Abstract
Herbal and dietary supplement consumption has rapidly expanded in recent years. Due to pharmacological and metabolic characteristics of some supplements, they can interact with prescription medications, potentially leading to clinically important and potentially preventable adverse reactions. Electronic health record (EHR) system provides a valuable source from which drug-supplement interactions can be mined and assessed for their clinical effects. A fundamental prerequisite is a functional understanding of supplement documentation in EHR and associated supplement coverage in major online databases. To address this, clinical notes and corresponding medication lists from an integrated healthcare system were extracted and compared with online databases. Overall, about 40% of listed medications are supplements, most of which are included in medication lists as nutritional or miscellaneous products. Gaps were found between supplement and standard medication terminologies, creating documentation difficulties in fully achieving robust supplement documentation in EHR systems. In addition, in the clinical notes we identified supplements which were not mentioned in the medication lists.
Introduction
The popularity of herbal and nutritional supplements in the United States (U.S.) has expanded rapidly in recent years.1 The American Botanical Council reported that nearly 6 billion dollars are spent on herbal supplements in the U.S. in 2013.2 The Center for Disease Control reports that around half of U.S. adults use some form of dietary supplements.3 Recently, the National Center for Complementary and Integrative Health (NCCIH, formerly the National Center for Complementary and Alternative Medicine) published the National Health Statistics Reports reporting that about 40% of Americans use some form of complementary and alternative medicine (CAM) and that nonvitamin/nonmineral dietary supplements were still the most commonly used complementary health approach among adults in 2002 (18.9%), 2007 (17.7%) and 2012 (17.7%).4 Among these, fish oil supplements (7.8%), glucosamine chondroitin, or a combination (2.6%) were found to be the most commonly consumed supplements (2012 National Health Interview Survey). The use of melatonin and probiotics has also dramatically increased from 0.6% and 0.4% in 2007 to 1.3% and 1.6% in 2012, respectively.4
CAM therapies are typically used to complement conventional medicine with the goal of reaching a better healthcare outcome, although less than 5% of U.S. adults exclusively used CAM therapy approaches4. One in four persons taking a prescription medicine also take an herbal supplement, increasing the possibility of drug-supplement interactions (DSIs). For example, warfarin can interact with various supplements such as Panax ginseng and Gingko biloba leading to severe adverse effects such as spontaneous postoperative bleeding.5 St. John’s Wort has also attracted attention as it lowers blood concentrations of cyclosporine, amitriptyline, digoxin, indinavir, and warfarin, potentially resulting in severe clinical syndromes.6 Another clinical study reported that volunteers receiving digoxin and St. John’s Wort had a 25% decrease in the concentration of digoxin in blood plasma.7 Unfortunately, the ability to readily identify adverse effects of herb and dietary supplements and their reactions with conventional Western medications is not well studied and the reports on such interactions occur less frequently in clinical practice.1
DSIs are most often due to an inhibitory or synergistic interactions between drugs and supplements with similar genes or molecular pathways within the body.8 Before market approval, new drugs are usually tested for interactions with other drugs through pharmacology experiments9 and clinical studies10. Testing for interactions between new drugs and supplements, however, are not required due to the differences in regulatory requirements for supplements, as delineated in the Dietary Supplement Health and Education Act of 1994 (DSHEA). The DSHEA rules designate supplements as food, which requires appropriate labeling and adherence to food safety rules. However, since supplements are not regulated as typical medications, they do not need to meet the usual clinical approval trials for safety and effectiveness, which includes assessment of medication pharmacology and potential drug-drug interaction characteristics. As such, post-market surveillance through pharmacoepidemiology studies11 and other standard mechanisms which are often used to detect adverse events in a given population are not required. These methods are limited, moreover, as they can only focus on a small set of drugs or supplements.
Electronic Health Record (EHR) systems are the main communication and documentation platform for healthcare providers. They store a large amount of data on drug prescribing behaviors, adverse drug events, and patient symptoms, thus providing a rich source of observational data offering the potential for active surveillance.12 Most current efforts use structured data such as diagnoses to find drug interactions and adverse drug events.13 These experiments often miss important information in clinical notes that can be leveraged for further clinical research and knowledge discovery. Unstructured texts can be analyzed using natural language processing (NLP) techniques for patient cohort identification14, phenotype extraction15, and drug-related information extraction16.
To facilitate effective mining of EHRs for DSIs, a better understanding of how the EHR represents the supplements and how effective the term coverage is for both structured clinical data and unstructured clinical notes is needed to extract accurate and comprehensive supplement information from the EHR. To the best of our knowledge, the investigation of supplement term representation and coverage on EHR systems is limited and deserves further investigation. One possible reason for the lack of extensive studies on DSIs is an absence of a standard and accepted terminology for herbal supplements. In a recent study comparing terms with different resources, we found that none of five major online databases covered all supplement terms.17 In this study, we sought to evaluate supplement term coverage between online supplement databases and EHR patient data. We also sought to investigate the adequacy of standard terminologies for representing supplements existing in the EHR.
Background
In this section, we introduce five online databases used to form a supplement list and the standard terminologies we evaluated for representing supplements.
Supplement databases
Natural Standard Authority Database (NSAD)18
NSAD is a database set up by Natural Standard, a research collaboration including physicians and researchers. There is a grade given to each entry, which reflects the level of evidence-based literature available about each product and its use. Each entry has an overview of the supplements, common names all over the world, uses, warnings, contraindications, mechanism of action, and a literature review. In addition to having entries for each of the supplements, there is an adverse reaction checker, an effectiveness checker, interactions display and a search for pregnancy related information.
Medscape19
Medscape is managed by the WebMD health professional network; the material is provided by physicians and authorities in that field. Medscape provides author attribution and sources of information making it a reliable source. Medscape also features a list of searchable entries and a tool which allows users to check for drug or supplement interactions.
Natural Medicines Comprehensive Database (NMCD)20
NMCD is managed by the therapeutic research center. The database has over 1,000 terms, but allows users to search in several other languages. Under each product, the effectiveness of the supplement, safety, known interactions with drugs, mechanism of action, and adverse reactions are listed. The information is targeted at physicians, researchers, and pharmacists. There is a separate advanced search for physicians which can used to make clinical decisions. This database also has an interactions checker and a search tool which lets users search with common names, scientific names or brand names making it one of the most useful and comprehensive databases.
MedlinePlus21
MedlinePlus is a web-based information service provided by the U.S. National Library of Medicine (NLM). It has a health topic section, a drug and supplements section and several other tools. This service including the drug and supplement database is maintained by the National Institutes of Health and is aimed at the general public. The information is provided by the National Center for Complementary and Alternative Medicine, National Toxicology Program, and the Office of Dietary Supplements. Each entry is followed by a journal style list of references for that supplement.
Drugs.com22
Drugs.com provides popular supplements with efficacy, side effects and interactions with drugs. The information is targeted at consumers and the format is user-friendly. Drugs.com also has a pill identifier and an interactions checker that lets users search for every potential interaction a drug will have with other drugs, supplements or food. Interactions are further classified as severe, moderate or mild. Information is derived from Wolters Kluwer Health Inc.
Unified Medical Language system (UMLS) and MetaMap
UMLS is a repository that integrates over 100 medical vocabularies from many sources and provides a unified platform which can be used to develop or enhance applications. One of the components in the UMLS is a Metathesaurus which has over 2 million terms and codes from many different vocabularies like Systematized Nomenclature of Medicine - Clinical Terms (SNOMED CT), Current Procedural Terminology (CPT), Logical Observation Identifiers Names and Codes (LOINC), etc. For this paper, UMLS was used to normalize data from different sources (i.e., EHR and online databases). Each concept in the UMLS Metathesaurus has a Concept Unique Identifier (CUI), which can be used to compare data from different sources.
MetaMap is a natural language processing tool developed by the NLM to automatically map biomedical texts to UMLS Metathesaurus. It is able to lexically and syntactically analyze the texts and provide a list of mapping concept candidates with mapping scores.
RxNorm, NDF-RT, and RxMix
The NLM creates a standardized nomenclature of clinical drugs called RxNorm from a source of 12 drug vocabularies. RxNorm has unique identifiers for each drug along with the drug’s dosage, generic name, chemical components, and dosage forms.23
National Drug File - Reference Terminology (NDF-RT) is a part of the Veteran Health Administration’s National Drug File. It is able to classify drugs into formal categories in addition to giving information about their molecular interactions, kinetics, therapeutic categories, and dose forms.24
RxMix is a web application that allows users to combine various functions from the RxNorm and NDF-RT application program interfaces (APIs) to create custom applications that can be run interactively or in a batch mode.25 We used this tool for mapping terms to RxNorm and NDF-RT concepts.
Methods
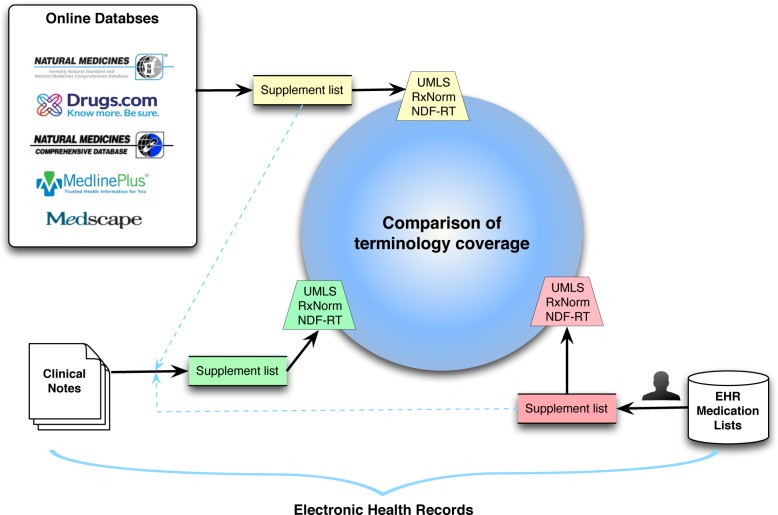
We first extracted supplement terms from both online databases and structured medication lists in EHRs, and then formed a comprehensive list of supplements followed by a search for the terms in clinical texts. All terms from online resources, medication lists, and clinical texts were mapped to UMLS, RxNorm and NDF-RT. Then, we compared the term coverage for the three resources (Figure 1).
Figure 1.
Illustration of methods to compare supplement terms between online databases and EHR.
Extraction of supplement list from online databases
To identify the selected databases, we performed an Internet search for the top herbal supplements databases to identify the selected databases. Any databases without support by evidence or with very short (<50) supplement lists were excluded. Databases no longer updated or maintained were also rejected for this study. We finally selected the five popular online databases: NSDA, Medscape, NMCD, MedlinePlus, and Drugs.com as the sources of herbal and dietary supplement terms. We extracted all supplement terms from databases and manually excluded the non-English words from online databases. A combined list of supplements was finally generated from online databases for further analysis.
Extraction of supplement terms from clinical data
We collected patients’ medication lists from the University of Minnesota Medical Center, Fairview Health Services (FHS) during a four-year period of 2011–2014. University of Minnesota institutional review board approval was obtained and informed consent waived for this minimal risk study.
The collected medication list from FHS were processed by using the following steps:
Step 1: A physician (coauthor EA) manually reviewed medication lists to select supplement terms;
Step 2: Supplements related information such as the frequency of supplement usage and their assigned pharmaceutical class (e.g., Nutritional Products), were extracted and analyzed to provide a better understanding of how supplements are represented in the medication lists;
Step 3: The list of supplements was mapped to the UMLS by using MetaMap. Only exact matches with a perfect score of 1000 were considered and retrieved;
Step 4: The supplement list was also mapped to standard terminologies RxNorm and NDF-RT by using RxMix25. We specifically used the RxNorm:findRxcuiByString and NDF-RT:findConceptsByName functions to extract the concepts.
Collection of supplement terms from clinical texts
We collected clinical texts from FHS during the same period, and analyzed the clinical texts using the following steps:
Step 1: Instead of manually reviewing charts to find the supplements, we first combined the supplement lists extracted from both online databases and medication lists to form a comprehensive list;
Step 2: The comprehensive list (blue dashed lines in Figure 1) was then used as a dictionary to search all clinical texts to investigate if these terms existed in the clinical texts;
Step 3: To normalize the supplement terms, we mapped these supplement terms to UMLS, RxNorm and NDF-RT, the same as steps 3&4 when mapping medication list in the above section.
Evaluation of supplements term coverage
We evaluated the term representation in medication lists based on their frequency and assigned classes. After supplement terms were mapped to UMLS, RxNorm and NDF-RT, we then evaluated the gap between the current terminologies to the existing terms. We used Venn diagrams to compare the term coverage of these concepts to see how they overlap with each other to provide a visual and quantitative assessment.
Results
Extraction of supplement list from online databases, clinical data and clinical texts
We extracted 3,115 unique supplement terms from the five online databases, 3,720 unique terms from medication lists, and 4,717 terms from clinical notes.
In the whole medication list in the EHR, we found about 40% of the listed medications are related to supplements. Among these supplements extracted from the EHR medication lists, most of them were classified as Nutritional Product (41.7%) and Miscellaneous Products (40.7%). Other types include Gastrointestinal Agents, Hematological Agents, Gastrointestinal Agents, and other types (Table 1). The top frequent supplements in the medication list include Fish oil, Glucosamine chondroitin, Probiotics, Melatonin, Coenzyme Q10, etc.
Table 1.
The supplement representation in medication list in EHR; percentages and classes.
| Class | Percentage |
|---|---|
| NUTRITIONAL PRODUCTS | 41.7% |
| MISCELLANEOUS PRODUCTS | 40.7% |
| GASTROINTESTINAL AGENTS | 8.0% |
| HEMATOLOGICAL AGENTS | 7.7% |
| GASTROINTESTINAL AGENTS | 1.2% |
Evaluation of supplements term coverage
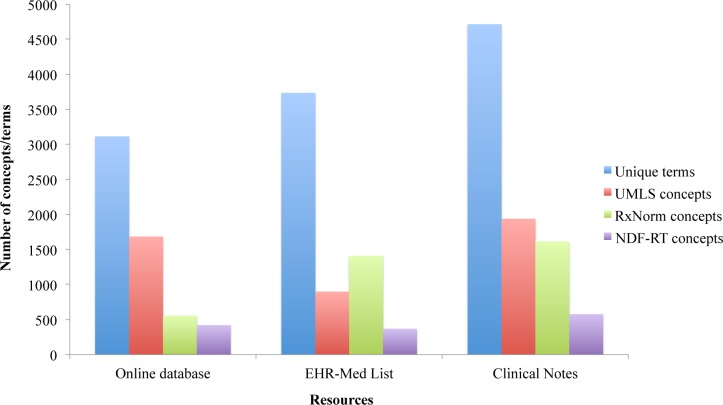
As shown in Figure 2, after mapping these terms to UMLS and specific standard terminologies, we obtained 1,683, 897, and 1,937 UMLS concepts, 556, 1,410, and 1,733 RxNorm concepts, and 421, 367, and 576 NDF-RT concepts from online databases, medication lists and clinical texts.
Figure 2.
Number of terms or concepts in each resource after mapping to the UMLS, RxNorm and NDF-RT.
For all three resources, over 60% of terms cannot be mapped to UMLS concepts, and even much less to RxNorm and NDF-RT. The number of mapped UMLS concepts is larger than RxNorm and NDF-RT for online databases and clinical notes, but the EHR medication list returns more RxNorm concepts than UMLS and NDF-RT concepts.
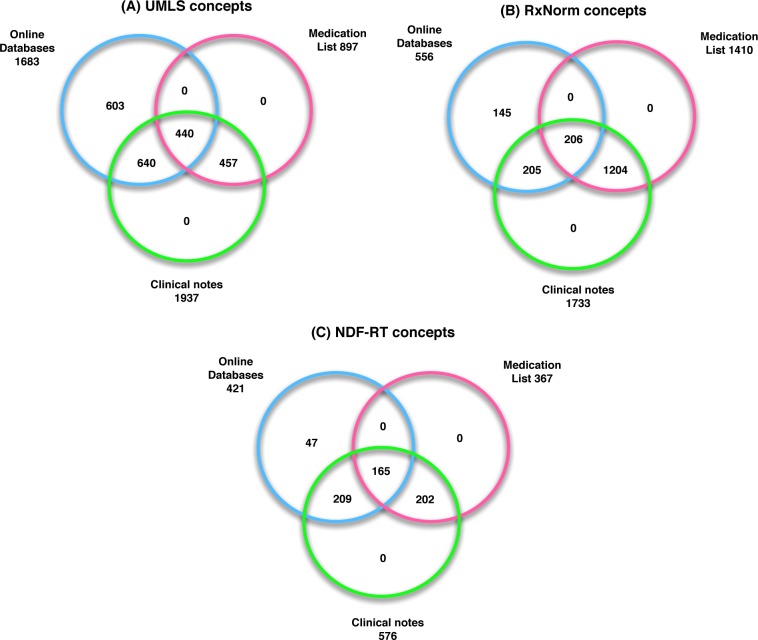
After mapping to the various terminologies and comparing the concepts coverage between three resources, we found that all concepts extracted from the medication list were a subset of those in the clinical notes. The concepts in both the medication list and the clinical notes have different degrees of overlap with those in online databases. In other words, clinical notes contain all supplement concepts in the medication list and part of the online databases while online databases do not contain all concepts existing in the EHR.
We further investigated those supplements, in clinical notes but did not include them in the supplement list (shown in Figure 3), and listed a few selected supplements with their occurrences in example sentences (Table 2). Many of them are related to history of food consumption, allergy problems and recommendation to take or avoid a specific supplement either due to risk of potential drug interactions or due to the known physiologic effects of the supplements. But we also found that patient was actually taking or using that supplement, such as Goat’s rue.
Figure 3.
Venn diagram showing the supplement term coverage between online databases, medication list, and clinical notes after mapping to (A) UMLS concepts, (B) RxNorm concepts, and (C) NDF-RT concepts.
Table 2.
Supplements only mentioned in clinical texts and not in the medication lists. Supplements are underlined in the examples.
| Supplements | Examples |
|---|---|
| Aloe vera | We discussed avoiding aloe vera for the potential interaction with cyclosporine. |
| … patient notes ‘irritation and sensitivity’ of skin, discussed use of 100% aloe vera gel mixed with Aquaphor, Mepilex given to patient for comfort along left axilla and instructed on use. | |
| … pt has also started on aloe vera juice. | |
|
| |
| Goat’s rue | she is taking her goat’s rue and mother’s milk plus supplements. |
| Discussed herbals and mom is taking both Fenugreek and goat’s rue to increase alveoli. | |
| She tried herbals including goat’s rue to increase alveoli without success. | |
|
| |
| White oak | Allergens positive for cat and dog dander, white oak and ragweed. |
| … a 2/6 reaction to American elm (white), and a 1/6 reaction to white oak and ragweed. | |
| Approximately 3 days ago he scraped his left anterior leg with a piece of white oak. | |
|
| |
| Marshmallow | Continue aloe vera and marshmallow root tea supplements. |
| SORE THROAT 1) gargle with salt or apple cider vinegar mixed with water 2) tea (“throat coat” tea includes licorice, marshmallow root and slippery elm or you can make cinnamon and honey tea - cinnamon has analgesic properties). | |
|
| |
| Adenosine | We then performed thrombectomy of the distal RCA after using nicardipine, nitroglycerine and adenosine. |
| Should and EKG that showed no other acute abnormalities he was given 6 mg of adenosine with resolution of the SVT. | |
| No significant abnormalities were noted with infusion of adenosine. | |
|
| |
| Buckwheat | Honey has anti-inflammatory properties (darker honey such as buckwheat is the best, give it on the spoon or make chamomile tea (which is also anti-inflammatory) with LOTS of honey). |
|
| |
| Hazelnuts | Have spinach (cooked or raw), colorful fruits, walnuts, hazelnuts, almonds in your diet. |
| Food intolerance Oral allergy syndrome: with hazelnut and birch pollen–pt eats all other nuts without a problem. | |
| … 1–2 ounces (a small handful) of almonds, walnuts, hazelnuts or pecans once a day in place of other less healthy snacks. | |
|
| |
| Spearmint | Applied seaband, spearmint aromatherapy, Reiki therapy |
| Therapies tried and outcome: Baby lotion no relief, Mary Kay lotion with spearmint with moderate relief. | |
| Try limiting chocolate, peppermint, and spearmint. | |
|
| |
| Black tea | Patient took some oral liquids while in clinic (black tea), and tolerated this well. |
| Drinking green tea, will switch to black tea. | |
|
| |
| Sitostanol | Plant sterols such as Benecol: Three servings per day (1.5 g sitostanol per 1 1/2 teaspoon [8 g] serving)… |
| Consuming plant sterols such as beta-sitosterol and -sitostanol (typically found in margarine spreads such as Promise activ or Benecol). | |
|
| |
| Poppy seeds | His wife works with natural foods and he does eat a lot of seeds including poppy seeds. |
| It is reasonable that the low level of morphine in his urine could be from high levels of seed intake including poppy seeds and this does not change my plan for opiate analgesics. | |
|
| |
| Quinoa | … organic meat, brown rice or quinoa or oats (gluten free), amaranth, plain steamed veggies, apples, grapes. |
| Try whole grains such as 7-grain breads, whole-wheat pasta, brown rice and other grains such as quinoa, barley, oats, and millet. | |
Discussion
DSIs are attracting more attention recently due to their known and potential adverse effects on patient safety. Many clinical studies have found potential interactions, although they focused on a small subpopulation. In addition, use of EHR data for DDI and drug adverse effects has been widely investigated, while only limited study has focused on mining EHR data to explore DSIs. This research investigates how EHR data represent herbal and dietary supplements and evaluates existing terminologies to represent the supplements in EHR systems. This paper studies DSIs by first establishing a list of supplements from a variety of sources and then identifying the coverage of the supplements in the medication lists and notes of an EHR system.
It is not required to document supplement usage in the EHR system, so it is surprising to find that about 40% of the medications documented in the EMR are herbal and dietary supplements. Unsurprisingly, the most frequently used nonmineral/nonvitamin supplements in the medication lists are consistent with the top 10 supplements listed in a recent report of US consumption of supplements.4 In the medication list, there is no easy way to identify the supplements other than manual review. The reason is that there is not a class called herb or dietary supplements or similar, and most of them are classified as nutritional or miscellaneous products. There are still 3% of nutritional products and 40% of miscellaneous products that are non-supplements.
The supplement names are similar to drugs in that they have many ways to represent them with common names or trade names. Many names in the medication lists are the combinations of two or more ingredients, connected by either “-“, “w/o” and/or “w/”, such as “Alpha Lipoic Acid-Cr-Cinnamon”, “Zinc Citrate-Phytase”, “Prenat-FeFum-FePo-FA-Omega 3”, “Prenat w/o A-FeCbGl-DSS-FA-DHA”, and “B-Complex w/C-Biotin-D-Zinc & Folic Acid”. Many names also contain information of enteral formulation such as “Tab” (i.e., Tablet), “Cap” (i.e., Capsule), parenteral formulations, such as “(Bulk) Powder”, “(Bulk) Granules“, “Crystals”, “Liquid”, and dosage information such as “200 MG”, “100 MG/ML”. These supplements with dosage and formulation information and those containing multiple ingredients were neither mapped to the UMLS concepts nor mapped to other terminologies. We did not perform a normalization step before mapping to keep some detailed information which can be recognized by RxNorm. Moreover, separating the combined supplement names into each of the ingredients is not meaningful. The RxNorm was designed to identify drugs with detailed information, so the terms with different dosages can be linked to their unique RxNorm concepts, although missed mappings still exist. For example, two supplements with the same ingredients and different dosages “Alpha-Lipoic Acid (Thioctic Acid) Cap 200 MG” and “Alpha-Lipoic Acid (Thioctic Acid) Cap 300 MG” were mapped to the same UMLS concept “C0023791:.ALPHA.-LIPOIC ACID (Thioctic Acid) [Organic Chemical,Pharmacologic Substance,Vitamin]”, but they have their own unique RxCUIs: 313841 and 333831, respectively. This is one reason why the number of mapped unique UMLS concepts is less than RxNorm concepts for supplements in the medication lists. Another reason is that many terms cannot find suitable UMLS concepts, but can be related to RxNorm concepts. For example, “Cinnamon Tab 500 MG” does not have an exact match in UMLS but does have a unique RxCUI of 6459.
A gap between terminologies and supplements in EHR was also observed. Terms with multiple ingredients cannot be mapped and were treated differently from those listed with one of these ingredients. Many terms with detailed dosage information can only be partly mapped to UMLS concepts with a mapping score lower than 1000, in which cases we treated them as non-mapped terms. NDF-RT has less coverage than the other two for the supplements. Not only for those terms with multiple ingredients or detailed information but also some general supplement terms such as “Chia Oil” and “Garlic” which cannot be mapped to NDF-RT. Thus, none of these terminologies can be used to represent all the supplement terms in the EHR system.
Considering many forms of a single supplement name, we mapped and normalized them to UMLS, RxNorm, and NDF-RT concepts before comparing the term coverage. By searching supplement lists both in online databases and medication lists, we found that all terms in the medication list were included in the list generated from clinical notes, which makes sense as clinicians usually import the current medication list as part of the clinical notes. About 64% of terms in the online databases were mentioned in the clinical notes, indicating the wide consumption of supplements. The lower overlap of UMLS concepts between the medication lists and major online databases may be due to the mapping issues mentioned above. But they have higher overlap 37% in RxNorm than 26% in UMLS concepts. The possible reason is that RxNorm is more sensitive to the supplements with detailed information than UMLS.
There are some supplements in clinical notes that are not mentioned in the medication lists. For example, “We discussed avoiding aloe vera for the potential interaction with cyclosporine” indicates the clinician realized the potential DSI between aloe vera and cyclosporine and suggested the patient to not use aloe vera. A mother also tried an herb supplement called goat’s rue to increase alveoli. The supplement marijuana was also mentioned as a clinical history component and relates to the patient’s strange behavior. Adenosine shows up in the notes but not in the medication list as it is a provider administered medication used as an acute therapy. Such medications may only show in the documentation as provider orders or as clinical note documentation as they would not have a role as a chronic medication.
We also found similar terms but not exactly the same names in both clinical notes and medication list. One example, “Cranberry juice” is in the clinical notes, but the medication list only has “CRANBERRY JUICE EXTRACT PO”, which does not exactly match with the UMLS concept “C1572601:CRANBERRY JUICE [Food]” (score of 694). Since we only include supplements with exact matches, we excluded this from the supplement list extracted from the medication list. Although some supplements existed in both sources but notes provide additional information; for example, “[ginkgo] increased risk for bleed especially since taking aspirin - reviewed with pt.” infers potential interactions between ginkgo and aspirin. Other examples include “Ginkgo has been shown to inhibit hepatic glucuronidation of MPA in vitro.” and “Medication Changes: He had been taking ginkgo but read an article that said it should not be taken with warfarin so he stopped taking it about 2 days ago.” This also suggests to us that it is necessary to complement unstructured data with structured data for obtaining comprehensive information from the EHR.
This pilot study has multiple limitations. We only collected clinical data from one site – FHS, during the limited period of 4 years, which may underestimate the term coverage in the EHR. When mapping to UMLS, we only considered the exact matches, so this brings lower returned concepts from UMLS. We did not distinguish reported alimentary facts from medical facts at the current stage. Moreover, it may have bias when we searched supplements in clinical notes by comparing with the supplements generated from the other two sources. The best way is to manually review, but that would be costly in terms of time and labor.
Conclusion
We evaluated supplement term coverage in the EHR by comparing with the existing online resources. Forty percent of medication lists are supplements and they are mostly categorized as Nutritional Products or Miscellaneous Products. All supplements in the medication lists were also mentioned in the clinical notes. We found there is a gap between standard terminologies and supplements in the EHR and not a single standard terminology can cover all supplements in the EHR. Moreover, clinical notes contain additional information such as suggestion or discussion of supplements existing in the mediation lists as well as those supplements not mentioned in the medication lists.
Acknowledgments
This research was supported by the University of Minnesota Informatics Institute on the Horizon grant (RZ), the Agency for Healthcare Research & Quality grant (R01HS022085) (GM), and University of Minnesota Clinical and Translational Science Institute supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000114) (Blazar). The authors thank Fairview Health Services for their support of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Health Research & Quality.
References
- 1.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Herbal Dietary Supplement Retail Sales Up 7.9% in 2013. Available from: http://cms.herbalgram.org/press/2014/2013_Herb_Market_Report.html?ts=1426104678&signature=bc1892b328e34cf97b908bdbfbefc416&ts=1426104768&signature=4a3a4506b7f6cc87beafeac57aba6557.
- 3.Ventola CL. Current issues regarding complementary and Alternative medicine in the United States. P&T. 2010;35(8):461–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. National Health Statistics Reports. 2015:1–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Milic N, Milosevic N, Golocorbin Kon S, Bozic T, Abenavoli L, Borrelli F. Warfarin interactions with medicinal herbs. Natural product communications. 2014 Aug;9(8):1211–6. [PubMed] [Google Scholar]
- 6.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001;61(15):2163–75. doi: 10.2165/00003495-200161150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum) Clinical pharmacology and therapeutics. 1999 Oct;66(4):338–45. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 8.Li AP. Primary hepatocyte cultures as an in vitro experimental model for the evaluation of pharmacokinetic drug-drug interactions. Advanced Pharmocology. 1997;43:103–30. doi: 10.1016/s1054-3589(08)60203-3. [DOI] [PubMed] [Google Scholar]
- 9.Quinney SK, Zhang X, Lucksiri A, Gorski JC, Li L, Hall SD. Physiologically based pharmacokinetic model of mechanism-based inhibition of CYP3A by clarithromycin. Drug metabolism and disposition: the biological fate of chemicals. 2010 Feb;38(2):241–8. doi: 10.1124/dmd.109.028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda K, Ikeda Y, Fujita T, Yoshida K, Azuma Y, Haruyama Y, et al. Identification of the rate-determining process in the hepatic clearance of atorvastatin in a clinical cassette microdosing study. Clinical pharmacology and therapeutics. 2011 Oct;90(4):575–81. doi: 10.1038/clpt.2011.142. [DOI] [PubMed] [Google Scholar]
- 11.Schelleman H, Bilker WB, Brensinger CM, Han X, Kimmel SE, Hennessy S. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clinical pharmacology and therapeutics. 2008 Nov;84(5):581–8. doi: 10.1038/clpt.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, Reich C, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Annals of internal medicine. 2010 Nov 2;153(9):600–6. doi: 10.7326/0003-4819-153-9-201011020-00010. [DOI] [PubMed] [Google Scholar]
- 13.van Puijenbroek EP, Egberts AC, Heerdink ER, Leufkens HG. Detecting drug-drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. European journal of clinical pharmacology. 2000 Dec;:56, 9–10, 733–8. doi: 10.1007/s002280000215. [DOI] [PubMed] [Google Scholar]
- 14.Kho AN, Pacheco JA, Peissig PL, Rasmussen L, Newton KM, Weston N, et al. Electronic medical records for genetic research: results of the eMERGE consortium. Science translational medicine. 2011 Apr 20;3(79):79re1. doi: 10.1126/scitranslmed.3001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullo IJ, Fan J, Pathak J, Savova GK, Ali Z, Chute CG. Leveraging informatics for genetic studies: use of the electronic medical record to enable a genome-wide association study of peripheral arterial disease. J Am Med Inform Assoc. 2010 Sep-Oct;17(5):568–74. doi: 10.1136/jamia.2010.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XY, Hripcsak G, Markatou M, Friedman C. Active Computerized Pharmacovigilance Using Natural Language Processing, Statistics, and Electronic Health Records: A Feasibility Study. J Am Med Inform Assoc. 2009 May-Jun;16(3):328–37. doi: 10.1197/jamia.M3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manohar N, Adam TJ, Pakhomov S, Melton GB, Zhang R. Evaluation of herbal and nutritional supplement resource term coverage. MEDINFO. 2015 [PMC free article] [PubMed] [Google Scholar]
- 18.Natural Standard Authority database. Available from: https://naturalmedicines.therapeuticresearch.com/
- 19.Medscape. Available from: https://naturalmedicines.therapeuticresearch.com/
- 20.Natural Medicine Comprehensive database. Available from: https://naturalmedicines.therapeuticresearch.com/
- 21.Medline Plus. Available from: https://naturalmedicines.therapeuticresearch.com/
- 22. Drugs.com. Available from: https://naturalmedicines.therapeuticresearch.com/
- 23.Nelson SJ, Zeng K, Kilbourne J, Powell T, Moore R. Normalized names for clinical drugs: RxNorm at 6 years. J Am Med Inform Assoc. 2011 Jul-Aug;18(4):441–8. doi: 10.1136/amiajnl-2011-000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NDF-RT. Available from: http://www.nlm.nih.gov/research/umls/sourcereleasedocs/current/NDFRT/
- 25.RxMix. Available from: http://mor.nlm.nih.gov/RxMix/