Summary
Background
Standard-of-care antiretroviral therapy (ART) uses a combination of drugs deemed essential to minimise treatment failure and drug resistance. Protease inhibitors are potent, with a high genetic barrier to resistance, and have potential use as monotherapy after viral load suppression is achieved with combination treatment. We aimed to assess clinical risks and benefits of protease inhibitor monotherapy in long-term clinical use: in particular, the effect on drug resistance and future treatment options.
Methods
In this pragmatic, parallel-group, randomised, controlled, open-label, non-inferiority trial, we enrolled adults (≥18 years of age) positive for HIV attending 43 public sector treatment centres in the UK who had suppressed viral load (<50 copies per mL) for at least 24 weeks on combination ART with no change in the previous 12 weeks and a CD4 count of more than 100 cells per μL. Participants were randomly allocated (1:1) to maintain ongoing triple therapy (OT) or to switch to a strategy of physician-selected ritonavir-boosted protease inhibitor monotherapy (PI-mono); we recommended ritonavir (100 mg)-boosted darunavir (800 mg) once daily or ritonavir (100 mg)-boosted lopinavir (400 mg) twice daily, with prompt return to combination treatment if viral load rebounded. All treatments were oral. Randomisation was with permuted blocks of varying size and stratified by centre and baseline ART; we used a computer-generated, sequentially numbered randomisation list. The primary outcome was loss of future drug options, defined as new intermediate-level or high-level resistance to one or more drugs to which the patient's virus was deemed sensitive at trial entry (assessed at 3 years; non-inferiority margin of 10%). We estimated probability of rebound and resistance with Kaplan-Meier analysis. Analyses were by intention to treat. This trial is registered with the International Standard Randomised Controlled Trial Number registry, number ISRCTN04857074.
Findings
Between Nov 4, 2008, and July 28, 2010, we randomly allocated 587 participants to OT (291) or PI-mono (296). At 3 years, one or more future drug options had been lost in two participants (Kaplan-Meier estimate 0·7%) in the OT group and six (2·1%) in the PI-mono group: difference 1·4% (−0·4 to 3·4); non-inferiority shown. 49 (16·8%) participants in the OT group and 65 (22·0%) in the PI-mono group had grade 3 or 4 clinical adverse events (difference 5·1% [95% CI −1·3 to 11·5]; p=0·12); 45 (six treatment related) and 56 (three treatment related) had serious adverse events.
Interpretation
Protease inhibitor monotherapy, with regular viral load monitoring and prompt reintroduction of combination treatment for rebound, preserved future treatment options and did not change overall clinical outcomes or frequency of toxic effects. Protease inhibitor monotherapy is an acceptable alternative for long-term clinical management of HIV infection.
Funding
National Institute for Health Research.
Introduction
Current HIV treatment guidelines recommend a combination of two drug classes for initiation and maintenance of antiretroviral therapy (ART).1, 2 The principle of combining drugs with different mechanisms of action to increase potency and reduce selection of drug-resistant mutants is common to the treatment of many infectious diseases. However, in HIV, the need for combination treatment might decrease once viral load has been suppressed.
Protease inhibitors are potent, with a high genetic barrier to resistance, and are the only drugs that act at many steps of the HIV lifecycle, thus having potential for use alone as monotherapy.3 Protease inhibitor monotherapy could be an attractive therapeutic option because of its potential to reduce renal, CNS, and other toxic effects associated with drugs widely used in standard ART combinations. The high genetic barrier to resistance might reduce the risk of resistance during periods of suboptimum treatment adherence. Furthermore, use of a single drug might decrease treatment costs. Although inadequate for initial treatment,4 findings from previous randomised trials of maintenance protease inhibitor monotherapy5, 6, 7, 8, 9 have shown high levels of short-term viral load suppression. However, these trials have not been of sufficient size and duration to address definitively the effects on long-term drug resistance, clinical disease progression, and drug toxic effects in clinical practice.5, 6, 7, 8, 9 Furthermore, investigators have mostly restricted the standard-of-care treatment to a specific protease inhibitor regimen and mandated use of a particular protease inhibitor for monotherapy, neither of which takes account of the diversity of regimen selection in routine clinical practice.
Research in context.
Evidence before this study
We searched PubMed for reports published between Jan 1, 1998, and Jan 1, 2008, with no language restrictions, using terms including “protease inhibitors”, “monotherapy”, and the individual drug names, and reviewed relevant HIV conference abstracts to identify randomised controlled trials that compared protease inhibitor monotherapy with triple antiretroviral therapy (ART) in patients who had previously achieved viral load suppression. We identified three trials, all investigating lopinavir monotherapy, and, overall, these trials supported the hypothesis of virological non-inferiority of monotherapy to triple therapy. Since then, authors of a meta-analysis of ten trials noted an overall risk ratio of 0·94 for viral load suppression at 48 weeks with protease inhibitor monotherapy compared with triple ART, with, at most, a 13% increase in the absolute risk of virological failure with protease inhibitor monotherapy.
Added value of this study
To our knowledge, this trial is the largest and longest-duration protease inhibitor monotherapy trial done so far, with more than three times the randomly allocated person follow-up time of previous trials, and thus it provides more precise estimates of important but uncommon efficacy and safety outcomes than did previous trials. By contrast with previous studies that restricted the choice of drugs in both the monotherapy (single specified protease inhibitor) and combination ART (usually to a protease-inhibitor-containing combination, and with the same protease inhibitor as specified for monotherapy) groups, this trial used a pragmatic design representative of routine clinical care, with flexibility of drug selection in both treatment groups. Also, whereas previous trials compared predefined regimens with short-term viral load endpoints, this trial compared treatment strategies with a primary efficacy endpoint (preservation of drug options) relevant to long-term clinical management. In this trial we noted an increase of about 32% in absolute risk of virological failure with protease inhibitor monotherapy (much greater than that previously reported), but all patients with rebound resuppressed spontaneously or with reintroduction of combination ART. Findings from this trial showed that the protease inhibitor monotherapy strategy was non-inferior to triple ART in preservation of future treatment options and did not change overall clinical outcomes or frequency of toxic effects.
Implications of all the available evidence
Protease inhibitor monotherapy does not increase the risk of drug resistance and is an acceptable alternative for long-term clinical management of HIV infection. Clinical benefits, if any, seem slight, and some patients might need to switch back to combination ART. Nevertheless, this approach could appeal to patients who are stable on treatment but who wish to minimise their exposure to specific drugs or more than one drug class.
We did a pragmatic randomised controlled trial comparing a protease inhibitor monotherapy treatment strategy with clinician-selected standard combination treatment, aiming to assess effects on drug resistance, future treatment options, and other, long-term, clinically relevant outcomes.
Methods
Study design and patients
In this pragmatic, parallel-group, randomised, controlled, open-label, non-inferiority trial, we enrolled participants attending 43 public sector hospital-based HIV treatment centres in the UK. Eligible participants were adults aged 18 years or older who were HIV positive, had been on ART consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-NRTI (NNRTI) or protease inhibitor for at least 24 weeks with no change in the previous 12 weeks, had a viral load of less than 50 copies per mL at screening and for at least 24 weeks before screening (one blip to less than 200 copies per mL allowed during this period if followed by at least two results less than 50 copies per mL), had a CD4 count of more than 100 cells per μL at screening, and who were willing to continue current ART or change according to the randomised allocation. Exclusion criteria were known major protease inhibitor resistance mutations at previous resistance testing (if done; not mandated), previous ART change for unsatisfactory virological response (ie, slow initial virological suppression, incomplete suppression, or rebound; change for convenience or toxic effect prevention or management allowed), protease inhibitor allergy, concomitant drugs with protease inhibitor interactions, present or anticipated need for radiotherapy or cytotoxic chemotherapy, treatment for acute opportunistic infection within the previous 3 months, present or planned pregnancy, active substance misuse or psychiatric illness, history of HIV encephalopathy with a present deficit of more than 1 in any domain of the Neuropsychiatric AIDS Rating Scale,10, 11 history of cardiovascular disease or a 10 year absolute coronary heart disease risk of more than 30% or more than 20% with diabetes or a family history of premature ischaemic heart disease or stroke,12 insulin-dependent diabetes mellitus, active or planned hepatitis C virus treatment, hepatitis B surface antigen positive at screening or since HIV diagnosis (unless hepatitis B DNA of less than 1000 copies per mL taken while off drugs active against hepatitis B), or a fasting plasma glucose of more than 7·0 mmol/L at screening.
The protocol was approved by the Cambridgeshire 4 Research Ethics Committee and Medicines and Healthcare Products Regulatory Agency. All participants provided written informed consent.
Randomisation and masking
Participants were randomly assigned (1:1) to maintain ongoing triple therapy (OT) or switch to a protease inhibitor monotherapy strategy (PI-mono). Randomisation was stratified by centre (nine groups, based on the eight largest sites and one group for the remaining sites) and baseline ART regimen (protease inhibitor or NNRTI). The computer-generated, sequentially numbered randomisation list (random permuted blocks of varying size) was preprepared by WS. Sites faxed screening forms to the trial coordinating centre, where trial staff confirmed eligibility and did the randomisation (they could access the next number on the list, but not the whole list). Treatment allocation was open label.
Procedures
In the OT group, we managed patients using triple combination treatment. In the PI-mono group, we switched patients to a single ritonavir-boosted protease inhibitor selected by the physician: we recommended darunavir (800 mg) boosted with ritonavir (100 mg) once daily or lopinavir (400 mg) boosted with ritonavir (100 mg) twice daily. Drugs were taken orally. Patients switching from NNRTI-based regimens continued NRTIs for the first 2 weeks. Protease inhibitor substitution was allowed in the event of toxic effects or for convenience. The strategy required prompt reintroduction of NRTIs (switch of protease inhibitor to NNRTI optional) for protocol-defined confirmed viral load rebound and management with combination treatment for the remainder of the trial (subsequent switches for toxic effects, convenience, and viral load failure allowed, as in OT group).
Study visits at baseline, weeks 4 and 8 (PI-mono group only), week 12, and every 12 weeks thereafter included assessment of clinical status, drug adherence (standardised questions), viral load, CD4 cell count, and safety blood tests (measured at site laboratory). Visits at baseline, week 12, week 48, and every 48 weeks thereafter included additional assessments of cardiovascular disease risk (Framingham equation),13 neurocognitive function (standard five-test battery),14, 15 symptomatic peripheral neuropathy (Brief Peripheral Neuropathy Screen),16 and quality of life (self-completed Medical Outcomes Study HIV Health Survey questionnaire17, 18). We classified clinical and laboratory events with protocol-defined diagnostic criteria (on the basis of Centers for Disease Control and Prevention criteria for AIDS,19 INSIGHT criteria for serious non-AIDS events,20 and Division of AIDS criteria for adverse events21), and an independent physician at the coordinating centre reviewed them. We calculated estimated glomerular filtration rate (eGFR) with the Chronic Kidney Disease Epidemiology Collaboration equation.22
If viral load was detectable at 50 copies per mL or higher at any visit, we repeated the test (on the same sample if available or a fresh sample draw if not). If viral load was less than 50 copies per mL on the repeat test, we continued routine follow-up, but if higher, we did adherence counselling and patients returned for a confirmatory test at least 4 weeks from the date that the first sample was taken. If viral load was less than 50 copies per mL with the confirmatory test, we resumed routine follow-up, but if higher (ie, on the third consecutive test), this result met the protocol definition of confirmed rebound and so the patient was required to change treatment, and we did a repeat test 4 weeks later.
We did genotypic resistance testing on all viral load rebound samples that were confirmed or preceded treatment switch. We did genotypic testing at site laboratories, repeated at the central laboratory if local sequencing was unsuccessful. We used the Stanford algorithm for drug susceptibility prediction. If we identified resistance mutations, we compared them with any genotypic testing reports from before the trial.
Outcomes
The primary outcome was loss of future drug options, defined as new intermediate-level or high-level resistance to one or more drugs in contemporary use to which we deemed patient's virus to be sensitive at trial entry (assessed at 3 years). We defined contemporary use on the basis of inclusion in present UK treatment guidelines,1 with saquinavir added because this drug was taken by some participants during the trial.
Secondary outcomes were occurrence of serious drug-related or disease-related complications, including death or serious AIDS-defining (excluding oesophageal candidiasis or chronic mucocutaneous herpes simplex virus infection) or non-AIDS-defining illness; total number of grade 3 and 4 adverse events; confirmed virological rebound; CD4 cell count change from baseline; neurocognitive function change from baseline; cardiovascular risk change from baseline; and health-related quality of life change from baseline (mental and physical health summary scores). Additional specified safety outcomes were facial lipoatrophy, abdominal fat accumulation, peripheral neuropathy, and estimated glomerular filtration rate.
Statistical analysis
We defined non-inferiority of the PI-mono group by the upper limit of the two-sided 95% CI for the difference in proportions of patients who maintain all future drug options during 3 years (OT group minus the PI-mono group) being less than 10%. We chose the 10% non-inferiority margin on the basis of US Food and Drug Administration guidance 23, 24 and with reference to other protease inhibitor monotherapy trials.9 Using a survival analysis approach (on a hazard ratio scale) to allow inclusion of all follow-up data for estimation of the primary endpoint, and assuming that 97% of patients in the OT group would meet the primary endpoint,7, 25, 26 with an 85% power and a 10% loss to follow-up, we estimated that about 280 patients per group would be needed to show non-inferiority. This approach gives a conservative estimate of the sample size, which has the additional advantage of allowing precise estimates of important secondary safety outcomes. On the basis of a conventional analysis of proportions, the power of the study to show non-inferiority for the primary endpoint with use of the same parameters would be more than 99%.
All comparisons were as randomised (intention to treat). We deemed a per-protocol analysis not relevant for this pragmatic trial in view of the fact that a switch back to combination treatment in the PI-mono group was an intrinsic element of the clinical strategy being assessed. Statistical tests are two-sided and test the hypotheses of no difference between randomised groups. We estimated the absolute difference between groups in reduction of future drug options using Kaplan-Meier analysis, with the 95% CI (two-sided) derived with bootstrap methods. In this analysis and other time-to-event analyses, we censored participants at the time of death, loss to follow-up, or withdrawal. The primary analysis included all new resistance mutations noted that conferred intermediate-level or high-level drug resistance. We predefined a sensitivity analysis in which we restricted loss of drug options to classes to which the patient was exposed during the trial, thus excluding mutations that were probably archived (ie, acquired at transmission or during treatment before enrolment, but unknown at trial entry).
For secondary endpoints, we compared binary outcome variables between groups using χ2 or Fisher's exact tests, with conventional or Agresti-Caffo 95% CIs for the risk difference and logistic regression models for adjusted analyses. For continuous variables, we compared groups by use of mean change from baseline and t tests or linear regression; we estimated change from baseline to the last available visit at which a measurement was done at or after week 144 (we did not include patients without such data). We estimated the proportion of patients who had viral load rebound with Kaplan-Meier analysis and compared groups by use of a log-rank test. We compared incidence rates with Poisson regression. We compared adherence during the entire follow-up period between groups with generalised estimating equations (independent correlation structure; binomial distribution).
We used Stata version 12.1 for all analyses. A data monitoring committee reviewed interim data annually. This trial is registered with the International Standard Randomised Controlled Trial Number registry, number ISRCTN04857074.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
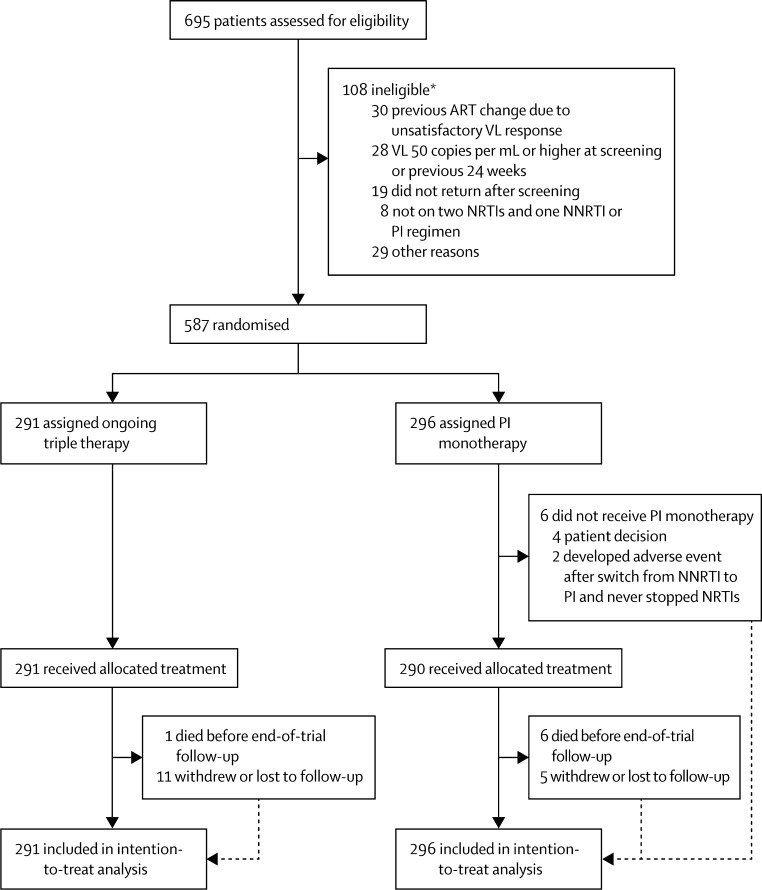
Between Nov 4, 2008, and July 28, 2010, we randomly allocated 587 participants to OT (291) or PI-mono (296; figure 1). One (<1%) died and 11 (4%) withdrew or were lost to follow-up in the OT group, whereas six (2%) died and five (2%) withdrew or were lost to follow-up in the PI-mono group. Study visits ended on Nov 1, 2013. The median duration of trial follow-up was 44 months (maximum 59 months). Participant characteristics were similar between groups (table 1).
Figure 1.
Trial profile
ART=antiretroviral therapy. PI=protease inhibitor. NNRTI=non-nucleoside reverse transcriptase inhibitor. NRTI=nucleoside reverse transcriptase inhibitor. VL=viral load. *Six patients had more than one reason for ineligibility.
Table 1.
Baseline characteristics
| OT (n=291) | PI-mono (n=296) | Total (n=587) | ||
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age (years) | 43 (37–49, 23–75) | 45 (39–50, 23–67) | 44 (38–49, 23–75) | |
| Route of infection | ||||
| Homosexual | 175 (60%) | 176 (59%) | 351 (60%) | |
| Heterosexual | 108 (37%) | 108 (36%) | 216 (37%) | |
| Other | 8 (3%) | 12 (4%) | 20 (3%) | |
| Female | 64 (22%) | 73 (25%) | 137 (23%) | |
| Ethnic origin | ||||
| White | 206 (71%) | 195 (66%) | 401 (68%) | |
| Black | 73 (25%) | 90 (30%) | 163 (28%) | |
| Other | 12 (4%) | 11 (4%) | 23 (4%) | |
| Hepatitis C virus antibody positive | 7 (2%) | 14 (5%) | 21 (4%) | |
| HIV disease status | ||||
| Previous AIDS-defining illness | 59 (20%) | 57 (19%) | 116 (20%) | |
| Nadir CD4 count (cells per μL) | 181 (90–258) | 170 (80–239) | 178 (86–250) | |
| Baseline CD4 count (cells per μL) | 512 (386–658) | 516 (402–713) | 513 (392–682) | |
| Undetectable baseline HIV viral load | 276 (95%) | 279 (94%) | 555 (95%) | |
| Duration of undetectable viral load (months) | 36 (17–62) | 38 (22–66) | 37 (20–63) | |
| ART history | ||||
| Years since ART started | 3·9 (2·0–6·4) | 4·2 (2·4–6·9) | 4·0 (2·2–6·7) | |
| On first ART combination | 91 (31%) | 96 (32%) | 187 (32%) | |
| Number of drugs ever received | 5 (3–6) | 4 (3–6) | 4 (3–6) | |
| NNRTI at entry | ||||
| Any | 157 (54%) | 157 (53%) | 314 (53%) | |
| Efavirenz | 115 (40%) | 115 (39%) | 230 (39%) | |
| Nevirapine | 42 (14%) | 39 (13%) | 81 (14%) | |
| Etravirine | 0 | 3 (1%) | 3 (1%) | |
| Protease inhibitor at entry | ||||
| Any | 134 (46%) | 139 (47%) | 273 (47%) | |
| Atazanavir | 59 (20%) | 59 (20%) | 118 (20%) | |
| Lopinavir | 28 (10%) | 49 (17%) | 77 (13%) | |
| Darunavir | 24 (8%) | 13 (4%) | 37 (6%) | |
| Saquinavir | 16 (5%) | 15 (5%) | 31 (5%) | |
| Fosamprenavir | 7 (2%) | 3 (1%) | 10 (2%) | |
| NRTIs at entry | ||||
| Any | 291 (100%) | 296 (100%) | 587 (100%) | |
| Emtricitabine and tenofovir | 190 (65%) | 180 (61%) | 370 (63%) | |
| Lamivudine and abacavir | 80 (27%) | 82 (28%) | 162 (28%) | |
| Other | 21 (7%) | 34 (11%) | 55 (9%) | |
| Resistance | ||||
| Resistance test result available before trial | 181 (62%) | 165 (56%) | 346 (59%) | |
| Intermediate-level or high-level resistance to NRTI or NNRTI before trial* | 4 (2%) | 7 (4%) | 11 (3%) | |
Data are median (IQR, range), median (IQR), or n (%). OT=ongoing triple therapy. PI-mono=protease inhibitor monotherapy. ART=antiretroviral therapy. NNRTI=non-nucleoside reverse transcriptase inhibitor. NRTI=nucleoside reverse transcriptase inhibitor.
Percentages are of the numbers of patients with resistance test results available before the trial.
In the PI-mono group, initial drug choices were darunavir in 233 (80%) of patients, lopinavir in 40 (14%), atazanavir in 16 (6%), and saquinavir in 1 (<1%); 58% were still taking monotherapy at trial end. Combination treatment was reintroduced for the following reasons: 69 (23%) for protocol-defined confirmed viral rebound (appendix p 8), 11 (4%) for viral rebound not meeting protocol criteria, 16 (5%) for toxic effects, and 22 (7%) for other or unknown reasons; 6 (2%) never started monotherapy. Overall, 72% of follow-up time was spent on monotherapy. Self-reported adherence to study drugs was high: participants reported not missing any ART doses in the past 2 weeks at 4301 (93%) of 4635 visits in the OT group and 4376 (92%) of 4748 visits in the PI-mono group (p=0·52).
The number of patients with loss of future drug options at 3 years (the primary outcome) was two patients (Kaplan-Meier estimate 0·7%) in the OT group and six (2·1%) in the PI-mono group (difference 1·4% [95% CI −0·4 to 3·4]), therefore meeting the non-inferiority criterion. PI-mono was also non-inferior with prespecified analyses of loss of future drug options during the full trial follow-up period (OT 1·8%; PI-mono 2·1%; difference 0·2% [–2·5% to 2·6]) and loss of future drug options during the full trial period but excluding mutations that were probably archived (OT 1·5%; PI-mono 1·0%; difference −0·4% [–2·9% to 1·4%]). One (<1%) participant on atazanavir monotherapy developed the Ile50Leu mutation (as a mixture with wild-type), conferring predicted high-level atazanavir resistance. We detected an isolated Leu90Met mutation in two (<1%) patients on darunavir monotherapy; both resuppressed with reintroduction of NRTIs. This mutation, possibly archived, does not affect darunavir sensitivity, but confers resistance to saquinavir and thus meets the endpoint definition. Three (1%) patients in the PI-mono group had NRTI or NNRTI mutations detected, probably archived from previous treatment. In the OT group, three (1%) patients had loss of future drug options to drug classes that they were taking and one (<1%) taking a protease-inhibitor-based regimen had NNRTI mutations that were probably archived (table 2; appendix p 2–3).
Table 2.
Individual patients with loss of future drug options by end of trial
| Drugs received during trial | Reverse transcriptase mutations | Protease mutations | Lost drug options | |
|---|---|---|---|---|
| OT | ||||
| 1* | ABC, 3TC, ATV | Val118Ile, Val179Asp, Met184Val | Ile84Val | 3TC, FTC, ATV, SQV, FPV, TPV |
| 2 | TDF, FTC, RPV, DRV | Leu100Ile, Lys103Asn, Met184Val | Ala71Val | 3TC, FTC, NVP, EFV, ETV, RPV |
| 3 | TDF, FTC, ETV, NVP, EFV, DRV | Lys65Arg, Glu138Ala, Tyr181Cys, Met184Val/Ile, His221Tyr, Met230Leu | .. | 3TC, FTC, ABC, TDF, NVP, EFV, ETV, RPV |
| 4* | TDF, FTC, DRV | Val106Ala | .. | NVP,‡ EFV‡ |
| PI-mono | ||||
| 5* | ATV | .. | Lys20Thr, Ile50Leu/Ile, Ala71Thr | ATV |
| 6* | DRV | .. | Leu90Met | SQV† |
| 7* | DRV | .. | Ala71Thr, Leu90Met | SQV† |
| 8* | DRV | Lys103Asn | .. | NVP,‡ EFV‡ |
| 9* | DRV | Lys103Asn | .. | NVP,‡ EFV‡ |
| 10* | DRV | Met41Leu, Thr215Asp | .. | ZDV‡ |
OT=ongoing triple therapy. ABC=abacavir. 3TC=lamivudine. ATV=atazanavir. FTC=emtricitabine. SQV=saquinavir. FPV=fosamprenavir. TPV=tipranavir. TDF=tenofovir. RPV=rilpivirine. NVP=nevirapine. EFV= efavirenz. ETV=etravirine. DRV=darunavir. PI-mono=protease inhibitor monotherapy. ZDV=zidovudine.
Met primary outcome at 3 years.
Possibly archived resistance.
Probably archived resistance (excluded in sensitivity analysis).
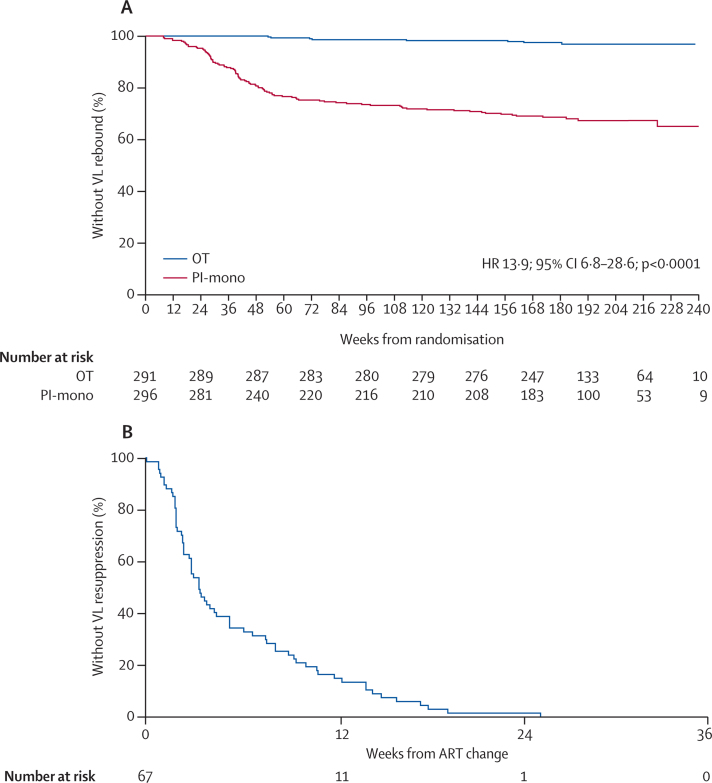
We noted one or more episodes of confirmed viral load rebound in eight (Kaplan-Meier estimate 3·2%) patients in the OT group and 95 (35·0%) in the PI-mono group during all follow-up: absolute risk difference 31·8% (95% CI 24·6–39·0; p<0·0001). Rebound while actually on monotherapy (occurred in 93 patients) was more common in the first year (24 per 100 person-years) than in subsequent years (six per 100 person-years; figure 2). Findings were similar with use of an extended definition of viral load rebound (appendix, p 9) and across the different protease inhibitors used as monotherapy. The median peak viral load at first episode of rebound on monotherapy was 526 copies per mL (peak was less than 400 copies per mL in 39 [42%] of 93 patients); of the 91 (98%) patients with subsequent tests available, 22 (24%) had resuppressed spontaneously and 69 (76%) resuppressed after changing ART: 47 (68%) by adding NRTIs only, 18 (26%) by adding NRTIs and changing the protease inhibitor to an NNRTI, two (3%) by changing the protease inhibitor monotherapy drug, and two (3%) other changes (appendix p 8). Viral load was resuppressed to less than 50 copies per mL after a median of 3·5 weeks (figure 2).
Figure 2.
Viral load rebound and resuppression
(A) Time to viral rebound. (B) Time to viral resuppression after change of ART in the PI-mono group. The time to first viral load of less than 50 copies per mL (midpoint between last test 50 copies per mL or more and first test of less than 50 copies per mL) for patients at the time of first rebound who were taking protease inhibitor monotherapy (excluding two patients in the PI-mono group who rebounded while not on monotherapy) is shown. Outcomes by type of treatment switch are shown in the appendix (p 8). ART=antiretroviral therapy. HR=hazard ratio. OT=ongoing triple therapy. PI-mono=protease inhibitor monotherapy. VL=viral load.
We noted no differences in serious drug-related or disease-related complications (death, AIDS, or serious non-AIDS) between groups (table 3). Causes of the one death in the OT group and six in the PI-mono group were diverse, and we did not deem any to be related to treatment strategy (appendix p 4). CD4 cell count increased similarly in both groups (table 3). Serious adverse events and clinical grade 3 and 4 adverse events did not differ between groups (table 3, appendix p 5–7). Fewer patients in the PI-mono group had a serum phosphate concentration of less than 0·65 mmol/L (p<0·0001, appendix p 6) and an eGFR of less than 60 mL/min per 1·73 m2 during follow-up (table 3). However, mean change in eGFR did not differ between groups (table 3), and only one case of end-stage renal failure occurred: a patient in the PI-mono group with pre-existing chronic renal impairment. Proportions of patients with symptomatic peripheral neuropathy, facial lipoatrophy, or abdominal fat accumulation during follow-up, and changes in neurocognitive function summary, cardiovascular disease risk, or quality of life summary scores did not differ between groups (table 3).
Table 3.
Secondary outcomes
| OT (n=291) | PI-mono (n=296) | Difference (95% CI)* | p value | ||
|---|---|---|---|---|---|
| Serious drug-related or disease-related complications | |||||
| Total | 8 (2·7%) | 15 (5·1%) | 2·3% (−0·8 to 5·4) | 0·15 | |
| Death† | 1 (0·3%) | 6 (2·0%) | 1·7% (−0·3 to 3·6) | 0·12 | |
| AIDS-defining event | 1 (0·3%) | 1 (0·3%) | 0·0% (−1·3 to 1·3) | 1·00 | |
| Serious non-AIDS event | 7 (2·4%) | 12 (4·1%) | 1·6% (−1·2 to 4·5) | 0·26 | |
| CD4 change (cells per mm3) | 93 (10) | 109 (9) | 16 (−11 to 42) | 0·30 | |
| Serious adverse event | 45 (15·5%) | 56 (18·9%) | 3·5% (−2·6 to 9·6) | 0·27 | |
| Total grade 3 or 4 adverse event | 159 (54·6%) | 137 (46·3%) | −8·4% (−16·4 to −0·3) | 0·043 | |
| Clinical grade 3 or 4 adverse event | 49 (16·8%) | 65 (22·0%) | 5·1% (−1·3 to 11·5) | 0·12 | |
| EGFR | |||||
| <60 mL/min per 1·73 m2‡ | 28/290 (9·7%) | 15/296 (5·1%) | −4·6% (−8·8 to −0·4) | 0·033 | |
| Change (mL/min per 1·73 m2) | −5·13 (0·67) | −3·83 (0·66) | 1·30% (−0·55 to 3·15) | 0·092 | |
| Symptomatic peripheral neuropathy§ | 44/283 (15·5%) | 46/289 (15·9%) | 0·4% (−5·6 to 6·3) | 0·90 | |
| Facial lipoatrophy¶ | 23/282 (8·2%) | 35/289 (12·1%) | 4·0% (−1·0 to 8·9) | 0·12 | |
| Abdominal fat accumulation¶ | 47/274 (17·2%) | 57/277 (20·6%) | 3·4% (−3·1 to 10·0) | 0·30 | |
| 10 year cardiovascular disease risk change | 1·32 (0·31) | 1·59 (0·31) | 0·27 (−0·58 to 1·12) | 0·52 | |
| Neurocognitive function change (NPZ-5 score) | 0·53 (0·04) | 0·52 (0·04) | −0·01 (−0·11 to 0·09) | 0·94 | |
| Quality of life change | |||||
| Mental health score | −0·75 (0·57) | −1·82 (0·54) | −1·07 (−2·61 to 0·47) | 0·17 | |
| Physical health score | −0·76 (0·53) | −1·17 (0·46) | −0·41 (−1·79 to 0·98) | 0·56 | |
Data are n (%), n/N (%), or mean (SE). Means are predicted means adjusted for baseline values. The numbers of patients are the numbers of patients having the specified event during the entire trial follow-up period. OT=ongoing triple therapy. PI-mono=protease inhibitor monotherapy. EGFR=estimated glomerular filtration rate.
Difference between PI-mono and OT. Absolute differences for binary outcomes or mean differences for continuous outcomes shown.
Causes of death were metastatic adenocarcinoma in the OT group and suicide, pulmonary embolism, breast carcinoma (recurrent), small-cell lung carcinoma, glioblastoma, and anal carcinoma in the PI-mono group (details in appendix p 4).
New episodes after baseline.
Symptomatic peripheral neuropathy at one or more of the post-baseline-scheduled follow-up assessments (irrespective of status at baseline).
Facial lipoatrophy present at one or more of the post-baseline-scheduled follow-up assessments (irrespective of status at baseline), assessed by a doctor or nurse; abdominal fat accumulation at last available assessment compared with baseline (irrespective of status at baseline), self-assessed by the patient.
Discussion
In patients who have achieved viral load suppression with combination treatment, a maintenance strategy of protease inhibitor monotherapy, with reintroduction of combination treatment in the event of viral load rebound, was non-inferior to continuous combination treatment for preservation of future treatment options during 3–5 years. Furthermore, and perhaps more relevant than is non-inferiority for understanding of the clinical implications of this approach, the findings showed that the absolute number of patients who lost future drug options with protease inhibitor monotherapy was very low. Only one patient developed clinically significant resistance to a protease inhibitor taken as monotherapy, an Ile50Leu mutation with atazanavir monotherapy; clinical negative effects are mitigated by an increase in sensitivity to other protease inhibitors conferred by this mutation.27 None of the 277 patients taking darunavir or lopinavir (the recommended options for monotherapy in this trial, taken by almost all patients) developed resistance affecting their efficacy. The very low amount of resistance concurs with previous trials of monotherapy (and trials with protease-inhibitor-based combinations),5, 6, 7, 8, 9 but extend findings from these trials to provide the crucial long-term randomised outcome data from a large pragmatic trial needed to provide confidence in use of protease inhibitor monotherapy in clinical practice.
We sought resistance fastidiously, testing confirmed viral rebound samples irrespective of level of viraemia with standard population sequence genotypic testing. Although protease inhibitor resistance could occur in other regions of the virus, such as gag or env genes, or in minority species that are not detected by population assays,3 previous minority species sequencing in patients with viral rebound on monotherapy has shown only a small excess of resistance compared with that detected by population sequencing.28, 29 Furthermore, our finding that patients with viral load rebound in the PI-mono group, without exception, achieved full viral load suppression when switched to combination treatment (usually by reintroduction of NRTIs) provides an important assurance that any mutations that were not detected by population sequencing did not affect subsequent treatment efficacy. The follow-up period of 3–5 years, long for an HIV treatment trial, is sufficient to have confidence that preservation of treatment options is likely to be durable in the long term.
A higher proportion of patients in the PI-mono group had viral rebound, as expected, although the difference was much greater than the 10–13% noted in a previous systematic review.9 Viral suppression is the traditional outcome variable for comparison of treatment regimens in clinical trials, but is less informative in long-term strategy trials such as this one, especially in view of the different effect of viral rebound on risk of drug resistance for different drug classes. Aside from risk of resistance (shown to be negligible in this study), these brief episodes of low-level viraemia, rapidly reversed by reintroduction of combination treatment, are unlikely to have adverse long-term clinical effects: much higher levels of viraemia are needed than those noted in this study to drive HIV disease progression,30 and CD4 cell count increases and total HIV disease-related clinical events were similar between the two groups. Risk of transmission to others arising from such short episodes of low-level viraemia is also likely to be negligible in view of the fact that transmission is exceedingly rare in patients with a low viral load, whether on or off ART.31, 32 Although more deaths occurred in the PI-mono group, the difference was not statistically significant and the causes were diverse, without a plausible link to the drugs taken or HIV disease progression. This finding is probably a chance imbalance, and follow-up of the cohort continues. Further analyses of predictive factors are planned that might enable identification of patients at low risk of rebound. However, selection factors are unlikely to be able to remove the excess risk entirely, and rebound and resuppression should be viewed as an integral part of the strategy for some patients. After the first year of suppression on monotherapy, the risk of rebound is much reduced, and this factor might increase acceptability for patients.
We noted fewer episodes of renal impairment in the PI-mono group as expected in view of the well-recognised renal toxic effects of NRTIs (especially tenofovir). However, the risk of serious drug toxic effects can be minimised by close laboratory monitoring and pre-emptive treatment changes, and no patients in the OT group developed end-stage renal disease.33 This pragmatic trial in which clinicians were allowed to switch drugs at clinical discretion (rather than being restricted to single-protocol-mandated regimens) provides a realistic estimate of the effect of protease inhibitor monotherapy in routine clinical practice, suggesting that benefits in terms of renal toxic effects would be small. We did not find any other clinical advantages of the monotherapy strategy. Treatment costs might be reduced, partly offset by increased monitoring costs, but considerations are complex, and detailed cost-effectiveness analyses will be reported in a separate paper.
A concern with protease inhibitor monotherapy is that suboptimum drug penetration into the CNS might lead to harm.34, 35 However, we noted no difference in neurocognitive function between groups, nor any significant excess of neurological events in the PI-mono group. We used a small battery of neurocognitive tests (for feasibility in view of the large numbers of patients and repeated assessments), possibly less sensitive than a comprehensive neurocognitive assessment would be. Although we cannot rule out delayed effects on neurocognitive function or neurological events after longer periods on monotherapy than those in this study, the size and duration of our trial nevertheless provides reassurance that even if monotherapy were to have less brain penetration, this factor is unlikely to have important clinical negative effects.
The strengths of this trial are its size and duration (more than three times the randomly allocated person follow-up time of previous studies, allowing precise estimates of important but uncommon efficacy and safety outcomes); low withdrawal and loss-to-follow-up; pragmatic design set in routine clinical care, with flexibility in drug selection in both treatment groups; and use of a primary efficacy endpoint relevant to long-term management. The open-label design, a possible limitation, is unlikely to have affected detection of viral rebound or resistance (and hence the primary endpoint) because the protocol-mandated frequency of viral load testing was identical in the two groups (apart from two extra tests at weeks 4 and 8 in the monotherapy group), and we did resistance testing in everyone with viral rebound. Likewise, the open-label design is unlikely to have affected adverse event detection because the frequency of laboratory safety monitoring was standardised and adverse events were graded with standardised diagnostic criteria. Another possible limitation is the absence of before-treatment resistance tests that would help rule out resistance acquired before the trial. Although entry criteria were broad, patients entering this trial were established for years on a stable effective regimen, yet needed to be willing to change to an alternative regimen (in some cases adding new drugs with risk of side-effects or increased pill burden) if randomly allocated to do so. In view of the absence of any certainty of benefit, only a few eligible patients would therefore have been likely to be prepared to participate. Such motivated patients might have better outcomes (perhaps mediated by adherence) than the general clinic population would have, and this notion is consistent with the very low amount of rebound and resistance noted in the OT group. The main study finding that, despite high levels of viral load rebound, protease inhibitor monotherapy does not jeopardise future treatment options is likely to be generalisable internationally to settings where treatment is individualised and regular viral load monitoring done. However, protease inhibitor monotherapy is not appropriate for resource-limited settings delivering treatment without regular viral load monitoring.36
Protease inhibitor monotherapy is an acceptable alternative to standard combination ART for long-term HIV management, with a slight benefit in terms of a reduction of renal toxic effects. The need for regular viral load monitoring and a possible switch back to combination treatment might be perceived as drawbacks, but this approach could nevertheless appeal to patients who are stable on treatment but wish to minimise their exposure to specific drugs or more than one drug class. Broadly, the trial challenges the notion that combination treatment is essential for management of chronic HIV infection.
Acknowledgments
Acknowledgments
This study was funded by the National Institute for Health Research Health Technology Assessment programme (project number 06/403/90). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Health Technology Assessment programme, National Institute for Health Research, National Health Service, or the Department of Health. We thank all patients and staff from all the centres participating in the Protease Inhibitor Monotherapy Versus Ongoing Triple Therapy trial, and the UK Community Advisory Board and African Eye for community support.
Contributors
NIP and DTD designed the study. AA-P, MF, IW, MJ, CO, FC, VL, AW, MG, and JF enrolled participants into the study. NIP, WS, AA-P, KS, and DTD coordinated and oversaw the study. WS and DTD did the statistical analysis. All authors interpreted data. NIP, WS, AA-P, and DTD drafted the report. All authors provided input into the report and approved the final version of the report.
Protease Inhibitor Monotherapy Versus Ongoing Triple Therapy (PIVOT) Trial Team
Participating UK sites: M Fisher, A Clarke, W Hadley, D Stacey (Elton John Centre, Brighton). M Johnson, P Byrne (Royal Free Hospital, London). I Williams, N De Esteban, P Pellegrino, L Haddow, A Arenas-Pinto (Mortimer Market Centre, London). C Orkin, J Hand, C De Souza, L Murthen, A Crawford-Jones (Barts & the London Hospital, London). F Chen, R Wilson, E Green, J Masterson (Royal Berkshire Hospital, Reading). V Lee, K Patel, R Howe (Manchester Royal Infirmary, Manchester). A Winston, S Mullaney (St Mary's Hospital, London). M Gompels, L Jennings (Southmead Hospital, Bristol). N Beeching, R Tamaklo (Royal Liverpool University Hospital, Liverpool). J Fox, A Teague, I Jendrulek, J Tiraboschi (Guy's and St Thomas' Hospital, London). E Wilkins, Y Clowes, A Thompson (North Manchester General Hospital, Manchester). G Brook, M Trivedi (Central Middlesex Hospital, London). K Aderogba, M Jones (Avenue House Clinic, Eastbourne). A DeBurgh-Thomas, L Jones (Gloucester Royal Hospital, Gloucester). I Reeves, S Mguni (Homerton University Hospital, London). D Chadwick, P Spence, N Nkhoma (James Cook University Hospital, Middlesbrough). Z Warwick, S Price, S Read (Derriford Hospital, Plymouth). E Herieka, J Walker, R Woodward (Royal Bournemouth Hospital, Bournemouth). J Day, L Hilton (Southend University Hospital, Westcliff-on-Sea). V Harinda, H Blackman (St Mary's Hospital, Portsmouth). P Hay, W Mejewska, O Okolo (St George's Hospital, London). E Ong, K Martin, L Munro (Royal Victoria Infirmary, Newcastle). D Dockrell, L Smart (Royal Hallamshire Hospital, Sheffield). J Ainsworth, A Waters (North Middlesex University Hospital, London). S Kegg, S McNamara (Queen Elizabeth Hospital, Woolwich). S Taylor, G Gilleran (Birmingham Heartlands Hospital, Birmingham). B Gazzard, J Rowlands (Chelsea and Westminster Hospital, London). S Allan, R Sandhu (University Hospital of Coventry, Coventry). N O'Farrell, S Quaid (Ealing Hospital, London). F Martin, C Bennett (Harrogate District Hospital, Harrogate). M Kapembwa (Northwick Park Hospital, Harrow). J Minton, J Calderwood (St James' Hospital, Leeds). F Post, L Campbell, E Wandolo (King's College Hospital, London). A Palfreeman, L Mashonganyika (Leicester Royal Infirmary, Leicester). T Balachandran, M Kakowa (Luton and Dunstable Hospital, Luton). R O'Connell, C Tanawa (Newham University Hospital, London). S Jebakumar, L Hagger (Edith Cavell Hospital, Peterborough). S Quah, S McKernan (Royal Victoria Hospital, Belfast). C Lacey, S Douglas, S Russell-Sharpe, C Brewer (York Teaching Hospital, York). C Leen, S Morris (Western General Hospital, Edinburgh). S Obeyesekera, S Williams (Barking Hospital, London). N David (Norfolk and Norwich University Hospital, Norwich). M Roberts, J Wollaston (Worcester Royal Hospital, Worcester).
Medical Research Council Clinical Trials Unit at University College London: N Paton, W Stöhr, A Arenas-Pinto, K Scott, D Dunn, E Beaumont, S Fleck, M Hall, S Hennings, I Kummeling, S Martins, E Owen-Powell, K Sanders, F van Hooff, L Vivas, E White.
Independent event reviewer: B Angus.
Trial Steering Committee: A Freedman (chair), B Cromarty, D Mercey, S Fidler, E Torok, A Babiker, B Gazzard, C Orkin, N Paton.
Data Monitoring Committee: T Peto (chair), D Lalloo, A Phillips, R James.
Declaration of interests
NIP, WS, KS, and DTD report grants from the National Institute of Health Research Health Technology Assessment programme during the conduct of this study. AA-P, MF, IW, MJ, CO, FC, VL, AW, MG, and JF report non-financial support from the UK Clinical Research Network during the conduct of this study. NIP reports grants, personal fees, and non-financial support from AbbVie and Janssen; personal fees and non-financial support from Merck and Roche; grants and non-financial support from GlaxoSmithKline; and non-financial support from Gilead Sciences, all outside the submitted work. AA-P reports grants from Janssen and ViiV Healthcare outside the submitted work. MF reports personal fees from AbbVie, Bristol-Myers Squibb, and Janssen; and grants and personal fees from Gilead Sciences, all outside the submitted work. IW reports grants and personal fees from Gilead Sciences; and grants from Pfizer, Merck Sharp & Dohme, and Bristol-Myers Squibb, all outside the submitted work. CO reports grants, personal fees, and non-financial support from Gilead Sciences, Janssen, ViiV Healthcare, and Bristol-Myers Squibb; and grants from Abbvie and Boehringer Ingelheim, all outside the submitted work. VL reports grants from Gilead Sciences, Merck Sharp & Dohme, ViiV Healthcare, Abbvie, Boehringer Ingelheim, and Janssen, all during the conduct of this study. AW reports honoraria or research grants from or engagement as a consultant or investigator in clinical trials sponsored by Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, Roche, Pfizer, and ViiV Healthcare. MG reports speakers fees, meeting sponsorship, and educational grants from Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen.
Supplementary Material
References
- 1.Williams I, Churchill D, Anderson J. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012. HIV Med. 2012;13(suppl 2):1–85. doi: 10.1111/j.1468-1293.2012.01029.x. [DOI] [PubMed] [Google Scholar]
- 2.Thompson MA, Aberg JA, Hoy JF. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 3.Rabi SA, Laird GM, Durand CM. Multi-step inhibition explains HIV-1 protease inhibitor pharmacodynamics and resistance. J Clin Invest. 2013;123:3848–3860. doi: 10.1172/JCI67399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosn J, Flandre P, Cohen-Codar I. Long-term (96-week) follow-up of antiretroviral-naïve HIV-infected patients treated with first-line lopinavir/ritonavir monotherapy in the MONARK trial. HIV Med. 2010;11:137–142. doi: 10.1111/j.1468-1293.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 5.Arribas JR, Delgado R, Arranz A. Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and 2 nucleosides for maintenance therapy of HIV: 96-week analysis. J Acquir Immune Defic Syndr. 2009;51:147–152. doi: 10.1097/QAI.0b013e3181a56de5. [DOI] [PubMed] [Google Scholar]
- 6.Cameron DW, da Silva BA, Arribas JR. A 96-week comparison of lopinavir-ritonavir combination therapy followed by lopinavir-ritonavir monotherapy versus efavirenz combination therapy. J Infect Dis. 2008;198:234–240. doi: 10.1086/589622. [DOI] [PubMed] [Google Scholar]
- 7.Katlama C, Valantin MA, Algarte-Genin M. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24:2365–2374. doi: 10.1097/QAD.0b013e32833dec20. [DOI] [PubMed] [Google Scholar]
- 8.Arribas JR, Clumeck N, Nelson M, Hill A, van Delft Y, Moecklinghoff C. The MONET trial: week 144 analysis of the efficacy of darunavir/ritonavir (DRV/r) monotherapy versus DRV/r plus two nucleoside reverse transcriptase inhibitors, for patients with viral load <50 HIV-1 RNA copies/mL at baseline. HIV Med. 2012;13:398–405. doi: 10.1111/j.1468-1293.2012.00989.x. [DOI] [PubMed] [Google Scholar]
- 9.Mathis S, Khanlari B, Pulido F. Effectiveness of protease inhibitor monotherapy versus combination antiretroviral maintenance therapy: a meta-analysis. PLoS One. 2011;6:e22003. doi: 10.1371/journal.pone.0022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 11.Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis. 1988;158:1079–1083. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 13.D'Agostino RB, Sr, Vasan RS, Pencina MJ. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Winston A, Arenas-Pinto A, Stohr W. Neurocognitive function in HIV infected patients on antiretroviral therapy. PLoS One. 2013;8:e61949. doi: 10.1371/journal.pone.0061949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arenas-Pinto A, Winston A, Stohr W. Neurocognitive function in HIV-infected patients: comparison of two methods to define impairment. PLoS One. 2014;9:e103498. doi: 10.1371/journal.pone.0103498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans SR, Ellis RJ, Chen H. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25:919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu AW, Rubin HR, Mathews WC. A health status questionnaire using 30 items from the Medical Outcomes Study. Preliminary validation in persons with early HIV infection. Med Care. 1991;29:786–798. doi: 10.1097/00005650-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Wu AW, Hanson KA, Harding G. Responsiveness of the MOS-HIV and EQ-5D in HIV-infected adults receiving antiretroviral therapies. Health Qual Life Outcomes. 2013;11:42. doi: 10.1186/1477-7525-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 20.Lifson AR, Belloso WH, Davey RT. Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV Clin Trials. 2010;11:205–219. doi: 10.1310/hct1104-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutes of Health Division of AIDS Division of AIDS table for grading the severity of adult and pediatric adverse events. http://rsc.tech-res.com/document/safetyandpharmacovigilance/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf (accessed Jan 1, 2008).
- 22.Levey AS, Stevens LA, Schmid CH. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services . Guidance for industry. Non-inferiority clinical trials. US Department of Health and Human Services; Washington, DC: 2010. [Google Scholar]
- 24.US Department of Health and Human Services . Guidance for industry. Human Immunodeficiency Virus-1 infection: developing antiretroviral drugs for treatment. US Department of Health and Human Services; Washington, DC: 2013. [Google Scholar]
- 25.Pulido F, Arribas JR. Noninferiority and lopinavir/ritonavir monotherapy trials. AIDS. 2008;22:1696–1697. doi: 10.1097/QAD.0b013e32830a98ac. [DOI] [PubMed] [Google Scholar]
- 26.Arribas JR, Horban A, Gerstoft J. The MONET trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV RNA below 50 copies/ml. AIDS. 2010;24:223–230. doi: 10.1097/QAD.0b013e3283348944. [DOI] [PubMed] [Google Scholar]
- 27.Sista P, Wasikowski B, Lecocq P, Pattery T, Bacheler L. The HIV-1 protease resistance mutation I50L is associated with resistance to atazanavir and susceptibility to other protease inhibitors in multiple mutational contexts. J Clin Virol. 2008;42:405–408. doi: 10.1016/j.jcv.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 28.McKinnon JE, Delgado R, Pulido F, Shao W, Arribas JR, Mellors JW. Single genome sequencing of HIV-1 gag and protease resistance mutations at virologic failure during the OK04 trial of simplified versus standard maintenance therapy. Antivir Ther. 2011;16:725–732. doi: 10.3851/IMP1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert-Niclot S, Flandre P, Valantin MA. Resistant minority species are rarely observed in patients on darunavir/ritonavir monotherapy. J Antimicrob Chemother. 2012;67:1470–1474. doi: 10.1093/jac/dks052. [DOI] [PubMed] [Google Scholar]
- 30.Raffanti SP, Fusco JS, Sherrill BH. Effect of persistent moderate viremia on disease progression during HIV therapy. J Acquir Immune Defic Syndr. 2004;37:1147–1154. doi: 10.1097/01.qai.0000136738.24090.d0. [DOI] [PubMed] [Google Scholar]
- 31.Quinn TC, Wawer MJ, Sewankambo N. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 32.Supervie V, Viard JP, Costagliola D, Breban R. Heterosexual risk of HIV transmission per sexual act under combined antiretroviral therapy: systematic review and Bayesian modeling. Clin Infect Dis. 2014;59:115–122. doi: 10.1093/cid/ciu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryom L, Mocroft A, Kirk O. Predictors of advanced chronic kidney disease and end-stage renal disease in HIV-positive persons. AIDS. 2014;28:187–199. doi: 10.1097/QAD.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 34.Gutmann C, Cusini A, Gunthard HF. Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS. 2010;24:2347–2354. doi: 10.1097/QAD.0b013e32833db9a1. [DOI] [PubMed] [Google Scholar]
- 35.Paton NI, Meynard JL, Pulido F, Arenas-Pinto A, Girard PM, Arribas J. Inappropriate claim of ‘failure of ritonavir-boosted lopinavir monotherapy in HIV’ in the Monotherapy Switzerland/Thailand (MOST) trial. AIDS. 2011;25:393–394. doi: 10.1097/QAD.0b013e328342fb7b. [DOI] [PubMed] [Google Scholar]
- 36.Paton NI, Kityo C, Hoppe A. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014;371:234–247. doi: 10.1056/NEJMoa1311274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.