Abstract
Background and Aims There have been very few studies investigating the influence of light on the effects of hemiparasitic plants on their hosts, despite the fact that hemiparasites are capable of photosynthesis but also access carbon (C) from their host. In this study we manipulated light availability to limit photosynthesis in an established hemiparasite and its hosts, and determined whether this affected the parasite’s impact on growth and performance of two different hosts. We expected that limiting light and reducing autotrophic C gain in the parasite (and possibly increasing its heterotrophic C gain) would lead to an increased impact on host growth and/or host photosynthesis in plants grown in low (LL) relative to high light (HL).
Methods The Australian native host Leptospermum myrsinoides and the introduced host Ulex europaeus were either infected or not infected with the native stem hemiparasite Cassytha pubescens and grown in either HL or LL. Photosynthetic performance, nitrogen status and growth of hosts and parasite were quantified. Host water potentials were also measured.
Key Results In situ midday electron transport rates (ETRs) of C. pubescens on both hosts were significantly lower in LL compared with HL, enabling us to investigate the impact of the reduced level of parasite autotrophy on growth of hosts. Despite the lower levels of photosynthesis in the parasite, the relative impact of infection on host biomass was the same in both LL and HL. In fact, biomass of L. myrsinoides was unaffected by infection in either HL or LL, while biomass of U. europaeus was negatively affected by infection in both treatments. This suggests that although photosynthesis of the parasite was lower in LL, there was no additional impact on host biomass in LL. In addition, light did not affect the amount of parasite biomass supported per unit host biomass in either host, although this parameter was slightly lower in LL than HL for U. europaeus (P = 0·073). We also found no significant enhancement of host photosynthesis in response to infection in either host, regardless of light treatment.
Conclusions Despite lower photosynthetic rates in LL, C. pubescens did not increase its dependency on host C to the point where it affected host growth or photosynthesis. The impact of C. pubescens on host growth would be similar in areas of high and low light availability in the field, but the introduced host is more negatively affected by infection.
Keywords: Biomass, Cassytha pubescens, gas exchange, hemiparasite–host association, Leptospermum myrsinoides, light, nitrogen, photosynthesis, Ulex europaeus, water potential
INTRODUCTION
Parasitic plants are of global importance as they are found in almost all ecosystems and can have substantial effects on landscape processes, plant community structure and host populations (Pennings and Callaway, 1996; Press and Phoenix, 2005; Quested, 2008). For example, in a model European grassland the presence of the root hemiparasite Rhinanthus minor can increase nutrient cycling (likely through indirect means) and plant diversity, but also decrease community biomass (Bardgett et al., 2006). Such decreases can be explained by R. minor restricting the dominance of grasses, which thereby releases forbs from competitive exclusion and changes community structure (Bardgett et al., 2006; Mudrák and Lepš, 2010). Such outcomes may depend on some hosts showing resistance to infection, while others show a varying degree of tolerance (Press and Graves, 1995; Press and Phoenix, 2005). For instance, some forb species show resistance to R. minor (Cameron et al., 2006; Cameron and Seel, 2007; Rümer et al., 2007). Tolerance of infection by parasitic plants is often greater in native hosts infected with native parasites compared with introduced hosts (Li et al., 2012). For example, in Australia the native host Leptospermum myrsinoides shows greater tolerance of infection with the native stem hemiparasite Cassytha pubescens than the introduced host, Cytisus scoparius (Prider et al., 2009).
Hemiparasites often affect less tolerant hosts via a combination of resource removal and impacts on host photosynthesis (Graves et al., 1989; Press et al., 1999; Shen et al., 2006). While hemiparasites are capable of photosynthesis, they are also known to remove significant amounts of carbon (C) from the xylem of their host(s) (Marshall and Ehleringer, 1990; Press et al., 1991; Seel et al., 1992; Marshall et al., 1994; Těšitel et al., 2010). Restricting parasite photosynthesis may change this balance and result in increased dependency on host C. For example, Cechin and Press (1993) found that as nitrogen (N) supply decreased from 3 mol m−3 to 0·5 mol m−3, photosynthesis of Striga hermonthica decreased by around 50 % while the proportion of host C found in leaves of the parasite increased by 21 %. Another way of manipulating parasite photosynthesis is to change light availability. Těšitel et al. (2011) found that, when shaded, Rhinanthus alectorolophus had lower rates of photosynthesis and a significantly higher percentage of host C in its biomass, relative to unshaded R. alectorolophus. They also found that, relative to controls, shading the young parasite had no impact or a positive effect on host biomass. The latter was presumably a result of shaded parasites being much smaller and representing a smaller carbon sink for the host than unshaded parasites. Studies by Těšitel et al. (2011, 2015) investigating carbon relations of associations involving R. alectorolophus and subsequent effects on host growth were conducted over a relatively short term (∼1·5 months), using juvenile seedlings of an annual parasite with determinate growth. In fact, dry mass of R. alectorolophus was only 0·5–1·0 g even in unshaded plants, and in shaded seedlings was <0·1 g. Unlike R. alectorolophus, many hemiparasites are perennial, have indeterminate growth and can have much higher biomass that can represent a significant C sink for hosts (Marshall and Elheringer, 1990; Marshall et al., 1994). In this latter case, it is reasonable to speculate that when established parasites are shaded to an extent that results in lower photosynthesis (and thus autotrophic C gain), they may become more dependent on the host for C, and that this could be a sufficiently large enough demand to have an impact on the host’s growth and photosynthesis, particularly if host growth is also limited, e.g. by low light. Additionally, hosts that show some tolerance of infection may be less impacted than more susceptible ones, as parasites typically grow more vigorously on the latter (Prider et al., 2009) and thus should represent a larger sink for C on these hosts. However, to our knowledge there have been no studies on the influence of light on host:parasite systems such as these.
Here we report results of experiments investigating the effect of light on the performance of the Australian native stem hemiparasite C. pubescens and its effect on growth and physiology of the tolerant, native host L. myrsinoides and the more susceptible, introduced host Ulex europaeus (Prider et al., 2009). It was hypothesized that parasite photosynthesis would be lower in low light compared with high light and that this would increase the dependence of the parasite on its host. As a consequence, it was speculated that the parasite would have a greater relative effect on host photosynthesis and growth in low light than in high light.
MATERIALS AND METHODS
Study species
Cassytha pubescens (Lauraceae) is a perennial hemiparasitic coiling vine native to Australia (Kokubugata et al., 2012). It has indeterminate growth with photosynthetic stems that are 0·5−1·5 mm in diameter with reduced scale-like leaves. Cassytha pubescens spreads over its hosts and attaches to stems and leaves via multiple haustoria (McLuckie, 1924). Leptospermum myrsinoides (Myrtaceae) is a perennial evergreen shrub native to south-eastern Australia (Harden, 1991). It is abundant in open woodland and is a common, but tolerant, host for C. pubescens (Prider et al., 2009). Ulex europaeus (Fabaceae) is a perennial evergreen shrub native to central and western Europe and North Africa (Clements et al., 2001) that was introduced to Australia in the 19th century (Parsons and Cuthbertson, 2001). Ulex europaeus is frequently parasitized by C. pubescens, which has significant negative impacts on growth of this host (Britton, 2002).
Growth conditions and experimental design
In Experiment 1, 10-month-old L. myrsinoides plants were obtained from a local commercial nursery. They were individually transplanted into 140 mm diameter (1·65 L) pots containing sandy/loam (60/40) in early May 2010. Three months later they were individually re-potted into 200 mm diameter (4·7 L) pots of sandy/loam (60/40). Plants were supplied with slow-release fertilizer (Osmocote; Scotts-Sierra Horticultural Products, Marysville, OH, USA) for the remainder of the experiment according to the manufacturer’s recommended dosage.
In Experiment 2, U. europaeus (∼15 cm in height) were collected from the field in the Adelaide Hills (35°27ʹ41ʺ S, 138°43ʹ91″ E). Plants were excavated and individually potted in 140 mm diameter (1·65 L) pots containing sandy/loam (60/40) in mid-January 2011. Eleven months later they were individually transplanted into 200 mm diameter (4·7 L) pots of sandy loam (60/40). Throughout, they were provided with liquid fertilizer (Nitrosol; Rural Research Ltd, Auckland, New Zealand; NPK 8:3:6) in accordance with the manufacturer’s directions.
Both experiments were carried out in the same glasshouse (University of Adelaide) at a similar time of year, using the same shade cloth structures, and plants were well watered throughout each experiment. Synchronous infection with C. pubescens of randomly selected host individuals was achieved using the technique of Shen et al. (2010). Briefly, infected U. europaeus (donor plants) were placed next to the experimental plants. C. pubescens stems extending from the donor plant were allowed to coil and attach to stems of experimental hosts. After C. pubescens had successfully attached to the new hosts, the connection with the donor host was severed. The infection process of C. pubescens on hosts took 3 months for L. myrsinoides and 5 months for U. europaeus. Plants were monitored for a further week to ensure that C. pubescens had successfully established on the new hosts. Light treatments were implemented around 1 month after the infection process for both experiments.
Infected and non-infected plants were randomly arranged into two light treatments, high light (HL) or low light (LL), and two blocks, with each block on a separate bench (replicate numbers are mentioned under each parameter measured). Plants in the LL treatment were housed in a frame (2 m high × 1·5 m deep × 1·2 m wide) completely covered by neutral density shade cloth that allowed 35 % light penetration. Adjacent HL plants were grown in ambient light and plant position within treatment blocks was re-randomized fortnightly. Light treatments for the L. myrsinoides and U. europaeus experiments were imposed in mid-January 2011 and early January 2012 and ran until early May 2011 and mid-May 2012, respectively. Mean midday photosynthetic photon flux density (PPFD) was recorded with a quantum sensor (LI-190SA; LI-COR, Lincoln, NE, USA) and data logger (LI-1400) on sunny days during each experiment. The PPFDs for the HL treatment blocks were 1182 ± 66 μmol m−2 s−1 (±1 s.e.) in Experiment 1 and 1159 ± 11 μmol m−2 s−1 in Experiment 2. For the LL treatment blocks they were 351 ± 22 μmol m−2 s−1 in Experiment 1 and 300 ± 5 μmol m−2 s−1 in Experiment 2.
Physiological and growth measurements
As we were not evaluating acclimation in this experiment, but rather were interested in the in situ photosynthesis, we measured gas exchange and chlorophyll fluorescence under growth light conditions. Nevertheless, rapid light response curves were measured for parasite and hosts (Supplementary Data Fig. S1) using a chlorophyll fluorometer (MINI-PAM; Walz, Effeltrich, Germany) fitted with a leaf clip (2030-B; Walz, Effeltrich, Germany). Midday electron transport rates (ETRs) were obtained in situ using the chlorophyll fluorometer and were calculated as follows:
where yield is the photochemical efficiency of photosystem II (PSII) in the light, PAR is photosynthetically active radiation (measured as photon flux density in μmol quanta m−2 s−1), 0·5 is included as absorption of two quanta are needed to transport an electron, and 0·84 is a standard absorption factor for higher plants (White and Critchley, 1999; Strong et al., 2000). Measurements were made on a single fully mature leaf of L. myrsinoides and spine of U. europaeus, and also 15 cm from the growing tip of C. pubescens, on sunny days between 12:00 and 14:30 h in early April in both experiments. In situ measurements were made in HL and LL on L. myrsinoides (n = 10, except LL infected plants, n = 8) and C. pubescens (n = 5) 76 and 86 d after treatments had been imposed (DAT), respectively (Experiment 1); and for U. europaeus and C. pubescens (n = 8) at 125 DAT (Experiment 2). The PPFD (μmol m−2 s−1) values for ETR measurements for L. myrsinoides and C. pubescens in HL were 1188 ± 4 and 933 ± 67 while for LL they were 341 ± 5 and 292 ± 4, respectively. Values for U. europaeus and C. pubescens in HL were 1033 ± 13 and 1024 ± 20 while for LL they were 307 ± 5 and 307 ± 4, respectively.
In addition, photosynthesis (A) and stomatal conductance (gs) measurements were made on L. myrsinoides leaves (PLC6 U cuvette) and U. europaeus spine clusters (PLC5 C cuvette) using a portable Ciras-2 gas exchange system (PP Systems, Amesburg, MA). For both experiments cuvette temperature was 25 °C and the CO2 reference supply was maintained at ∼390 ppm. Cuvette leaf temperature was 24·5 ± 0·4 and 25·3 ± 0·1 °C for L. myrsinoides and U. europaeus, respectively. In situ measurements in HL and LL were made on uninfected and infected plants between 10:30 and 13:15 h on a sunny day in April, at 81 DAT for L. myrsinoides (n = 5) and 137 DAT for U. europaeus (n = 6, except HL uninfected plants, n = 5). The PPFD values (μmol m−2 s−1) during gas exchange measurement for L. myrsinoides were 1464 ± 10 and 535 ± 11 and those for U. europaeus were 1057 ± 18 and 313 ± 5 in HL and LL, respectively.
Midday shoot water potential (Ψ) was determined on freshly cut shoots of uninfected and infected plants. Immediately after excision, shoots were placed into a Scholander-type pressure bomb with a digital gauge (PMS Instrument Company, Albany, OR, USA) and balancing pressure was recorded when xylem sap first appeared at the cut end. Measurements were made between 12:00 and 13:40 h on a sunny day in April at 83 DAT for L. myrsinoides (n = 6) and 138 DAT for U. europaeus (n = 6, except HL uninfected n = 5 and infected plants n = 7).
A destructive harvest of uninfected and infected plants and parasite was conducted at 104 and 157 DAT for Experiment 1 and Experiment 2, respectively. Stems, leaves and roots of L. myrsinoides (Experiment 1, n = 5), stems, spines (Experiment 2, very few if any leaves) and roots of U. europaeus (n = 6) and stems of C. pubescens from Experiment 1 (n = 5) and Experiment 2 (n = 6) were collected and oven-dried at 70 °C for 3 d prior to weighing. Leaf area for both L. myrsinoides and U. europaeus was determined using the relationships between leaf area and dry weight obtained from a subsample of foliage from each treatment (Rolston and Robertson, 1976). For these positive relationships, R was ≥0·95 for all treatments in both experiments. Nitrogen concentration of oven-dried C. pubescens stems, L. myrsinoides leaves and U. europaeus spines (replication as above) was determined using the Elementar Rapid N III Nitrogen Analyzer Version J by Waite Analytical Services (University of Adelaide).
Statistical analyses
The variances of the data were homogeneous and Experiments 1 and 2 were analysed separately. The effects of light and infection on hosts were assessed using two-way ANOVA. When significant interactions between light and infection were detected, the analyses for the four combinations were continued. If no interaction was detected, we then considered independent effects of light (uninfected and infected HL plants pooled versus uninfected and infected LL plants pooled) and independent effects of infection (uninfected HL and LL plants pooled versus infected HL and LL plants pooled). One-way ANOVA was used to determine the effect of light on C. pubescens. When a significant effect for a parameter was detected by the model, a Tukey–Kramer HSD was then used for post hoc pairwise comparisons of means. All data were analysed with the software JMP version 4.0.3 (SAS Institute, 2000) with α = 0·05.
RESULTS
Parasite and host ETR
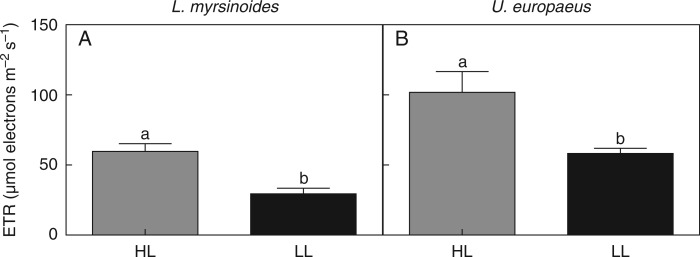
Our aim was to limit photosynthesis of the hemiparasite C. pubescens by growing plants in LL, and, as expected, midday ETR of C. pubescens on both L. myrsinoides and U. europaeus was significantly lower in LL than HL (Table 1). Midday ETRs of C. pubescens growing in HL were 51 and 43 % higher relative to those in LL when growing on L. myrsinoides or U. europaeus, respectively (Fig. 1A, B).
Table 1.
One-way ANOVA results (P values) for the effect of light on C. pubescens midday electron transport rate (ETR), biomass, biomass per gram host biomass and stem nitrogen concentration (N), when infecting L. myrsinoides or U. europaeus (each host species was analysed separately)
| Source of variation | ETR | Biomass | Grams dry weight of parasite per g dry weight of host | N |
|---|---|---|---|---|
| L. myrsinoides | ||||
| Light | 0·002 | 0·191 | 0·388 | 0·829 |
| U. europaeus | ||||
| Light | 0·012 | 0·001 | 0·073 | 0·0004 |
Significant effects are in bold.
F and sum of square values and d.f. are provided in Supplementary Data Table S1.
Fig. 1.
In situ midday electron transport rates (ETRs) of C. pubescens growing on L. myrsinoides (A) or U. europaeus (B) in high (HL, dark grey bars) or low light (LL, black bars). Letters indicate significant differences; bars are means (±1 s.e.) and n = 5 (A) and 8 (B).
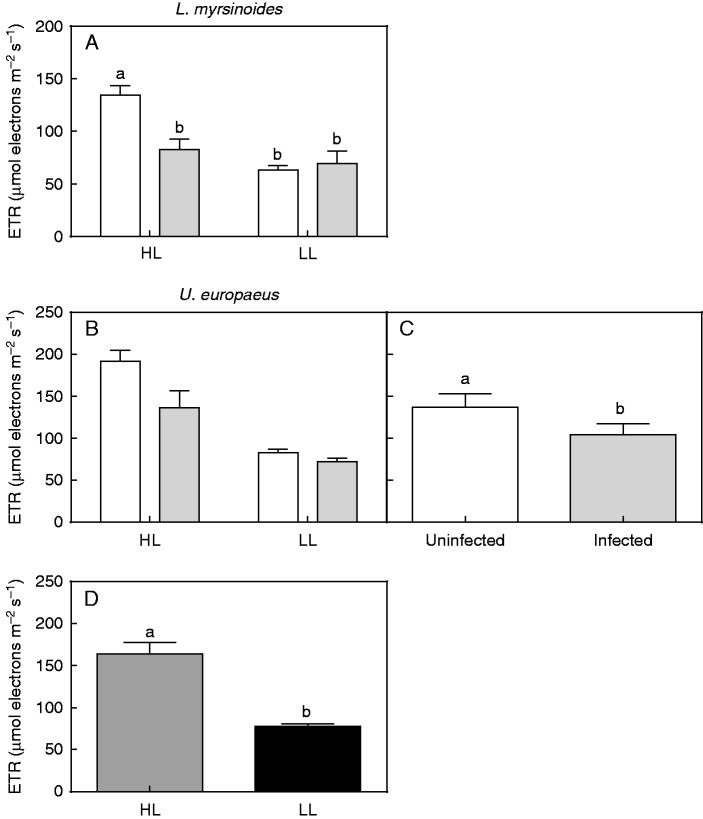
Midday ETR of L. myrsinoides was significantly affected by infection in HL but not in LL (significant interaction; Table 2, Fig. 2A). Midday ETR was 39 % lower in HL-grown infected plants relative to uninfected plants. By contrast, there was no significant interaction between light and infection for midday ETR of U. europaeus, but there were independent infection and light effects (Table 2, Fig. 2B–D). On average, midday ETR of infected plants was 24 % lower than that of uninfected plants, irrespective of light conditions (Fig. 2C). Midday ETR of HL-grown U. europaeus was 53 % higher, on average, than that of LL plants, regardless of their infection status (Fig. 2D).
Table 2.
Two-way ANOVA results (P values) for the effect of C. pubescens and light on midday electron transport rate (ETR), photosynthetic rates (A), stomatal conductance (gs), midday shoot water potentials (Ψ), total, shoot and root biomass, leaf or spine area (L/S A), shoot/root ratio (S/R) and leaf or spine nitrogen (N) concentration of L. myrsinoides and U. europaeus (each species was analysed separately)
| Parameter |
L. myrsinoides |
U. europaeus |
||||
|---|---|---|---|---|---|---|
| I × L | I | L | I × L | I | L | |
| ETR | 0·0009 | 0·018 | <0·0001 | 0·084 | 0·012 | <0·0001 |
| A | 0·450 | 0·011 | 0·939 | 0·178 | 0·908 | <0·0001 |
| gs | 0·727 | 0·010 | 0·176 | 0·010 | 0·825 | 0·262 |
| Ψ | 0·058 | 0·333 | 0·0009 | 0·371 | 0·651 | <0·0001 |
| Total | 0·006 | 0·774 | <0·0001 | 0·153 | <0·0001 | <0·0001 |
| Shoot | 0·016 | 0·421 | <0·0001 | 0·071 | <0·0001 | <0·0001 |
| Root | 0·015 | 0·249 | <0·0001 | 0·532 | 0·041 | <0·0001 |
| L/S A | 0·776 | 0·423 | 0·0002 | 0·261 | <0·0001 | 0·0002 |
| S/R | 0·115 | 0·385 | 0·003 | 0·928 | 0·034 | 0·003 |
| N | 0·040 | 0·714 | 0·0004 | 0·745 | 0·123 | 0·007 |
I, infection; L, light.
Significant effects are in bold.
F and sum of square values and d.f. are provided in Supplementary Data Tables S2 and S3.
Fig. 2.
In situ midday electron transport rates (ETRs) of L. myrsinoides (A) and U. europaeus (B) grown in high (HL) or low light (LL), and uninfected (open bars) or infected (grey bars) with C. pubescens. (C) Independent effect of infection on in situ midday ETR of U. europaeus (open bar, average of HL and LL uninfected plants pooled; grey bar, average of HL and LL infected plants pooled). (D) Independent effect of light on in situ midday ETR of U. europaeus in HL (dark grey bars, average of uninfected and infected HL plants pooled) versus LL (black bars, average of uninfected and infected LL plants pooled). Letters indicate significant differences; bars are means (±1 s.e.) and n = 8–10 (A), 8 (B) and 16 (C, D).
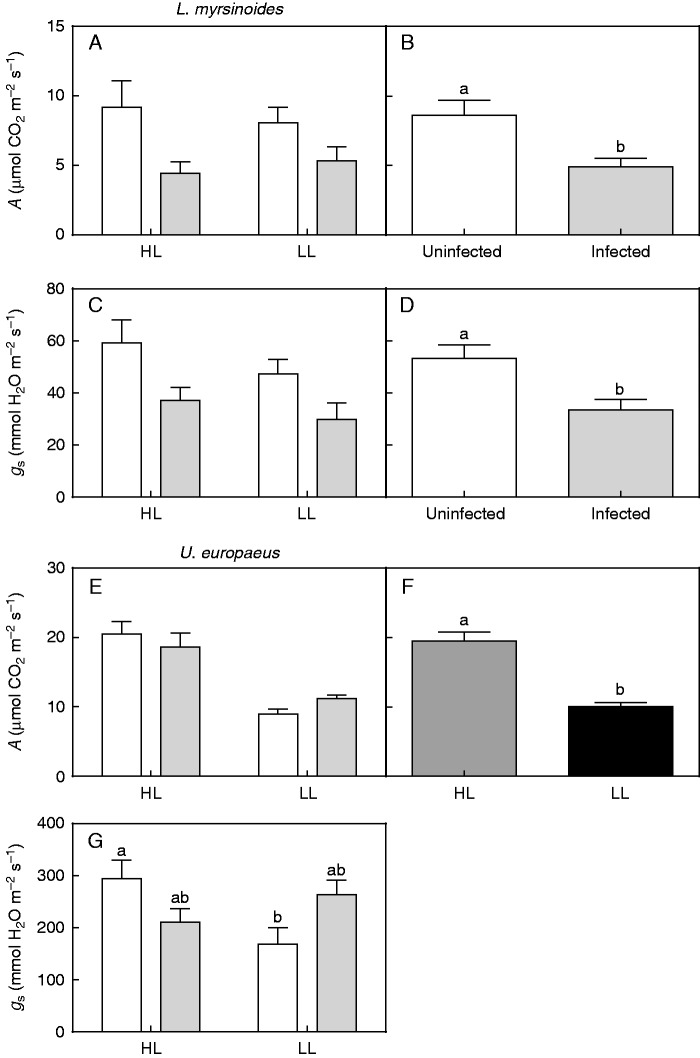
Host A, gs and Ψ
There was no interaction between light and infection for A in L. myrsinoides (Table 2, Fig. 3A). On average, photosynthetic rates of infected plants were 43 % lower compared with those of uninfected plants, irrespective of light conditions (significant infection effect; Table 2, Fig. 3B). Similarly, there was no significant interaction between light and infection for gs of L. myrsinoides, but this parameter was also independently affected by infection (Table 2, Fig. 3C, D). Stomatal conductance of infected L. myrsinoides was, on average, 37 % less compared with that of uninfected plants, across the light treatments (Fig. 3D).
Fig. 3.
In situ photosynthetic rates (A) and stomatal conductance (gs) of L. myrsinoides (A, C) and U. europaeus (E, G) grown in high (HL) or low light (LL) and uninfected (open bars) or infected (grey bars) with C. pubescens. Independent effect of infection on in situ A (B) and gs (D) of L. myrsinoides (open bars, average of HL and LL uninfected plants pooled; grey bars, average of HL and LL infected plants pooled). (F) Independent effect of light on in situ A of U. europaeus in HL (dark grey bars, average of uninfected and infected HL plants pooled) versus LL (black bars, average of uninfected and infected LL plants pooled). Letters indicate significant differences; bars are means (±1 s.e.) and n = 5 (A, C), 10 (B, D), 6 (E, G, except uninfected HL plants, n = 5) and 11–12 (F).
There was also no interaction between light and infection for A in U. europaeus (Table 2, Fig. 3E). Infection had no effect on this parameter, whereas light did (Table 2). On average, photosynthetic rates of U. europaeus in HL were 48 % higher than those in LL, regardless of their infection status (Fig. 3F). By contrast, there was a significant interaction between light and infection for gs of U. europaeus (Table 2). Stomatal conductance was unaffected by infection regardless of light treatment; there was a trend for gs of infected plants to be lower when grown in HL, but the opposite occurred in LL (Fig. 3G). Uninfected plants in HL had significantly higher gs than uninfected plants in LL (Fig. 3G).
There was no interaction for midday Ψ in L. myrsinoides (Table 2). There was no independent infection effect on this parameter but it was independently affected by light (Table 2). Midday Ψ in HL L. myrsinoides was 17 % lower relative to that in LL plants (Table 3). Likewise, there was no significant interaction between light and infection for midday Ψ of U. europaeus (Table 2). Infection also had no significant, independent effect on this parameter in U. europaeus, whereas light did (Table 2). Water potentials at midday of HL U. europaeus were 2-fold lower than those of LL plants (Table 3).
Table 3.
Midday shoot water potential (Ψ, MPa) of L. myrsinoides and U. europaeus in high (HL) or low light (LL), uninfected (−) or infected (+) with C. pubescens. The two species were analysed separately. L. myrsinoides: no interaction (n = 6), no infection but significant independent light effect (n = 12). U. europaeus: no interaction (n = 5−7), no infection but significant independent light effect (n = 12)
| Treatment | L. myrsinoides | U. europaeus |
|---|---|---|
| HL− | −1·98 ± 0·10 | −2·12 ± 0·07 |
| HL+ | −1·74 ± 0·07 | −2·08 ± 0·11 |
| LL− | −1·50 ± 0·10 | −0·98 ± 0·09 |
| LL+ | −1·58 ± 0·04 | −1·11 ± 0·07 |
| Infection effect | ||
| − | −1·74 ± 0·10 | −1·50 ± 0·19 |
| + | −1·66 ± 0·05 | −1·63 ± 0·15 |
| Light effect | ||
| HL | −1·86 ± 0·07a | −2·10 ± 0·07a |
| LL | −1·54 ± 0·05 b | −1·05 ± 0·06b |
Data are means (±1 s.e.) and letters denote significant differences.
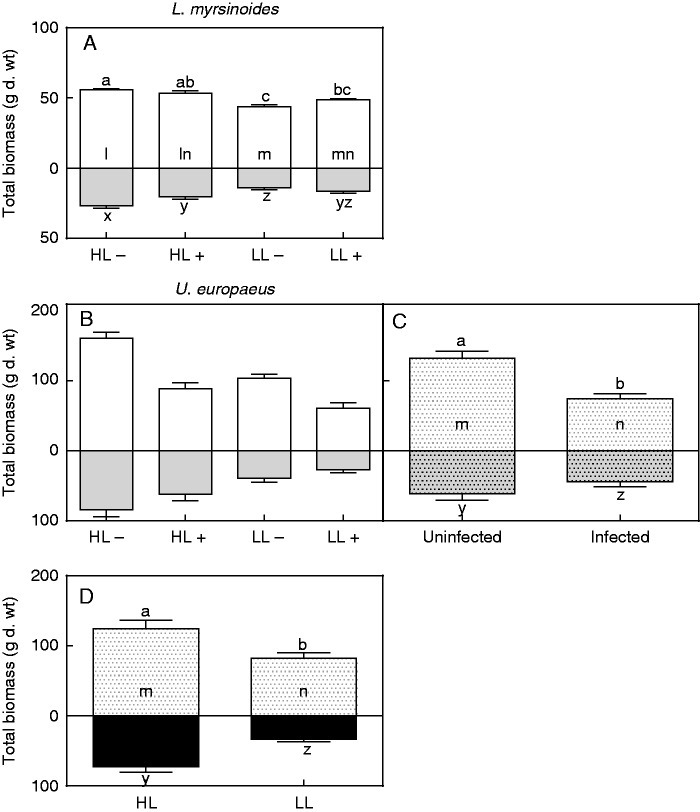
Host growth
Total and shoot biomass of L. myrsinoides was not significantly affected by infection in HL or LL; however, biomass of uninfected HL plants was significantly higher compared with that of uninfected LL plants (significant interaction for both total and shoot biomass; Table 2, Fig. 4A). Root biomass of L. myrsinoides was negatively affected by infection in HL but not in LL, and again that of uninfected HL plants was significantly higher than that of uninfected LL plants (significant interaction; Table 2, Fig. 4A). There was no significant interaction or infection effect on leaf area or shoot/root ratio of L. myrsinoides (Table 2). Light, however, did affect these parameters, and for LL plants leaf area and shoot/root ratio were 29 and 26 % higher, respectively, relative to those of HL plants (Tables 2 and 4).
Fig. 4.
Total, shoot (open bars) and root (grey bars) biomass of L. myrsinoides (A) and U. europaeus (B) grown in high (HL) or low light (LL), and uninfected (minus) or infected (plus) with C. pubescens. (C) Independent effect of infection on total, shoot (open dotted bar) and root biomass (dotted grey bars) of U. europaeus (left bar, average of uninfected HL and LL plants pooled; right bar, average of infected HL and LL plants pooled). (D) Independent effect of light on total, shoot (open dotted bar) and root biomass (black bars) of U. europaeus (left bar, average of uninfected and infected HL plants pooled; right bar, average of uninfected and infected LL plants pooled). Letters indicate significant differences for total (a–c), shoot (l–n) and root (x–z) biomass; bars are means (±1 s.e.) and n = 5 (A), 6 (B) and 12 (C, D).
Table 4.
Leaf or spine area (L/S A) (cm2), shoot/root ratio and leaf or spine nitrogen (N) concentration (%) of L. myrsinoides and U. europaeus in either HL or LL and either uninfected (−) or infected (+) with C. pubescens. The two species were analysed separately. L. myrsinoides: no interactions except for N (n = 5), no independent infection but significant light effect for leaf area and shoot/root ratio (n = 10). U. europaeus: no interactions (n = 6), but significant independent effect of infection on spine area and shoot/root ratio (n = 12) and significant independent effect of light on all three parameters (n = 11–12)
| Treatment | L/S area | Shoot/root | N |
|---|---|---|---|
| L. myrsinoides | |||
| HL− | 2816 ± 113 | 2·12 ± 0·134 | 1·84 ± 0·07a |
| HL+ | 2695 ± 234 | 2·66 ± 0·182 | 1·98 ± 0·07ab |
| LL− | 3983 ± 252 | 3·22 ± 0·208 | 2·19 ± 0·06b |
| LL+ | 3731 ± 257 | 3·06 ± 0·262 | 2·10 ± 0·03b |
| Infection effect | |||
| − | 3400 ± 234 | 2·67 ± 0·216 | – |
| + | 3213 ± 238 | 2·86 ± 0·164 | – |
| Light effect | |||
| HL | 2756 ± 124a | 2·39 ± 0·139a | – |
| LL | 3857 ± 175b | 3·14 ± 0·160b | – |
| U. europaeus | |||
| HL− | 1267 ± 73 | 2·06 ± 0·291 | 1·50 ± 0·10 |
| HL+ | 773 ± 109 | 1·51 ± 0·109 | 1·30 ± 0·07 |
| LL− | 827 ± 40 | 2·84 ± 0·291 | 1·78 ± 0·09 |
| LL+ | 512 ± 78 | 2·33 ± 0·146 | 1·65 ± 0·13 |
| Infection effect | |||
| − | 1047 ± 77a | 2·45 ± 0·229a | 1·66 ± 0·08 |
| + | 643 ± 75b | 1·92 ± 0·152b | 1·48 ± 0·09 |
| Light effect | |||
| HL | 1020 ± 97a | 1·78 ± 0·170a | 1·39 ± 0·06a |
| LL | 670 ± 63b | 2·59 ± 0·173b | 1·72 ± 0·08b |
Data are means (± 1 s.e.) and letters denote significant differences.
By contrast, there were no significant interactions between light and infection for any of the growth measures for U. europaeus (Table 2, Fig. 4B). Infection had a significant, independent impact on all growth parameters for this host (Table 2, Fig. 4C). Total biomass of infected plants was 40 % lower, on average, than that of uninfected plants (Fig. 4C), regardless of light treatment. Shoot and root biomass were 40 and 28 %, respectively, lower compared with values for uninfected plants (Fig. 4C). Leaf area and shoot/root ratio of infected U. europaeus were 40 and 22 %, respectively, lower than those of uninfected plants (Table 4). Light also significantly affected all growth parameters of U. europaeus (Table 2). Total biomass of plants grown in LL was 40 % lower, on average, relative to that of the HL-grown plants, regardless of infection (Fig. 4D). Shoot and root biomass of U. europaeus in LL were 34 and 55 %, respectively, lower than in HL plants (Fig. 4D). Leaf area and shoot/root ratio of LL U. europaeus were 34 % less and 31 % higher, respectively, compared with HL-grown plants (Table 4).
Parasite growth
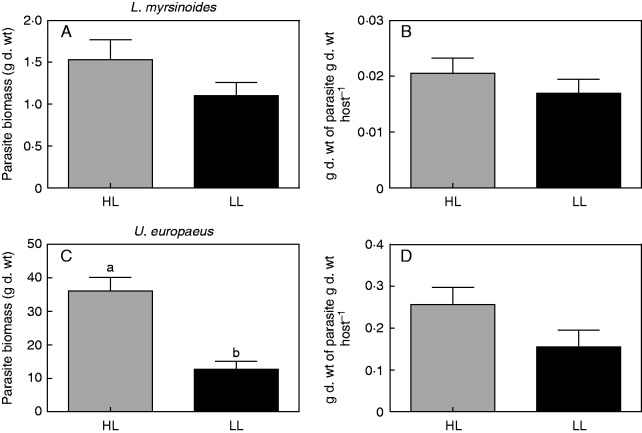
Final biomass of C. pubescens growing on L. myrsinoides was similar between light treatments (no significant light effect; Table 1, Fig. 5A). Likewise, there was no light effect on parasite biomass per unit dry weight of L. myrsinoides hosts (Table 1, Fig. 5B). By contrast, biomass of C. pubescens growing on U. europaeus in HL was 65 % higher than that in LL (significant light effect; Table 1, Fig. 5C). However, light did not affect parasite biomass per unit dry weight of U. europaeus hosts (Table 1, Fig. 5D).
Fig. 5.
Total biomass and grams of parasite dry weight per gram of host dry weight, respectively, of C. pubescens growing on L. myrsinoides (A, B) or U. europaeus (C, D) in high (HL, dark grey bars) or low light (LL, black bars). Letters indicate significant differences; bars are means (±1 s.e.) and n = 5 (A, B) and 6 (C, D).
Parasite and host N
There was no difference in N concentration of C. pubescens stems when growing on L. myrsinoides in HL (1·8 ± 0·08 %) or LL (1·8 ± 0·03 %) (Table 1). By contrast, N concentration of C. pubescens growing on U. europaeus in HL (1·8 ± 0·15 %) was 43 % lower compared with that in LL (3·2 ± 0·21 %) (Table 1).
With reference to L. myrsinoides, leaf N concentration of uninfected HL plants was not significantly different from that of infected HL plants but was significantly less than in LL uninfected and infected plants, which did not differ significantly from each other (significant interaction; Tables 2 and 4). By contrast, there was no interaction between light and infection for spine N of U. europaeus (Table 2). Infection had no significant independent effect on spine N of U. europaeus, while light did (Table 2). Nitrogen concentration of HL U. europaeus was 19 % less relative to that of LL plants (Table 4).
DISCUSSION
As predicted, photosynthesis (ETR) of C. pubescens was significantly lower in LL than HL. However, contrary to our hypothesis, this did not result in a greater relative impact of infection on biomass of either host in LL. Biomass of U. europaeus infected with C. pubescens was 40 % lower than that of uninfected plants, regardless of light treatment. In contrast, infection had no effect on total biomass of L. myrsinoides in either LL or HL. There was a trend for parasite biomass per unit U. europaeus biomass to be lower in LL compared with HL, but this was not significant.
Previous studies have also shown that photosynthesis of hemiparasites such as mistletoes is impacted by light (Strong et al., 2000; Matsubara et al., 2002), but to our knowledge only one study has investigated whether this also influences the parasite’s effect on host growth. A recent study by Borowicz and Armstrong (2012) found that light did not influence the effect of the perennial root hemiparasite Pedicularis canadensis on the grass Andropogon gerardii. Similarly, we found that light had no impact on the relative effect of the stem hemiparasite on host growth. Hemiparasites are known to remove significant amounts of C from their hosts (Press et al., 1991; Press and Whittaker, 1993; Těšitel et al., 2010), but our results suggest that, despite the lower potential for C fixation in LL, C. pubescens did not increase its dependency for C on either host to the point where it affected host growth.
We found no effect of light on the relative impact of C. pubescens on host growth; however, it is possible that the parasite’s demand for host C may still have increased in LL but that this was met by an increase in host photosynthesis. Stimulatory parasite effects on host photosynthesis have been reported for associations involving the root heimparasite S. hermonthica (Cechin and Press, 1993) and the stem and root holoparasites Cuscuta reflexa and Orobanche cernua, respectively (Jeschke et al., 1994, 1997; Jeschke and Hilpert, 1997; Hibberd et al., 1998, 1999). In contrast, several studies have found that parasites, including C. pubescens, can have deleterious effects on host photosynthesis (Gurney et al., 2002; Hwangbo et al., 2003; Meinzer et al., 2004; Shen et al., 2007, 2010; Mauromicale et al., 2008; Prider et al., 2009). Increases in host photosynthesis are explained by the parasite acting as an extra sink for C, thus reducing the accumulation of carbohydrate in host foliage, which would normally act as a signal to downregulate photosynthesis (Jeschke and Hilpert, 1997; Jeschke et al., 1997; Hibberd et al., 1998, 1999). We did find some evidence that photosythesis of infected U. europaeus may have been slightly stimulated in LL, as there were small but non-significant increases in both photosynthesis and stomatal conductance relative to uninfected plants (Fig. 3E, G). Similarly, infection appeared to have a greater negative effect on ETR of both hosts in HL than in LL (Fig. 2A, B and Supplementary Data Fig. S1).
While light did not alter the relative effect of C. pubescens on total biomass of either host, there were differences in the absolute impact of infection on each host. In Experiment 1, C. pubescens had no effect on total biomass of the native L. myrsinoides. In contrast, in Experiment 2 total biomass of the introduced U. europaeus infected with C. pubescens was 40 % lower than that of uninfected plants, in both HL and LL. These differences may be related to the evolutionary history of each host. Ulex europaeus was introduced to Australia in the late 19th century, whereas L. myrsinoides and C. pubescens are both native to Australia and co-occur across eastern and southern parts of the country. Other studies have also reported that native parasites have a greater effect on growth of introduced hosts compared with native hosts (Prider et al., 2009; Li et al., 2012). The longer association between native hosts and parasites could have resulted in the evolution of mechanisms of resistance or tolerance to infection in the native hosts. Consistent with this, L. myrsinoides appears to have evolved some tolerance to infection with C. pubescens, as it is a common host in the wild but seems not to be significantly impacted by infection (Prider et al., 2009). Mechanisms of tolerance may include preventing formation of effective haustorial connections between host and parasite, thus reducing the ability of the parasite to remove resources. For example, Tsang (2010) used 32P to demonstrate that transfer of phosphorus to C. pubescens was more effective from the introduced host C. scoparius than the native host Acacia myrtifolia. Thus, despite the fact that C. pubescens affected photosynthesis of L. myrsinoides (likely driven by a decrease in stomatal conductance; Fig. 3D), the lack of an effect of infection on total biomass in this host may be largely explained by a poor haustorial connection. Conversely, the negative effect of C. pubescens on U. europaeus may be primarily due to an effective haustorial connection and removal of resources from this host (as may be inferred from the vigorous growth of the parasite), in addition to effects on host photosynthesis.
A number of studies have shown that more vigorous parasite growth is generally associated with a greater effect on the host (Gibson and Watkinson, 1991; Matthies, 1996; Keith et al., 2004; Cameron et al., 2008; Prider et al., 2009; Li et al., 2012; but see Cameron et al., 2006). This is consistent with our results, where there was minimal parasite growth and effect on total biomass of L. myrsinoides. By contrast, U. europaeus supported a higher biomass of C. pubescens and was strongly affected by infection. Similarly, C. pubescens was also found to grow more vigorously and achieved significantly greater biomass on the introduced host, C. scoparius, compared with L. myrsinoides in the field (Prider et al., 2009). Vigorous growth of the parasite on U. europaeus might be partly due to the higher ETR of C. pubescens relative to that on L. myrsinoides. It may also be explained by a more effective haustorial connection as mentioned above. Whereas light had no effect on parasite biomass supported by L. myrsinoides, total parasite biomass on U. europaeus was much lower in LL than HL. This may be partly explained by LL significantly decreasing the ETR of the parasite and thus autotrophic contributions to its own growth. Further, U. europaeus hosts were smaller in LL relative to HL (Fig. 4D), and thus would have had a lower capacity for resource uptake and supply to the parasite in these conditions. There was also a trend for parasite biomass per unit U. europaeus biomass to be lower in LL relative to HL (P = 0·073). Thus, it is possible that resource uptake by C. pubescens was lower, per unit of host biomass, in LL versus HL on this host.
Despite the lower rates of photosynthesis in the parasite in LL, our results suggest that the parasite is removing a similar amount of C per unit host biomass in both light conditions, but this needs to be confirmed. Thus, growth of the parasite seems to be tightly coupled to host growth, suggesting that parasite growth is determined by the extent to which the host supplies resources. However, it is also possible that growth of the parasite is determined by its own ability to fix C. If this were so, however, we would have expected much greater biomass of C. pubescens on L. myrsinoides than we observed, as photosynthesis of the parasite on this host was half that of the parasite on U. europaeus, but parasite biomass was 10-fold greater on U. europaeus than L. myrsinoides.
Conclusions
It is concluded from our experiments that, despite having lower rates of photosynthesis in LL, the parasite did not increase its dependency on host C to the point where it affected host growth or photosynthesis. With reference to U. europaeus, there appears to be coordination between host and parasite, with a smaller infected host in LL supporting a smaller parasite. Such coordination in responses between host and parasite growth has also been suggested for associations involving mistletoes that access resources from the host xylem and the stem holoparasites Cuscuta campestris and Cuscuta reflexa (Marshall et al., 1994; Shen et al., 2013). In general, our studies demonstrated that growth of the introduced host U. europaeus, but not the native host L. myrsinoides, is negatively affected by the native stem hemiparasite C. pubescens and is independent of light. Finally, our data indicated that C. pubescens will have a similar negative effect on the growth of U. europaeus in areas of both high and low light availability in the field.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and comprise the following. Figure S1: rapid light response curves for both parasite and host from either experiment. Table S1: one-way ANOVA results (F, sum of square values and d.f.) for the effect of light on ETR, biomass, grams of parasite dry weight per gram of host dry weight and stem nitrogen concentration of parasite infecting either host. Table S2: two-way ANOVA results (F and sum of squares values and d.f.) for the effect of light and infection on ETR, A, gs and Ψ of either host. Table S3: two-way ANOVA results (F and sum of squares values and d.f.) for the effect of light and infection on total, shoot and root biomass, leaf or spine area, shoot/root ratio and leaf or spine nitrogen concentration of either host.
ACKNOWLEDGEMENTS
Special thanks to Dr Jane N. Prider, Hong T. Tsang, Elizabeth C. Maciunas, Associate Professor Robert J. Reid, Angela Cirocco and Michele Cirocco for all their assistance. This work was supported by the Nature Foundation SA Inc. (60106911) and the Field Naturalists Society of South Australia Lirabenda Endowment Fund (75108097).
LITERATURE CITED
- Bardgett RD, Smith RS, Shiel RS, et al. 2006. Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature 439: 969–972. [DOI] [PubMed] [Google Scholar]
- Borowicz VA, Armstrong JE. 2012. Resource limitation and the role of a hemiparasite on a restored prairie. Oecologia 169: 783–792. [DOI] [PubMed] [Google Scholar]
- Britton T. 2002. The impact of Cassytha pubescens R. Br. on the physiology and growth of gorse (Ulex europaeus L.) in South Australia. Honours Thesis, University of Adelaide, Australia. [Google Scholar]
- Cameron DD, Seel WE. 2007. Functional anatomy of haustoria formed by Rhinanthus minor: linking evidence from histology and isotope tracing. New Phytologist 174: 412–419. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Coats AM, Seel WE. 2006. Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Annals of Botany 98: 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DD, Geniez JM, Seel WE, Irving LJ. 2008. Suppression of host photosynthesis by the parasitic plant Rhinanthus minor. Annals of Botany 101: 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechin I, Press MC. 1993. Nitrogen relations of the sorghum-Striga hermonthica host-parasite association: growth and photosynthesis. Plant, Cell and Environment 16: 237–247. [DOI] [PubMed] [Google Scholar]
- Clements DR, Peterson DJ, Prasad R. 2001. The biology of Canadian weeds. 112. Ulex europaeus L. Canadian Journal of Plant Science 81: 325–337. [Google Scholar]
- Gibson CC, Watkinson AR. 1991. Host selectivity and the mediation of competition by the root hemiparasite Rhinanthus minor. Oecologia 86: 81–87. [DOI] [PubMed] [Google Scholar]
- Graves JD, Press MC, Stewart GR. 1989. A carbon balance model of the sorghum-Striga hermonthica host-parasite association. Plant, Cell and Environment 12: 101–108. [Google Scholar]
- Gurney AL, Press MC, Scholes JD. 2002. Can wild relatives of sorghum provide new sources of resistance or tolerance against Striga species? Weed Research 42: 317–324. [Google Scholar]
- Harden GJ. 1991. Flora of New South Wales, Vol. 2 Kensington: New South Wales University Press. [Google Scholar]
- Hibberd JM, Quick WP, Press MC, Scholes JD. 1998. Can source-sink relations explain responses of tobacco to infection by the root holoparasitic angiosperm Orobanche cernua? Plant, Cell and Environment 21: 333–340. [Google Scholar]
- Hibberd JM, Quick WP, Press MC, Scholes JD, Jeschke WD. 1999. Solute fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant, Cell and Environment 22: 937–947. [Google Scholar]
- Hwangbo J-K, Seel WE, Woodin SJ. 2003. Short-term exposure to elevated atmospheric CO2 benefits the growth of a facultative annual root hemiparasite, Rhinanthus minor (L.), more than that of its host, Poa pratensis (L.). Journal of Experimental Botany 54: 1951–1955. [DOI] [PubMed] [Google Scholar]
- Jeschke WD, Hilpert A. 1997. Sink-stimulated photosynthesis and sink-dependent increase in nitrate uptake: nitrogen and carbon relations of the parasitic association Cuscuta reflexa–Ricinus communis. Plant, Cell and Environment 20: 47–56. [Google Scholar]
- Jeschke WD, Räth N, Bäumel P, Czygan F-C, Proksch P. 1994. Modelling the flow and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb. and its host Lupinus albus L. I. Methods for estimating net flows. Journal of Experimental Botany 45: 791–800. [Google Scholar]
- Jeschke WD, Baig A, Hilpert A. 1997. Sink-stimulated photosynthesis, increased transpiration and increased demand-dependent stimulation of nitrate uptake: nitrogen and carbon relations in the parasitic association Cuscuta reflexa-Coleus blumei. Journal of Experimental Botany 48: 915–925. [Google Scholar]
- Keith AM, Cameron DD, Seel WE. 2004. Spatial interactions between the hemiparasitic angiosperm Rhinanthus minor and its host are species-specific. Functional Ecology 18: 435–442. [Google Scholar]
- Kokubugata G, Nakamura K, Forster PI, et al. 2012. Cassytha pubescens and C. glabella (Lauraceae) are not disjunctly distributed between Australia and the Ryukyu Archipelago of Japan—evidence from morphological and molecular data. Australian Systematic Botany 25: 364–373. [Google Scholar]
- Li J, Jin Z, Song W. 2012. Do native parasitic plants cause more damage to exotic invasive hosts than native non-invasive hosts? An implication for biocontrol. PLoS One 7: e34577 Doi: 10.1371/journal.pone.0034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JD, Ehleringer JR. 1990. Are xylem-tapping mistletoes partially heterotrophic? Oecologia 84: 244–248. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Ehleringer JR, Schulze E-D, Farquhar G. 1994. Carbon isotope composition, gas exchange and heterotrophy in Australian mistletoes. Functional Ecology 8: 237–241. [Google Scholar]
- Matsubara S, Gilmore AM, Ball MC, Anderson JM, Osmond CB. 2002. Sustained downregulation of photosystem II in mistletoes during winter depression of photosynthesis. Functional Plant Biology 29: 1157–1169. [DOI] [PubMed] [Google Scholar]
- Matthies D. 1996. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: heterotrophic benefit and parasite-mediated competition. Oikos 75: 118–124. [Google Scholar]
- Mauromicale G, Lo Monaco A, Longo AMG. 2008. Effect of branched broomrape (Orobanche ramosa) infection on the growth and photosynthesis of tomato. Weed Science 56: 574–581. [Google Scholar]
- McLuckie J. 1924. Studies in parasitism. I. A contribution to the physiology of the genus Cassytha, Part 1. Proceedings of the Linnean Society of New South Wales 49: 55–78. [Google Scholar]
- Meinzer FC, Woodruff DR, Shaw DC. 2004. Integrated responses of hydraulic architecture, water and carbon relations of western hemlock to dwarf mistletoe infection. Plant, Cell and Environment 27: 937–946. [Google Scholar]
- Mudrák O, Lepš J. 2010. Interactions of the hemiparasitic species Rhinanthus minor with its host plant community at two nutrient levels. Folia Geobotanica 45: 407–424. [Google Scholar]
- Parsons WT, Cuthbertson EG. 2001. Noxious weeds of Australia, 2nd edn Collingwood: CSIRO Publishing. [Google Scholar]
- Pennings SC, Callaway RM. 1996. Impact of a parasitic plant on the structure and dynamics of salt marsh vegetation. Ecology 77: 1410–1419. [Google Scholar]
- Press MC, Graves JD. 1995. Parasitic plants. London: Chapman & Hall. [Google Scholar]
- Press MC, Phoenix GK. 2005. Impacts of parasitic plants on natural communities. New Phytologist 166: 737–751. [DOI] [PubMed] [Google Scholar]
- Press MC, Whittaker JB. 1993. Exploitation of the xylem stream by parasitic organisms. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 341: 101–111. [Google Scholar]
- Press MC, Smith S, Stewart GR. 1991. Carbon acquisition and assimilation in parasitic plants. Functional Ecology 5: 278–283. [Google Scholar]
- Press MC, Scholes JD, Watling JR. 1999. Parasitic plants: physiological and ecological interactions with their hosts. In: MC Press, JD Scholes, MG Barker, eds. Physiological plant ecology. Oxford: Blackwell Science, 175–197. [Google Scholar]
- Prider JN, Watling JR, Facelli JM. 2009. Impacts of a native parasitic plant on an introduced and a native host species: implications for the control of an invasive weed. Annals of Botany 103: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quested HM. 2008. Parasitic plants—impacts on nutrient cycling. Plant and Soil 311: 269–272. [Google Scholar]
- Rolston MP, Robertson AG. 1976. Some aspects of the absorption of picloram by gorse (Ulex europaeus L.). Weed Research 16: 81–86. [Google Scholar]
- Rümer S, Cameron DD, Wacker R, Hartung W, Jiang F. 2007. An anatomical study of the haustoria of Rhinanthus minor attached to roots of different hosts. Flora 202: 194–200. [Google Scholar]
- Seel WE, Cechin I, Vincent CA, Press MC. 1992. Carbon partitioning and transport in parasitic angiosperms and their hosts. In: CJ Pollock, JF Farrar, AJ Gordon, eds. Carbon partitioning: within and between organisms. Oxford: BIOS Scientific Publishers, 199–223. [Google Scholar]
- Shen H, Ye W, Hong L, et al. 2006. Progress in parasitic plant biology: host selection and nutrient transfer. Plant Biology 8: 175–185. [DOI] [PubMed] [Google Scholar]
- Shen H, Hong L, Ye W, Cao H, Wang Z. 2007. The influence of the holoparasitic plant Cuscuta campestris on the growth and photosynthesis of its host Mikania micrantha. Journal of Experimental Botany 58: 2929–2937. [DOI] [PubMed] [Google Scholar]
- Shen H, Prider JN, Facelli JM, Watling JR. 2010. The influence of the hemiparasitic angiosperm Cassytha pubescens on photosynthesis of its host Cytisus scoparius. Functional Plant Biology 37: 14–21. [Google Scholar]
- Shen H, Xu S-J, Hong L, Wang Z-M, Ye W-H. 2013. Growth but not photosynthesis response of a host plant to infection by a holoparasitic plant depends on nitrogen supply. PLoS One 8: e75555 Doi: 10.1371/journal.pone.0075555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong GL, Bannister P, Burritt D. 2000. Are mistletoes shade plants? CO2 assimilation and chlorophyll fluorescence of temperate mistletoes and their hosts. Annals of Botany 85: 511–519. [Google Scholar]
- Těšitel J, Plavcová L, Cameron DD. 2010. Interactions between hemiparasitic plants and their hosts: the importance of organic carbon transfer. Plant Signaling and Behavior 5: 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Těšitel J, Lepš J, Vráblová M, Cameron DD. 2011. The role of heterotrophic carbon acquisition by the hemiparasitic plant Rhinanthus alectorolophus in seedling establishment in natural communities: a physiological perspective. New Phytologist 192: 188–199. [DOI] [PubMed] [Google Scholar]
- Těšitel J, Těšitelová T, Fisher JP, Lepš J, Cameron DD. 2015. Integrating ecology and physiology of root-hemiparasitic interaction: interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytologist 205: 350–360. [DOI] [PubMed] [Google Scholar]
- Tsang HTS. 2010. Cassytha pubescens: germination biology and interactions with native and introduced hosts. Masters Thesis, University of Adelaide, Australia. [Google Scholar]
- White AJ, Critchley C. 1999. Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynthesis Research 59: 63–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.