Abstract
Deep brain stimulation (DBS) of the thalamic centromedian/parafascicular (CM-Pf) complex has been reported as a promising treatment for patients with severe, treatment resistant Tourette syndrome (TS). In this study, safety and clinical outcomes of bilateral thalamic CM-Pf DBS were reviewed in a series of twelve consecutive patients with medically refractory TS, eleven of whom met the criteria of post-surgical follow-up at our institution for at least two months. Five subjects were followed for a year or longer. Consistent with many TS patients, all subjects had psychiatric co-morbidities. Tic severity and frequency was measured by the Yale Global Tic Severity Scale (YGTSS) over time (average 26 months) in ten subjects. One subject was tested at two weeks follow-up only and thus was excluded from group YGTSS analysis. Final YGTSS scores differed significantly from preoperative baseline. The average (n=10) improvement relative to baseline in the Total Score was 54% (95% confidence interval (CI): 37–70); average improvement relative to baseline in the YGTTS Motor tic, Phonic tic, and Impairment subtests was 46% (95% CI: 34–64), 52% (95% CI: 34–72), and 59% (95% CI: 39–78), respectively. There were no intraoperative complications. Following surgery, one subject underwent wound revision due to a scalp erosion and wound infection; the implanted DBS system was successfully salvaged with surgical revision and combined antibiotic therapy. Stimulation-induced adverse-effects did not prevent the use of the DBS system, although one subject is undergoing a trial period with the stimulator off. This surgical series adds to the literature on CM-Pf DBS and supports its use as an effective and safe therapeutic option for severe refractory TS.
Keywords: deep brain stimulation, thalamus, Tourette syndrome, centromedian/parafascicular complex
INTRODUCTION
Tourette syndrome (TS) is a neuropsychiatric disorder characterized by the presence of motor and phonic tics and often associated with comorbid conditions such as obsessive-compulsive disorder (OCD) and attention-deficit hyperactivity disorder (ADHD).1 In the majority of cases, tic onset occurs during childhood, increases in severity and frequency during adolescence and reaches peak severity in the second decade of life, after which most patients experience improvement.1,2 In a subset of individuals with TS, however, the symptoms continue across the life span in a waxing and waning pattern.1
The first-line treatment for TS includes medications such as alpha-adrenergic blockers, typical and atypical antipsychotics, and benzodiazepines. When first-line treatment fails, botulinum toxin injections and behavioral therapies may be attempted.3,4 In patients with medically refractory TS or intolerable adverse effects from standard medications, deep brain stimulation (DBS) may represent an alternative. The introduction in 1999 of DBS of the centromedian/parafascicular (CM-Pf) thalamic nuclear complex for TS was based on the success in tic reduction from lesioning procedures targeting CM-Pf.5 Since then, CM-Pf DBS for clinically indicated TS cases has been reported by nine additional groups,6–15 including a review of the first three cases we conducted at our institution.16 Based on the initial favorable results, we have continued to target the CM-Pf in patients with refractory TS, and the present report expands our series to include all patients implanted at our center.
MATERIALS AND METHODS
A consecutive series of patients treated with DBS for TS at our institution between December 13, 2006 and November 10, 2014 was reviewed for a retrospective study, approved by the Mayo Clinic Institutional Review Board (IRB). All candidates satisfied Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) 17 criteria for TS and were approved for DBS by the interdisciplinary Mayo Clinic Neuromodulation Committee. The subject group included three patients 18 years of age or younger who had significant tic-related functional impairment, self-injurious behaviors, and were unable to consistently attend school. Eleven of the twelve subjects met the inclusion criteria of clinical follow-up of two months or longer at our institution. The excluded subject underwent postoperative follow-up at another institution. The extended follow-up of three previously described subjects 16 is reported here. All eleven subjects had psychiatric co-morbidities. Table 1 summarizes subject demographics and presentation. Three years prior to thalamic lead implantation, subject 6 had undergone bilateral globus pallidus interna DBS for TS at another institution with no benefit.
Table 1.
Study subject characteristics.a
| Patient | Gender | Age at disease onset | Age at surgery | Psychiatric comorbidities | Education/job status | Familiarity |
|---|---|---|---|---|---|---|
| 1 | M | 6 | 17 | Anxiety disorder NOS, depression, OCD, PDD, previously hospitalized for suicidal ideation | Home-schooled, part-time job | ADHD, Asperger syndrome, dystonia, tic disorder (brother); depression, OCD, TS (father) |
| 2 | F | 10 | 35 | ADHD, anxiety disorder NOS, OCD, self-harm attempts | High school graduate, occasional jobs | / |
| 3 | M | 7 | 17 | ADHD, OCD | Home-schooled | N/A (patient adopted) |
| 4 | M | 4 | 18 | GAD, OCD | High-school honor student, part-time job | ADHD (father); anxiety NOS (multiple family members on both sides); mood disorder and TS (paternal grandfather); hoarding disorder (paternal grandmother) |
| 5 | M | 6 | 46 | Depression, resolved OCD | Full-time job | Tic disorder (father, son, brother) |
| 6 | M | 9 | 27 | Benzodiazepine and narcotics dependence | Full-time job | / |
| 7 | M | 9 | 27 | Depression, OCD | Unemployed | / |
| 8 | F | 14 | 32 | ADHD, bipolar disorder, PTSD, substance abuse, suicide attempts, borderline personality disorder | Employed | / |
| 9 | F | 6 | 22 | ADHD, single episode of major depression | Home-schooled, unemployed | / |
| 10 | M | 12 | 27 | Depression, dysthymia | College graduate, unemployed | Tic disorder (paternal great uncle) |
| 11 | M | 4 | 27 | OCD | College graduate, unemployed | Alcohol abuse, anxiety NOS (mother, sister, multiple family members on maternal side), depression (sister, multiple family members on both sides), tic disorder (father) |
ADHD = attention-deficit hyperactivity disorder; GAD = generalized anxiety disorder; NOS = not otherwise specified; OCD = obsessive compulsive disorder; PDD = pervasive developmental disorder; PTSD = post-traumatic stress disorder; TS = Tourette syndrome.
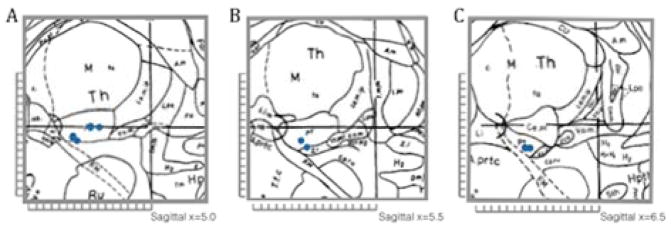
All subjects underwent bilateral quadripolar CM-Pf DBS electrode implantation (3387 model DBS lead, Medtronic, Minneapolis, MN; contacts 0 to 4, with contact 0 at target) with contact 0 located 5–7mm lateral, 8–11mm posterior, and 0–3mm inferior to the midpoint of the anterior commissure-posterior commissure plane (Figure 1). Electrode and stimulator were implanted in a single procedure under general anesthesia (n=10) or under local anesthesia during lead implantation and general anesthesia during stimulator placement (n=1).
Figure 1.
Stimulating lead location.
(A, B, C) Targeting of the right centromedian-parafascicular complex. Blue dots mark the targeted position of the lead tip (3387 model DBS lead, Medtronic, Minneapolis, MN) in 11 patients on a sagittal plane (A) 5.00mm, (B) 5.50mm, and (C) 6.50 lateral off midline. [Adapted from Schaltenbrand G & Wahren W (1977). Atlas for Stereotaxy of the Human Brain. Thieme, New York,18 with permission]
Intraoperative microelectrode recordings along the planned trajectory to aid in targeting were conducted in four patients (subjects 1, 2, 4 and 5), and intraoperative macrostimulation to identify potential motor side-effects was conducted in three patients (subjects 1, 2, and 5).
Preoperative targeting was accomplished by fusing the stereotactic images with the Shaltenbrand and Wahren atlas,18 and intraoperative fluoroscopy was used to verify lead location. All subjects underwent postoperative imaging (MRI, n=5; CT, n=6) for analysis of lead location and detection of any intracranial complications. Lead extensions were tunneled subcutaneously, connecting the intracranial electrodes to a stimulator implanted in a thoracic subcutaneous pocket.
As per our standard clinical practice, stimulator programming was conducted two weeks post-surgery and at subsequent visits as needed (average maximum follow-up 26 months; range: 2–91 months). During the first session, the efficacy and potential adverse effects associated with each contact were investigated relative to increased stimulation amplitude and, if necessary, pulse width and frequency. During each session, stimulation settings were modified as deemed necessary by the programmer and the patient.
The Yale Global Tic Severity Scale (YGTSS) was administered at pre-operative screening and at follow-up; lower YGTSS scores represent a reduced tic severity. Bootstrapping was used to calculate 95% confidence intervals (CI) of the average percent improvement in YGTSS scores. A paired sample t-Wilcoxon signed-rank test (p<.05) was used to compare baseline preoperative and final follow-up YGTSS test results (n=10, subjects 1–4, 6–11). Subject 5 was excluded from group YGTSS analysis because his only available YGTSS scores were collected two weeks post-surgery, and thus may have reflected a microlesional effect. However, we included subject 5 in our series, because he had a 21-month clinical follow-up and because he had an infectious complication, information we feel important to report in this patient population. The YGTSS was not administered at every follow-up visit for every subject. Table 2 summarizes the length of time between pre-surgical baseline and the last available YGTSS and the date of last clinical follow-up for each subject.
Table 2.
| Patient | Preoperative test scores | Follow up test scores | Latest available YGTSS (months) | Last available clinical follow-up (months) | Tics at presentation | Tics at last follow-up | Absolute score change (% change) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Motor | Phonic | Impairment | Total | Motor | Phonic | Impairment | Total | Motor | Phonic | Impairment | |||||
| 1 | 93 | 23 | 20 | 50 | 22 | 12 | 0 | 10 | 91 | 95 | SM, CM, SP, CP, SIB, coprolalia | SM, CM | −71 (−76) | −11 (−48) | −20 (−100) | −40 (−80) |
| 2 | 80 | 17 | 23 | 40 | 59 | 13 | 16 | 30 | 59 | 68 | SM, CM, SP, CP | SM, CM, SP, CP | −21 (−26) | −4 (−24) | −7 (−30) | −10 (−25) |
| 3 | 77 | 19 | 8 | 50 | 0 | 0 | 0 | 0 | 31 | 62 | SM, CM, SP, CP | / | −77 (−100) | −19 (−100) | −8 (−100) | −50 (−100) |
| 4 | 71 | 20 | 21 | 30 | 18 | 10 | 8 | 0 | 41 | 41 | SM, CM, SP, CP, SIB | SM, CM, SP | −53 (−75) | −10 (−50) | −13 (−62) | −30 (−100) |
| 5 | 79 | 21 | 18 | 40 | 44 | 13 | 11 | 20 | 0.5 | 21 | SM, CM, SP | SM, CM, SP, CP | −35 (−44) | −8 (−38) | −7 (−39) | −20 (−50) |
| 6 | 95 | 25 | 20 | 60 | 30 | 14 | 6 | 10 | 12 | 29 | SM, CM, SP, SIB | SM, CM, SP, CP | −65 (−68) | −11 (−44) | −14 (−70) | −50 (−83) |
| 7 | 83 | 22 | 21 | 40 | 54 | 12 | 12 | 30 | 7 | 11 | SM, CM, SP, CP, SIB | SM, CM, SP | −29 (−35) | −10 (−45) | −9 (−43) | −10 (−25) |
| 8 | 94 | 23 | 21 | 50 | 70 | 15 | 15 | 40 | 6 | 7 | SM, CM, SP, CP | SM, CM, SP, CP | −24 (−26) | −8 (−35) | −6 (−29) | −10 (−20) |
| 9 | 96 | 23 | 23 | 50 | 45 | 13 | 12 | 20 | 2 | 8 | SM, CM, SP, CP, SIB, copropraxia, coprolalia | SM, CM, SP, copropraxia | −51 (−53) | −10 (−43) | −11 (−48) | −30 (−60) |
| 10 | 91 | 22 | 19 | 50 | 80 | 20 | 20 | 40 | 3 | 3 | SM, CM, SP, CP, copropraxia, coprolalia | SM, CM, SP, CP, coprolalia, copropraxia | −11 (−12) | −2 (−9) | +1 (+5) | −10 (−20) |
| 11 | 80 | 22 | 18 | 40 | 29 | 9 | 10 | 10 | 3 | 3 | SM, CM, SP, CP, copropraxia, coprolalia | SM, SP, CP | −51 (−64) | −13 (−59) | −8 (−44) | −30 (−75) |
CM = complex motor; CP = complex phonic; N/A = not applicable; SM = simple motor; SP = simple phonic; YGTSS = Yale Global Tic Severity Scale.
Lower scores represent symptom reduction and improved function.
RESULTS
Intraoperative recordings and stimulation
The results of intraoperative microelectrode recordings (left recordings in subject 1; bilateral recordings in subjects 2, 4, and 5) showed bursting neuronal firing consistent with thalamic neurons. Due to lack of perceived clinical utility the procedure was not performed in the remaining patients. In Subject 2, the only patient who underwent the procedure while awake, microelectrode recordings at the planned right-sided target evoked a neuronal sensory response to tactile stimuli.
During macrostimulation, subject 2 reported transient ipsilateral thumb and hand numbness during left stimulation (contacts 3-0+, 120μs pulse width, 100Hz frequency, 2-4V amplitude). During right macrostimulation (120μs pulse width, 100Hz frequency, 3V amplitude), the patient reported contralateral hand and face paresthesias that were transient when stimulating contacts 3-0+ and persistent when stimulating contacts 3-1+. These effects led to repositioning the right lead 2cm more anteriorly. Macrostimulation at contacts 3-0+ (120μs pulse width, 100Hz frequency again elicited contralateral hand and face paresthesias that were transient at 2.5V amplitude and persistent at 3V amplitude. Additionally, the patient reported a decrease in tic urges and in facial and vocal tics during left stimulation (contacts 3-0+, 120μs pulse width, 100Hz frequency, 1.5V amplitude).
There were no adverse motor effects during intraoperative macrostimulation in subject 2, nor in subjects 1 and 5, who underwent the procedure in the absence of muscle relaxation.
Clinical Outcomes
The final YGTSS was administered an average of 26 months post-surgery (range: 2–91 months) (n=10; subjects 1–4 and 6–11). The YGTSS Total Score improved by an average percent change of 54% compared to baseline (range: 12% – 100%; 95% CI: 37–70). Motor scores improved by an average percent change of 46% (range: 9% – 100%; 95% CI: 34–64), Phonic scores by an average percent change of 52% (range: 5% increase to 100% decrease; 95% CI: 34–72), and Impairment scores by an average percent change of 59% (range: 20% – 100%; 95% CI: 39–78) (Table 2). The average Total, Motor, Phonic, and Impairment scores at the last follow-up differed significantly from baseline scores (p=.005, p=.005, p=.007, p=.005, respectively).
Clinical follow-up averaged 29 months (range: 2–95 months) at the end of which all but two patients (subjects 8 and 10) reported marked reduction in tic severity and/or frequency and improvement in quality of life. Following implantation, patients were able to be employed (n=7), enjoy an improved social life (n=5), drive (n=3), and go to college (n=1). Subject 8 reported no symptom improvement; however, switching the stimulator off for one week, five months after lead implantation, resulted in worsening of tics, and he requested that it be turned back on. Subject 10 experienced recurrence of symptoms five months after lead implantation and is currently undergoing a trial period off stimulation.
Complications and adverse effects
Neurological examination and postoperative imaging revealed no intraoperative complications. There was one post-operative surgical complication. Three months after lead implantation, subject 5 developed a left parietal scalp erosion at the location of the intracranial electrodes, with external protrusion of the wires and purulent drainage. He underwent a surgical revision, with excision of the skin erosion, creation of a surgical pocket for the reinserted wires, bacitracin irrigation, and insertion of a catheter for drainage and vancomycin delivery to the surgical site. The catheter was removed prior to hospital dismissal; cultures from the surgical wound grew methicillin-sensitive Staphylococcus Aureus. He was started on intravenous piperacillin-tazobactam (3.375 gr q.i.d. for 3 days) and vancomycin (1.550 gr b.i.d. for 7 days), followed by oral levofloxacin (500mg for 10 days), and the implant was successfully salvaged. At last follow-up, 17 months after the surgical wound revision, the patient had not developed any other hardware-related infectious complications.
Post-surgical adverse effects found on neuropsychological evaluations included: mild processing speed and response inhibition impairment (subject 2); decreased memory (subject 3); and mild decline on some measures of attention and mental flexibility (subject 4). Other adverse effects included: shock-like sensations around the battery site (subject 2); neck tightness along the lead extension (subjects 4 and 8); temporary anterograde amnesia (subject 4); and recurrent new-onset occipital headache associated with left facial allodynia, nausea, vomiting, photo- and phonophobia (subject 5).
Stimulation-induced adverse effects included recurrent tension headache, worsening of pre-existing tremor, and transient blurring of vision at the initiation of stimulation (subject 1). Voltage increase resulted in dizziness (subject 5) and mild paresthesias (subject 7) lasting a few days. Adverse effects occurring upon voltage increase that resolved with voltage decrease included dysarthria, involuntary movements of the tongue and jaw, and mouth opening (subject 8) and a single seizure-like episode (speech interruption and involuntary eye movements) (subject 4). Three years after surgery, subject 4 reported recurrent suicidal thoughts of 2-week duration.
DISCUSSION
DBS for TS has been performed in over 100 patients, with the CM-Pf complex the most widely investigated target. Other targets include the globus pallidus, the anterior limb of the internal capsule, the nucleus accumbens, and the subthalamic nucleus.19 The role of the CM-Pf in motor and executive functions such as attention and motor control20–22 and its connections with many subcortical regions22,23 may contribute to the efficacy of CM-Pf DBS for TS. The literature suggests good tic control with CM-Pf DBS.7–11,13,15,24–26 The largest series of CM-Pf DBS for TS (34 patients)26 reports significant tic and comorbidity reduction at both long- and short-term follow-up. Double-blinded randomized trials further support CM-Pf DBS as an effective TS treatment.7,8,11,25
Ours was an open label study of CM-Pf DBS for severe, medication refractory TS. This case series found that nine out of the eleven subjects experienced clinically relevant tic reduction and higher levels of social functioning. The average improvement on the YGTSS was significant. The five subjects (1–4, 6) who were followed for one year or longer had a greater average decrease in their YGTSS scores (average decrease of 69% in YGTSS Total Score compared to baseline) than those followed for less than one year (subjects 7–11), who had an average of decrease of 38% in YGTSS Total Score compared to baseline. Of those, only subject 2 had a Total Score and subscore improvement of 30% or less. This suggests that it may take at least a year to optimize control of TS symptoms.
The highest stimulation amplitude for achieving tic control without adverse effects was 4.05V. Paresthesias and dizziness were the main adverse effects of amplitudes above 4.05V, and there was no improvement in efficacy above that level.
As Porta and colleagues observed,12 DBS outcomes for TS are often interpreted differently by patients and clinicians, a phenomenon we also experienced. This may be related to patient comorbidities and anxiety or to a mismatch between therapeutic results and patient expectations. Thus, before undergoing DBS surgery, patients should be carefully counseled about the potential limitations of DBS therapy. In some cases, an ethical specialist or neuropsychologist may be needed to assure the medical team that the patient has the capacity to understand the nature of the surgery and potential outcomes. Pre-and postsurgical measures of tic severity and frequency should also be administered.
Three subjects were 18 years or younger and had an average reduction in the YGTSS Total Score of 75% or higher and a tic reduction of 56% or higher. At the time of surgery, two of these three pediatric patients presented tics with severe cervical spine involvement and dental fractures, and none were able to attend school full-time. At follow-up, they reported improved social functioning and were able to hold jobs or attend college. Our results and those from other case series13,26 concordant with new inclusion criteria recommendations for TS DBS,19 lead us to support DBS in selected pediatric patients to promote normal development, social functioning, and educational achievement.
A higher incidence of system damage or infection has been reported in DBS for TS compared to DBS for other movement disorders.13,26,27 In our series, no hardware needed to be explanted, and the single patient with an infectious complication continued to benefit from stimulation.
Limitations and future directions
As a retrospective study, it was difficult to control all variables. Long-term (three or more years) follow-up YGTSS scores were available for only three patients, and follow-up time varied across our series. In part, the limited subject pool and limited available follow-up reflects the small number of TS patients for whom DBS is appropriate and available as an off-label treatment It should be noted as well that although double-blinded cross-over trials support the use of DBS in TS, open label studies report better clinical outcomes,28 which may have been a factor in the positive outcomes reported in this open label series.
Finally, as a retrospective study, formal neuropsychological or psychiatric evaluations were not consistently performed at follow-up in all patients, and our estimates of positive or adverse effects in these domains depended on subjective evaluation, observation, patient reports, and modification of treatment for existing comorbidities. This may be of relevance because the literature includes reports of both TS DBS-related cognitive decline 7,29 as well as maintenance of cognitive functions.12,25
CONCLUSION
In this retrospective case series of CM-Pf DBS for medically refractory TS, we observed good overall tic control and improvement of general functioning. Our positive clinical outcomes and a single instance of an adverse event directly related to surgery support the current literature in favor of expanded adoption of this treatment modality in a selected TS patient population.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the National Institutes of Health (R01 NS070872 award to KHL). Dr. Bryan T. Klassen receives research support from Medtronic unrelated to this study.
We thank Dr. Jamie J. Van Gompel for performing one of the surgeries in this series, Bruce A. Kall and Laura M. Haugen for providing patient targeting images, and Dr. Shinho Cho for performing part of the statistical analysis.
Abbreviations
- CM-Pf
centromedian-parafascicular
- DBS
deep brain stimulation
- TS
Tourette syndrome
- YGTSS
Yale Global Tic Severity Scale
Footnotes
Conflict of interest disclosure: The authors report no further financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leckman JF, Bloch MH, Scahill L, King RA. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642–649. doi: 10.1177/08830738060210081001. [DOI] [PubMed] [Google Scholar]
- 2.Leckman JF, Zhang H, Vitale A, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102(1 Pt 1):14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Malaty IA, Akbar U. Updates in medical and surgical therapies for Tourette syndrome. Curr Neurol Neurosci Rep. 2014;14(7):458. doi: 10.1007/s11910-014-0458-4. [DOI] [PubMed] [Google Scholar]
- 4.Singer HS. The treatment of tics. Curr Neurol Neurosci Rep. 2001;1(2):195–202. doi: 10.1007/s11910-001-0016-8. [DOI] [PubMed] [Google Scholar]
- 5.Hassler R, Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s disease. Rev Neurol (Paris) 1970;123(2):89–100. [PubMed] [Google Scholar]
- 6.Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353(9154):724. doi: 10.1016/s0140-6736(98)05964-9. [DOI] [PubMed] [Google Scholar]
- 7.Ackermans L, Duits A, van der Linden C, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011;134(Pt 3):832–844. doi: 10.1093/brain/awq380. [DOI] [PubMed] [Google Scholar]
- 8.Maciunas RJ, Maddux BN, Riley DE, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107(5):1004–1014. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- 9.Bajwa RJ, de Lotbiniere AJ, King RA, et al. Deep brain stimulation in Tourette’s syndrome. Mov Disord. 2007;22(9):1346–1350. doi: 10.1002/mds.21398. [DOI] [PubMed] [Google Scholar]
- 10.Lee MW, Au-Yeung MM, Hung KN, Wong CK. Deep brain stimulation in a Chinese Tourette’s syndrome patient. Hong Kong Med J. 2011;17(2):147–150. [PubMed] [Google Scholar]
- 11.Okun MS, Foote KD, Wu SS, et al. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA Neurol. 2013;70(1):85–94. doi: 10.1001/jamaneurol.2013.580. [DOI] [PubMed] [Google Scholar]
- 12.Porta M, Servello D, Zanaboni C, et al. Deep brain stimulation for treatment of refractory Tourette syndrome: long-term follow-up. Acta Neurochir (Wien) 2012;154(11):2029–2041. doi: 10.1007/s00701-012-1497-8. [DOI] [PubMed] [Google Scholar]
- 13.Motlagh MG, Smith ME, Landeros-Weisenberger A, et al. Lessons Learned from Open-label Deep Brain Stimulation for Tourette Syndrome: Eight Cases over 7 Years. Tremor Other Hyperkinet Mov (N Y) 2013:3. doi: 10.7916/D8M32TGM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields DC, Cheng ML, Flaherty AW, Gale JT, Eskandar EN. Microelectrode-guided deep brain stimulation for Tourette syndrome: within-subject comparison of different stimulation sites. Stereotact Funct Neurosurg. 2008;86(2):87–91. doi: 10.1159/000112429. [DOI] [PubMed] [Google Scholar]
- 15.Kaido T, Otsuki T, Kaneko Y, Takahashi A, Omori M, Okamoto T. Deep brain stimulation for Tourette syndrome: a prospective pilot study in Japan. Neuromodulation. 2011;14(2):123–128. doi: 10.1111/j.1525-1403.2010.00324.x. discussion 129. [DOI] [PubMed] [Google Scholar]
- 16.Savica R, Stead M, Mack KJ, Lee KH, Klassen BT. Deep brain stimulation in tourette syndrome: a description of 3 patients with excellent outcome. Mayo Clin Proc. 2012;87(1):59–62. doi: 10.1016/j.mayocp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, text revision) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 18.Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the Human Brain. 2. George Thieme Verlag; Stuttgart, Germany: 1977. [Google Scholar]
- 19.Schrock LE, Mink JW, Woods DW, et al. Tourette syndrome deep brain stimulation: A review and updated recommendations. Mov Disord. 2015;30(4):448–471. doi: 10.1002/mds.26094. [DOI] [PubMed] [Google Scholar]
- 20.Kim JP, Min HK, Knight EJ, et al. Centromedian-parafascicular deep brain stimulation induces differential functional inhibition of the motor, associative, and limbic circuits in large animals. Biol Psychiatry. 2013;74(12):917–926. doi: 10.1016/j.biopsych.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78(2–3):60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckert U, Metzger CD, Buchmann JE, et al. Preferential networks of the mediodorsal nucleus and centromedian-parafascicular complex of the thalamus--a DTI tractography study. Hum Brain Mapp. 2012;33(11):2627–2637. doi: 10.1002/hbm.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadikot AF, Rymar VV. The primate centromedian-parafascicular complex: anatomical organization with a note on neuromodulation. Brain Res Bull. 2009;78(2–3):122–130. doi: 10.1016/j.brainresbull.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Houeto JL, Karachi C, Mallet L, et al. Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76(7):992–995. doi: 10.1136/jnnp.2004.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welter ML, Mallet L, Houeto JL, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65(7):952–957. doi: 10.1001/archneur.65.7.952. [DOI] [PubMed] [Google Scholar]
- 26.Servello D, Sassi M, Brambilla A, Defendi S, Porta M. Long-term, post-deep brain stimulation management of a series of 36 patients affected with refractory gilles de la tourette syndrome. Neuromodulation. 2010;13(3):187–194. doi: 10.1111/j.1525-1403.2009.00253.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim W, Pouratian N. Deep brain stimulation for Tourette syndrome. Neurosurg Clin N Am. 2014;25(1):117–135. doi: 10.1016/j.nec.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Shahed J. Design challenges for stimulation trials of Tourette’s syndrome. Lancet Neurol. 2015;14(6):563–565. doi: 10.1016/S1474-4422(15)00043-5. [DOI] [PubMed] [Google Scholar]
- 29.Schoenberg MR, Maddux BN, Riley DE, et al. Five-Months-Postoperative Neuropsychological Outcome From a Pilot Prospective Randomized Clinical Trial of Thalamic Deep Brain Stimulation for Tourette Syndrome. Neuromodulation. 2014 doi: 10.1111/ner.12233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.