Abstract
Prolonged diarrhea is usually defined as acute-onset diarrhea lasting 7 days or more, but less than 14 days. Its trend has been declining in recent years because of improvement in the management of acute diarrhea, which represents the ideal strategy to prevent prolonged diarrhea. The pathogenesis of prolonged diarrhea is multifactorial and essentially based on persistent mucosal damage due to specific infections or sequential infections with different pathogens, host-related factors including micronutrient and/or vitamin deficiency, undernutrition and immunodeficiency, high mucosal permeability due to previous infectious processes and nutrient deficiency with consequential malabsorption, and microbiota disruption. Infections seem to play a major role in causing prolonged diarrhea in both developing and developed areas. However, single etiologic pathogens have not been identified, and the pattern of agents varies according to settings, host risk factors, and previous use of antibiotics and other drugs. The management of prolonged diarrhea is complex. Because of the wide etiologic spectrum, diagnostic algorithms should take into consideration the age of the patient, clinical and epidemiological factors, and the nutritional status and should always include a search for enteric pathogens. Often, expensive laboratory evaluations are of little benefit in guiding therapy, and an empirical approach may be effective in the majority of cases. The presence or absence of weight loss is crucial for driving the initial management of prolonged diarrhea. If there is no weight loss, generally there is no need for further evaluation. If weight loss is present, empiric anti-infectious therapy or elimination diet may be considered once specific etiologies have been excluded.
Keywords: Prolonged Diarrhea, Persistent Diarrhea, Children, Malnutrition
Introduction and context
Diarrheal disorders are a major health problem in pediatrics worldwide. Accounting for more than 750,000 deaths in children under the age of 5 per year, they are the second leading cause of death in this population according to the World Health Organization (WHO) 1. Definitions of diarrheal episodes are usually based on the duration of symptoms rather than etiology. However, there is little consistency and agreement on the definition of acute, prolonged, persistent, and chronic diarrhea in pediatric and adult subjects and in developed and developing countries. In a systematic review on 138 trials, Johnston et al. identified 64 different definitions of diarrhea and 69 definitions of diarrhea resolution. The definitions provided by the WHO were the most commonly used ( Table 1) 2.
Table 1. Definitions of diarrheal illnesses.
| Diarrhea | The most commonly recognized definition of diarrhea is based on World Health Organization parameters and
define diarrhea by the passage of 3 or looser than normal stools in the preceding 24-hour period. An episode of diarrhea is defined as lasting 1 day or more and usually ends after at least 2 days without diarrhea. |
| Acute diarrhea | Episode of self-limiting diarrhea with acute onset, typically lasting 5 to 7 days. In most cases, it is due to an
intestinal infection and may be combined to fever and vomiting, meeting the definition of acute gastroenteritis. Acute diarrhea may be also related to extra-intestinal infections (i.e. urinary infection, viral respiratory infections), food-poisoning, iatrogenic intestinal damage (i.e. chemotherapy, radiotherapy) or other intestinal and extra-intestinal diseases such as acute appendicitis. |
| Prolonged diarrhea | Acute onset diarrhea lasting from 7 to 14 days not covering the definition of persistent diarrhea. It is usually
due to persistent infections or to post-infectious intestinal damage (i.e. carbohydrate malabsorption, small intestine bacterial overgrowth) that may prolong the duration of diarrhea behind the expected time. Some experts refer to this as acute-protracted diarrhea. |
| Persistent diarrhea | Diarrhea lasting 14 days or more, usually associated with weight loss, ultimately leading to severe nutritional
impairment and that may require clinical nutrition. The classical definition of persistent diarrhea was intended to exclude some causes of chronic diarrhea such as celiac disease or inflammatory bowel diseases. |
| Chronic diarrhea | In many contexts chronic diarrhea is a synonymous of persistent diarrhea. The World Health Organization
uses this definition rather than persistent diarrhea. However, chronic diarrhea usually does not have an acute onset and is the manifestation of structural and inflammatory bowel disorders. Some experts refer to chronic diarrhea in case of episodes lasting more than 4 weeks. |
| Post-infectious diarrhea | Acute onset diarrhea lasting 7 to 14 days and following an episode of acute gastroenteritis. This definition is
covered by prolonged diarrhea. |
| Intractable diarrhea | Non-infectious diarrhea lasting more than 14 days, intractable despite extensive hospital therapy.
Typical of young infants, usually below 3 months (but not only). Typically needs intravenous fluids or clinical nutrition and is related to high mortality. |
| Congenital diarrhea | Congenital diarrhea is an inherited enteropathy with a typical onset early in life. For many of these conditions,
severe chronic diarrhea represents the main clinical manifestation, while in others, diarrhea is only a component of a more complex multi-organ or systemic disease. |
Most diarrheal illnesses last 5–7 days and are due to self-limiting intestinal infections. These episodes are usually defined as acute diarrhea (AD). According to WHO, episodes of diarrhea are usually classified as AD when they last up to a maximum of 14 days and “persistent or chronic diarrhea” when they last >14 days 3. However, a subset of children experiences an acute-onset diarrhea lasting 7 days or more, but less than 14 days; this may be defined as prolonged diarrhea (ProD) and usually indicates an episode that continues beyond the expected duration of a typical acute infectious diarrhea 4. This entity accounts for a relatively small but consistent number of diarrheal episodes that, especially in developing countries, is associated with a high risk of mortality and morbidity 4. In this review, we define ProD as an episode of diarrhea of acute onset lasting 7 to 13 days and persistent diarrhea (PD) as diarrhea lasting 14 or more days.
Epidemiology and risk factors
Data on the etiology and epidemiology of ProD are limited because of the huge variability in defining diarrheal disorders. Some evidence may be extrapolated from studies reporting data on PD 5– 7.
In a study in Israeli children, about a quarter of the study population presented with ProD (lasting 8–13 days) and 14–18% with PD (14 or more days’ duration) 6. However, Moore et al. firstly proposed the definition of ProD as a specific disorder and reported an incidence of 12% of all diarrheal cases in a large Brazilian cohort, accounting for a quarter of all days of diarrhea recorded in the 10-year study period 4. In the same population, less than 5% presented with PD. It should be noted that when a diarrheal episode progresses from acute to ProD, there is a 6-fold higher risk that the episode will evolve into PD 4.
ProD is more common in children aged 6 to 24 months and peaks in the second semester of life 4. Children who developed ProD in their first year of life have a doubled risk of developing PD at pre-school age 4. In addition, children experiencing severe diarrhea and dysenteric illnesses with blood and mucus in their stools are more likely to present with a course longer than those who present with mild-to-moderate diseases 8, 9.
These findings, that demonstrate a close relationship between ProD and PD, may be due to different mechanisms: on one hand, ProD affects child growth and mucosal immunity and impacts on gut microflora and intestinal barrier functions; on the other hand, the increased risk of subsequent episodes may be related to specific individual features or to genetic, nutritional, or environmental characteristics that predispose to persistent intestinal illnesses. Mainly in developing areas, ProD is linked with malnutrition in a complex cause-effect relationship, implicating a multifactorial “vicious cycle” involving intestinal infections, microflora disruption, micronutrient deficiency, and immunodeficiency. The role of malnutrition is supported by the evidence that non-breast-fed children and those who are weaned early or recently exposed to formula, as well as children with underlying malnutrition, vitamin deficiency, and wasting, are at increased risk of developing ProD 4, 8– 10.
Environmental factors also contribute to ProD, since living in poor areas with poor hygiene conditions and low mothers’ education expose children to a doubled risk of developing ProD 4.
Finally, the risk of ProD is reduced by half for 10 years’ increase in maternal age, and if a mother completes primary school, the risk of ProD and PD in her child decreases 6.
Irrespective of the etiology and risk factors, children with ProD have a higher risk of nutritional derangement, micronutrient deficiency, risk of developing PD, infections, and immunodeficiency.
Etiology and pathophysiology
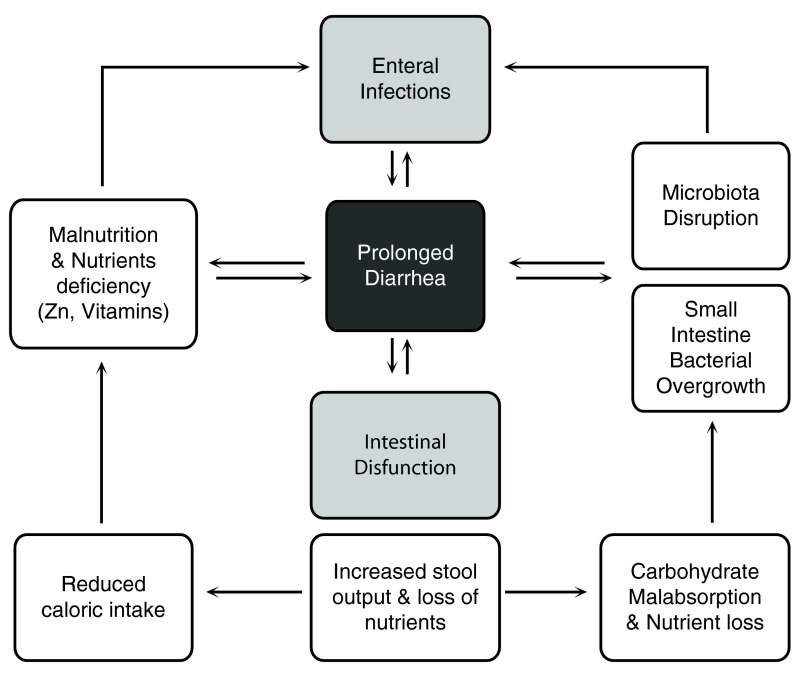
The pathogenesis of ProD is multifactorial and essentially based on 1) persistent mucosal damage due to specific agents or sequential infections with different pathogens, 2) host-related factors including micronutrient and/or vitamin deficiency, undernutrition, and immunodeficiency, 3) high mucosal permeability due to previous infectious processes and nutrient deficiency with consequent malabsorption, and 4) microbiota disruption ( Figure 1). In some cases, ProD may represent the onset of chronic intestinal disorders including celiac disease, inflammatory bowel disease (IBD), and autoimmune enteropathies that are usually characterized by PD.
Figure 1. Multifactorial etiology of prolonged diarrhea.
“Vicious cycle” of prolonged diarrhea involves intestinal infections, microflora disruption, micronutrient deficit, undernutrition, and immunodeficiency.
Table 2. Etiology of Prolonged Diarrhea in children.
HIV: human immunodeficiency virus.
| Infections |
|---|
| Viruses |
| Rotavirus
Norovirus Sapovirus Astrovirus Cytomegalovirus (HIV-infected children) |
| Bacteria |
| Shigella
Enteroaggregative E. coli (EAgg EC) Enteropathogenic E. coli (EPEC) Clostridium difficile Campylobacter Yersinia Mycobacterium avium complex (HIV-infected children) |
| Parasites |
| Cryptosporidium
Giardia lamblia Cyclospora Entamoeba histolytica |
| Small Intestine Bacterial Overgrowth – SIBO |
| Underlying malnutrition |
| Vitamins and Micronutrients deficiency (Zinc, Vitamin A) |
| Food-induced diarrhea |
| Lactose intolerance
Carbohydrate malabsorption Cow-milk protein intolerance Food allergy Celiac disease |
| Antibiotic-associated diarrhea |
In children with PD, electron microscopy shows shortening of villi, decrease in number and height of microvilli, blunting of borders of enterocytes, loss of glycocalyx, and presence of mucous pseudomembranes coating the epithelial surface. In addition, children with PD often have marked mucosal inflammation when compared to children with AD, presenting higher interferon-gamma response, significant elevation of fecal lactoferrin, interleukin (IL)-8, and IL-1β, and a higher percentage of CD8 + T cells 11– 13. More than half of children with severe AD or ProD develop protein-losing enteropathy 14. Also, children with ProD share some of these immunological characteristics.
Microbial agents
Although infections are a major cause of ProD, there is no clear evidence of a role for selected pathogens in inducing ProD 6, 8, 14.
In developing countries, many of the bacterial pathogens responsible for AD and dysentery also cause PD. Invasive diarrhea due to Shigella contributes to ProD and PD, and the prevalence of Shigella among children with ProD and PD is higher than in those with AD 6, 8, 15– 17. Enteropathogenic Escherichia coli have been found in at least 25% of children tested in low- and middle-income countries and may cause ProD in some settings 5, 7, 8.
Selected viruses may be responsible for ProD. Rotavirus, norovirus, and sapovirus have been found in as many as 50% of diarrheal episodes resolving within the third week in children living in the United States 18. In some cases, such as rotavirus infection, ProD could be due to susceptibility to other infections or to nutrient malabsorption rather than being the direct result of the pathogenic agent 11. About 10% of astrovirus infections may complicate AD with prolonged episodes of diarrhea, even in non-immunocompromised patients 19.
Parasites including Giardia, Cryptosporidium, and Cyclospora have been related to ProD and PD in developing areas. Cryptosporidium, a common agent of ProD and PD in HIV-infected children, was frequently isolated also in immunocompetent children 4.
Although 35–70% of children with PD tested positive for at least one pathogen, multiple isolations are common, making it difficult to distinguish bystanders from agents causing illness 5, 6.
In addition, the pattern of microorganisms that tends to be associated with ProD is different in developed and developing countries. In the former, viruses are more frequently found, whereas in developing countries, specific bacteria and protozoa are more common.
Small intestinal bacterial overgrowth
A further mechanism that may cause ProD is so-called small intestinal bacterial overgrowth (SIBO). It involves the colonization of the small intestine by bacteria that are usually found in the colonic microbiota or an increase in their number. SIBO is more common in children with underlying intestinal diseases, such as blind loop syndrome, dysmotility, or inflammatory diseases of the intestine. However, SIBO may be a possible complication of AD due to recent infection, the presence in the intestinal lumen of carbohydrates and short-chain fatty acids, the temporary alteration of gut motility, or previous antimicrobial use. It has been associated with antacid therapy. The bacterial overgrowth in the small intestine causes inflammation and malabsorption of liposoluble vitamins and steatorrhea with further worsening of diarrhea and malnutrition.
Malnutrition and malabsorption
ProD and PD are commonly seen in association with malnutrition and micronutrient deficiencies in developing areas. The latter conditions cause the impairment of immunological mechanisms for clearing infections as well as delayed intestinal repair, thereby contributing to the vicious cycle between diarrhea and malnutrition 20.
PD is also due to malabsorption of select nutrients. Food intolerance and food allergy may be responsible for ProD. The mucosal damage secondary to infection leads to loss of lactase activities with a consequent malabsorption of carbohydrates that, due to their osmotic power, worsen and perpetuate diarrhea.
In addition, the increased permeability related to both acute infection and underlying malnutrition may potentially lead to food-antigen sensitization.
Post-infectious irritable bowel syndrome
Some children and adolescents may develop functional gastrointestinal symptoms, including PD, following a single infectious episode of diarrhea. The clinical picture is characterized by diarrhea and abdominal pain without loss of body weight. This is a syndrome defined as chronic non-specific diarrhea of childhood or “toddler’s diarrhea” and is currently named post-infectious irritable bowel syndrome. The Rome III criteria define the type of symptoms and their duration in age groups 21. Based on age-related criteria, the diagnosis can be made without further investigation.
Diagnostic approach
The management of ProD is complex because the etiology and pathogenesis are complex. Furthermore, the majority of available data on management and treatment are focused on PD rather than on ProD and very few studies have examined ProD as a distinct category 4.
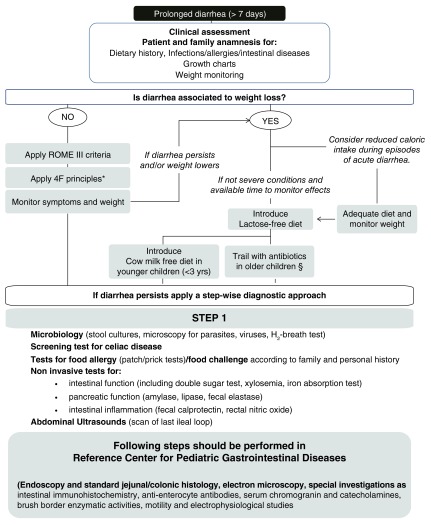
In developing countries, the main consequences of ProD are nutritional derangement, morbidity (with an increased risk of hospital admission), and even death 14. In this setting, it is difficult to perform expensive and time-consuming tests to identify the etiology of ProD. Optimal management of AD is the ideal strategy to prevent ProD. It includes appropriate fluid replacement, zinc treatment, and optimal nutrition in developing countries 22. Rotavirus vaccination, promotion of breast-feeding, promotion of hand washing, improved water supply and quality, and community-wide sanitation indirectly prevent ProD 23, 24. However, the diagnostic approach to and the treatment of ProD is a challenge. Because of the wide etiologic spectrum, diagnostic algorithms of ProD should also include the age of the patient, clinical and epidemiological factors, and the results of microbiological investigations, when available. An algorithm for ProD is provided in Figure 2. The algorithm is intended as a general tool, and decisions must take into consideration medical history and clinical features of individual cases as described below.
Figure 2. Diagnostic and therapeutic approach to prolonged diarrhea.
*Feeding pattern should be normalized according to the “4F” role: fat (increase dietary lipids to at least 35–40% of total daily energy intake), fiber (normalize fiber intake by introduction of fruits and wholegrain bread), fluid (restrict fluid intake if history is significant for high fluid consumption), and fruit juice (discourage overconsumption of fruit juices, especially those containing sorbitol or a high fructose/glucose ratio).
§ Empiric antibiotic treatment should cover most probable enteric infections ( Shigella and enteropathogenic Escherichia coli) and/or small intestine bacterial overgrowth.
Clinical evaluation
Specific clues in the family and personal history (previous episodes of AD or ProD, history of chronic diseases or food allergy, and immunological status) may provide useful indications.
Initial clinical examination should include the evaluation of general and nutritional status and the presence of weight loss. Dehydration requires prompt supportive interventions to stabilize the child. Malnutrition may precede the onset of diarrhea, contributing to its duration, or it could be the consequence of the disease. The presence or absence of weight loss may help drive the subsequent diagnostic and therapeutic approach ( Figure 2).
Some children may lose weight as a consequence of poor caloric input after an episode of AD, and in these cases, reintroduction of a free diet is generally effective. However, most children with ProD and weight loss deserve specific medical interventions and careful follow up. If first-line therapeutic interventions do not result in a clear clinical improvement and diarrhea persists, a referral to the gastroenterologist is needed ( Figure 2).
If there is no evidence of weight loss, in most cases there is no need for further investigation. However, a clinical re-evaluation with assessment of the trend of symptoms and weight and revision of nutritional regimens may be considered. Children with persistence of symptoms may need to be referred to the gastroenterologist for further care.
Laboratory and other investigations
The initial diagnostic workup for children with ProD should include stool cultures and a search for parasites and enteric viruses. However, the wide pattern of microorganisms potentially involved requires high-level techniques.
Although traditional culture is still an invaluable tool in clinical settings, in some instances other techniques are needed for the identification and differentiation of bacterial species 25. For other pathogens, it is more important to identify the toxins than the organisms themselves, as in the case of enterotoxigenic E. coli, Shiga toxin of enterohemorrhagic E. coli, and Clostridium difficile 26.
Light microscopy represents the traditional technique used to diagnose intestinal parasites. However, its sensitivity depends on the burden of infection, the stage and delivery to the labs of the specimen, and the experience of the observer 26. More sensitive and specific enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) analysis are used to detect protozoa in fecal samples, but these assays are still not routinely available.
Enteric viruses are commonly searched for by means of ELISA and latex agglutination analysis. However, molecular genetic techniques detect a wider spectrum of pathogens than conventional techniques and provide information about their molecular epidemiology 25. Again, these techniques are not readily available in primary care settings and in resource-limited areas.
A search for SIBO should also be included in the diagnostic work-up of ProD. Breath hydrogen test may identify an abnormal bacterial proliferation in the small bowel; it can also be used to detect carbohydrate malabsorption 27. However, the sensitivity and specificity of this test is low and it is difficult to perform, mainly in young children. Alternatively, a simple measurement of stool pH or a test for reducing substances (Clinitest) may be easily done at the bedside to investigate carbohydrate (usually lactose) malabsorption. Other specific diagnostic tests, including evaluation of intestinal function and inflammation, imaging, and endoscopy, should be performed in case of persistence of diarrhea in the setting of pediatric gastroenterology.
Non-invasive diagnostic tests for intestinal and pancreatic function (including dual absorption test, xylosemia, iron oral load, lipase, and fecal elastase) and inflammation (fecal calprotectin and last ileal loop abdominal ultrasound) may provide useful information for diagnosis, when available 27, 28. Although abdominal ultrasound may be affected by subjective evaluation if an expert consultant is available, it may be of support in management. The association of a negative scan of last ileal loop with negative fecal calprotectin significantly reduces the possibility of IBD 28. The use of noninvasive diagnostic tests in the diagnostic algorithm may reduce invasive procedures. However, if diagnosis is not obtained otherwise, endoscopy with biopsy should be considered.
In young children with a familial history of atopy and suggestive personal history and clinical features, skin tests for the screening of food allergy (including patch tests and prick tests) may be performed and elimination diet with subsequent challenge should be considered 29.
Treatment
Empiric treatment
Expensive laboratory evaluations usually are of little benefit in guiding the successful treatment of ProD, and an empirical therapeutic approach may be effective in the majority of cases ( Figure 2).
If there is no weight loss, the approach should be conservative and no investigations are generally needed. A review of the diet is worthwhile, including a check on a possible excess of sugar-containing drinks as a cause of diarrhea. Feeding pattern should be normalized according to the “4F” rule: fat (increase dietary lipids to at least 35–40% of total daily energy intake), fiber (normalize fiber intake by introduction of fruits and wholegrain bread), fluid (restrict fluid intake if history is significant for high fluid consumption), and fruit juice (discourage overconsumption of fruit juices, especially those containing sorbitol or with a high fructose/glucose ratio).
A lactose-free diet can be started if weight loss is present. Some children with ProD who do not have weight loss at first presentation may benefit from a lactose-free diet since disaccharidase deficiency secondary to AD is relatively common. Exclusion diets are usually administered with the double purpose of overcoming food intolerance, which may be the primary cause of ProD, or its complication. The sequence of elimination should be graded from less (e.g. cow’s milk protein hydrolysate) to more restricted diets (amino acid-based formula) according to the child’s clinical conditions. This approach should be reserved for infants and young children.
Antimicrobials
ProD is often an infection-induced illness in the majority of cases 30. However, considering that pathogens associated with ProD are also often found in healthy children without diarrhea 7, even when an enteric pathogen is detected, it is not always clear that this is the cause of the illness.
Antimicrobial agents are indicated for the treatment of selected parasites 31 and selected enteropathogenic bacteria, such as enteropathogenic E. coli and enteroaggregative E. coli 14. Pathogens such as Shigella and Cryptosporidium are commonly associated with ProD in tropical, developing countries and should be treated in case of ProD 4.
Nitazoxanide is a broad-spectrum antimicrobial agent with activity against protozoa, nematodes, cestodes, trematodes, and bacteria, with a favorable safety profile 32, 33. It is effective in childhood cryptosporidiosis 34 but not consistently in undernourished children or in HIV-infected patients 35. Anecdotal cases of children successfully treated with nitazoxanide because of PD (<30 days) have been reported 36. This strategy seems to be effective in select situations, saving time-consuming tests to identify the cause of diarrhea 36.
Metronidazole can be used for Giardia, and trimethoprim-sulfamethoxazole can be used for Cyclospora and as a second-line antibacterial drug for a number of pathogens 14.
Very few data on the treatment of viral ProD are available. Human immunoglobulin, available for intravenous use, may be administered orally (300 mg/kg of body weight) in a single dose. The rationale of passive immunotherapy is based on the demonstration of neutralizing antibodies against all viruses in a medical preparation of immunoglobulins 37. They are found in the stools after administration, and this treatment reduces the duration of stay in severe and/or immunocompromised patients with AD and in patients with severe diarrheal episodes due to rotavirus 38, 39. This treatment seems to also have a potential role in immunocompromised patients with norovirus enteritis 40. In this population, a positive trend towards resolution of diarrhea and decreased stool output in the treatment group compared with placebo was found, but no benefit was reported for length of hospital stay or hospital cost 40. However, no data on oral immunoglobulins and viral-ProD is available, with the exception of rotavirus 41.
Ciprofloxacin is effective in cases of diarrhea associated with enteroaggregative E. coli in HIV-infected adults 42. However, its efficacy needs to be proven in a large-scale trial in children with ProD.
The efficacy of antimicrobials in children with ProD in whom etiology is unknown is even more controversial. In a recent systematic review in young children with PD of unknown or non-specific cause from developing countries, no difference was demonstrated for oral gentamicin or metronidazole compared with placebo, whereas sulfamethoxazole-trimethoprim was more effective than placebo for diarrhea at 7 days and for total stool volume 43. To date, the evidence to recommend the use of antibiotics in ProD of unknown or non-specific causes is still limited.
Probiotics
Some data support the use of probiotics in ProD. Lactobacillus spp. and Saccharomyces boulardii significantly reduced the number of stools and duration of diarrhea in children with PD 44.
A recent Cochrane review showed that probiotics shortened the duration of diarrhea and reduced stool frequency and hospital stay. However, only four trials with a small number of participants were available for meta-analysis. The authors concluded that, although probiotics appear to hold promise as adjunctive therapy, there is insufficient evidence to recommend their routine use in children with ProD 45.
Nutritional interventions
Dietary treatment is crucial in ProD. However, nutritional interventions are often expensive and poorly applicable in developing countries. Locally available, inexpensive foods and vitamin and mineral supplementation have been proposed 46– 48. Locally tailored nutritional interventions are ideal in the developing world: they are inexpensive and culturally acceptable, provide sufficient levels of energy and nutrients to malnourished children, and allow the continuation of dietary therapy at home 46, 49.
A multicenter study in severely ill children aged 4–36 months with PD showed a high success rate of a dietary regimen using inexpensive, locally available foods (variable association of rice, maize, lentils, chicken, yoghurt, milk, sucrose or glucose, and oil) and vitamin and mineral supplementation 46. Although a recent systematic review underlined the low-quality evidence of these studies, the authors found no evidence to support the use of proprietary formulas or specialized ingredients over the use of locally produced and readily available foods in the treatment of either AD or PD 50.
Other approaches that have been evaluated include the use of amylase-rich flours in cereal-based porridges to decrease viscosity and thus increase nutrient density and children’s nutrient intake. However, these trials have been performed only in children with AD 51. Also, mixed diets including specific ingredients thought or known to have antidiarrheal properties, such as green banana, have been tested. These reports showed a significantly higher cumulative probability of recovery in children 52, 53.
Children with ProD and malnutrition may present with deficiency of selected micronutrients such as vitamin A, zinc, folic acid, copper, and selenium 54. Zinc was found to have therapeutic efficacy (typically resolution of small bowel damage and shortening duration of diarrhea) in several trials of PD 55. Zinc and vitamin A in combination seem to be even more effective than either vitamin A or zinc alone in reducing PD in developing countries 56.
Conclusion
In children with severe malnutrition, ProD may be the direct result of secondary immunodeficiency and the consequence of a reduction of intestinal absorptive-digestive surface. In this condition, nutritional interventions (nutritional regimen review/optimization and micronutrient supplementation) should be considered with the aim of optimizing the use of residual functioning intestine over time. Enteral nutrition given continuously by feeding tube may be used in severe cases of reduction of intestinal functional surface. However, anti-infectious therapy should also be considered.
Overall, the treatment of ProD is a balance between a possible infectious etiology and a nutritional approach. When, however, the features of irritable bowel syndrome (defined according to the Rome III criteria) are met, no specific medication should be prescribed. However, parental reassurance and diet tailoring (e.g. an increase in fiber intake) may be helpful, and counseling/behavioral therapy may be indicated to reduce anxiety that may trigger gastrointestinal symptoms. If a specific cause of diarrhea is detected, such as celiac disease or IBD, an appropriate therapy should be started.
Abbreviations
WHO, World Health Organization; AD, acute diarrhea; ProD, prolonged diarrhea; PD, persistent diarrhea; IBD, inflammatory bowel disease; SIBO, small intestinal bacterial overgrowth.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Jorge Amil Dias, Unit of Paediatric Gastroenterology, Hospital S. João, Porto, Portugal
Hans Hoekstra, Department of Pediatrics,, Hieronymus Bosch Hospital, Hertogenbosch, Netherlands
Sean Moore, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. World Health Organization: Diarrhoeal disease.2013. Reference Source [Google Scholar]
- 2. Johnston BC, Shamseer L, da Costa BR, et al. : Measurement issues in trials of pediatric acute diarrheal diseases: a systematic review. Pediatrics. 2010;126(1):e222–31. 10.1542/peds.2009-3667 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization: Persistent diarrhoea in children in developing countries: memorandum from a WHO meeting. Bull World Health Organ. 1988;66(6):709–17. [PMC free article] [PubMed] [Google Scholar]
- 4. Moore SR, Lima NL, Soares AM, et al. : Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139(4):1156–64. 10.1053/j.gastro.2010.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Abba K, Sinfield R, Hart CA, et al. : Pathogens associated with persistent diarrhoea in children in low and middle income countries: systematic review. BMC Infect Dis. 2009;9:88. 10.1186/1471-2334-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fraser D, Dagan R, Porat N, et al. : Persistent diarrhea in a cohort of Israeli Bedouin infants: role of enteric pathogens and family and environmental factors. J Infect Dis. 1998;178(4):1081–8. 10.1086/515662 [DOI] [PubMed] [Google Scholar]
- 7. Lima AA, Moore SR, Barboza MS, Jr, et al. : Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J Infect Dis. 2000;181(5):1643–51. 10.1086/315423 [DOI] [PubMed] [Google Scholar]
- 8. Das SK, Faruque AS, Chisti MJ, et al. : Changing trend of persistent diarrhoea in young children over two decades: observations from a large diarrhoeal disease hospital in Bangladesh. Acta Paediatr. 2012;101(10):e452–7. 10.1111/j.1651-2227.2012.02761.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Lima AA, Guerrant RL: Persistent diarrhea in children: epidemiology, risk factors, pathophysiology, nutritional impact, and management. Epidemiol Rev. 1992;14:222–42. [DOI] [PubMed] [Google Scholar]
- 10. Mathai J, Raju B, Bavdekar A, et al. : Chronic and persistent diarrhea in infants and young children: status statement. Indian Pediatr. 2011;48(1):37–42. 10.1007/s13312-011-0018-9 [DOI] [PubMed] [Google Scholar]
- 11. Azim T, Ahmad SM, Sefat-E-Khuda, et al. : Immune response of children who develop persistent diarrhea following rotavirus infection. Clin Diagn Lab Immunol. 1999;6(5):690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taniguchi K, Rikimaru T, Yartey JE, et al. : Immunological background in children with persistent diarrhea in Ghana. Pediatr Int. 1999;41(2):162–7. 10.1046/j.1442-200X.1999.4121034.x [DOI] [PubMed] [Google Scholar]
- 13. Steiner TS, Lima AA, Nataro JP, et al. : Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177(1):88–96. 10.1086/513809 [DOI] [PubMed] [Google Scholar]
- 14. Ochoa TJ, Salazar-Lindo E, Cleary TG: Management of children with infection-associated persistent diarrhea. Semin Pediatr Infect Dis. 2004;15(4):229–36. 10.1053/j.spid.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 15. Baqui AH, Black RE, Sack RB, et al. : Epidemiological and clinical characteristics of acute and persistent diarrhoea in rural Bangladeshi children. Acta Paediatr Suppl. 1992;381(Supplement s383):15–21. 10.1111/j.1651-2227.1992.tb12366.x [DOI] [PubMed] [Google Scholar]
- 16. Mahalanabis D, Alam AN, Rahman N, et al. : Prognostic indicators and risk factors for increased duration of acute diarrhoea and for persistent diarrhoea in children. Int J Epidemiol. 1991;20(4):1064–72. 10.1093/ije/20.4.1064 [DOI] [PubMed] [Google Scholar]
- 17. Ahmed F, Ansaruzzaman M, Haque E, et al. : Epidemiology of postshigellosis persistent diarrhea in young children. Pediatr Infect Dis J. 2001;20(5):525–30. 10.1097/00006454-200105000-00011 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Vernacchio L, Vezina RM, Mitchell AA, et al. : Characteristics of persistent diarrhea in a community-based cohort of young US children. J Pediatr Gastroenterol Nutr. 2006;43(1):52–8. 10.1097/01.mpg.0000228094.74207.39 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Caballero S, Guix S, El-Senousy WM, et al. : Persistent gastroenteritis in children infected with astrovirus: association with serotype-3 strains. J Med Virol. 2003;71(2):245–50. 10.1002/jmv.10476 [DOI] [PubMed] [Google Scholar]
- 20. Bhutta ZA: Post-infectious persistent diarrhea in developing countries. Textbook of Pediatric Gastroenterology and Nutrition Editor: Stefano Guandalini. Taylor & Francis.2004;193–200. Reference Source [Google Scholar]
- 21. Rasquin A, Di Lorenzo C, Forbes D, et al. : Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–37. 10.1053/j.gastro.2005.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickinson B, Surawicz CM: Infectious diarrhea: an overview. Curr Gastroenterol Rep. 2014;16(8):399. 10.1007/s11894-014-0399-8 [DOI] [PubMed] [Google Scholar]
- 23. UNICEF/WHO: Diarrhoea: why children are still dying and what can be done. Geneva: WHO Press,2009;1–68. Reference Source [Google Scholar]
- 24. Guarino A, Winter H, Sandhu B, et al. : Acute gastroenteritis disease: Report of the FISPGHAN Working Group. J Pediatr Gastroenterol Nutr. 2012;55(5):621–6. 10.1097/MPG.0b013e318272b5e2 [DOI] [PubMed] [Google Scholar]
- 25. Guarino A, Giannattasio A: New molecular approaches in the diagnosis of acute diarrhea: advantages for clinicians and researchers. Curr Opin Gastroenterol. 2011;27(1):24–9. 10.1097/MOG.0b013e3283413750 [DOI] [PubMed] [Google Scholar]
- 26. Pawlowski SW, Warren CA, Guerrant R: Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology. 2009;136(6):1874–86. 10.1053/j.gastro.2009.02.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guarino A, De Marco G: Persistent diarrhea. In RE Kleinman, IR Sanderson, O Goulet, PM Sherman, G Mieli-Vergani, & BL Shneider (Eds.), Walker's pediatric gastrointestinal disease: Pathology, diagnosis, management ISBN 978-1-55009-364-3. 5th ed.,2008;265–274. [Google Scholar]
- 28. Canani RB, de Horatio LT, Terrin G, et al. : Combined use of noninvasive tests is useful in the initial diagnostic approach to a child with suspected inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2006;42(1):9–15. 10.1097/01.mpg.0000187818.76954.9a [DOI] [PubMed] [Google Scholar]
- 29. Koletzko S, Niggemann B, Arato A, et al. : Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221–9. 10.1097/MPG.0b013e31825c9482 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Nataro JP, Sears CL: Infectious causes of persistent diarrhea. Pediatr Infect Dis J. 2001;20(2):195–6. [DOI] [PubMed] [Google Scholar]
- 31. Farthing MJ: Diarrhoea: a significant worldwide problem. Int J Antimicrob Agents. 2000;14(1):65–9. 10.1016/S0924-8579(99)00149-1 [DOI] [PubMed] [Google Scholar]
- 32. Diaz E, Mondragon J, Ramirez E, et al. : Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am J Trop Med Hyg. 2003;68(4):384–5. [PubMed] [Google Scholar]; F1000 Recommendation
- 33. White AC, Jr: Nitazoxanide: an important advance in anti-parasitic therapy. Am J Trop Med Hyg. 2003;68(4):382–3. [PubMed] [Google Scholar]
- 34. Rossignol JF, Ayoub A, Ayers MS: Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J Infect Dis. 2001;184(1):103–6. 10.1086/321008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Sears CL, Kirkpatrick BD: Is nitazoxanide an effective treatment for patients with acquired immune deficiency syndrome-related cryptosporidiosis? Nat Clin Pract Gastroenterol Hepatol. 2007;4(3):136–7. 10.1038/ncpgasthep0737 [DOI] [PubMed] [Google Scholar]
- 36. Cohen SA: Use of nitazoxanide as a new therapeutic option for persistent diarrhea: a pediatric perspective. Curr Med Res Opin. 2005;21(7):999–1004. 10.1185/030079905X50534 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Guarino A, Canani RB, Russo S, et al. : Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics. 1994;93(1):12–6. 10.1097/00005176-199311000-00053 [DOI] [PubMed] [Google Scholar]
- 38. Guarino A, Ashkenazi S, Gendrel D, et al. : European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132–52. 10.1097/MPG.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 39. Guarino A, Albano F, Canani RB, et al. : HIV, fatal rotavirus infection, and treatment options. Lancet. 2002;359(9300):74. 10.1016/S0140-6736(02)07287-2 [DOI] [PubMed] [Google Scholar]
- 40. Florescu DF, Hermsen ED, Kwon JY, et al. : Is there a role for oral human immunoglobulin in the treatment for norovirus enteritis in immunocompromised patients? Pediatr Transplant. 2011;15(7):718–21. 10.1111/j.1399-3046.2011.01556.x [DOI] [PubMed] [Google Scholar]
- 41. Guarino A, Guandalini S, Albano F, et al. : Enteral immunoglobulins for treatment of protracted rotaviral diarrhea. Pediatr Infect Dis J. 1991;10(8):612–4. 10.1097/00006454-199108000-00010 [DOI] [PubMed] [Google Scholar]
- 42. Wanke CA, Gerrior J, Blais V, et al. : Successful treatment of diarrheal disease associated with enteroaggregative Escherichia coli in adults infected with human immunodeficiency virus. J Infect Dis. 1998;178(5):1369–72. 10.1086/314443 [DOI] [PubMed] [Google Scholar]
- 43. Abba K, Sinfield R, Hart CA, et al. : Antimicrobial drugs for persistent diarrhoea of unknown or non-specific cause in children under six in low and middle income countries: systematic review of randomized controlled trials. BMC Infect Dis. 2009;9:24. 10.1186/1471-2334-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Gaón D, García H, Winter L, et al. : Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children. Medicina (B Aires). 2003;63(4):293–8. [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Bernaola Aponte G, Bada Mancilla CA, Carreazo NY, et al. : Probiotics for treating persistent diarrhoea in children. Cochrane Database Syst Rev. 2013;8:CD007401. 10.1002/14651858.CD007401.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Evaluation of an algorithm for the treatment of persistent diarrhoea: a multicentre study. International Working Group on Persistent Diarrhoea. Bull World Health Organ. 1996;74(5):479–89. [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. de Mattos AP, Ribeiro TC, Mendes PS, et al. : Comparison of yogurt, soybean, casein, and amino acid-based diets in children with persistent diarrhea. Nutr Res. 2009;29(7):462–9. 10.1016/j.nutres.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 48. Valentiner-Branth P, Steinsland H, Santos G, et al. : Community-based controlled trial of dietary management of children with persistent diarrhea: sustained beneficial effect on ponderal and linear growth. Am J Clin Nutr. 2001;73(5):968–74. [DOI] [PubMed] [Google Scholar]
- 49. Brown KH: Appropriate diets for the rehabilitation of malnourished children in the community setting. Acta Paediatr Scand Suppl. 1991;374(Supplement s374):151–9. 10.1111/j.1651-2227.1991.tb12018.x [DOI] [PubMed] [Google Scholar]
- 50. Gaffey MF, Wazny K, Bassani DG, et al. : Dietary management of childhood diarrhea in low- and middle-income countries: a systematic review. BMC Public Health. 2013;13(Suppl 3):S17. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Mitra AK, Rahman MM, Mahalanabis D, et al. : Evaluation of an energy-dense meal liquefied with amylase of germinated wheat in children with acute watery diarrhoea: a randomized controlled clinical trial. Nutr Res. 1995;15(7):939–51. 10.1016/0271-5317(95)00056-O [DOI] [Google Scholar]
- 52. Rabbani GH, Larson CP, Islam R, et al. : Green banana-supplemented diet in the home management of acute and prolonged diarrhoea in children: a community-based trial in rural Bangladesh. Trop Med Int Health. 2010;15(10):1132–9. 10.1111/j.1365-3156.2010.02608.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Rabbani GH, Teka T, Zaman B, et al. : Clinical studies in persistent diarrhea: dietary management with green banana or pectin in Bangladeshi children. Gastroenterology. 2001;121(3):554–60. 10.1053/gast.2001.27178 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Bhan MK, Bhandari N, Bahl R: Management of the severely malnourished child: perspective from developing countries. BMJ. 2003;326(7381):146–51. 10.1136/bmj.326.7381.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Black RE: Zinc deficiency, infectious disease and mortality in the developing world. J Nutr. 2003;133(5 Suppl 1):1485S–9S. [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Rahman MM, Vermund SH, Wahed MA, et al. : Simultaneous zinc and vitamin A supplementation in Bangladeshi children: randomised double blind controlled trial. BMJ. 2001;323(7308):314–8. 10.1136/bmj.323.7308.314 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation