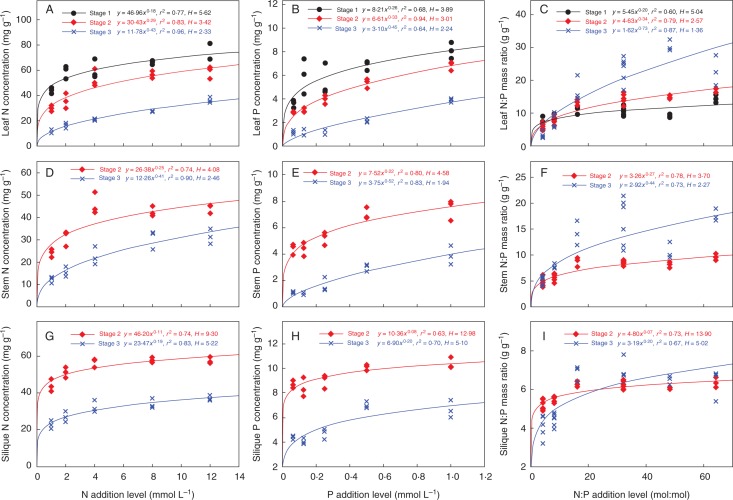
Homeostasis control over N and P concentrations and N : P ratios were estimated by calculating the regulation coefficient (H) according to the power equation (Sterner and Elser, 2002): y = c*x1/H, where y is the plant N or P concentration (mg g−1 dry mass) or N : P ratio, x is the N or P concentration (mmol L−1) or N : P ratio of nutrient solutions and c is a constant. Higher H implies stronger homeostatic regulation capacity. H for N (HN) reflects changes in tissue N concentration when N was added at the constant P supply level, and H for P (HP) reflects changes in tissue P concentration when P was added at the constant N supply level and H for N : P ratio (HNP) reflects changes in tissue N : P ratios with N : P addition levels across all treatments. The heterogeneity of H among groups was indicated by analysing the overlap level of the 95 % confidence interval (CI) among different groups (Deng et al., 2015). There would be a significant difference in H between two groups if there was not any overlap of their 95 % CIs.
To examine the covariation in N and P concentrations and N : P ratio across plant organs at the same stage, and across developmental stages in the same organ, we investigated the stoichiometric scaling relationship using the reduced major axis (RMA) regression (Warton et al., 2006), which was expressed by log10 Y = α*(log10 X) + β, where X and Y represent tissue N or P concentration or N : P ratio across all pairwise combinations, and α and β are the slope (i.e. scaling exponent) and intercept of the regression line, respectively. The scaling relationship between Y and X was assigned as isometric when the 95 % CI of the exponent α contains 1, whereas a 95 % CI of α above (or below) 1 indicates that Y increases faster (or slower) than linearly with X. All statistical analyses were performed using R 2.15.2 with the package ‘smatr’ (R Development Core Team, 2012).
RESULTS
Overall patterns of HN, HP and HNP
Tissue N and P concentrations, and N : P ratio increased with their corresponding higher N, P and N : P ratio addition levels in the solutions. These patterns showed a rapid increase at lower addition levels and then levelled off, which could be well characterized by the power equation: y = c*x1/H (Table 2; Fig. 1). The power equation could explain 74–96 % of the variation in N concentration, 63–94 % of the variation in P concentration and 60–89 % of the variation in N : P ratio (Table 2). HN, HP and HNP exhibited large variations, primarily ranging from approx. 2·33 to 9·30, 1·94 to 12·98, and 1·36 to 13·9, respectively (Table 2).
Table 2.
Homeostasis model statistics and stoichiometric homeostasis coefficients (H) for N (HN), P (HP) and N : P ratio (HNP)
| Types | N |
P |
N : P ratio |
|||
|---|---|---|---|---|---|---|
| HN (L95 %; U95 %) | r2 | HP (L95 %; U95 %) | r2 | HNP (L95 %; U95 %) | r2 | |
| Stage 1 | ||||||
| Leaf | 5·62 (4·21; 8·43) | 0·76 | 3·89 (2·76; 6·61) | 0·68 | 5·04 (3·76; 7·64) | 0·60 |
| Stage 2 | ||||||
| Leaf | 3·42 (2·70; 4·69) | 0·83 | 3·01 (2·61; 3·56) | 0·94 | 2·57 (2·24; 3·01) | 0·89 |
| Stem | 4·08 (3·02; 6·30) | 0·74 | 4·58 (3·54; 6·51) | 0·80 | 3·70 (3·03; 4·76) | 0·78 |
| Silique | 9·30 (6·86; 14·50) | 0·74 | 12·98 (8·90; 23·93) | 0·63 | 13·90 (11·10; 18·55) | 0·73 |
| Stage 3 | ||||||
| Leaf | 2·33 (2·10; 2·68) | 0·96 | 2·24 (1·66; 3·45) | 0·74 | 1·36 (1·18; 1·61) | 0·87 |
| Stem | 2·46 (2·05; 3·08) | 0·90 | 1·94 (1·52; 2·68) | 0·83 | 2·27 (1·82; 3·03) | 0·73 |
| Silique | 5·22 (4·11; 7·18) | 0·83 | 5·10 (3·67; 8·34) | 0·70 | 5·02 (3·89; 7·06) | 0·67 |
L95 % and U95 % indicate lower and upper 95 % confidence intervals of H, respectively. Statistical equations were all significant at P < 0·001.
FIG. 1.
N and P concentrations and N : P mass ratios of different organs at the three stages along the N and P and N : P ratio addition levels. The homeostasis model equation y = c*x1/H is used to determine the regression line with an estimate of H for stoichiometric homoeostasis. All lines are fit by an equation with P < 0·001.
Patterns of H and stoichiometric scaling relationships across organs at the same stage
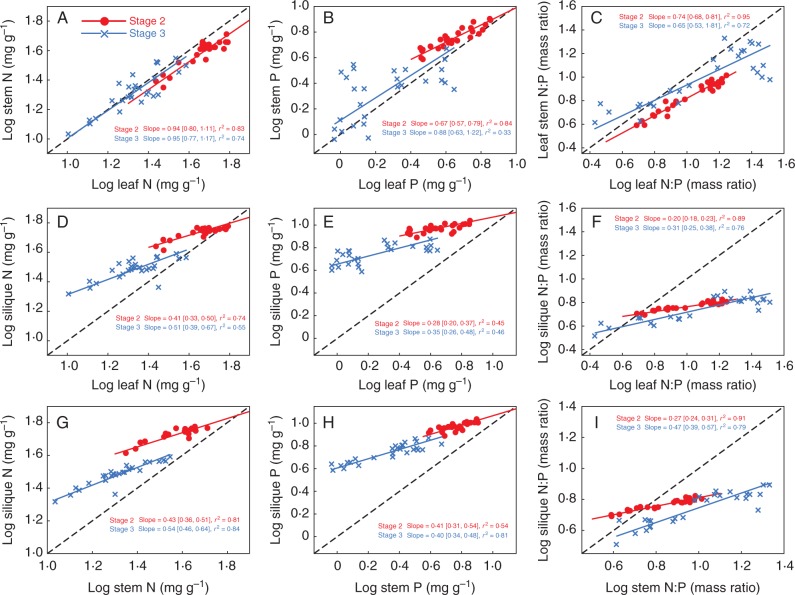
All H parameters for silique differed significantly from leaf and stem at the same stage (Table 2; Fig. 1). HN, HP and HNP were all highest in silique, followed by stem and leaf at stage 2 (9·30, 4·08 and 3·42 for HN; 12·98, 4·58 and 3·01 for HP; 13·9, 3·7 and 2·57 for HNP) and at stage 3 (5·22, 2·46 and 2·33 for HN; 5·10, 1·94 and 2·24 for HP; 5·02, 2·27 and 1·36 for HNP) (Table 2). In the case of stem versus leaf at stage 2 or at stage 3, the scaling exponents of P concentration and N : P ratio were significantly lower than 1·0, whereas the scaling exponent of N concentration was indistinguishable from 1·0 (Fig. 2A–C), suggesting that leaf showed faster increases than stem in P concentration and N:P ratio in addition to N concentration. In the case of silique versus leaf (or stem) at stage 2 or at stage 3, scaling exponents of N and P concentrations and N : P ratio were all significantly and consistently lower than 1·0 (Fig. 2D–I), suggesting that elemental compositions in silique relative to leaf and stem changed more slowly with the alterations of nutrient availabilities.
Fig. 2.
Scaling relationships of N and P concentrations and N : P ratios across pairwise organ combinations at the same stage (stages 2 and 3). Reduced major axis (RMA) regression was used to determine the significant line (P < 0·05). Numbers in square brackets are the lower and upper 95 % confident intervals of the RMA slopes. All data have been log10-transformed before analysis.
Patterns of H and stoichiometric scaling relationships across stages in the same organ
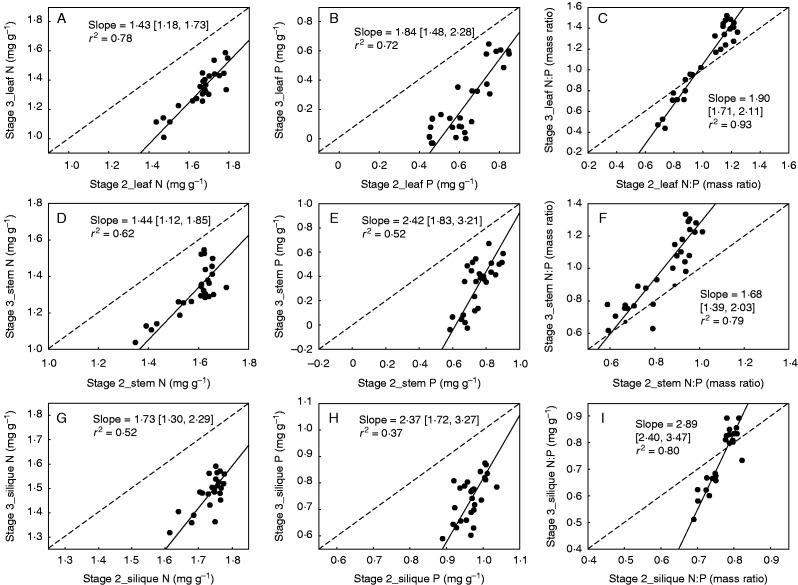
All H parameters decreased significantly over the developmental stages (Table 2; Fig. 1). Leaf HN, HP and HNP were significantly highest at stage 1, followed by stage 2 and 3 (5·62, 3·42 and 2·33 for HN; 3·89, 3·01 and 2·24 for HP; 5·04, 2·57 and 1·36 for HNP) (Table 2). Stem had significantly higher HN (4·08 vs. 2·46), HP (4·58 vs. 1·94) and HNP (3·70 vs. 2·27) at stage 2 than at stage 3, respectively (Table 2). Similarly, silique also showed higher HN (9·30 vs. 4·65), HP (12·98 vs. 5·10) and HNP (13·90 vs. 5·02) at stage 2 than at stage 3, respectively (Table 2). These results suggested that each plant organ was required to maintain a relatively constant elemental composition in young tissues at earlier developmental stages. In the case of each organ at stage 3 versus stage 2, scaling exponents of tissue N and P concentrations and N : P ratio were all significantly and consistently higher than 1·0 (Fig. 3), further indicating that the elemental composition of each organ at later stages varied much faster with the changes in nutrient availability.
FIG. 3.
Scaling relationships of N and P concentrations and N : P ratios and across pairwise stage combinations within each organ. Reduced major axis (RMA) regression was used to determine the significant line (P < 0·05). Numbers in square brackets are the lower and upper 95 % confident intervals of the RMA slopes. All data were log10-transformed before analysis.
DISCUSSION
Young tissues have higher stoichiometric homeostasis than old tissues
Stoichiometric characteristics are tightly related to the ontogenesis of plant tissue (Güsewell, 2004). In general, tissue N and P concentrations decreased but N : P ratios increased over ontogenesis, because old tissue often translocated nutrients to young tissues (Chapin et al., 1980; Olsen and Bell, 1990; Santa Regina et al., 1997; Ågren, 2008; Zhang et al., 2013). Previous studies showed that old leaves (or senesced-leaves) had higher variances of N and P stoichiometry and were more sensitive to soil nutrient availability than newly expanded leaves (or mature green-leaves) (Han et al., 2013; Schreeg et al., 2014; González-Zurdo et al., 2015). Similarly, we found that old tissues of A. thaliana tended to alter their elemental composition more easily than young tissues under changing nutrient conditions. The shift from homeostasis to heterostasis in leaves, stems and siliques may partly come from the relocation of N and P to regenerative tissue. Young tissues probably require N and P that are given by the stoichiometry of essential metabolic processes (Güsewell, 2004), maintaining a stable chemical composition. By contrast, old tissues may maintain the basic physiological processes without any further increase in size, and then translocate their nutrients to young tissues that are actively growing (Chapin et al., 1980; Usuda, 1995), resulting in the higher variability of elemental composition in response to the changes in nutrient availability.
Another potential cause of this higher stoichiometric homeostasis in young tissues of A. thaliana may be the higher growth rate in these tissues, relative to old ones. Each organ of A. thaliana at an earlier stage has higher N and P concentrations but lower N : P ratios (Table 3). According to the growth rate hypothesis (Sterner and Elser, 2002), plants with faster growth rate tend to have higher P concentration but lower N : P ratios. Thus, we could speculate that organs at an earlier stage should have relatively higher growth rate and mitotic activity because of rapid cellular growth (Ryan and Bormann, 1982). Persson et al. (2010) documented that stoichiometric homeostasis of algae was tightly associated with growth rate. Algae under fast growth required relatively stable biochemical allocations and then constrained their elemental compositions to the greatest extent possible (Elrifi and Turpin, 1985; Shafik et al., 1997; Persson et al., 2010). This may also be attributed to the lack of excessive uptake and nutrient storage for algae under rapid growth due to the high nutrient demands (Elrifi and Turpin, 1985). Accordingly, we suggest that a similar explanation might be applied to the higher stoichiometric homeostasis in young tissues of A. thaliana associated with rapid growth rate.
Table 3.
N and P concentrations and N : P mass ratios for each organ across developmental stages
| Group | n | N (mg g−1) | P (mg g−1) | N : P mass ratio |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Stage 1 | ||||
| Leaf | 27 | 59·4 ± 9·0a | 6·49 ± 1·65b | 9·7 ± 2·5b |
| Stage 2 | ||||
| Leaf | 27 | 46·8 ± 9·5c | 4·56 ± 1·33d | 11·3 ± 4·2b |
| Stem | 27 | 39·2 ± 7·2d | 5·73 ± 1·12c | 7·1 ± 2·0c |
| Silique | 27 | 54·4 ± 4·6b | 9·32 ± 0·74a | 5·9 ± 0·5c |
| Stage 3 | ||||
| Leaf | 27 | 22·5 ± 6·9f | 2·04 ± 1·17e | 15·7 ± 9·8a |
| Stem | 27 | 21·7 ± 6·4f | 2·35 ± 1·00e | 11·1 ± 5·5b |
| Silique | 27 | 30·8 ± 4·8e | 5·63 ± 1·03c | 5·7 ± 1·3c |
Mean and SD are the arithmetic mean and standard deviation, respectively, and n is sample number across the nine treatments with three replicates.
* Different lower-case letters indicate significant difference (P < 0·05) based on one-way ANOVA and least significant difference post-hoc test among seven groups.
Reproductive tissue has the highest stoichiometric homeostasis across organs
Our results show that reproductive tissue has a lower variability in elemental composition in response to nutrient availabilities, relative to leaf and stem. This result corresponds well with the finding in Senecio vulgaris that seeds relative to shoots have higher N and P concentrations but lower variabilities (Fenner, 1986). Seed nutrients determine the important reproductive processes (Lee and Fenner, 1989; Naegle et al., 2005; De Frenne et al., 2011; Carón et al., 2014), which presumably depend on high nutrient concentrations and favourable elemental compositions (Fenner, 1986). Within the reproductive phase of plants, nutrients previously invested in vegetative structures are remobilized to the developing reproductive organs, leading to a rapid decline and strong fluctuation in nutrient composition in the vegetative components (Lambers et al., 2008; Marschner and Marschner, 2012).
Conversely, it was previously observed that reproductive tissue nutrient content varied isometrically with leaf nutrient content for woody plants (Kerkhoff et al., 2006; Minden and Kleyer, 2014). This difference might be attributed to various sampling processes. For woody species as perennial plants, reproductive tissues are sampled with concurrent newly expanded leaves (Pérez-Harguindeguy et al., 2013). Both reproductive tissues and leaves in these woody plants could be regarded as growth centres associated with active growth, which requires high nutrient concentrations and stable elemental compositions in these tissues and thus results in the isometric relationship of nutrient content between reproductive tissues and leaves. By contrast, for A. thaliana as an annual herb, reproductive tissues were sampled with concurrent mature green-leaves at stage 2, and with concurrent senesced leaves at stage 3. In this case, we could regard the reproductive tissue as the relatively younger tissue in contrast to leaf because of its later occurrence in A. thaliana (Meinke et al., 1998), and then expect that reproductive tissue performs a higher stoichiometric homeostasis than leaf at the same stage.
Lower homeostatic control over leaf N : P relative to stem N : P at the same stage
In this study, we found that N : P ratio changed faster in leaf than in stem at the same stage, in contrast to results from previous studies. It has been widely proposed that leaf shows a lower variation in elemental composition than stem for woody plants (Kerkhoff et al., 2006; Garrish et al., 2010; Minden and Kleyer, 2014; Schreeg et al., 2014; Yang et al., 2014). Both Garrish et al. (2010) and Schreeg et al. (2014) concluded that stem N : P ratio of tree seedlings was more sensitive to soil nutrient availability than leaf N : P ratio, and speculated that a similar pattern could be extrapolated to herbaceous species due to the analogous structures between tree seedlings and herbs. Similarly, metabolic organs (e.g. leaf and reproductive tissue) showed lower variations in their elemental composition than structural organs (e.g. stem) (Kerkhoff et al., 2006). Stems of A. thaliana have significantly different structures and functions from those of woody plants. Stem of A. thaliana is freshly produced with active meristems but low structural materials, and transports water, carbohydrates and nutrients to reproductive tissues (Meinke et al., 1998). In contrast, the stem of woody plants contains large amounts of structural materials and low nutrient content, provides mechanical support and transports water, carbohydrates and nutrients to leaves and also fruits (Pallardy, 2010). In this case, we could regard the stem of A. thaliana rather as a metabolic organ. Furthermore, relative to leaf, stem could be assigned as a younger tissue because of its later occurrence in A. thaliana (Meinke et al., 1998), and thus shows a higher stoichiometric homeostasis according to the above explanations.
CONCLUSIONS
This study demonstrates organ-specific and stage-dependent variations in stoichiometric homeostasis of A. thaliana, and emphasizes the influence of ontogenetic changes. Our results reveal that reproductive tissues relative to other organs and young tissues relative to old tissues have more constrained elemental composition under changing nutrient availability, probably because of their higher metabolic activity and cellular growth rate requiring N and P based on the stoichiometry of essential metabolic processes (Güsewell, 2004). The previous finding that stem N : P ratio is a more responsive indicator of soil nutrient availability than young leaf N : P ratio (Garrish et al., 2010; Schreeg et al., 2014) could not be found in A. thaliana, related to the distinct structure and function of stems between this annual forb and woody plants. Our results could have pronounced implications for diagnosing relative availabilities of N and P in ecosystems, suggesting that the N and P stoichiometry of old tissues might be stronger indicators of nutrient status for plants than that of young tissues. Additional research is required to gain a deeper understanding of the implication of this across species with multiple functional traits.
ACKNOWLEDGEMENTS
We thank N. Y. Kim and H. Zhang for assistance with laboratory work. This study was supported by the National Natural Science Foundation of China (Project nos. 31321061, 31330012 and 41173083) and the National Science Foundation for Fostering Talents in Basic Research (Project nos. J1103406 and J0105).
LITERATURE CITED
- Aerts R, Chapin FS. 2000. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research 30: 1–67. [Google Scholar]
- Ågren GI. 2008. Stoichiometry and nutrition of plant growth in natural communities. Annual Review of Ecology, Evolution, and Systematics 39: 153–170. [Google Scholar]
- Carón MM, De Frenne P, Brunet J, et al. 2014. Latitudinal variation in seeds characteristics of Acer platanoides and A. pseudoplatanus. Plant Ecology 215: 911–925. [Google Scholar]
- Cernusak LA, Winter K, Turner BL. 2010. Leaf nitrogen to phosphorus ratios of tropical trees: experimental assessment of physiological and environmental controls. New Phytologist 185: 770–779. [DOI] [PubMed] [Google Scholar]
- Chapin FS. 1980. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics 11: 233–260. [Google Scholar]
- Chapin FS, Johnson DA, McKendrick JD. 1980. Seasonal movement of nutrients in plants of differing growth form in Alaskan tundra ecosystems: implications for herbivory. Journal of Ecology 68: 189–209. [Google Scholar]
- De Frenne P, Kolb A, Graae BJ, et al. 2011. A latitudinal gradient in seed nutrients of the forest herb Anemone nemorosa. Plant Biology 13: 493–501. [DOI] [PubMed] [Google Scholar]
- Deng Q, Hui D, Luo Y, et al. 2015. Down-regulation of tissue N : P ratios in terrestrial plants by elevated CO2. Ecology doi.org/10.1890/15-0217.1. [DOI] [PubMed] [Google Scholar]
- Dijkstra FA, Pendall E, Morgan JA, et al. 2012. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytologist 196: 807–815. [DOI] [PubMed] [Google Scholar]
- Elrifi IR, Turpin DH. 1985. Steady-state luxury consumption and the concept of optimum nutrient ratios: a study with phosphate and nitrate limited Selenastrum minutum (Chlorophyta). Journal of Phycology 21: 592–602. [Google Scholar]
- Elser JJ, Fagan WF, Kerkhoff AJ, Swenson NG, Enquist BJ. 2010. Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytologist 186: 593–608. [DOI] [PubMed] [Google Scholar]
- Fenner M. 1986. The allocation of minerals to seeds in Senecio vulgaris plants subjected to nutrient shortage. Journal of Ecology 74: 385–392. [Google Scholar]
- Fujita Y, Venterink HO, van Bodegom PM, et al. 2014. Low investment in sexual reproduction threatens plants adapted to phosphorus limitation. Nature 505: 82–86. [DOI] [PubMed] [Google Scholar]
- Güsewell S. 2004. N : P ratios in terrestrial plants: variation and functional significance. New Phytologist 164: 243–266. [DOI] [PubMed] [Google Scholar]
- Garrish V, Cernusak LA, Winter K, Turner BL. 2010. Nitrogen to phosphorus ratio of plant biomass versus soil solution in a tropical pioneer tree, Ficus insipida. Journal of Experimental Botany 61: 3735–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Zurdo P, Escudero A, Mediavilla S. 2015. N resorption efficiency and proficiency in response to winter cold in three evergreen species. Plant and Soil 394: 87–98. [Google Scholar]
- Han W, Tang L, Chen Y, Fang J. 2013. Relationship between the relative limitation and resorption efficiency of nitrogen vs phosphorus in woody plants. PLoS ONE 8: e83366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Berkeley, CA: Circular 347, California Agricultural Experiment Station, College of Agriculture, University of California. [Google Scholar]
- Jones JB. 2001. Laboratory guide for conducting soil tests and plant analysis. New York: CRC Press. [Google Scholar]
- Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. 2006. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. The American Naturalist 168: E103–E122. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin III S, TL P. 2008. Plant physiological ecology. New York: Springer. [Google Scholar]
- Lee W, Fenner M. 1989. Mineral nutrient allocation in seeds and shoots of twelve Chionochloa species in relation to soil fertility. Journal of Ecology 77: 704–716. [Google Scholar]
- Marschner H, Marschner P. 2012. Marschner’s mineral nutrition of higher plants. London: Academic Press. [Google Scholar]
- Meinke DW, Cherry JM, Dean C, Rounsley SD, Koornneef M. 1998. Arabidopsis thaliana: a model plant for genome analysis. Science 282: 662–682. [DOI] [PubMed] [Google Scholar]
- Minden V, Kleyer M. 2014. Internal and external regulation of plant organ stoichiometry. Plant Biology 16: 897–907. [DOI] [PubMed] [Google Scholar]
- Naegle E, Burton J, Carter T, Rufty T. 2005. Influence of seed nitrogen content on seedling growth and recovery from nitrogen stress. Plant and Soil 271: 329–340. [Google Scholar]
- Obeso JR. 2012. Mineral nutrient stoichiometric variability in Hedera helix (Araliaceae) seeds. Annals of Botany 109: 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J, Bell L. 1990. A glasshouse evaluation of ‘critical’ N and P concentrations and N : P ratios in various plant parts of six Eucalypt species. Australian Journal of Botany 38: 281–298. [Google Scholar]
- Pallardy SG. 2010. Physiology of woody plants. London: Academic Press. [Google Scholar]
- Persson J, Fink P, Goto A, Hood JM, Jonas J, Kato S. 2010. To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119: 741–751. [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Ryan D, Bormann F. 1982. Nutrient resorption in northern hardwood forests. BioScience 32: 29–32. [Google Scholar]
- Santa Regina I, Rico M, Rapp M, Gallego H. 1997. Seasonal variation in nutrient concentration in leaves and branches of Quercus pyrenaica. Journal of Vegetation Science 8: 651–654. [Google Scholar]
- Schreeg LA, Santiago LS, Wright SJ, Turner BL. 2014. Stem, root, and older leaf N : P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 95: 2062–2068. [DOI] [PubMed] [Google Scholar]
- Shafik H, Herodek S, Presing M, Vörös L, Balogh K. 1997. Growth of Cyclotella meneghiniana Kutz. II. Growth and cell composition under different growth rates with different limiting nutrient. Annals of Limnology 33: 223–233. [Google Scholar]
- Sterner RW, Elser JJ. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press. [Google Scholar]
- Usuda H. 1995. Phosphate deficiency in maize. V. Mobilization of nitrogen and phosphorus within shoots of young plants and its relationship to senescence. Plant and Cell Physiology 36: 1041–1049. [Google Scholar]
- Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biological Reviews 81: 259–291. [DOI] [PubMed] [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino RM. 1998. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Molecular Biology 37: 455–469. [DOI] [PubMed] [Google Scholar]
- Yan ZB, Kim NY, Han WX, et al. 2015. Effects of nitrogen and phosphorus supply on growth rate, leaf stoichiometry, and nutrient resorption of Arabidopsis thaliana. Plant and Soil 388: 147–155. [Google Scholar]
- Yang X, Tang ZY, Ji CJ, et al. 2014. Scaling of nitrogen and phosphorus across plant organs in shrubland biomes across Northern China. Scientific Reports 4: 5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Elser JJ, He NP, et al. 2011. Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 166: 1–10. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wu HH, Yu Q, et al. 2013. Sampling date, leaf age and root size: implications for the study of plant C:N : P stoichiometry. PloS ONE 8: e60360. [DOI] [PMC free article] [PubMed] [Google Scholar]