Abstract
The secondary use of EHR data for research is expected to improve health outcomes for patients, but the benefits will only be realized if the data in the EHR is of sufficient quality to support these uses. A data quality (DQ) ontology was developed to rigorously define concepts and enable automated computation of data quality measures. The healthcare data quality literature was mined for the important terms used to describe data quality concepts and harmonized into an ontology. Four high-level data quality dimensions (“correctness”, “consistency”, “completeness” and “currency”) categorize 19 lower level measures. The ontology serves as an unambiguous vocabulary, which defines concepts more precisely than natural language; it provides a mechanism to automatically compute data quality measures; and is reusable across domains and use cases. A detailed example is presented to demonstrate its utility. The DQ ontology can make data validation more common and reproducible.
Introduction and Background
The healthcare system in the United States continues to adopt electronic health records (EHR) at a rapid pace.1 The EHR is designed to replace a paper chart and to document and facilitate the delivery of care. Since this electronic data is now much more easily accessed than abstracting from paper charts, it is frequently used for other purposes such as clinical effectiveness research, predictive modeling, population health management and healthcare quality improvement. Secondary use of EHR data is expected to improve health outcomes for patients, but the benefits will only be realized if the data that is captured in the EHR is of sufficient quality to support these secondary uses.2 Investigators have shown that EHR data often contain errors that can impact research results, yet only 24% of clinical studies that use EHR data had a data validation section.3 In order to measure the quality of data there must be an understanding of how the data will be used.4
There is no generally accepted quantitative measure of data quality, but Juran gives an often cited qualitative definition as “…high-quality data are data that are fit for use in their intended operational, decision-making, planning, and strategic roles.”5(p.34–8) Data quality may be adequate when used for one task, but not for another. For example, a higher level of data quality is needed to count the number of diabetic patients with controlled HgA1C than to just count the number of patients. A task refers to concepts in a clinical domain and those concepts are represented by the data. For each task, a set of data quality measures must be developed that determine if the data are adequate to perform the task. The healthcare data quality literature provides terminology and definitions and attempts to organize data quality measures, but there is no general agreement on what these measures should be.6 This terminology-based approach defines measures using natural language, which does not adequately represent the relationships between concepts and is too loosely defined to yield a quantifiable measure of data quality. A better approach is to use an ontology which provides a sufficiently rigorous foundation for concept definitions that enable automated methods for calculating data quality measures.
An ontology is a formal, explicit specification of a shared conceptualization.7 Each concept (also called a “class”) in the ontology has a name, attributes, properties (relations to other concepts) and constraints that must always be true for a concept. The key benefits of defining data quality measures in terms of an ontology are that an ontology is: 1) a specification, written in a formal language and able to represent semantics, 2) a shared vocabulary that everyone can use to precisely refer to an aspect of the world, and 3) a sufficiently rigorous specification that can be used for logical inference and computation.8 An ontology is a logical theory about a part of the world and it defines interrelationships between concepts and axioms that should be true about that world. Automated reasoning can be applied to check internal consistency and make inferences beyond what was explicitly stated in the ontology.9 This automation eliminates the need for redefining the data quality measures for every task in every domain.
No formal healthcare data quality ontology currently exists, but there is research that examines core data quality concepts. Wang and Strong10 proposed a framework that consolidates 118 different general data quality characteristics into 20 categories. Kahn11 proposed a healthcare specific framework using a “fit-for-use” data quality model in which he proposes five high-level dimensions. Liaw6 performed an extensive literature review looking for commonalities on data quality dimensions. He found consensus on the five most common occurring dimensions were “accuracy”, “completeness”, “consistency”, “correctness” and “timeliness”. While there is some agreement among investigators on these high-level dimensions, there is little agreement or consistency in definitions of more granular data quality concepts such as “validity”, “reliability” and “believability”.12 In a 2012 paper, Weiskopf13 defined five high-level dimensions of data quality and listed synonyms for each (Table 1).
Table 1:
Weiskopf Five Dimensions of Data Quality with Synonyms
| Dimension | Synonyms |
|---|---|
| Completeness | Accessibility, Accuracy, Availability, Missingness, Omission, Presence, Quality, Rate of recording, Sensitivity, Validity |
| Correctness | Accuracy, Corrections made, Errors, Misleading, Positive predictive value, Quality, Validity |
| Concordance | Agreement, Consistency, Reliability, Variation |
| Plausibility | Accuracy, Believability, Trustworthiness, Validity |
| Currency | Recency, Timeliness |
While these dimensions capture orthogonal aspects of data quality, they are defined using natural language descriptions and synonyms. As can be seen from Weiskopf’s descriptions, the same terms may be used multiple times to mean different things (i.e. “Accuracy” occurs 3 times), introducing confusion regarding what aspect of data quality is being described. To provide better conceptual clarity and precision, an ontology is needed.
This paper describes the development of a healthcare data quality ontology (DQ ontology) which provides rigorous definitions and can automate the computation of data quality measures. Given formal ontologies for a clinical domain and for a task, the DQ ontology enables measures to be reused without having to reinvent new data quality assessments for every research project. Ontologies for some clinical domains14 and tasks15 already exist and researchers can focus on creating additional ontologies that can be used by the DQ ontology to yield quantified measures. This can make it easier to incorporate data quality validation as a standard component of research results. The DQ ontology was developed from a comprehensive list of data quality terms present in the literature. The terms were organized into an ontology and constraints were defined that precisely describe a data quality measure better than natural language and enable quantification of the measure. It makes explicit which data quality concepts depend on the use of the data and which depend on the clinical domain. A detailed example demonstrates the utility of this ontology for quantifying measures and for discussing aspects of data quality.
Materials and Methods
There are a number of methodologies for developing an ontology,8 but the method described by Noy and McGuiness16 was selected due to its simplicity and effectiveness. This methodology advocates a seven-step process that takes a list of terms and definitions and turns them into a formal ontology. The first step is to define the scope of the ontology. For this study, the scope is a shared vocabulary of data quality concepts with formal definitions that are automatically computable to quantify data quality. The software development community has had success adopting the approach of a common vocabulary to allow researchers to spend less time defining concepts and more time applying it in research.17 Next, the reuse of existing ontologies was considered. No formal healthcare data quality ontology exists; but ontologies that describe clinical domains and tasks do exist and will be reused and referenced by the DQ ontology.14,15
In order to enumerate the important terms in the ontology, an extensive PubMed search for articles published between January 1995 and January 2015 was performed to obtain a comprehensive list of terms and definitions that are used to describe healthcare data quality. The goal was to find literature reviews and meta-analyses of papers about healthcare data quality to identify as many core concepts as possible. Also, all articles about informal healthcare data quality frameworks or ontologies were examined for key terms and definitions. Keywords included in the query were: (“data quality”) and (“health” or EHR) and (“literature review” or framework or ontology or assessment or model) and (dimensions or accuracy or consistency or completeness or correctness).
There were 181 articles identified, which were manually reviewed by the first author and narrowed to five meta-analyses from Liaw6, Weiskopf13, Kahn11, Chen18, and Lima19. These papers were either reviews of other papers about healthcare data quality or they proposed an informal data quality framework. They all attempted to categorize data quality concepts into semi-orthogonal dimensions. The references from these papers were also reviewed, which yielded an additional five sources: Wang10, Wand20, Chan21, CIHI22, Stvilia23. Collectively, these 10 meta-analyses reviewed 412 papers looking for common aspects of healthcare data quality. There was similarity on high-level concepts such as “correctness”, “consistency” and “completeness”, but there were limited definitions for important terms such as “dataset”, “data”, “measurement”, “metric” and “measure”. Additional papers from the information science literature were found to further define these important concepts24–26.
Ontologies can be specified using a number of methods including OWL27, first order logic, or as UML28. For this paper, the ontology is documented using a UML diagram and a table that lists constraints. A bottom-up approach was taken in which terms and definitions from the meta-analyses were matched and harmonized into equivalent concepts and these concepts where grouped into higher-level categories. Each concept has properties and relationships with other concepts that were discerned from reading the description in the articles. The cardinality of relationships was also defined. Cardinality indicates whether an associated concept is optional, must always occur, or can occur multiple times. For example, a patient must always have a gender, but a blood pressure reading is an optional observation. Constraints were also defined for each concept, describing what should always be true for a concept. The constraints evaluate to a Boolean (true/false) result and can be written in a number of languages including, Object Constraint Language (OCL), first order predicate logic (FOPL), pseudo-code or openEHR constraint language.8,29 For this study, pseudo-code was chosen because it succinctly captures the important aspects of the constraint without introducing a specific, complex syntax.
Results
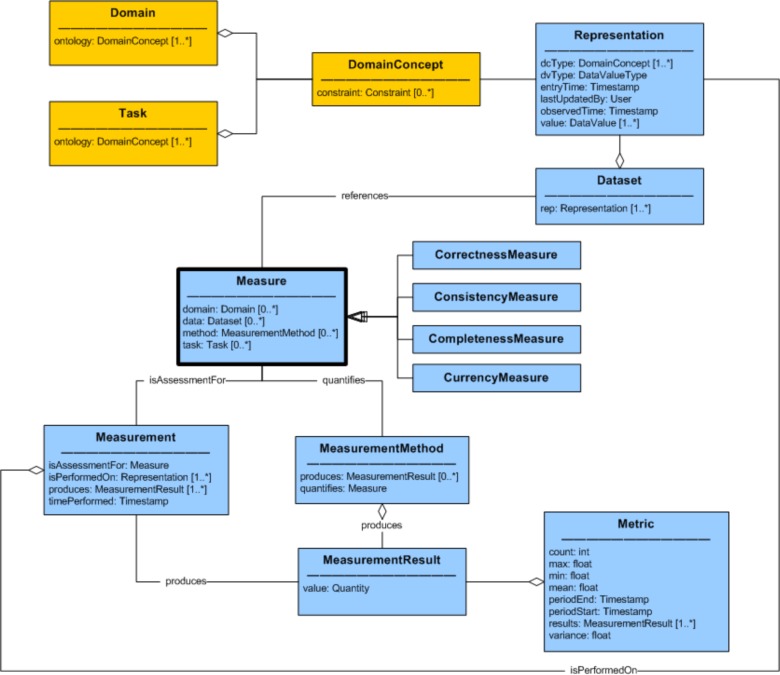
There were 96 terms and definitions extracted from the literature as a basis for the data quality measures of the ontology. Terms that described the same concept were matched based on their definition and use within the articles. Concepts that appeared in less than three of the articles were deemed non-core and were left out of this version of the DQ ontology. The resulting data quality ontology is shown in Figure 1 as a UML diagram depicting the relationships, attributes, and cardinality of the concepts. For readability, the 19 lower-level Measures were not included in the diagram and are listed in Table 2, which also provides a definition of the measure and a reference to equivalent terms from the meta-analyses. A bold font is used to indicate that a term refers to a concept from an ontology.
Figure 1:
Data Quality Ontology
Table 2:
Data Quality Ontology - Measure Detail
| Concept | Definition | References / Synonyms |
|---|---|---|
| CorrectnessMeasure | ||
| RepresentationIntegrity | Aspects of the Representation that reassure that data was not corrupted or subject to data entry errors. | Correctness: Credibility of source6, Accuracy: …free of error11, Integrity18, Repeatability18, Structural Consistency23 |
| RelativeCorrectness | Assesses the quality of a Representation by comparing it to its counterpart in another Dataset which is a “relative standard”, computed as PPV. | Accuracy: …conformity with actual value6, Correctness13, Believability11, Validity13,19, Comparability20,21, Accuracy10,13,18,23, Corrections made13, Errors13, Misleading13, PPV13, Quality13 |
| RepresentationCorrectness | A correct Representation has high accuracy and is complete. | Correctness: …accuracy and completeness6, Accuracy20,21 |
| Reliability | The data is correct and suitable for the Task. | Reliability6,18–20, Accuracy: Measurement Error22 |
| ConsistencyMeasure | ||
| RepresentationConsistency | The data is a valid value and format for its Data Value Type and all of the Representations for the same information have the same values. | Consistency: …values and physical representation of data6, Concordance13, Format11, Internal Consistency18, Consistency13, Precision20, Format11,20, Reliability13, Variation13, Accuracy: Edit and Imputation22, Representational Consistency10 |
| DomainConsistency | Concepts in the Domain are represented in the data and the data satisfies syntactic and semantic rules. Constraints for the Domain are satisfied. | Accuracy: Refers to values and representation6, Correctness: …format and types are valid6, Plausibility13, Believability10,13, Relational Integrity Rules11, Consistency18–20, Measure validity21, Accuracy13, Trustworthiness13, Validity13,23 |
| CodingConsistency | Representations that are of coded text data type must be correctly mapped to an enumerated list or a terminology. | Consistency: …codes/terms…mapped to a reference terminology6, Valid values11, Comparability: Equivalency22, Semantic Consistency23 |
| DomainMetadata | Meta-data exists to describe the Domain and it is logically consistent. | Methodological Clarity19, Metadata Documentation18, Comparability: Data dictionary standards22, Interpretability10 |
| CompletenessMeasure | ||
| RepresentationComplete | Domain independent extent to which data is not missing. | Completeness: …information is not missing6, Completion19, Completeness18,21, Accuracy: Item Non-22 |
| DomainComplete | The extent to which information is present or absent as expected. | Appropriate amount of data: Data are present or absent as expected13, Optionality11, Content20 |
| RelativeCompleteness | The extent to which a truth about the world is represented in the data. This is computed as sensitivity relative to another Dataset. | Completeness: Is a truth…in the EHR?13, Accessibility10,13,19, Accuracy13, Availability13, Missingness13, Omission13, Presence13, Quality13, Rate of Recording13, Sensitivity13, Validity13 |
| Sufficiency | The data has sufficient Representations along a given dimension (i.e. time, patient, encounter) to perform the Task. | Completeness: …sufficient breadth and depth for the task6, Appropriate amount of data11, Representativeness18, Sufficiency20, Accuracy: Coverage22, Granularity11,18, Continuity11, Level of Detail20, Completeness10,23, Precision23 |
| DomainCoverage | The data can represent the values and concepts required by the Domain. | Completeness: …represent every meaningful state of the […] real world6, Completeness: All values for a variable are recorded6, Coverage19, Completeness20 |
| TaskCoverage | The data contains all of the information required by the Task. | Completeness: …depict every possible state of the task6, Usableness18,20, Usability18, Utility18, Importance20, Usefulness20, Value-added10 |
| Flexibility | The extent to which the data is sufficient to be used by many Tasks. | Consistency: …information…appl[ies] to different tasks6, Flexibility10,20, Relevance: Adaptability22 |
| Relevance | The data is sufficient for the Task and conforms to the Domain. | Relevance6,18,20,23, Relevance: Value22, Relevancy10 |
| CurrencyMeasure | ||
| RepresentationCurrent | Calculation for time difference between when an observation was made and when it was entered into the system. | Timeliness: delay between a change of the real-world state and…the information system6, Currency13,18,23, Timeliness13,18,20, Up-datedness18, Recency13 |
| DatasetCurrent | Time difference between when a Dataset was updated and when it was made available. For example, periodic updates to a repository. | Timeliness: …availability of output is on time6, Opportunity19, Periodicity18, Currency11,20, Timeliness: Data currency22, Timeliness10 |
| TaskCurrency | The Data is sufficiently up-to-date for the requirements of the Task. | Timeliness: …information is up to date for task6, Timeliness: …age of the data is appropriate for the task11, Timeliness (external)20 |
The meta-analyses articles make pervasive reference to concepts such as “data”, “information” and “value”. In the DQ ontology, a more precise concept, Representation, defines the lowest level, atomic piece of information that exists in the data being assessed (synonyms for this concept are data field, observation, value, etc). Representations have a DataValue (the part that is stored somewhere) as well as a DataValueType that specifies a format to which the DataValue must conform (i.e. numeric quantity, string, choice field, etc). DataValueTypes put constraints on the DataValue of the Representation, and can only refer to intrinsic information about the value itself and not to relationships with other Representations. Formal semantics about concepts represented in the data are defined in a separate Domain ontology. Representations have an attribute, DomainConcept, which maps data to a concept in the clinical Domain ontology. There can be multiple Representations for each concept in the Domain. For example, a systolic blood pressure value can be represented as a single number (i.e. 123) or it can be encoded as the first part of a string (i.e. “123/92”). DomainConcepts can also have multiple synonyms in the Domain ontology (i.e. “BP” and “Blood Pressure”), but for the purpose of assessing data quality, they can all be mapped to a single, primary DomainConcept (i.e. “Blood Pressure”). The Task designates the context or the specific use of the data and is necessary for assessing fitness-for-purpose. The Domain and Task are separate, formal ontologies to which the DQ ontology refers. A Dataset is an arbitrary grouping of Representations of interest. For example, a Dataset can be all of the Representations in the entire EHR.
One of the key concepts in the DQ ontology is the Measure, which is defined as “a quantity that characterizes a quality of the data”. Other possible terms considered were “dimension”, “aspect”, “measurement”, “metric”. Measure was chosen because it captured the notion of quantifying an aspect of interest. The word is used as a noun, not a verb. A Measure is quantified using a MeasurementMethod. A Measurement is a process that performs a MeasurementMethod on a specific Representation (or Dataset) at a point in time that yields a MeasurementResult which is a quantity, usually numeric (but possibly a boolean or text value). A Metric is a statistic about a series of MeasurementResults along a dimension such as time or across patients. For example, a MeasurementResult could indicate that there were 72 data format errors in a Dataset. But a Metric for that situation would be that there were an average of 5.5 data format errors per day or per patient. This part of the DQ ontology was based in part on core concepts from the Ontology for Software Measurement24.
Four high-level data quality dimensions (CorrectnessMeasure, ConsistencyMeasure, CompletenessMeasure and CurrencyMeasure) categorize 19 lower level Measures. “Accuracy” is one of the terms that had many definitions in the literature. In Weiskopf13, she lists at least 3 different ways that the term is used. It sometimes means only correctness but it is also used to represent completeness or plausibility. For that reason, the term “accuracy” has been avoided in the DQ ontology because it is too overloaded. Instead, the term “correctness” was selected to represent this core concept.
Illustrative Example of Using the DQ Ontology
In what follows, an example is provided to illustrate the utility of the DQ ontology concepts. Table 3 lists constraints (using pseudo-code) for some of the Measures. These will be used to show how data quality measures can be computed for a sample Dataset (Table 4) with respect to the task of calculating an eMeasure. An eMeasure30 is a ratio for a health outcome of interest. For example, NQF 0018, “Controlling High Blood Pressure”, is defined to be “The percentage of patients 18–85 years of age who had a diagnosis of hypertension and whose blood pressure was adequately controlled (<140/90mmHg) during the measurement period.”
Table 3:
Examples of Data Quality Measure Constraints
| Measure | Constraint |
|---|---|
| RepresentationConsistency | Representation is valid format |
| DomainConsistency | RepresentationConsistency and (Representation DomainConcepts are in Domain) and DomainComplete and Representation’s DomainConcept Constraints are satisfied |
| CodingConsistency | if Representation is coded text then Representation should have valid code |
| DomainMetadata | Domain ontology is consistent |
| RepresentationComplete | Representation value is not empty |
| DomainComplete | RepresentationComplete or Representation’s DomainConcept cardinality is satisfied |
| Sufficiency | Task SufficiencyConstraint is satisfied |
| DomainCoverage | Domain’s DomainConcepts are subset of Dataset’s DomainConcepts |
| TaskCoverage | DomainCoverage and (Task’s DomainConcepts are subset of Dataset’s DomainConcepts) |
Table 4:
Example Patient Data
| Domain Concept | Patient | ||||
|---|---|---|---|---|---|
| MRN | Age | Encounter | |||
| Diagnosis | BloodPressureObservation | ||||
| Systolic | Diastolic | ||||
| Data Value Type | numeric | numeric | coded text | numeric | numeric |
| Data Value | 1 | 72 | “ICD9:401.0” | 147 | 92 |
| 2 | 81 | “ICD9:401.0” | 142 | “High” | |
| 3 | 77 | “ICD9:401.1” | 140 | ||
| 4 | 60 | “ICD9:xxx” | 92 | 100 | |
| 5 | 44 | “ICD9:401.9” | |||
For the DQ ontology to be applicable, a Domain and a Task need to be defined. In this case, the Task is to calculate the eMeasure defined above and the Domain consists of concepts related to blood pressure as well as some information about the patient and the encounter. To make the example more concrete, a minimalist (and incomplete) Domain and Task ontology will be defined. A portion of a blood pressure (Domain) ontology is shown below (patterned after the openEHR blood pressure clinical model14):
BloodPressureDomain (portion) is an instance of a Domain ontology consisting of:
Patient is a Structure and has 1 MRN, [0 or more] Encounter, 1 Age
Age is a Quantity with a constraint of “Age > 0 and < 120”
Encounter is a Structure with [0 or more] Diagnosis, [0 or more] BloodPressureObservation
BloodPressureObservation has [0 or 1] Systolic, [0 or 1] Diastolic
Systolic is a Quantity with a constraint of “value > 0 and < 1000, Systolic > Diastolic”
Diastolic is a Quantity with a constraint of “value > 0 and < 1000, Systolic > Diastolic”
The Task usually has a formal ontology, but for simplicity’s sake a task definition serves to illustrate how concepts in the Domain are referenced to specify the criteria for the patient population of interest. It defines the semantics of “diagnosis of hypertension” which, in this example, is a value set of codes from the ICD9 terminology. A portion of an example Task instance, TaskNQF0018 is shown below. It is patterned after the eMeasure Quality Data Model (QDM)15.
TaskNQF0018 (portion) is an instance of a Task ontology consisting of:
PatientPopulation refers to Patients Age and Diagnosis:
InclusionCriteria: Diagnosis in {401.0, 401.1, 401.9} and Age ≥ 18 and Age ≤ 85 SufficiencyConstraint: At least 1 BloodPressureObservation per Encounter
Numerator refers to the most recent BloodPressureObservation: Formula is count(BloodPressureObservation.Systolic > 140 and BloodPressureObservation.Diastolic > 90)
Denominator refers to PatientPopulation: Formula is count(PatientPopulation)
Sample patient data is shown in Table 4. Each of the cells in the table shows the value of an instance of a Representation. The topmost column headers indicate the DomainConcept to which each of the cells map. The lower column headers show the DataValueType for the cells in the column. For brevity, other Representation information (entryTime, observedTime, etc.) is not shown.
To assess the quality of the sample data, Measurements that quantify some of the Measures were performed. For this example, the MeasurementMethod evaluates the class constraint of a Measure for all of the Representations in a Dataset and produces a MeasurementResult, which is the proportion of constraints that were satisfied. These results are shown in Table 5. The quantity in the table cell is a fraction where the numerator is the number of constraints that are satisfied and the denominator is the number of Representations for each concept. The cell also shows the decimal equivalent for the fraction. As an example, to compute RepresentationConsistency for the Diastolic DomainConcept, the three Representations in the last column of Table 4 are examined. It can be seen that these Representations have a DataValueType of numeric. But the value for Patient2 is not valid. Therefore, only two of the three Representations have RepresentationConsistency. The rest of the MeasurementResults are shown in the table.
Table 5:
Measurement Process Summary for Some Measures
| Measure | Measurement Process Summary | MeasurementResult | ||||
|---|---|---|---|---|---|---|
| Systolic | Diastolic | BloodPressure Observation | Encounter | Patient | ||
| Measures that involve only the Representation | ||||||
| RepresentationConsistency | Satisfied if all Representations conform to their DataValueTypes. Patient2. Encounter.Blood PressureObservation.Diastolic is an invalid value. | 4/4 1.0 |
2/3 .67 |
3/4 .75 |
4/5 .80 |
4/5 .80 |
| RepresentationComplete | Patient3.Encounter.BloodPressureObservation.Diastolic has a missing value so it is not RepresentationComplete. | 4/4 1.0 |
2/3 .67 |
3/4 .75 |
4/5 .80 |
4/5 .80 |
| Measures that involve the Representation and Domain | ||||||
| DomainConsistency |
DomainConsistency is satisfied if all of the concepts in the Domain exist in the data (true for this example). Also, the data must have RepresentationConsistency (Patient2 does not) and all of the constraints for all of the Domain concepts must be satisfied. Patient4 has a diastolic blood pressure value that is higher than the systolic value, so the constraint is not satisfied. But Patient5’s missing BloodPressureObservation is allowed by the Domain. |
3/4 .75 |
1/3 .33 |
1/4 .25 |
2/5 .40 |
2/5 .40 |
| CodingConsistency | True if all coded text Representations have valid values. Patient4.Encounter.Diagnosis is invalid in the ICD9 terminology. |
4/5 .80 |
4/5 .80 |
|||
| DomainMetadata | The Domain ontology is defined and contains no logical inconsistencies. It would be considered inconsistent if it contained another rule that stated patient age was optional (i.e. “Patient has [0 or 1] Age”). | 4/4 1.0 |
3/3 1.0 |
4/4 1.0 |
5/5 1.0 |
5/5 1.0 |
| DomainComplete | Even though Patient5.Encounter.BloodPressureObservation.Diastolic is missing, the Domain ontology indicates that it is optional, so the constraint is satisfied. | 4/4 1.0 |
4/4 1.0 |
4/4 1.0 |
5/5 1.0 |
5/5 1.0 |
| DomainCoverage | Satisfied since all of the Domain concepts are represented in the data. | 4/4 1.0 |
3/3 1.0 |
4/4 1.0 |
5/5 1.0 |
5/5 1.0 |
| Measures that involve the Representation, Domain and Task | ||||||
| Sufficiency | The Task specifies a SufficiencyContraint that requires at least 1 BloodPressureObservation must exist during the assessment period. Patient5 and Patient3 don’t have valid blood pressure observations recorded. |
3/5 .60 |
3/5 .60 |
|||
| TaskCoverage | TaskCoverage is satisfied if the Task concepts are a subset of the concepts represented in the Dataset. In this case, only the data at the Patient level has all of the Task concepts represented. Therefore, the eMeasure can only be calculated when all the data from the Patient level and below is available. | 0/4 0.0 |
0/3 0.0 |
0/4 0.0 |
0/5 0.0 |
5/5 1.0 |
This example shows how the DQ ontology enables a meaningful discussion of data quality characteristics required for computing an eMeasure. It also illustrates a method for quantifying each Measure by evaluating the proportion of constraints satisfied by the Representations.
Discussion
The DQ ontology presented in this study harmonized data quality concepts from the literature and provides a practical framework to evaluate data quality in health care through explicit definitions using constraints and relationships between concepts. The ontological approach provides more precise definitions of concepts than simply relying on natural language, it enables computation of a quantity for a Measure (MeasurementResult) and it makes explicit the relationship between the DQ ontology and the Task and Domain ontologies. This allows the DQ ontology to be reused for different Domains and for different Tasks without having to devise new Measures. The benefit of specifying these as separate ontologies was demonstrated in the previous section. For example, when calculating the DomainConsistency Measure, constraints from the Domain ontology (i.e. “Systolic > Diastolic”) can be referenced when computing MeasurementResults without having to change the definition of the MeasurementMethod (or the computer program that implements it). The same benefit is true when calculating the Sufficiency Measure. A SufficiencyConstraint can be evaluated for different Task ontologies to yield a MeasurementResult without having to change how Measures are defined. Not having to invent a new data quality framework for every research project should make validating data quality more common and reproducible.
Precisely defining both the Domain and Task ontology are very important in accurately describing what each data quality Measure means. Some of the Measures have constraints that reference the Task; these are clearly context dependent. Other Measures reference only the Representation or the Domain and are task independent. The constraints make clear exactly how aspects of each are related and help sharpen definitions. An example will illustrate this. DomainConsistency and RepresentationConsistency often get intertwined in definitions found in the literature. Liaw6 listed a number of sub-meanings under his “Consistency” dimension. One sub-definition (“Consistency: Representation of data values is same in all cases”) is equivalent to RepresentationConsistency, but he did not list an exact equivalent to the concept of DomainConsistency. The closest mapping is “Accuracy: Refers to values and representation of output data”. On the other hand, Weiskopf13 separated and clearly defined these differences. The concept of RepresentationConsistency is embodied as “Concordance: Is there agreement between elements in the EHR, or between the EHR and another data source?” and the concept of DomainConsistency is well defined as “Plausibility: Does an element in the EHR makes sense in light of other knowledge about what that element is measuring?” But there is an issue in the “Concordance” definition in that the last part of her definition “…or between the EHR and another data source” includes reference to another Measure (RelativeCorrectness). A Representation can have RepresentationConsistency without having DomainConsistency, but the reverse is not true. This is reflected in the constraint for DomainConsistency by explicitly referring to RepresentationConsistency as part of the definition. This also highlights the usefulness of a shared vocabulary for data quality. It makes it possible to discuss nuances of data quality characteristics.
Another issue that occurs frequently in the literature is the term “accuracy;” there is an assumption that it is possible to know what is absolutely true about the world. For EHR data, there are no true gold standards for comparison. There are only other sets of data whose “accuracy” is unknown which can be referred to as relative gold standards.31 Comparing one dataset to another to yield a positive predictive value (PPV) and sensitivity measure are a useful way to characterize the data.32 The concept of RelativeCorrectness measures whether data is likely correct by matching a Representation to its counterpart in another Dataset. The matches are considered true positives and are divided by the number of Representations in the Dataset to yield a PPV as a CorrectnessMeasure. Similarly, RelativeCompleteness looks to see which “truths” of the world are captured in the EHR data. If a Representation is present in one Dataset and is also present in the other “relative gold standard”, then these true positives are divided by the number of Representations in the other Dataset to yield sensitivity as a measure of how complete the first Dataset is.
There are a number of limitations to the current research. Data quality concepts described in the meta-analyses were harmonized and mapped to concepts in the DQ ontology. Care was taken to map based on meaning or context of use, but since the meaning was from an interpretation of a definition (or sometimes, a single term), the mapping might not represent what the author of the meta-analyses intended. This research depended heavily on the core data quality concepts contained in the meta-analyses. The literature search may not have been exhaustive in finding all of the meta-analyses or there may be important data quality concepts that were not discussed in those papers. Since many data quality concepts are repeated amongst the papers, it is likely that the most important ones were captured. It is expected that additional data quality concepts will be added to the DQ ontology as the need for having a formal definition for the concept arises. Concepts that did not appear in at least three of the papers were not included in the DQ ontology. This includes concepts such as objectivity, non-duplication, security and privacy. Future work is needed to incorporate these into the DQ ontology. The concept of DomainComplete is currently too simplistic. It will need to be expanded to better define types of missing data as missing completely at random (MCAR), missing at random (MAR) and missing not at random (MNAR).
The DQ ontology is applicable to structured EHR data. Additional research is needed to extend the DQ ontology to notes and other unstructured data present in EHRs. Natural language processing (NLP) techniques may be used to parse relevant DomainConcepts from the unstructured information. In that case, the DQ assessment techniques described in this paper could be used to characterize that portion of the data.
The next phase of this research is to use the DQ ontology to perform data quality Measurements on actual EHR data. A Domain ontology for a clinical area will be developed in full and mapped through Representations to EHR DataValues. Similarly, a formal Task ontology will be created and referenced by the data quality Measures. The constraints for the DQ ontology Measures will be written in a formal language, which can then directly be used to compute MeasurementResults and Metrics for a real-world Dataset.
Conclusion
The healthcare data quality literature was mined for the important terms used to describe data quality concepts. These terms were harmonized into a DQ ontology that represents core data quality concepts. Four high-level data quality dimensions (CorrectnessMeasure, ConsistencyMeasure, CompletenessMeasure and CurrencyMeasure) categorize 19 lower level Measures. These concepts serve as an unambiguous vocabulary when discussing healthcare data quality. The class constraints precisely define concepts better than using natural language and provide a mechanism to automatically compute MeasurementResults to quantify data quality. The DQ ontology can be reused with different clinical Domain and Task ontologies to make validating data quality more common and reproducible.
References
- 1.King J, Patel V, Furukawa MF. Physician Adoption of Electronic Health Record Technology to Meet Meaningful Use Objectives: 2009–2012. 2012. [Google Scholar]
- 2.Ancker JS, Shih S, Singh MP, et al. Root Causes Underlying Challenges to Secondary Use of Data. AMIA Symp Proc. 2011:57–62. [PMC free article] [PubMed] [Google Scholar]
- 3.Dean BB, Lam J, Natoli JL, Butler Q, Aguilar D, Nordyke RJ. Review: use of electronic medical records for health outcomes research: a literature review. Med Care Res Rev. 2009;66(6):611–638. doi: 10.1177/1077558709332440. [DOI] [PubMed] [Google Scholar]
- 4.Orr K. Data Quality and Systems Theory. Commun ACM. 1998;41(2):66–71. [Google Scholar]
- 5.Juran JM, Godfrey AB. Juran’s Quality Control Handbook. 5th ed. New York: McGraw-Hill; 1999. [Google Scholar]
- 6.Liaw S, Rahimi A, Ray P, et al. Towards an ontology for data quality in integrated chronic disease management: a realist review of the literature. Int J Med Inform. 2013;82(1):10–24. doi: 10.1016/j.ijmedinf.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Studer R, Benjamins R, Fensel D. Knowledge engineering: Principles and methods. Data Knowl Eng. 1998;25(1–2):161–198. [Google Scholar]
- 8.Staab S, Studer R. Handbook on Ontologies. Springer; 2010. [Google Scholar]
- 9.Horrocks I. What Are Ontologies Good For? Evol Semant Syst. 2013:175–188. [Google Scholar]
- 10.Wang RY, Strong DM. Beyond Accuracy: What Data Quality Means to Data Consumers. J Manag Inf Syst. 1996;12(4):5–33. [Google Scholar]
- 11.Kahn MG, Raebel MA, Glanz JM, Riedlinger K, Ascp MT, Steiner JF. ANALYTIC METHODS: A Pragmatic Framework for Single-site and Multisite Data Quality Assessment in Electronic Health. Med Care. 2012;50(7):21–29. doi: 10.1097/MLR.0b013e318257dd67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almutiry O, Wills G, Crowder R. Toward a Framework for Data Quality in Electronic Health Record. 2013. [Google Scholar]
- 13.Weiskopf NG, Weng C. Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Informatics Assoc. 2012:2–8. doi: 10.1136/amiajnl-2011-000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heard S. openEHR Clinical Knowledge Manager. openEHR. 2015. [Accessed January 1, 2015]. http://openehr.org/ckm/
- 15.National Quality Forum . Quality Data Model December 2013. 2013. [Google Scholar]
- 16.Noy NF, McGuinness DL. Ontology Development 101: A Guide to Creating Your First Ontology. 2001. [Google Scholar]
- 17.Gamma E, Helm R, Johnson R, Vlissides J. Design Patterns: Elements of Reusable Object-Oriented Software. Pearson Education; 1994. [Google Scholar]
- 18.Chen H, Hailey D, Wang N, Yu P. A review of data quality assessment methods for public health information systems. Int J Environ Res Public Health. 2014;11:5170–5207. doi: 10.3390/ijerph110505170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima CRDA, Schramm JMDA, Coeli CM, Silva MEM Da. Revisão das dimensões de qualidade dos dados e métodos aplicados na avaliação dos sistemas de informação em saúde. Cad Saúde Públ. 2009;25(10):2095–2109. doi: 10.1590/s0102-311x2009001000002. [DOI] [PubMed] [Google Scholar]
- 20.Wand Y, Wang RY. Anchoring Data Quality Dimensions in Ontological Foundations. Commun ACM. 1996;39(11):86–95. [Google Scholar]
- 21.Chan KS, Fowles JB, Weiner JP. Electronic Health Records and the Reliability and Validity of Quality Measures: A Review of the Literature. Med Care Res Rev. 2010;67(5):503–527. doi: 10.1177/1077558709359007. [DOI] [PubMed] [Google Scholar]
- 22.Canadian Institute of Health . The CIHI Data Quality Framework. 2009. [Google Scholar]
- 23.Stvilia B, Gasser L, Twidale MB, Smith LC. A Framework for Information Quality Assessment. J Am Soc Info Sci Tech. 2007;58(12):1720–1733. doi: 10.1002/asi. [DOI] [Google Scholar]
- 24.Bertoa M, Vallecillo A. An Ontology for Software Measurement. In: Calero C, Ruiz F, Piattini M, editors. Ontologies for Software Engineering and Software Technology. Heidelberg: Springer; 2006. pp. 175–196. [Google Scholar]
- 25.Pipino LL, Lee YW, Wang RY. Data quality assessment. Commun ACM. 2002;45(4):211. doi: 10.1145/505248.506010. [DOI] [Google Scholar]
- 26.Fox C, Levitin A, Redman T. The notion of data and its quality dimensions. Inf Process Manag. 1994;30(1):9–19. [Google Scholar]
- 27.W3C OWL Working Group OWL 2 Web Ontology Language Document Overview. 2012. http://www.w3.org/TR/owl2-overview/
- 28.Object Management Group Ontology Definition Metamodel. 2014. http://www.omg.org/spec/ODM/1.1/
- 29.Beale ET, Heard S. Architecture Overview. 2008:1–79. [Google Scholar]
- 30.Centers for Medicare & Medicaid Services The CMS EHR Incentive Programs: Small-Practice Providers and Clinical Quality Measures. 2011. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/CQM_Webinar_10-25-2011.pdf.
- 31.Kahn MG, Eliason BB, Bathurst J. Quantifying clinical data quality using relative gold standards. AMIA Annu Symp Proc. 2010;2010:356–360. [PMC free article] [PubMed] [Google Scholar]
- 32.Hogan WR, Wagner MM. Accuracy of data in computer-based patient records. J Am Med Inform Assoc. 1997;4(5):342–355. doi: 10.1136/jamia.1997.0040342. [DOI] [PMC free article] [PubMed] [Google Scholar]