Highlight
Dominant negative form of OsLSK1 (Large spike S-domain receptor like Kinase 1) leads to the improvement of yield components, resulting in significantly increased grain yield in rice.
Key words: Panicle architecture, rice, S-domain receptor-like kinase.
Abstract
The S-domain receptor kinase (SRK) comprises a highly polymorphic subfamily of receptor-like kinases (RLKs) originally found to be involved in the self-incompatibility response in Brassica. Although several members have been identified to play roles in developmental control and disease responses, the correlation between SRKs and yield components in rice is still unclear. The utility of transgenic expression of a dominant negative form of SRK, OsLSK1 (Large spike S-domain receptor like Kinase 1), is reported here for the improvement of grain yield components in rice. OsLSK1 was highly expressed in nodes of rice and is a plasma membrane protein. The expression of OsLSK1 responded to the exogenous application of growth hormones, to abiotic stresses, and its extracellular domain could form homodimers or heterodimers with other related SRKs. Over-expression of a truncated version of OsLSK1 (including the extracellular and transmembrane domain of OsLSK1 without the intracellular kinase domain) increased plant height and improve yield components, including primary branches per panicle and grains per primary branch, resulting in about a 55.8% increase of the total grain yield per plot (10 plants). Transcriptional analysis indicated that several key genes involved in the GA biosynthetic and signalling pathway were up-regulated in transgenic plants. However, full-length cDNA over-expression and RNAi of OsLSK1 transgenic plants did not exhibit a detectable visual phenotype and possible reasons for this were discussed. These results indicate that OsLSK1 may act redundantly with its homologues to affect yield traits in rice and manipulation of OsLSK1 by the dominant negative method is a practicable strategy to improve grain yield in rice and other crops.
Introduction
A rapid increase in world human population and a reduction in agricultural resources has promoted many researchers to carry out more biotechnology research to increase grain yield in cereal crops. Rice (Oryza sativa L.) is one of the most important staple cereal crops as it feeds more than half of the world’s population. Grain yield is an essential agronomic trait of rice which consists of many components including panicles per plant, grains per panicle, and grain weight (Xing and Zhang, 2010). To date, a number of genes have been reported to regulate this complex trait.
Plant receptor-like protein kinases (RLKs) comprise one of the largest and most diverse superfamily of plant proteins with 610 and 1 131 members in the Arabidopsis and rice genomes, respectively (Shiu et al., 2004). The RLK gene superfamily has been implicated in the prevention of self-pollination, the pathogen defence response, hormone perception, developmental regulation, adaptation to abiotic stresses, and quantitative yield components (Haderlein et al., 2001; Bai et al., 2009; Lehti-Shiu et al., 2009; Den Herder et al., 2012). Plant RLKs are composed of an extracellular N-terminal domain which acts as a ligand-binding site, a single membrane-spanning region, and a conserved C-terminal cytoplasmic protein kinase domain (Castells and Casacuberta, 2007). According to the structural feature of the extracellular domain, RLKs are classified into 44 subfamilies, such as leucine rich repeat (LRR), self-compatibility domain (S-domain), wall-associated kinase (WAK), lectin (Shiu and Bleecker, 2001; Tor et al., 2009), and many more. Among these subclasses one or only a small number of proteins have been characterized in detail.
The first plant RLK gene (ZmPK1) was isolated in maize (Walker and Zhang, 1990), and thereafter many RLK genes with diverse functions have been identified in many plant species (Becraft, 2002). For example, In the WAK RLK subfamily, WAK is required for cell expansion throughout plant development (Wagner and Kohorn, 2001). The function of the Lectin RLK protein, Pi-d2, is to enhance resistance to the rice blast fungus (Chen et al., 2006). The calmodulin-binding receptor-like protein kinase (CBRLK1) acts as a negative regulator of pathogen resistance in Arabidopsis (Kim et al., 2009). In the largest LRR RLK subfamily, CLAVATA1 (CLV1) controls meristem development (Clark et al., 1993). Rice Xanthomonas resistance 21 (Xa21) and Xa26 are associated with enhanced tolerance to Xanthomonas oryzae pv.oryzae (Lee et al., 2003; Wang et al., 2006). BR insensitive 1 (BRI1) and BRI1 associated kinase 1 (BAK1) work together to perceive and modulate BR signaing (Li and Chory, 1997). HAESA is related to floral organ abscission (Jinn et al., 2000); flagellin sensitive 2 (FLS2) can recognize the bacterial elicitor flagellin (Danna et al., 2011; Gómez-Gómez and Boller, 2000); AvrPphB susceptible 1 (PBS1) confers resistance to Pseudomonas syringae strains (Swiderski and Innes, 2001); ERECTA1 regulates organ shape (Shpak et al., 2003; van Zanten et al., 2009). In addition, LRR-RLK subfamily can affect the yield components. Over-expression the LRR-RLK gene LRK1 improves yield components of rice, including the panicles per plant, spikelets per panicle, grains per plant, 1000-grain weight and plant height (Zha et al., 2009).
S-domain RLKs (SRKs) represent the second largest subfamily of RLKs, with 147 members in rice. The well-characterized Brasssica S-domain RLK (SRK) is a self-incompatibility receptor which auto-phosphorylated when pollinated with incompatible pollen (Goring and Rothstein, 1992; Takasaki et al., 2000; Cabrillac et al., 2001). Expression pattern analysis showed that many genes encoding S-domain RLKs were broadly expressed in many tissues and rapidly accumulated in response to wounding and pathogen invasion, suggesting possible roles in both developmental control and disease responses (Dwyer et al., 1994; Pastuglia et al., 1997, 2002). The newly identified member OsSIK2 increases stress tolerance and delays dark-induced leaf senescence in transgenic rice plants (Chen et al., 2013).
In this study, an S-domain RLK OsLSK1 associated with abiotic stress sensitivity and increased grain yield was characterized. Over-expression of the extracellular domain of OsLSK1, rather than the full length, improved the yield components in rice. The OsLSK1 extracellular domain can form dimers with itself or with five of the most homologous SRKs, suggesting ectopic expression of the extracellular domain of OsLSK1 may cause a dominant negative effect to alter the yield components in rice. Further investigation showed that the GA biosynthetic and signalling pathway genes may be involved in the improvement of the yielding traits. Our study provides a new approach for yield improvement in cereal crops.
Materials and methods
Plant materials and growth conditions
Rice plants were cultivated at the Experimental Station of the Chinese Academy of Agricultural Sciences in Beijing (39°54′ N, 116°23’ E) during the summer. The field test experiments were performed at two locations with different soil fertility levels. Each location consisted of three replicates and each replicate included 10 individuals for each material. The relevant agronomical traits were analysed at the heading and mature stages. Statistical analysis was performed with independent samples using the least significance difference (LSD) software.
Generation of transgenic rice plants
To generate the OXOsLSK1-t and OXOsLSK1-f vectors, the extracellular domain (1–1 590bp) and the full length of OsLSK1 were cloned into the gateway entry vector pDONR 201 and then recombined into the pCAMBIA1301-Bar-FLAG vector, driven by the ubiquitin promoter. To construct the RNAi vector, a 309bp fragment (from 380–689bp) of OsLSK1 was amplified and cloned into the gateway entry vector pDONR 201, and then recombined into pANDA vector. The primer sequences are listed in Supplementary Table S1 at JXB online. All constructs were introduced into Agrobacterium tumefaciens strain EHA105 and then transformed into Kitaake wild-type plants (Gao et al., 2013).
GUS histochemical staining
To obtain pOsLSK1::GUS transgenic plants, a 2 249bp promoter region of OsLSK1 was amplified using the forward primer 5′-TATTTTCGGTACAATGGAGGTCG-3′ and hte reverse primer 5′-CGTTTCAACTATAGCAGTTTGGC-3′ from the genome of Nipponbare, and inserted into the HindIII and BamHI sites of the pCAMBIA3301-GUS vector. GUS histochemical staining assays were performed according to the method of Jefferson et al. (1987) and Laubinger et al. (2006).
RNA isolation and qRT-PCR analysis
RNA was isolated using TRIZOL (Invitrogen) and treated with DNase I (Invitrogen). The cDNA was synthesized from 3.0 μg total RNA using TransScript ® II One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech). LightCycler 480 SYBR Green I Master (Roche) was used for the quantitative PCR reaction. The mRNA level of Actin was used as the internal control. All the primers used are listed in Supplementary Table S1 at JXB online.
Hormone treatments and abiotic stresses
To investigate the response of OsLSK1 mRNA to hormone and abiotic stresses, the 3-week-old rice seedlings (Kitaake) were treated with 20 μM GR-24, 20% PEG, 1% H2O2, 200mM NaCl, and 0.1mM ABA, GA, BR, ABA, and JA as described in previously (Tang et al., 2012; Chu et al., 2013). Leaves samples were collected at 0, 2, 4, 8, 12, 24, 36, and 48h after the treatment. The OsLSK1 expression was monitored by qRT-PCR. Actin was used as the internal control.
Immunoblots
One-week-old seedlings of the over-expression lines and the wild-type controls were ground to extract protein. Immunoblots analysis were performed essentially as described by Meng et al. (2013).
Subcellular localization of OsLSK1
Full-length OsLSK1 was amplified and cloned into the gateway entry vector pDONR 201 and then fused in-frame at both the N- and C-terminus of YFP in the Gateway system (Invitrogen) vector pENSG-YFP and pEXSG-YFP under the control of the 35S CaMV promoter (Laubinger et al., 2006). The YFP-OsLSK1 and the OsLSK1-YFP constructs were transiently expressed in Arabidopsis leaf protoplasts (Abel and Theologis, 1994). The protoplasts were examined under a Leica TCS-SP4 confocal microscope after 12h incubation at 30 °C in the dark. The fluorescent lipophilic styryl dye FM4-64 was used to label the plasma membrane (Fischer-Parton et al., 2000). Empty vectors were used as a control.
Yeast two-hybrid assay
The yeast two-hybrid assay was performed according to the manufacturer’s instructions (ProQues two-hybrid system with Gateway technology, Invitrogen). The OsLSK1 extracellular domain was fused in-frame to the GAL4 DNA binding domain in the bait vector pDEST32 (Invitrogen). The extracellular domain of OsLSK1, LOC_Os01g47810, LOC_Os01g47820, LOC_Os01g48000, LOC_Os01g48020, and LOC_Os01g48040 were fused in-frame to the GAL4 DNA transcription activation domain in the prey vector pDEST22 (Invitrogen). The bait and prey plasmids were co-transformed into the yeast strain AH109 and grown on Trp-/Leu- plates at 28 °C for 2–3 d before being transferred to SD/Trp-/Leu-/His-/Ade plates at 28 °C for 2–3 d. The β-Gal activity was analysed by a colony-lift filter assay, using X-gal (5-bromo-4-chloro-3-indolyl-β-d-galacto-pyranoside) for blue colour development, according to the Yeast Protocols Handbook (Clontech). The yeast two-hybrid assay primers are listed in Supplementary Table S1 at JXB online.
BiFC assays
The N-terminal (including the extracellular domain and the transmembrane domain) of OsLSK1, LOC_Os01g47810, LOC_Os01g47820, LOC_Os01g48000, LOC_Os01g48020, and LOC_Os01g48040 were cloned into the pSPYNE (R) 173 or the SPYCE(MR) vector (Waadt and Kudla, 2008) and then transferred into Agrobacterium tumefaciens. The BiFC experiments were performed using Nicotiana benthamiana leaves through Agrobacterium tumefaciens infiltration as described by Meng et al. (2013). The YFP fluorescence was excited by a 514-nm laser and captured at 523–600nm. The construct primers are listed in Supplementary Table S1 at JXB online.
Results
Rice OsLSK1 encodes a typical S-domain receptor-like protein
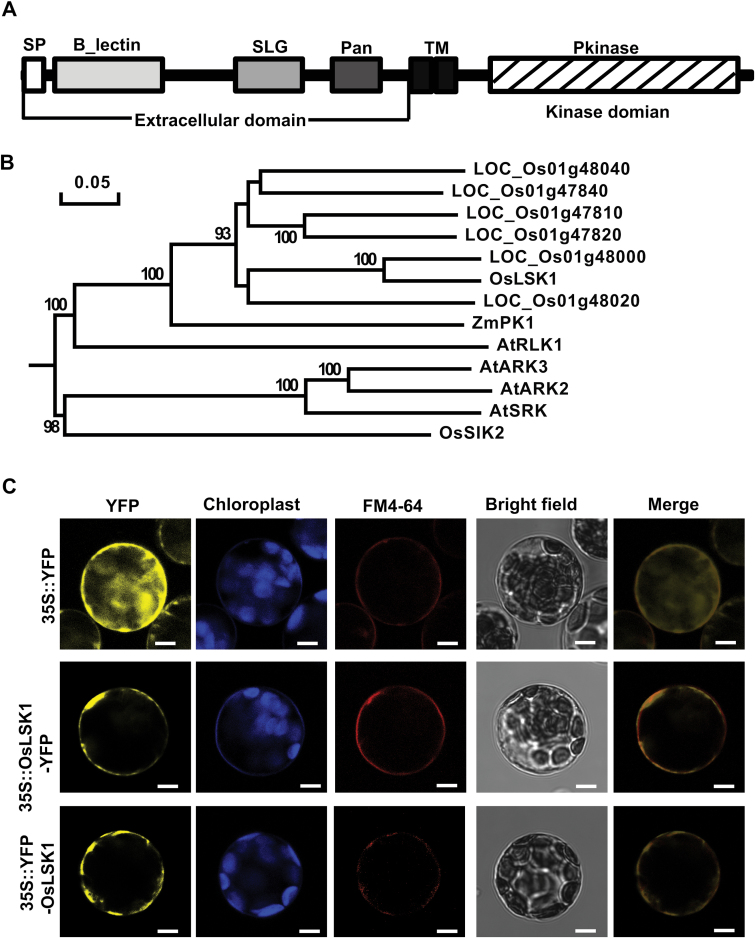
The complete sequence of OsLSK1 (2 460bp, GENBANK accession no: NM_001050355 GI: 115439080) was isolated and cloned by a reverse transcriptase-polymerase chain reaction (RT-PCR) from Oryza sativa ssp. japonica cv. Nipponbare seedlings. OsLSK1 encodes a protein of 819 amino acid residues with a predicted molecular mass of 89.9kDa. Protein analysis of the deduced amino acid sequence revealed that OsLSK1 contains a typical Type III S-receptor kinase structure (Zhang et al., 2011), including a B-Lectin domain (amino acids 76–188), an SLG domain (S_locus_glycop domain) (amino acids 238–313), a PAN like domain (amino acids 347–404), a transmembrane domain (amino acids 435–485), and a kinase domain at the C-terminal cytoplasmic region (amino acids 524–805) (Fig. 1A). Further analysis showed that the extracellular region had a membrane localization signal peptide composed of 27 amino acids at the N terminal; the intracellular domain contained the typical ATP-binding motif and a kinase active site (see Supplementary Fig. S1 at JXB online).
Fig. 1.
OsLSK1 encodes an S-domain RLK. (A) Schematic representation of the OsLSK1 protein. SP, signal peptide; SLG, S-locus glycoprotein; TM, transmembrane domain. (B) Rooted phylogenetic tree. The S-domain RLK sequences selected contain the conserve domain including B_Lectin, SLG(S locus glycoprotein), and Pan in the extracellular region. The amino acid sequences were aligned using Clustal X, and the phylogenetic tree was constructed by MEGA5.1 using the Neighbor–Joining method and bootstrap support was based on 1 000 replicates. (C) Subcellular localization of OsLSK1. The OsLSK1-YFP and YFP-OsLSK1 fusion proteins were mainly located in the plasma membrane. YFP was used as a control. Bar=5 μm.
Amino acid alignment analysis showed that OsLSK1 shares high similarity at both the intercellular and the extracellular domains with the homologues in rice (OsSIK2, LOC_Os01g47810, LOC_Os01g47820, LOC_Os01g48000, LOC_Os01g48020, and LOC_Os01g48040), maize (ZmPK1), and Arabidopsis (AtRLK1, AtSRK, AtARK2, and AtARK3) (see Supplementary Fig. S1 at JXB online). Phylogenetic analysis showed that OsLSK1 shares 55–81% high identity with rice homologues, 73% identity with the maize homologue (ZmPK1), and only about 33% identity with the Arabidopsis genes (Fig. 1B).
The membrane localizing signal peptide and transmembrane domain of the OsLSK1 protein suggest that it may be a membrane protein. To test this, two constructs harbouring the fusion proteins YFP-OsLSK1 and OsLSK1-YFP were generated, in which YFP was fused to the 5′ and 3′ ends of the OsLSK1 protein, respectively. Transient expression in the Arabidopsis mesophyll protoplasts showed that both YFP-OsLSK1 and OsLSK1-YFP were exclusively located on the plasma membrane, co-localizing especially with the plasma membrane fluorescent lipophilic styryl dye FM4-64. By contrast, the control protein YFP was only detectable in the intracellular region (Fig. 1C). Together, these data suggest that OsLSK1 is a plasma membrane-localized protein.
OsLSK1 forms dimers at the extracellular domain
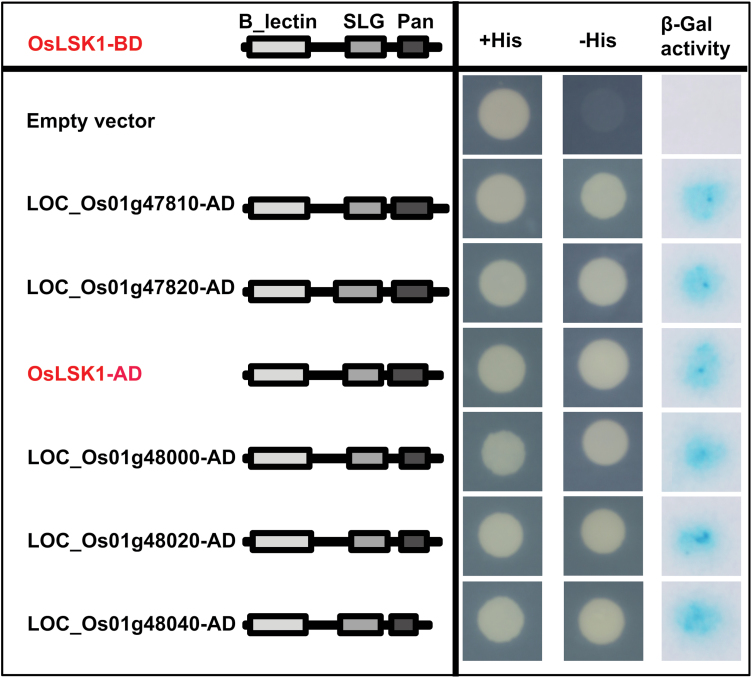
Rice genome annotation analysis (http://rice.plantbiology.msu.edu/index.shtml) showed that OsLSK1 is located on chromosome 1 and clustered with other six homologous genes within a 130kb region, which was separated by ten retrotransposons (see Supplementary Fig. S2B at JXB online). A previous report indicated that the PAN-like domain in the SRK extracellular region can serve as the interaction part of the molecule (Naithani et al., 2007). As shown in Fig. 1A, and in Supplementary Fig. S2A at JXB online, both OsLSK1 and its six homologues in rice contained the PAN-like domain. This prompted us to investigate whether OsLSK1 interacts with itself or with other homologues. cDNAs of the extracellular region of OsLSK1 and five homologues in rice (LOC_Os01g47840 could not be cloned) were cloned into two yeast expression vectors (pDEST22 and pDEST32), respectively, and co-transformed into a yeast strain for the yeast two-hybrid experiment. Results showed that the extracellular domain of OsLSK1 could interact with itself and also with all five homologues (Fig. 2).
Fig. 2.
OsLSK1 extracellular domain interacts with itself and its homologues in yeast. A histidine autotrophy assay and a colony-lift filter assay showed that the constructs (left panel) were also able to transactivate the expression of the lacZ reporter genes (right panel). The homologues include LOC_Os01g47810, LOC_Os01g47820, LOC_Os01g48000, LOC_Os01g48020, and LOC_Os01g48040.
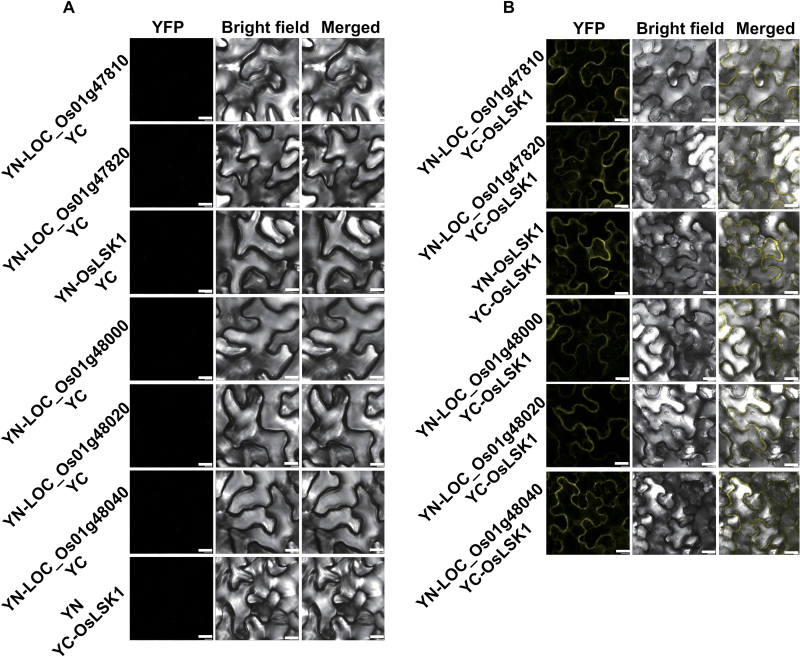
This result was then confirmed in planta using a bimolecular fluorescence complementation (BiFC) assay. The extracellular domain (including the transmembrane domain) of OsLSK1 and its five homologues were fused with the N-terminal of YFP (YN) or the C-terminal of YFP (YC) and co-expressed in N. benthamiana leaves using the Agrobacterium-mediated infiltration method. Strong fluorescence signals were detected in the N. benthamiana epidermal cell plasma membrane which was co-transfected with Agrobacterium tumefaciens strains harbouring YC-OsLSK1/YN-OsLSK1, YC-OsLSK1/YN-LOC_Os01g47810, YC-OsLSK1/YN-LOC_Os01g47820, YC-OsLSK1/YN-LOC_Os01g48000, YC-OsLSK1/YN-LOC_Os01g48020 or YC-OsLSK1/YN-LOC_Os01g48040. By contrast, the control which was co-transfected with YC/YN-OsLSK1, YC/YN-LOC_Os01g47810, YC/YN-LOC_Os01g47820, YC/YN-LOC_Os01g48000, YC/YN-LOC_Os01g48020 or YC/YN-LOC_Os01g48040 exhibited no signal (Fig. 3). Both the yeast two-hybrid and BiFC analysis indicated that OsLSK1 could interact with itself and also with five homologues, suggesting that OsLSK1 can form homodimers (maybe polymers) with itself and heterodimers with five homologues, respectively.
Fig. 3.
OsLSK1 extracellular domain interacts with itself or its homologues in planta. Epifluorescence and bright-field images of N. benthamiana epidermal leaf cells (scale bars=20 μm).
Expression pattern analysis of OsLSK1
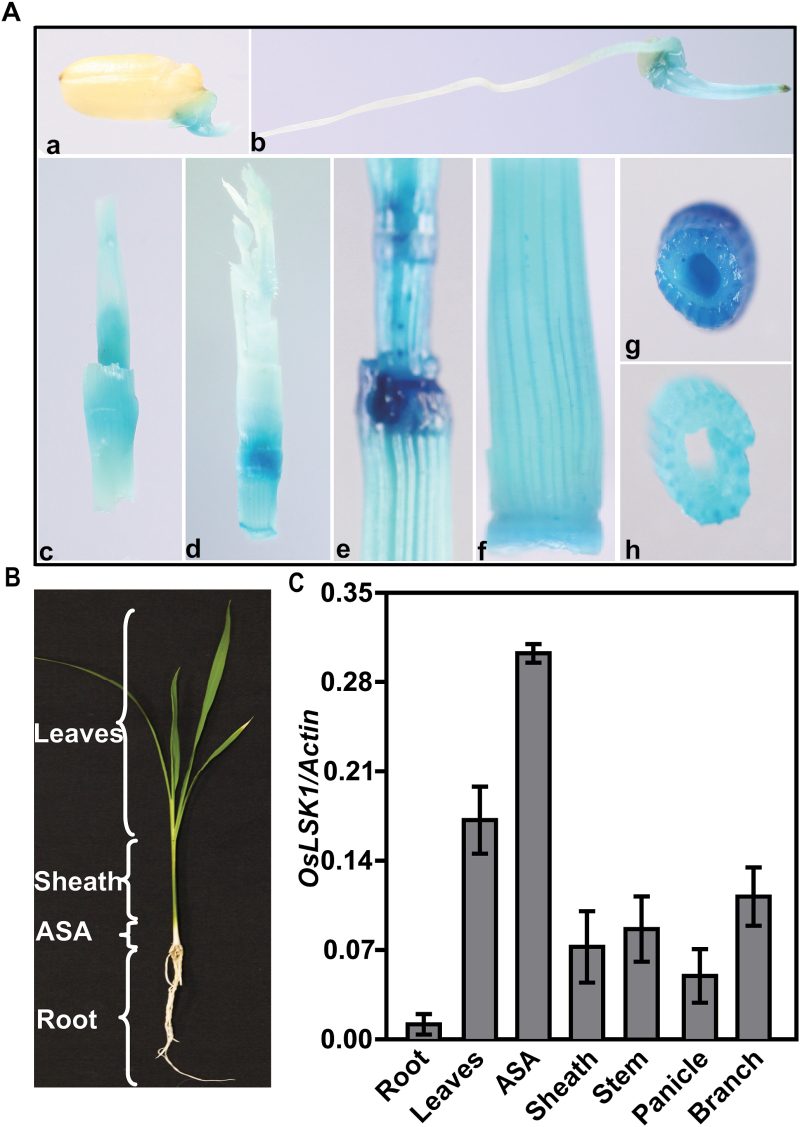
To investigate the tissue-specific expression pattern of OsLSK1, rice transgenic pOsLSK1::GUS reporter lines were used, in which the 2 249bp OsLSK1 promoter region is fused to the GUS gene (Shapiro and Zhang, 2001). As shown in Fig. 4A, histochemical staining revealed that OsLSK1 is expressed in the plumule of the germinating seed, the coleoptile, leaf, node, and sheath, but not in the young spike or root. OsLSK1 expression was also monitored from seeding to mature caryopses and our results showed an extremely high expression level of OsLSK1 in the stem, especially in the nodes, suggesting OsLSK1 may play important roles in node development. The OsLSK1 mRNA expression pattern was also analysed using qRT-PCR. Similar to the GUS staining results, OsLSK1 was expressed in nearly all the tissues but only weakly in roots and panicles. By contrast with the GUS staining results, OsLSK1 was highly expressed in young leaves and ‘Around the Shoot Apex’ region (ASA) (Fig. 4B, C), rather than in the stem. The difference between qRT-PCR and GUS staining may be due to posttranscriptional regulation.
Fig. 4.
OsLSK1 expression pattern in rice. (A) OsLSK1 expression revealed by GUS staining in OsLSK1 promoter–GUS transgenic plants.(a) Germinated seed (2 d), (b) a small seedling(5 d), (c) a shoot tip, (d) a young panicle (1.5cm long), (e) a stem node, (f) a sheath, (g) a cross-section of the stem node, and (h) a cross-section of the sheath. (B) Schematic diagram showing the root, leaves, and ASA (around the shoot apex) of a 30-d-old seeding. (C) The transcript level of OsLSK1 in various tissues and organs by quantitative RT-PCR. Data are shown as means ±SD (n=3). Root, leaves, and ASA (around the shoot apex) of a 30-d-old seeding and the stem (without the stem node), panicle (2cm length), branch (4cm length panicle without grains) of heading stage wild-type plants (Kitaake) were used.
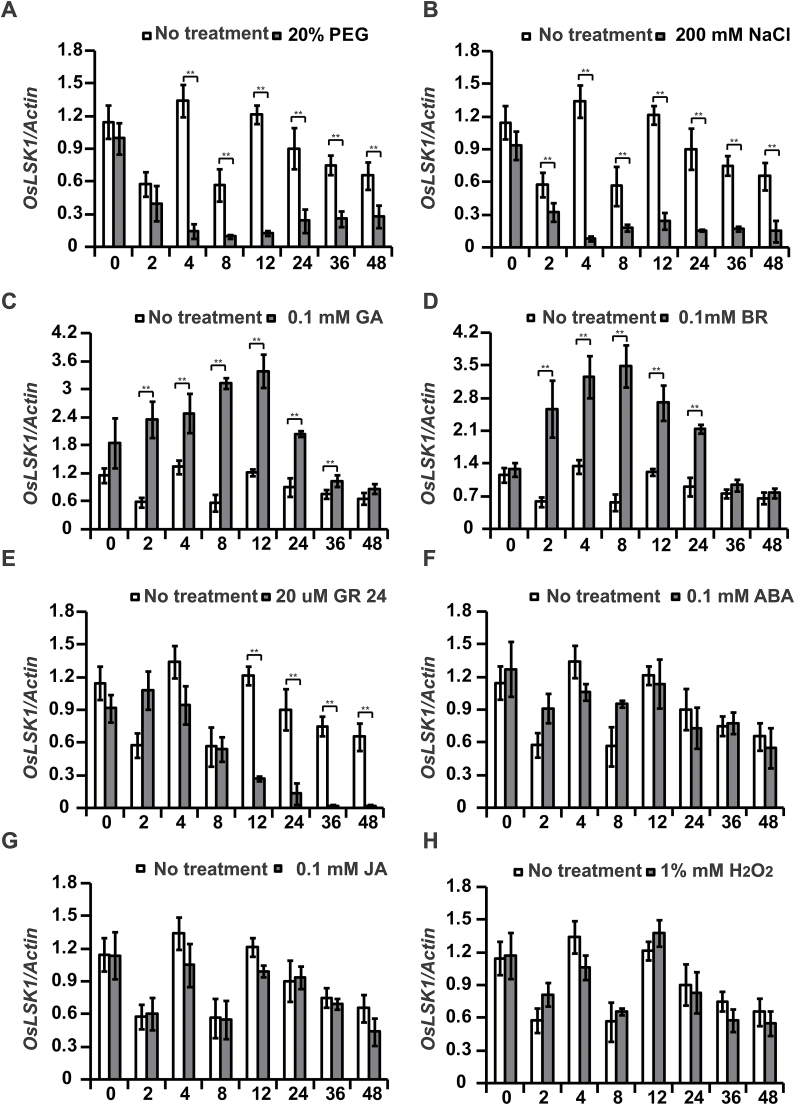
It has been reported that RLKs are transcriptionally regulated in response to hormone and abiotic stresses (Gish and Clark, 2011; Marshall et al., 2012; Lin et al., 2013; Wu and Zhou, 2013; Xu et al., 2013). Therefore, expression of OsLSK1 was also monitored under the phytohormone and abiotic stress treatments and the results indicated that OsLSK1 responded differently to the various phytohormone treatments. OsLSK1 was apparently induced by treatments with GA (0.1mM) and BR (0.1mM), with expression peaks at 4–8h after treatment. Thereafter, expression was gradually reduced (Fig. 5C, D). However, OsLSK1 expression was inhibited by GR24 (20 μM) 8h after the treatment and reached the lowest level 48h later (Fig. 5E). In addition, no significant differences in OsLSK1 expression were detected after the ABA (0.1mM) and JA (0.1mM) (Fig. 5F, G) treatments. The responses of OsLSK1 to abiotic stresses such as polyethylene glycol (PEG) and salinity (NaCl) and H2O2 were also analysed. OsLSK1 expression rapidly declined when treated with polyethylene glycol (PEG 20%) and NaCl (200mM) stress, but showed no obvious change when treated with H2O2 (1%) treatments (Fig. 5A, B, H).
Fig. 5.
OsLSK1 responds to hormones and abiotic stresses. Expression of OsLSK1 in wild type (Kitaake) seedlings in response to (A) PEG, (B) NaCl, (C) gibberellin (GA), (D) brassinosteroid (BR), (E) strigolactone anologue GR 24, (F) ABA, (G) jasmonic acid (JA), and (H) H2O2 treatment by quantitative RT-PCR. Data are shown as means ±SD. (Student’s t tests, **P <0.01, n=3).
Over-expressing the extracellular domain of OsLSK1 improves the quantitative yield components in rice
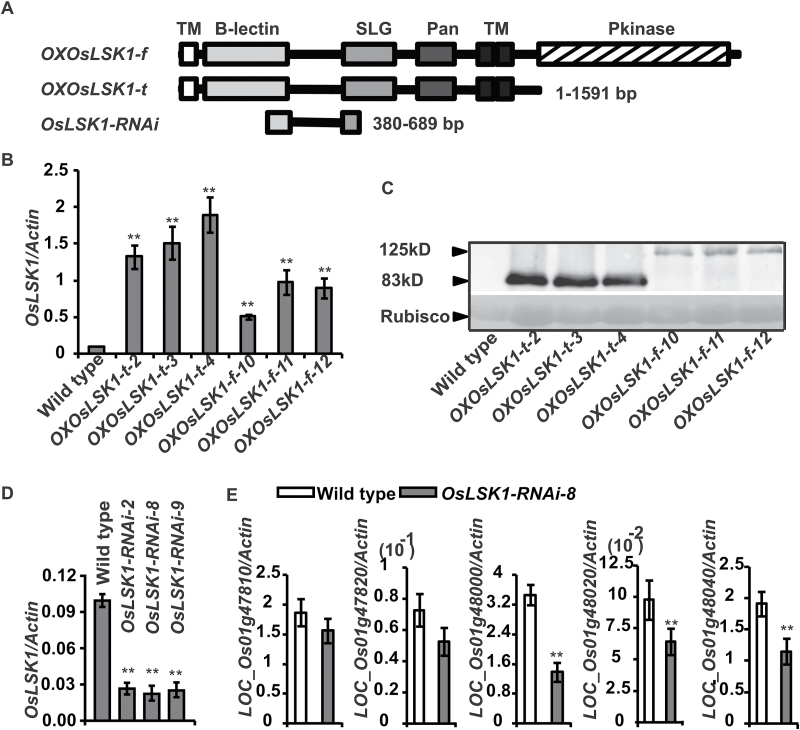
To examine the biological function of OsLSK1, multiple independent lines of T0 transgenic rice were obtained by Agrobacterium-mediated transformation, in which the full-length gene (OsLSK1-f) and the truncated gene (OsLSK1-t) (Fig. 6A) were over-expressed, respectively. Molecular analysis results showed that both OsLSK1-f and OsLSK1-t were highly expressed at both the RNA and protein levels in most of the transgenic lines tested (Fig. 6B, C). In addition, OsLSK1-RNAi T0 transgenic rice was also obtained. qRT-PCR results showed that OsLSK1 was dramatically reduced in most of the T0 OsLSK1-RNAi lines (Fig. 6D). The expression of the OsLSK1 homologues was also examined. Among them, LOC_Os01g48000 mRNA was reduced to a significantly low level, while others were only slightly down-regulated (Fig. 6E). Phenotype analysis indicated that OXOsLSK1-t, but not the OXOsLSK1-f and OsLSK1-RNAi transgenic plants, showed obvious phenotypes during the entire rice growth period compared with wild-type rice (see Supplementary Fig. S3 at JXB online). Thereafter, two lines (OXOsLSK1-t-3 and OXOsLSK1-t-4) which accumulated the highest expression levels of RNA and protein in all of the transgenic lines tested were selected for further study (Fig. 7).
Fig. 6.
Identify of OsLSK1 transgenic plants. (A) Schematic diagram showing the position of the OsLSK1 transgenic construct including ‘OXOsLSK1-f’, over-expressing full-length OsLSK1; ‘OXOsLSK1-t’, over-expressing the extracellular domain of OsLSK1 and OsLSK1-RNAi. (B) The expression of OsLSK1 determined by quantitative RT-PCR. (C) Immunoblots showing the level of OsLSK1-t-Flag fusion protein in OXOsLSK1-t plants and OsLSK1-f-Flag fusion protein in OsLSK1-f plants. (D) Transcriptional abundance of OsLSK1 homologous genes in wild type and OsLSK1-RNAi transgenic lines. Data are shown as means ±SD. (Student’s t tests, **P <0.01, n=3).
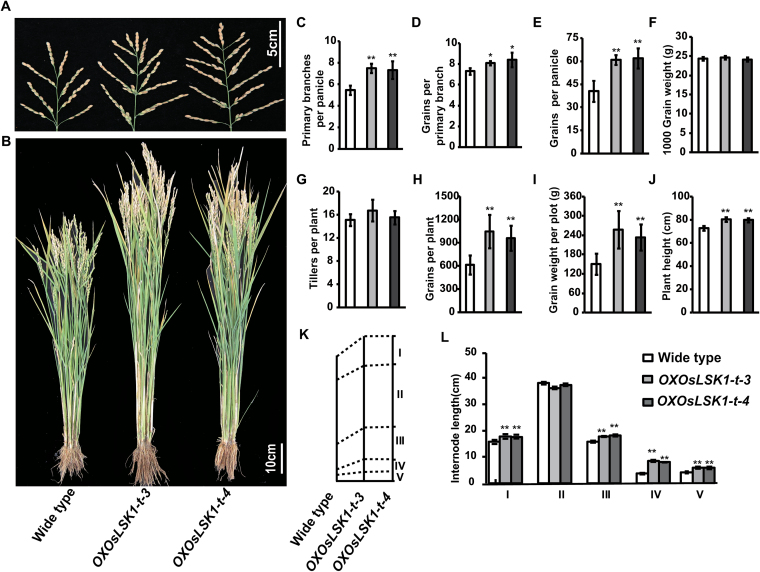
Fig. 7.
Phenotype analysis of OsLSK1-t over-expressing plants. (A) Panicle morphology of wild type and OXOsLSK1-t. (B) Gross morphology of wild-type and OXOsLSK1-t transgenic rice. (C–I) Comparison of (C) primary branches per panicle, (D) grains per primary branch, (E) grains per panicle, (F) 1 000 grain weight, (G) tillers per plant, (H) grains per plant, (I) grain weight per plot, and (J) plant height between the wild type and OXOsLSK1-t transgenic rice. Data are shown as means ±SD. (Student’s t tests, *P <0.05, **P <0.01, n=60). (K, L) Schematic representation (K) and comparison of the various elongation patterns of internodes (L) in the wild type and OXOsLSK1 transgenic rice. The first to fifth internodes are indicated as I–V from top to bottom. Data are shown as means ±SD. (Student’s t tests, **P <0.01, n=10).
Compared with the wild-type plant, OXOsLSK1-t-3 and OXOsLSK1-t-4 showed larger panicles, higher grain yields, and taller culm phenotypes (Fig. 7). To analyse the larger panicle phenotype, the branching pattern of OXOsLSK1-t-3 and OXOsLSK1-t-4 was examined in more detail. On average, OXOsLSK1-t-3 and OXOsLSK1-t-4 panicles possessed 7.50 and 7.33 primary branches, respectively, while the wild-type panicle only possessed 5.48 primary branches. This indicates that the primary branches per panicle were significantly more abundant in OXOsLSK1-t-3 and OXOsLSK1-t-4 than in the wild type (Fig. 7C). The number of grains produced on the primary branches (Fig. 7D) was also determined. The results showed that the number of grains per primary branch of the OXOsLSK1-t-3 and OXOsLSK1-t-4 transgenic lines were 8.11 and 8.42, respectively, significantly higher than that of the wild type (7.33) (Fig. 7D). As a result, the increase in primary branches and grains produced on the primary branches led to the dramatic increase in grain number per panicle in OXOsLSK1-t-3 and OXOsLSK1-t-4 which were 60.84 and 61.80, respectively, whereas it was only 40.46 in wild-type plants (Fig. 7E).
Other components of the rice yield trait were also examined such as tiller number, seed setting rate (SSR), and 1000-grain weight. Statistical analysis revealed that there was no difference between OXOsLSK1-t-3, OXOsLSK1-t-4, and wild-type plants (see Supplementary Table S2 at JXB online; Fig. 7F, G) for seed setting rate (SSR), 1000-grain weight, and tiller number. The actual grain yield at plot level (10 plants per plot) was also measured; the results showed that the grain yield of OXOsLSK1-t-3 and OXOsLSK1-t-4 increased more than 55.8% compared with wide-type plants (Fig. 7G). The increased grain number per panicle appears to explain why the OXOsLSK1-t-3 and OXOsLSK1-t-4 plants have a higher grain yield.
To investigate culm phenotype, internode length at a late stage of rice growth was measured. It was found that all the internodes of OXOsLSK1-t-3 and OXOsLSK1-t-4, except for the second (II) internode, were significantly increased compared with that of the wild type. This increase in internode length was particularly obvious in the fourth and fifth internodes (Fig. 7J, K, L).
GA biosynthetic and signalling pathway genes were up-regulated in the OXOsLSK1-t plant
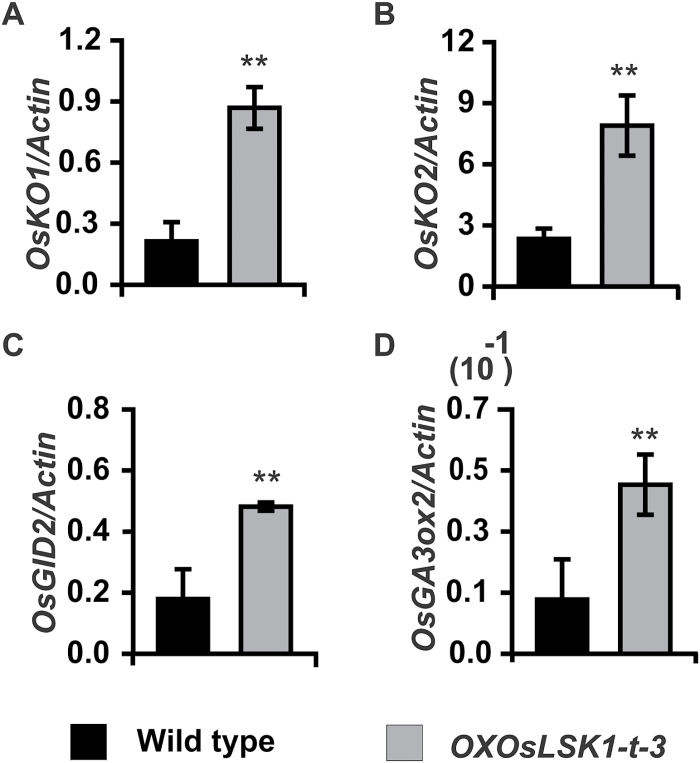
Our data indicated that OsLSK1 is positively responsive to the GA and BR treatments, which suggests that the GA and BR biosynthetic or signalling pathways may associate with the phenotype of the OXOsLSK1-t transgenic plants. To test this speculation, qRT-PCR was used to compare the mRNA expression of several key genes involved in the GA (OsKO1, OsKO, and GA20ox2) or BR (OsBRI1 and OsGSR1) biosynthesis and GA signal transduction pathways (OsGID2) between OXOsLSK1-t-3 and wild-type seedlings at the six-leaf stage. qRT-PCR results showed that the expression of genes (OsKO1, OsKO2, GA20ox, and OsGID2) involved in the GA biosynthesis and signalling transduction pathways were significantly up-regulated (2–4-fold) in OXOsLSK1-t transgenic seedlings compared with their respective expression in the wild type (Fig. 8). However, there were no significantly different expression changes observed for the BR biosynthesis genes between the wild-type and OXOsLSK1-t transgenic seedlings (see Supplementary Fig. S4 at JXB online). These data suggest that the phenotypes of OXOsLSK1-t transgenic plants might be related to the expression changes of genes involved in the GA biosynthetic and signalling pathways.
Fig. 8.
Quantitative RT-PCR analysis of plant morphologically related genes in OsLSK1-t over-expressing plants. (A): OsKO1, (B): OsKO2, (C): OsGID2, (D): OsGA3ox2. Data are shown as means ±SD. (Student’s t tests, **P <0.01, n=3).
Discussion
Rapid population growth has made food shortage a serious problem in the world, prompting more studies on biotechnology to improve grain yield in crops. Rice grain yield is mainly determined by three quantitative component traits, including panicle per plant, grains per panicle, and grain weight (Bai et al., 2009; Xing and Zhang, 2010). Grains per panicle is a highly variable trait and depends on the structural features of the panicle including the number of primary and secondary branches, panicle length, and the percentage of filled grains. Although several genes have been reported to regulate these traits, the gene networks that control rice yield remained elusive. In this study, an S-domain receptor-like kinase gene, OsLSK1, is reported which localizes in the plasma membrane and is broadly expressed in various tissues and organs (Figs 1, 4). Over-expression of the OsLSK1 extracellular domain affects various pleiotropic phenotypes such as plant height, primary branch number, grain number per primary branch, and grain number per panicle, resulting in a significant increase in the grain yield per plant (Fig. 7; see Supplementary Fig. S3 at JXB online).
The plant hormone GA includes a large group of tetracyclic diterpenoid compounds and regulates diverse biological processes such as seed germination, flowering, and plant height (Sun, 2008). Physiological studies and phenotypic characterization of dwarf mutants indicated that GA determines plant height primarily through increased internode elongation (Sun, 2008; Salas Fernandez et al., 2009). A number of studies also show that the exogenous application of GA could lead to significantly increases in various growth characters, namely, plant height, number of tillers, grains per plant,and yield attributes in rice (Pan et al., 2013). Our data proved that OsLSK1 was up-regulated under high concentrations of GA. Moreover, over-expression of the extracellular domain of OsLSK1 showed a similar phenotype to that of the exogenous application of GA. Further investigations showed that OsKO1, OsKO2, GA20ox2, and OsGID2 were up-regulated in the OXOsLSK1-t plant. OsKO1 and OsKO2 are ent-kaurene oxidases and encode the key branch-point enzyme involved in the first step of GA biosynthesis. GA20ox2 is a gibberellin 20-oxidase, a key oxidase enzyme in the biosynthesis of gibberellin that catalyses the conversion of GA12 and GA53 to GA9 and GA20, respectively. OsGID2 (GA-insensitive dwarf2) is a component of the SCF complex which mediates GA-dependent DELLA protein degradation to positively regulate gibberellin (GA) signalling. These results suggested that the phenotype of OXOsLSK1-t transgenic plants might be caused by both increasing the endogenous GA concentration and by enhanced GA signal transduction.
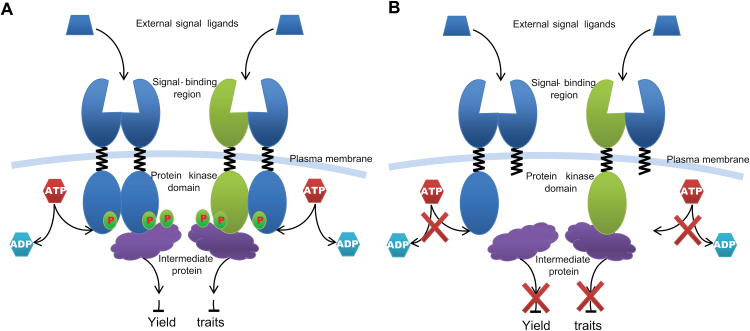
Receptor-like kinase are one of the largest protein families in plants with over 1000 members in rice (Shiu et al., 2004). In general, a RLK contains three functional domains: an extracellular domain, a transmembrane domain, and an intracellular serine/threonine kinase domain. The activation of RLKs is phosphorylation-dependent. External signal ligands are recognized by the extracellular domain which triggers phosphorylation activity of the intracellular cytoplasmic kinase domain. The intracellular cytoplasmic kinase domain then phosphorylates substrate proteins within the cell which activates the downstream signalling pathways. In this process, the dimerization of RLKs is required for its function (Stokes and Gururaj Rao, 2008) (Fig. 9A). For example: the Arabidopsis CRINKLY4, is functional as a homodimer to control development of the integument and seed coat (Stokes and Gururaj Rao, 2008); the formation of a heterodimer between BRI1 and BAK1 is critical for their function in BR signal transduction(Nam and Li, 2002). The extracellular domain of the S-domain receptor kinase possesses a PAN-like domain, which was reported to be a protein interaction domain (Naithani et al., 2007). The S-receptor kinase OsLSK1 has six homologous genes in the rice genome, sharing 55– 81% identity. These seven S-domain RLK genes clustered within a 130kb region and were separated by ten retrotransposons (see Supplementary Fig. S2 at JXB online). The yeast two-hybrid and BiFC data showed that OsLSK1 could form homo-/heterodimers with itself or its homologous proteins at the extracellular domain (Figs 2, 3). In this study, full-length OsLSK1 over-expressing plants and RNAi plants did not show any visible phenotype. However, over-expression of a truncated version of OsLSK1, in which the intracellular kinase domains were deleted, improved the plant height and yield components. It is proposed that dimerization of OsLSK1 and its homologous proteins could underlie these surprising observations (Fig. 9B). When the truncated versions of the OsLSK1 proteins were introduced into plants, they formed the functionless heterodimers with endogenous OsLSK1 and its homologues to block the phosphorylation activity of the intracellular cytoplasmic kinase domain, therefore disrupting external signal transduction. In the meantime, the non-functional OsLSK1-t homodimers and heterodimers compete with the internal functional OsLSK1 homodimers and heterodimers for the external signal ligands which are required for signal transduction. As a consequence, the truncated versions of OsLSK1 exhibited dominant negative phenotypes that positively affect the yield components in transgenic rice.
Fig. 9.
A working model of OsLSK1. (A) A working model of RLKs. (B) Dominant negative working model of RLKs.
The expression levels of OsLSK1 and its homologues were examined in RNAi plants. Although the expression of OsLSK1 was dramatically decreased in RNAi transgenic lines, the homologous genes were not severely affected. The functional redundancy might explain why no phenotype was observed in OsLSK1-RNAi transgenic lines (see Supplementary Fig. S3 at JXB online). For the full-length OsLSK1 transgenic plants, there are two possibilities: one is that there were insufficient signal molecules for the excess exogenous and endogenous OsLSK1 proteins to show the phenotype; the other is that the appropriate trigger of the phenotype was not found. Fortunately, a new approach has been found by using the over-expression of the extracellular domain of OsLSK1 to improve the yield components in rice.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Amino acid sequence alignment of the OsLSK1 homologous proteins.
Supplementary Fig. S2. The protein structures and genome distribution of OsLSK1 homologous genes.
Supplementary Fig. S3. Phenotype analysis of OXOsLSK1-f and OsLSK1-RNAi plants.
Supplementary Fig. S4. Quantitative RT–PCR analysis of plant morphologically related genes in OXOsLSK1-t plants.
Supplementary Table S2. Primers.
Supplementary Table S2. Yield traits of transgenic plants.
Acknowledgements
This work was supported in part by a Ministry of Agriculture Transgenic Research Grant (2010ZX08010-002) to the Institute of Crop Science, Chinese Academy of Agricultural Sciences, by the National Natural Science Foundation of China (31000577 to TZ, 31301298 to JL), and the CAAS Innovation Program.
References
- Abel S, Theologis A. 1994. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. The Plant Journal 5, 421–427. [DOI] [PubMed] [Google Scholar]
- Bai L, Zhang G, Zhou Y, Zhang Z, Wang W, Du Y, Wu Z, Song CP. 2009. Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca signalling, is required for abscisic acid responses in Arabidopsis thaliana . The Plant Journal 60, 314–327. [DOI] [PubMed] [Google Scholar]
- Becraft PW. 2002. Receptor kinase signaling in plant development. Annual Review of Cell and Developmental Biology 18, 163–192. [DOI] [PubMed] [Google Scholar]
- Cabrillac D, Cock JM, Dumas C, Gaude T. 2001. The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 410, 220–223. [DOI] [PubMed] [Google Scholar]
- Castells E, Casacuberta JM. 2007. Signalling through kinase-defective domains: the prevalence of atypical receptor-like kinases in plants. Journal of Experimental Botany 58, 3503–3511. [DOI] [PubMed] [Google Scholar]
- Chen L-J, Wuriyanghan H, Zhang Y-Q, Duan K-X, Chen H-W, Li Q-T, Lu X, He S-J, Ma B, Zhang W-K. 2013. An S-domain receptor-like kinase, OsSIK2, confers abiotic stress tolerance and delays dark-induced leaf senescence in rice. Plant Physiology 163, 1752–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shang J, Chen D, Lei C, Zou Y, Zhai W, Liu G, Xu J, Ling Z, Cao G. 2006. A B‐lectin receptor kinase gene conferring rice blast resistance. The Plant Journal 46, 794–804. [DOI] [PubMed] [Google Scholar]
- Chu J, Yao X, Yue Z, Li J, Zhao J. 2013. The effects of selenium on physiological traits, grain selenium content and yield of winter wheat at different development stages. Biological Trace Element Research 151, 434–440. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. 1993. CLAVATA1, a regulator of meristem and flower development in Arabidopsis . Development 119, 397–418. [DOI] [PubMed] [Google Scholar]
- Danna CH, Millet YA, Koller T, Han S-W, Bent AF, Ronald PC, Ausubel FM. 2011. The Arabidopsis flagellin receptor FLS2 mediates the perception of Xanthomonas Ax21 secreted peptides. Proceedings of the National Academy of Sciences, USA 108, 9286–9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Herder G, Yoshida S, Antolin-Llovera M, Ried MK, Parniske M. 2012. Lotus japonicus E3 ligase SEVEN IN ABSENTIA4 destabilizes the symbiosis receptor-like kinase SYMRK and negatively regulates rhizobial infection. The Plant Cell 24, 1691–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer KG, Kandasamy MK, Mahosky DI, Acciai J, Kudish BI, Miller JE, Nasrallah ME, Nasrallah JB. 1994. A superfamily of S locus-related sequences in Arabidopsis: diverse structures and expression patterns. The Plant Cell 6, 1829–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer‐Parton S, Parton R, Hickey P, Dijksterhuis J, Atkinson H, Read N. 2000. Confocal microscopy of FM4‐64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. Journal of Microscopy 198, 246–259. [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. 2000. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Molecular Cell 5, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Gao H, Zheng X-M, Fei G, Chen J, Jin M, Ren Y, Wu W, Zhou K, Sheng P, Zhou F. 2013. Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLoS Genetics 9, e1003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish LA, Clark SE. 2011. The RLK/Pelle family of kinases. The Plant Journal 66, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goring DR, Rothstein SJ. 1992. The S-locus receptor kinase gene in a self-incompatible Brassica napus line encodes a functional serine/threonine kinase. The Plant Cell 4, 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haderlein L, Jensen TL, Dowbenko RE, Blaylock AD. 2001. Controlled release urea as a nitrogen source for spring wheat in Western Canada: yield, grain N content, and N use efficiency. ScientificWorldJournal 1, Suppl ement 2, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal 6, 3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn T-L, Stone JM, Walker JC. 2000. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes and Development 14, 108–117. [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Jung MS, Lee K, Kim KE, Yoo JH, Kim MC, Kim DH, Cho MJ, Chung WS. 2009. An S-locus receptor-like kinase in plasma membrane interacts with calmodulin in Arabidopsis . FEBS Letters 583, 36–42. [DOI] [PubMed] [Google Scholar]
- Laubinger S, Marchal V, Gentilhomme J, Wenkel S, Adrian J, Jang S, Kulajta C, Braun H, Coupland G, Hoecker U. 2006. Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 133, 3213–3222. [DOI] [PubMed] [Google Scholar]
- Lee JH, Takei K, Sakakibara H, et al. 2003. CHRK1, a chitinase-related receptor-like kinase, plays a role in plant development and cytokinin homeostasis in tobacco. Plant Molecular Biology 53, 877–890. [DOI] [PubMed] [Google Scholar]
- Lehti-Shiu MD, Zou C, Hanada K, Shiu SH. 2009. Evolutionary history and stress regulation of plant receptor-like kinase/pelle genes. Plant Physiology 150, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J. 1997. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90, 929–938. [DOI] [PubMed] [Google Scholar]
- Lin W, Ma X, Shan L, He P. 2013. Big roles of small kinases: the complex functions of receptor-like cytoplasmic kinases in plant immunity and development. Journal of Integrative Plant Biology 55, 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A, Aalen RB, Audenaert D, et al. 2012. Tackling drought stress: receptor-like kinases present new approaches. The Plant Cell 24, 2262–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Li H, Wang Q, Liu B, Lin C. 2013. Blue light–dependent interaction between cryptochrome2 and CIB1 regulates transcription and leaf senescence in soybean. The Plant Cell Online 25, 4405–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naithani S, Chookajorn T, Ripoll DR, Nasrallah JB. 2007. Structural modules for receptor dimerization in the S-locus receptor kinase extracellular domain. Proceedings of the National Academy of Sciences,USA 104, 12211–12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J. 2002. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110, 203–212. [DOI] [PubMed] [Google Scholar]
- Pan S, Rasul F, Li W, Tian H, Mo Z, Duan M, Tang X. 2013. Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice (N Y) 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuglia M, Roby D, Dumas C, Cock JM. 1997. Rapid induction by wounding and bacterial infection of an S gene family receptor-like kinase gene in Brassica oleracea . The Plant Cell 9, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuglia M, Swarup R, Rocher A, Saindrenan P, Roby D, Dumas C, Cock JM. 2002. Comparison of the expression patterns of two small gene families of S gene family receptor kinase genes during the defence response in Brassica oleracea and Arabidopsis thaliana . Gene 282, 215–225. [DOI] [PubMed] [Google Scholar]
- Salas Fernandez MG, Becraft PW, Yin Y, Lübberstedt T. 2009. From dwarves to giants? Plant height manipulation for biomass yield. Trends in Plant Science 14, 454–461. [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Zhang C. 2001. The role of NDR1 in avirulence gene-directed signalling and control of programmed cell death in Arabidopsis . Plant Physiology 127, 1089–1101. [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. 2001. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proceedings of the National Academy of Sciences, USA 98, 10763–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH. 2004. b Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. The Plant Cell 16, 1220–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, Lakeman MB, Torii KU. 2003. Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor–like kinase signaling pathway that regulates organ shape. The Plant Cell Online 15, 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KD, Gururaj Rao A. 2008. Dimerization properties of the transmembrane domains of Arabidopsis CRINKLY4 receptor-like kinase and homologs. Archives of Biochemistry and Biophysics 477, 219–226. [DOI] [PubMed] [Google Scholar]
- Sun T-p. 2008. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. The Arabidopsis Book 6, e0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiderski MR, Innes RW. 2001. The Arabidopsis PBS1 resistance gene encodes a member of a novel protein kinase subfamily. The Plant Journal 26, 101–112. [DOI] [PubMed] [Google Scholar]
- Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K. 2000. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403, 913–916. [DOI] [PubMed] [Google Scholar]
- Tang N, Zhang H, Li X, Xiao J, Xiong L. 2012. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiology 158, 1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tor M, Lotze MT, Holton N. 2009. Receptor-mediated signalling in plants: molecular patterns and programmes. Journal of Experimental Botany 60, 3645–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten M, Snoek LB, Proveniers MC, Peeters AJ. 2009. The many functions of ERECTA. Trends in Plant Science 14, 214–218. [DOI] [PubMed] [Google Scholar]
- Waadt R, Kudla J. 2008. In planta visualization of protein interactions using Bimolecular Fluorescence Complementation (BiFC). Cold Spring Harbor Protocols 2008, pdb prot4995. [DOI] [PubMed] [Google Scholar]
- Wagner TA, Kohorn BD. 2001. Wall-associated kinases are expressed throughout plant development and are required for cell expansion. The Plant Cell 13, 303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Zhang R. 1990. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica . Nature 345, 743–746. [DOI] [PubMed] [Google Scholar]
- Wang Y-S, Pi L-Y, Chen X, Chakrabarty PK, Jiang J, De Leon AL, Liu G-Z, Li L, Benny U, Oard J. 2006. Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. The Plant Cell Online 18, 3635–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhou JM. 2013. Receptor-like kinases in plant innate immunity. Journal of Integrative Plant Biology 55, 1271–1286. [DOI] [PubMed] [Google Scholar]
- Xing Y, Zhang Q. 2010. Genetic and molecular bases of rice yield. Annual Review of Plant Biology 61, 421–442. [DOI] [PubMed] [Google Scholar]
- Xu P, Xu SL, Li ZJ, Tang W, Burlingame AL, Wang ZY. 2013. A brassinosteroid-signaling kinase interacts with multiple receptor-like kinases in Arabidopsis. Molecular Plant 7, 441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X, Luo X, Qian X, He G, Yang M, Li Y, Yang J. 2009. Over-expression of the rice LRK1 gene improves quantitative yield components. Plant Biotechnology Journal 7, 611–620. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang L, Yuan Y, Tian D, Yang S. 2011. Rapid copy number expansion and recent recruitment of domains in S-receptor kinase-like genes contribute to the origin of self-incompatibility. The FEBS Journal 278, 4323–4337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.