Abstract
Coumarins are naturally-occurring compounds with diverse and interesting biological activities. In the present study, we evaluated the in vitro cytotoxic effect of 8-(acetyloxy)-3-[4-(acetyloxy)phenyl]-2-oxo-2H-chromen-7-yl acetate (6); 8-(acetyloxy)-3-(4-methanesulfonyl phenyl)-2-oxo-2H-chromen-7-yl acetate (7); 4-(2-oxo-2H-chromen-3-yl)phenyl acetate (8); 3-(4-methanesulfonylphenyl)-2H-chromen-2-one (9); 4-(4-methyl-2-oxo-2H-chromen-3-yl)phenyl acetate (10); 3-(4-methanesulfonylphenyl)-4-methyl-2H-chromen-2-one (11); 8-(acetyloxy)-3-[4-(acetyloxy)phenyl]-4-methyl-2-oxo-2H-chromen-7-yl acetate (12); and 5-(acetyloxy)-3-[4-(acetyloxy) phenyl]-2-oxo-2H-chromen-7-yl acetate (13) in human lung (A549) cancer and normal lung (MRC-9) cell lines at different concentrations for 48 h using crystal violet dye binding assay. The cytotoxic effect of these coumarin derivatives were compared to the standard drug, docetaxel. Furthermore, the effect of the most active compound on the cell-cycle using propidium iodide, mitochondrial membrane potential (MMP) using tetramethyl rhodamine methyl ester (rhodamine-123) and reactive oxygen species (ROS) production using 2′,7′-dichlorofluorescin diacetate (PCFDA) were also evaluated.
Results
Compound 7 had the greatest cytotoxic effect (cytotoxic concentration, CC50=24 μM) and selectivity (MRC-9; CC50 >100 μM; inactive) in the A549 cell line, and caused cells to arrest in the S phase of the cell cycle, loss of MMP and increased ROS production in a concentration-dependent manner.
Conclusion
These findings suggest that compound 7 could serve as a new lead for the development of novel synthetic compounds with enhanced anticancer activity.
Keywords: 3-Arylcoumarin derivatives, cytotoxic effect, cell cycle phase, mitochondrial transmembrane potential, reactive oxygen species production
Coumarins are an important class of naturally-occurring heterocyclic compounds known as benzo-α-pyrones (1, 2). They occur as secondary metabolites in fruits, seeds, roots and leaves of many plant species, functioning as growth regulators, bacteriostats, fungistats, as well as in defense against infection (3, 4). Coumarins exhibit a diverse array of pharmacological and biochemical properties. For example, the simplest coumarin (compound 1; Figure 1) and its metabolite, 7-hydroxycoumarin (compound 2; Figure 1) demonstrate strong antitumor activities against various cancer cell types such as A549 (lung), ACHN (renal), H727 (lung), MCF-7 (breast) and HL-60 (leukemia) (5). Other reported properties of coumarins include photochemotherapy, anti-coagulant, anti-HIV, anti-inflammatory, anti-microbial, and anti-helmintic activities (2, 6, 7). In addition to their biological activities, coumarins are used as additives in food and cosmetics, and as optical brightening agents (8). These properties of coumarin and derivatives have led to considerable interest in their synthesis and possible applications as therapeutic agents for the treatment of various diseases.
Figure 1.
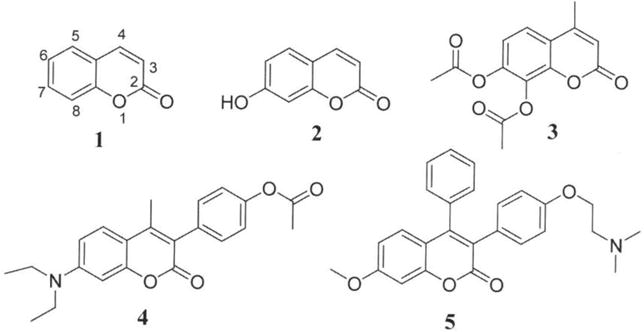
Chemical structure of 1: coumarin; 2: 7-hydroxycoumarin: 3: 7,8-diacetoxy-4-methylcoumarin (DAMC); 4: 4-(7-diethylamino)-4-methyl-2-oxo-2H-chromen-3-yl)-phenylacetate: 5: 3-(4-(2-(dimethylamino)ethoxy)phenyl)-7-methoxy-4-phenylcoumarin.
Certain studies have shown that the therapeutic applications, and pharmacological and biochemical properties of coumarins depend on the nature of the group(s) present and their pattern of substitution on the core chemical structure (9–11). For example, acetoxycoumarin such as 7,8-diacetoxy-4-methylcoumarin (DAMC; compound 3; Figure 1) exhibits anticancer and prooxidant activities against human tumor cell lines (12, 13); and phenylcoumarins such as 3-arylcoumarins exhibit different biological properties such as anti-oxidant, anti-inflammatory, anticancer, anti-HIV, monoamine oxidase (MAO) inhibitory and anti-microbial activities (14, 15) etc. As part of our ongoing investigation on coumarin derivatives as anticancer agents, we recently showed that coumarin containing either p-acetoxyphenyl (compound 4) or 4-(2-(dimethylamino)ethoxy)phenyl groups (compound 5) at the carbon-3 position on the coumarin ring exhibits cytotoxic activity against different cancer cell lines (9, 16). These studies and others strongly support the potential therapeutic-applications of coumarins, making them attractive for further evaluation as novel therapeutic agents for cancer treatment.
The antitumor activities of a variety of coumarins have been extensively examined (4, 17). For example, the acetyl moieties of polyphenolic acetates such as compound 3 (Figure 1) play an important role in identifying effective therapeutic new drugs for improving cancer therapy based on biological protein acetylation (18, 19). Additionally, heterocyclic compound containing methylsulfonyl (-SO2Me) pharmacophore also offers great potential for the development of novel anticancer agents (20–22). These findings aroused our interest in cytotoxicity studies of 3-arylcoumarin derivatives (compounds 6–11; Table I) containing either acetoxy or methylsulfonyl groups with the aim of obtaining more potent biologically active compounds. We herein report the cytotoxic effect of compounds 6–11 in A549 (cancer) and MRC (normal) cell lines in comparison to the reference drug docetaxel, and the effect of the most active compound on cell-cycle phases, MMP and ROS production.
Table I.
The cytotoxic concentration (CC50) values for compounds 6–11 and docetaxel tested in human lung (A549) cancer and normal lung (MRC-9) cell lines for 48 h.
| Compound | CC50(μM) mean ± SD
|
||
|---|---|---|---|
| A549 | MRC-9 | ||
| 6 |
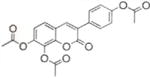
|
75.9±0.65 | 83.7±0.0 |
| 7 |
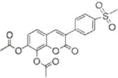
|
24.2±0.70 | >100 |
| 8 |
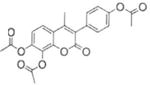
|
85.0±3.8 | 79.8±0.20 |
| 9 |
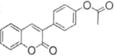
|
>100 | ND |
| 10 |
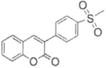
|
>100 | ND |
| 11 |
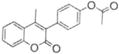
|
>100 | ND |
| 12 | Docetaxel (standard drug) |
9.40±0.07 | ND |
Data represent the average of triplicate values. The cytotoxic concentration (CC50) values were determined from Graph pad Prism using mean values of data points at different concentrations. Drug effects were determined after 48 h exposure.
ND: Not determined.
Materials and Methods
Chemicals
F12 K medium, dulbecco’s modified eagle medium (DMEM), penicillin-streptomycin anti-biotic solution (100×), fetal bovine serum (FBS), 0.25% Trypsin-0.02% EDTA solution (1×), phosphate buffer (PBS), 50% glutaraldehyde, crystal violet. IGEPAL CA-630, propidium iodide, 2′,7′-dichlorofluorescein diacetate (DCFDA), tetramethyl rhodamine methyl ester (rhodamine-123) and RNase were obtained from Sigma-Aldrich Company (St. Louis, MO, USA). Potassium phosphate, EDTA, D-glucose, ethanol were obtained from Thomas Scientific Company (Swedesboro, NJ. USA).
Cell line maintenance
Human lung (A549) cancer and normal lung (MRC-9) cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured as per the guidelines supplied.
Cell viability assay
The cells (A549 or MRC-9) were plated at a density of 5×104 cells per well in polystyrene, flat-bottom 24-well microtiter plates (Corning Costar, Rochester, NY. USA) in cell-type specific medium containing 10% FBS, 100 units of penicillin/ml, 100 μg of streptomycin/ml and allowed to stabilize overnight in a CO2 incubator at 37°C. Afterwards, the cells were treated with compounds at different concentrations (0, 10, 25, 50, 75 and 100 μM) in a final volume of 1 ml per well in triplicate wells for each treatment for 48 h at 37°C in a 5% CO2 incubator. All studies were repeated at least thrice.
At the end of the incubation period, the viability was evaluated by dye uptake assay according to our previously reported method (9, 16, 23). Glutaraldehyde (400 μl of 0.25%) was added to each well and plates were incubated for 30 min at room temperature to fix the cells. The glutaraldehyde (0.07% final concentration in the well) in the crystal violet dye staining assay procedure fixed the viable cells after the treatment with compound. The plates were rinsed with water to wash off dead cells and dried under airflow inside a laminar hood for 5–10 min. Crystal violet (400 μl of 0.1%) was added to each well, plates were incubated for 15 min, washed and dried. Finally, 1 ml of 0.05 M sodium phosphate solution (monobasic) in 50% ethyl alcohol was added to each well and the plates were read at 540 nm in a plate reader (Bio-Tek EL800 Plate Reader, Winooski, VT. USA). The mean absorbance value of the untreated cells (control) was considered as 100% and the treated sample percentages were calculated by comparing the treated samples absorbance with the mean absorbance of the control. The concentration of all tested drugs where 50% viability was observed in comparison to the untreated control (CC50) was calculated according to Ipsen and Feigl, 1970 (24).
Cell-cycle analysis
Cells at a density of 0.65×106 cells per T-25 cm2 flask in complete culture medium were plated and incubated overnight in a CO2 incubator at 37°C. The cells were then treated with active compound 7 at different concentrations (0, 10 and 20 μM) in a final volume of 5 ml per flask in triplicate flasks for 48 h at 37°C in a 5% CO2 incubator. The effect of the most active and selective compound on cell-cycle phase in A549 cell line was studied using a C6 Accuri flow cytometer (Accuri Cytometers, Ann Arbor, MI, USA) according to our previously reportal method (9, 16, 23). At the end of incubation, the treated cells were trypsinized and pelleted, washed with PBS and resuspended in 1 ml of Vindelov’s reagent [Tris buffered saline (pH 7.6) containing ribonuclease A, propidium iodide and IGEPAL CA- 630]. The cells were then incubated at 4°C overnight and analyzed using a flow cytometer at a low flow rate (~150 cells or less).
Measurement of MMP
The MMP was measured according to our previously reported method (23). At the end of incubation, the treated cells were fixed with 400 μl of 0.25% aqueous glutaraldehyde, containing rhodamine-123 for 30 min at room temperature. The cells were washed with tap water and air dried in a hood. Once completely dry, 500 μl of 0.1% Triton X 100 in dPBS was added per well and incubated at 37°C for 1 h. The plates were then read with excitation filter set at 485 nm and emission filter at 538 nm on a microplate Fluorometer (Tecan, Seestrasse, Männedorf, Switzerland).
Measurement of ROS production
ROS was measured according to a previously reported method (25). The cells at 20,000 cells/well in final volume of 80 μl of medium were plated in a 96-well plate. DCFDA (10 μl of 50 μM) was then added to each well and incubated for 30 min and then cells were treated with 0, 10, 25, 50, 75, and 100 μM of compound for 30 min. The fluorescence was read with excitation filter set at 488 nm and emission filter at 530 nm on a microplate Fluorometer (Tecan, Seestrasse, Männedorf, Switzerland).
Statistical analysis
The viability values are presented as the mean±standard deviation (SD). All data for treated cells are presented as percentage values in comparison to the untreated control (100%). The viability and CC50 graphs were plotted in Prism 5.00 software (GraphPad Software, Inc., San Diego, CA, USA). The CC50 value was calculated from the graph where the lines for live and dead cells met (the dose at which 50% of the cells died) using Prism 5 software (GraphPad Software, Inc.).
Results
Cytotoxic effect of compounds 6–11 in A549 cancer cell line
In vitro cytotoxic effect of compounds 6–11 was evaluated at different concentrations (0, 25, 50, 75 and 100 μM) in A549 and MRC-9 cell lines for 48 h using crystal violet dye binding assay. The CC50 values for all tested compounds are listed in Table I. Results from this study indicate that compounds 6 (CC50=75.2 μM), 7 (CC50=24.2 μM) and 8 (CC50=84.7 μM) caused concentration-dependent cell death (Figure 2a), while compounds 9–11 did not cause cell death (inactive; CC50 ≥100 μM; Table 1) in A549 cell line after 48 h compared to the untreated control cells. Comparison of the cytotoxic effect of 7 (most active compound; CC50=24.2 μM) and docetaxel (CC50=9.47 μM) indicates a 2.5-fold decrease in potency (Figure 2b). Docetaxel is a class of cytotoxic agent known as taxanes and is used in the treatment of breast, lung, prostate, stomach, and head/neck cancer (26). It was used in the present study for comparative studies. Compound 7 is also the most selective compound (inactive; CC50 >100 μM) compared to 6 (CC50=83.3 μM) and 8 (CC50=76.6 μM) against MRC-9 cell line based on the calculated CC50 value (Table I).
Figure 2.
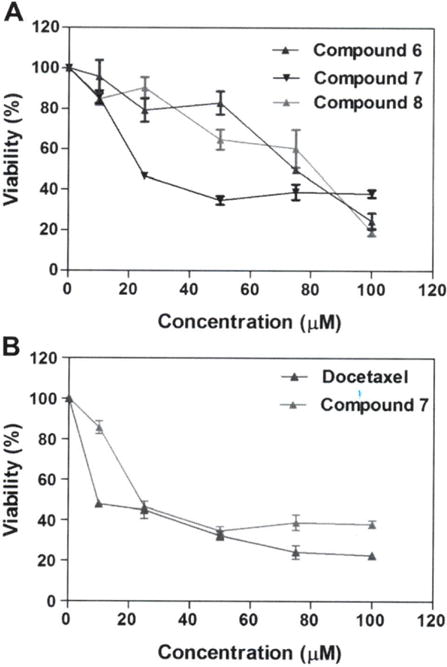
A: Effect of compounds 6–8 on A549 cell viability, B: Effect of compound 7 and docetaxel on A549 cell viability. Data are presented as the mean±SD, n = 9. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
Effect of compound 7 on the different phases of cell cycle
To evaluate compound 7 cytotoxic effect on cell-cycle phases. A549 cells were treated with 10 and 25 μM concentrations of compound for 48 h, followed by staining with propidium iodide. Compound 7 was selected for this investigation based on the in vitro result, indicating it as the most cytotoxic and selective compound. The percentage of cells in G0/G1, S and G2/M phases of the cell-cycle were analyzed using a flow cytometer. Results from this investigation indicate that compound 7 caused cell-cycle arrest in S phase at 25 μM (5.02%) compared to control cells (Figure 3).
Figure 3.
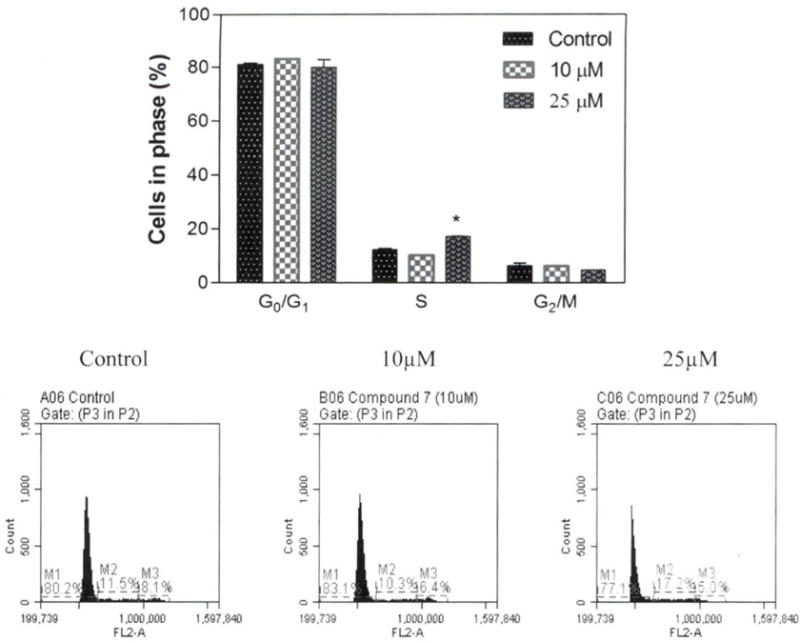
Effect of compound 7 on AS49 cell-cycle distribution. Data are presented as mean±SEM, n=3, *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
MMP
The effect of compound 7 on MMP was evaluated at different concentrations (0, 10, 25, 50, 75 and 100 μM) in A549 cells using rhodamine-123 dye. In this investigation, the results indicate a decrease in fluorescence intensity at 10 μM (96.4%), 25 μM (94.8%), 50 μM (93.4%), 75 μM (92.9%) and 100 μM (81.7%) in comparison to the untreated control cells (100%), indicating loss of MMP (Figure 4).
Figure 4.
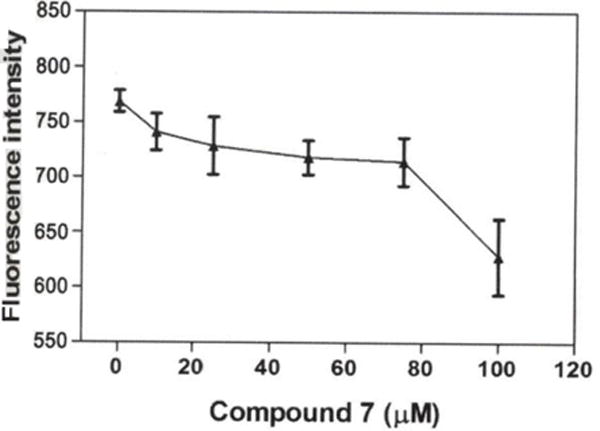
Effect of compound 7 on mitochondrial membrane potential (MMP) of A549 cells. Data are presented as the mean±SEM, n=3. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
ROS production
The effect of compound 7 on ROS production was evaluated at different concentrations (0, 25, 50, 75 and 100 μM) in A549 cells using DCFDA dye. The results indicate an increase in fluorescence intensity at 10 μM (107.1%), 25 μM (129.0%), 50 μM (136.6%), 75 μM (146.0%), and 100 μM (231.2%) in comparison to the untreated control cells (100%), indicating an increased in ROS production (Figure 5).
Figure 5.
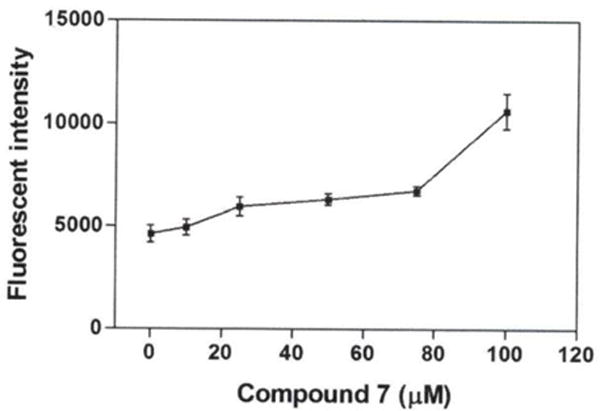
Effect of compound 7 on reactive oxygen species (ROS) production of A549 cells. Data are presented as the mean±SEM, n=3. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
Discussion
The acetoxy and methylsulfonyl groups are biologically proven anticancer pharmacophores, and thus their substitution in coumarin scaffolds may further enhance biological activity. As part of our ongoing investigation of coumarin derivatives aims at evaluating the role of substituent in modulating cytotoxicity, we herein report the in vitro cytotoxic effect of 3-arylcoumarin containing either acetoxy or methylsulfonyl groups (6–11) in the A549 cell line, and the effect of the most active compound 7 on cell cycle phases, MMP and ROS production. The in vitro cytotoxic results from this study indicate that compounds 6–8, bearing 7,8-diacetoxy groups, displayed differential concentration-dependent cytotoxic effect on A549 cancer cell line after 48 h (Table I). This observation is consistent with previous finding that the presence of 7,8-diacetoxy groups enhanced drug activity and specificity of compound 3 (13, 19). Interestingly, structure–activity relationship study of 6–11 (Table I) indicates that the presence of either p-acetoxy phenyl or p-(methylsulfonyl)phenyl group substituted at the carbon-3 position make no contribution to the observed cytotoxic effect of compounds 6–8. However, we may associate the selectivity of compound 7 in the MRC-9 cell line to p-(methylsulfonyl)phenyl group based on the structure–activity relationship study of compounds 6 and 7. The present finding reveals the beneficial effect of both 7,8-diacetoxy-and p-(methylsulfonyl(phenyl groups on the coumarin ring in modulating selective cytotoxic effect against the A549 cell line.
Studies have demonstrated that the toxic effect of compounds on different phases of the cell cycle can activate or inhibit cell growth and proliferation of cancer cells in vitro (27). The result from this investigation showed that compound 7 arrests A549 cells in the S phase at 25 μM, an indication of cells inhibiting DNA synthesis (Figure 3). This finding is consistent with previous investigations indicating that coumarins caused different cancer cell lines to arrest in the S phase (28, 29).
Mitochondria are known to play an important role in the process of cellular metabolism (30). In the present investigation, we used rhodamine-123 dye to determine whether compound 7 caused loss of MMP. Rhodamine-123 is a green-fluorescent dye used in numerous investigations to measure MMP changes in cells; the amount of dye taken up by the cells is directly proportional to the MMP (31, 32). Thus, evaluation of MMP changes is of critical importance in accessing the effect of chemicals on mitochondrial function in living cells. Our result indicates a decrease in fluorescence intensity compared to untreated control cells, suggesting that the elicited cytotoxic effect of compound 7 is associated with loss of MMP (Figure 4). This finding is in agreement with previous studies indicating that compound 3 led to loss of MMP in A549 cell line (33).
Mitochondria are an important source of ROS production in cells, contributing to mitochondrial damage in a range of disease conditions (34). To examine whether compound 7 caused ROS production in cell death of A549 cells, the intracellular ROS level was measured using DCFDA dye. Our result indicated an increase in fluorescence intensity compared to the untreated control cells; suggesting that the elicited cytotoxic effect of compound 7 is also associated with ROS production (Figure 5). This finding is in agreement with a previous study indicating that compound 3 elevated the level of ROS production (33).
In conclusion, our studies demonstrated that compound 7 exhibits selective cytotoxic effects, and caused cell arrest in the S phase of the cell cycle, loss of MMP and increase of ROS production in the A549 cell line. This investigation demonstrates the importance of 7,8-diacetoxy and p-(methylsulfonyl) phenyl groups on the coumarin ring in modulating selective cytotoxic effect in the A549 cell line. Furthermore, it suggests that the elicited cytotoxic effect of compound 7 in A549 cells is through mitochondrial dysfunction, which might be mediated by ROS production. However, further studies are required in order to achieve a better understanding of the exact mechanism of this coumarin-class compound.
Acknowledgments
The Authors gratefully acknowledge Florida A & M University TITLE III PROGRAM for their financial support.
Footnotes
Declaration of Interest
The Authors declare that they have no financial or non-financial competing interests in regard to this study.
References
- 1.Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr Med Chem. 2005;12(8):887–916. doi: 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- 2.Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15(26):2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray RDH, Mendez J, Brown SA. The Natural Coumarins: Occurrence, Chemistry and Biochemistry. John Wiley & Sons; New York: 1982. [Google Scholar]
- 4.Lacy A, O’Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des. 2004;10(30):3797–37811. doi: 10.2174/1381612043382693. [DOI] [PubMed] [Google Scholar]
- 5.Stanchev S, Momekov G, Jensen F, Manolov I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur J Med Chem. 2008;43(4):694–706. doi: 10.1016/j.ejmech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Jung J-C, Park O-S. Synthetic Approaches and Biological Activities of 4-Hydroxy coumarin derivatives. Molecules. 2009;14(11):4790–4803. [Google Scholar]
- 7.Jung J-C, Lee J-H, Oh S, Lee J-G, Park O-S. Synthesis and antitumor activity of 4-hydroxycoumarin derivatives. Bioorg Med Chem Lett. 2004;14:5527–5531. doi: 10.1016/j.bmcl.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 8.O’Kennedy R, Thornes RD. Coumarins – Biology, Applications and Mode of Action. John Wiley & Sons; Chichester, UK: 1997. [Google Scholar]
- 9.Musa MA, Badisa VL, Latinwo LM, Cooperwood J, Sinclair A, Abdullah A. Cytotoxic activity of new acetoxycoumarin derivatives in cancer cell lines. Anticancer Res. 2011;31(6):2017–2022. [PMC free article] [PubMed] [Google Scholar]
- 10.Kostova I. Studying plant-derived coumarins for their pharmacological and therapeutic properties as potential anticancer drugs. Expert Opin Drug Discov. 2007;2(12):1605–18. doi: 10.1517/17460441.2.12.1605. [DOI] [PubMed] [Google Scholar]
- 11.Kostova I, Raleva S, Genova P, Argirova R. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg Chem Appl. 2006:68274–68283. doi: 10.1155/BCA/2006/68274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshy L, Dwarakanath BS, Raj HG, Chandra R, Mathew TL. Suicidal oxidative stress induced by certain antioxidants. Indian J Exp Biol. 2003;41(11):1273–1278. [PubMed] [Google Scholar]
- 13.Khurana P, Kumari R, Vohra P, Kumar A, Seema, Gupta G, Raj HG, Dwarakanath BS, Parmar VS, Saluja D, Bose M, Vij A, Chaudhary NK, Adhikari JS, Tyagi YK, Kohli E. Acetoxy drug: protein transacetylase catalyzed activation of human platelet nitric oxide synthase by polyphenolic peracetates. Bioorg Med Chem. 2006;14(2):575–583. doi: 10.1016/j.bmc.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Yan B, Peterson LB, Blagg BS. 3-Arylcoumarin derivatives manifest anti-proliferative activity through Hsp90 inhibition. ACS Med Chem Lett. 2012;3(4):327–331. doi: 10.1021/ml300018e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matos MJ, Teran C, Perez-Castillo Y, Uriarte E, Santana L, Vina D. Synthesis and study of a series of 3-arylcoumarins as potent and selective monoamine oxidase B inhibitors. J Med Chem. 2011;54(20):7127–7137. doi: 10.1021/jm200716y. [DOI] [PubMed] [Google Scholar]
- 16.Musa MA, Badisa VL, Latinwo LM, Waryoba C, Ugochukwu N. In vitro cytotoxicity of benzopyranone derivatives with basic side chain against human lung cell lines. Anticancer Res. 2010;30(11):4613–4617. [PMC free article] [PubMed] [Google Scholar]
- 17.Kontogiorgis CA, Hadjipavlou-Litina DJ. Synthesis and antiinflammatory activity of coumarin derivatives. J Med Chem. 2005;48(20):6400–6408. doi: 10.1021/jm0580149. [DOI] [PubMed] [Google Scholar]
- 18.Raj HG, Parmar VS, Jain SC, Goel S, Singh A, Gupta K, Rohil V, Tyagi YK, Jha HN, Olsen CE, Wengel J. Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part II: Mechanism-based inhibition of rat liver microsome-mediated aflatoxin Bl-DNA binding by the candidate antimutagen 7,8-diacetoxy-4-methylcoumarin. Bioorg Med Chem. 1998;6(10):1895–1904. doi: 10.1016/s0968-0896(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 19.Raj HG, Singh BK, Kohli E, Dwarkanath BS, Jain SC, Rastogi RC, Kumar A, Adhikari JS, Watterson AC, Olsen CE, Parmar VS. Acetoxy drug: protein transacetylase: A novel enzyme-mediating protein acetylation by polyphenolic peracetates. Pure Appl Chem. 2005;77(1):245–250. [Google Scholar]
- 20.Zarghi A, Javid FS, Ghodsi R, Dadrass OG, Daraei B, Hedayati M. Design, synthesis and biological evaluation of new 5,5-diarylhydantoin derivatives as selective cyclooxygenase-2 inhibitors. Sci Pharm. 2011;79(3):449–460. doi: 10.3797/scipharm.1104-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foote KM, Blades K, Cronin A, Fillery S, Guichard SS, Hassall L, Hickson I, Jacq X, Jewsbury PJ, McGuire TM, Nissink JW, Odedra R, Page K, Perkins P, Suleman A, Tam K, Thommes P, Broadhurst R, Wood C. Discovery of 4-{4-[(3R)-3-Methylmorpholin-4-yl]-6-[1-(methylsulfonyl)cyclopropyl]pyrimidin-2-yl}-1H-indole (AZ20): a potent and selective inhibitor of ATR protein kinase with monotherapy in vivo antitumor activity. J Med Chem. 2013;56(5):2125–2138. doi: 10.1021/jm301859s. [DOI] [PubMed] [Google Scholar]
- 22.Shyam K, Hrubiec RT, Furubayashi R, Cosby LA, Sartorelli AC. 1,2-Bis(sulfonyl) hydrazines. 3. Effects of structural modification on antineoplastic activity. J Med Chem. 1987;30(11):2157–2161. doi: 10.1021/jm00394a040. [DOI] [PubMed] [Google Scholar]
- 23.Musa MA, Badisa VL, Latinwo LM. Cytotoxic activity of N, N′-Bis (2-hydroxybenzyl) ethylenediamine derivatives in human cancer cell lines. Anticancer Res. 2014;34(4):1601–1607. [PubMed] [Google Scholar]
- 24.Ipsen J, Feigl P. Bancroft’s Introduction to Biostatistics. 2nd. Harper and Row; New York: 1970. p. 164. [Google Scholar]
- 25.Russell LH, Mazzio E, Badisa RB, Zhu ZP, Agharahimi M, Oriaku ET, Goodman CB. Autoxidation of gallic acid induces ROS-dependent death in human prostate cancer LNCaP cells. Anticancer Res. 2012;32(5):1595–1602. [PMC free article] [PubMed] [Google Scholar]
- 26.Fauzee NJ, Wang YL, Dong Z, Li QG, Wang T, Mandarry MT, Lu X, Juan P. Novel hydrophilic taxane analogues inhibit growth of cancer cells. Asian Pac J Cancer Prev. 2012;13(2):563–567. doi: 10.7314/apjcp.2012.13.2.563. [DOI] [PubMed] [Google Scholar]
- 27.Sa G, Das T. Anti cancer effects of curcumin: cycle of life and death. Cell Div. 2008;3(14):1–14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasul A, Khan M, Yu B, Ma T, Yang H. Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in human gastric adenocarcinoma SGC-7901 cells. Asian Pac J Cancer Prev. 2011;12(5):1219–1223. [PubMed] [Google Scholar]
- 29.Ruiz-Marcial C, Chilpa RR, Estrada E, Reyes-Esparza J, Fariña GG, Rodríguez-Fragoso L. Antiproliferative, cytotoxic and antitumour activity of coumarins isolated from Calophyllum brasiliense. Journal of Pharmacy and Pharmacology. 2007;59:719–725. doi: 10.1211/jpp.59.5.0013. [DOI] [PubMed] [Google Scholar]
- 30.Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol Rev. 2002;54(1):101–127. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- 31.Scaduto RC, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76(1 Pt 1):469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millot JM, Sharonov S, Manfait M. Scanning microspectrofluorometry of rhodamine 123 in multidrug-resistant cells. Cytometry. 1994;17(1):50–58. doi: 10.1002/cyto.990170107. [DOI] [PubMed] [Google Scholar]
- 33.Goel A, Prasad AK, Parmar VS, Ghosh B, Saini N. Apoptogenic effect of 7,8-diacetoxy-4-methylcoumarin and 7,8-diacetoxy-4-methylthiocoumarin in human lung adenocarcinoma cell line: role of NF-kappaB, Akt, ROS and MAP kinase pathway. Chem Biol Interact. 2009;179(2–3):363–374. doi: 10.1016/j.cbi.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 34.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]


