Abstract
OBJECTIVES
To evaluate the effectiveness of penile vibratory stimulation for the management of retarded orgasm. Retarded orgasm, a condition characterized by difficulty achieving orgasm and ejaculation, is one of the most recalcitrant of the male sexual dysfunctions. Currently, no evidence-based treatments have been proven to ameliorate this condition.
METHODS
Men who had a complete inability to achieve an orgasm during sexual relations in the previous 3 months were instructed in the use of penile vibratory stimulation. The men’s responses were measured by self-report of orgasm function and using the orgasm and satisfaction domains of the International Index of Erectile Function. The responses were assessed at baseline (admission into the study) and at 3 and 6 months.
RESULTS
A total of 36 men met the inclusion criteria, and 72% reported the restoration of orgasm. These responders reported that orgasm during sexual relations occurred 62% of the time. A statistically and clinically significant increase occurred in the orgasm and satisfaction domains of the International Index of Erectile Function between the baseline visit and the 3-month follow-up visit. These gains were sustained at 6 months.
CONCLUSIONS
Penile vibratory stimulation is an effective treatment for retarded orgasm. Penile vibratory stimulation should be integrated into current cognitive-behavioral sex therapy techniques to achieve maximal effectiveness and satisfaction.
Retarded orgasm (ejaculation) is characterized by prolonged ejaculatory latency. This condition is one of the most poorly understood and pharmacologically recalcitrant of the male sexual dysfunctions. In general, a scarcity of original research has focused on this dysfunction.1 Almost all the published studies in this area have consisted of case reports or reviews that have defined the dysfunction and highlighted the types of treatments available for this disorder. Few reports, if any, have described original research that has systematically investigated and provided empirical evidence delineating the impact of this disorder or demonstrating the effectiveness of the available treatments.
The paucity of research is unfortunate because clinical reports have suggested this condition results in a significant reduction in sexual satisfaction and psychological well-being.2 For many men, this dysfunction results in the inability to achieve an orgasm during sexual relations. Master and Turek observed that men with this condition may seek partners who can accommodate a minimal sexual lifestyle.3 Jannini et al.2 suggest that retarded ejaculation can have significant deleterious effects on a man’s sexual satisfaction and a couple’s relationship. However, these are only clinical observations, and, without empirical evidence, we cannot verify the affect of this disorder or confirm the significance of the distress that this condition may cause.
Historically, the incidence rates of retarded orgasm have been relatively low, with rates in the general population between 1% and 4%.2,4 In the past decade, however, clinicians have increasingly identified retarded orgasm as a side effect of selective serotonin reuptake inhibitors (SSRIs). SSRIs increase serotonin (5-HT) neurotransmission and ejaculatory delay has been related to activation in the 5-HT2C receptors in animal and human studies.5,6 A number of studies have identified delayed orgasm as a side effect of SSRI medications, with rates generally ranging from 16% to 37%7,8; some studies have reported rates of this side effect as great as 60% to 70%.9,10
Other proposed etiologies for retarded ejaculation include neurologic disorders, as well as psychological and relationship issues. Most of the research that has examined the association between neurologic disorders and ejaculatory problems has investigated patients with spinal cord injuries. In terms of psychosocial etiologies, psychodynamic interpretations have suggested that the causes of retarded ejaculation range from the fear of castration to a strict religious background. Other investigators have taken a more systemic approach11 and viewed this problem as a result of attraction or relationship difficulties.4 In addition, in a large group of men, no overt etiology will be found (ie, idiopathic retarded orgasm). These men display no explicit physical or psychological difficulties (ie, relationship difficulties, attempting pregnancy) that would account for the extended ejaculation latency.
Currently, cognitive-behavioral sex therapy9,12 is the primary treatment for restoring orgasm during sexual relations. The available evidence on the effectiveness of these treatments is rather limited.1,13 Both successful and unsuccessful case reports have been cited.4,14 Although these types of reports are useful to help conceptualize the issues, they are limited in their scope and often overemphasize a single case instead of basing conclusions on a representative sample with empirical data.
No pharmacologic therapy has demonstrated consistent efficacy in managing retarded ejaculation. Researchers have explored the effects of yohimbine and cyproheptadine on male ejaculatory functioning in animals with some success.15–18 In general, however, this research has been confined to animal experiments, and researchers have not systematically investigated the impact these mediations have on ejaculation time in humans.15,16
Given the lack of published studies reporting empirical data on the treatment of this disorder, this study was undertaken to evaluate the utility of penile vibratory stimulation (PVS) in restoring orgasm in men with idiopathic retarded orgasm.
MATERIAL AND METHODS
Subjects
These subjects were consecutive patients seeking treatment for secondary anorgasmia from a Sexual Medicine Clinic in a major metropolitan area in the Midwestern United States. The subjects included men who self-reported anorgasmia and were in a committed relationship at study enrollment. No overt psychosocial causes were found for this anorgasmia (ie, reported relationship difficulties or attempting pregnancy), and the subject and his partner both reported an interest in addressing the anorgasmia. These men did not report nocturnal ejaculations. The eligibility requirements for the subjects included (a) the complete inability to achieve an orgasm at any time during sexual relations with a partner in the previous 3 months, (b) the ability to obtain functional erections without erectogenic pharmacotherapy, (c) normal neurologic history and examination conducted by a Board-certified urologist, and (d) normal penile biothesiometry (penile vibration sensation). Men were excluded from the study if they had a diagnosis of primary anorgasmia, were currently using or had used within the past 3 months an SSRI, had undergone pelvic surgery, had a sensory deficit as evidenced by abnormal biothesiometry values, or were using erectogenic pharmacotherapy. Men who met the inclusion criteria were informed of the risks and benefits of study participation and provided written informed consent. The institutional review board approved this study.
Assessments
The participants were instructed to use a commercially available vibrator (Pin Point Massager, Brookstone, Merrimack, NH) which provides a nonvariable vibratory amplitude and frequency. The exact vibratory amplitude and frequency is unknown. The patients were instructed to apply the vibrator to the frenular area of the penis for three 1-minute periods separated by 1-minute rest periods. At least three attempts using the vibrator were required for inclusion in the study. Study questionnaires included a demographic questionnaire and the International Index of Erectile Function (IIEF).8 The IIEF questionnaire contains 15 questions, subdivided into five domains: erectile function, libido, orgasmic function, sexual satisfaction, and overall satisfaction. The questionnaire addresses the patient’s sexual function during the 4-week period before completing the inventory. Each question is scored on a 5-point Likert scale, with higher scores indicating better function: a score of 5 indicates “always or nearly always,” 1 indicates “never or nearly never,” and 3 indicating “about half the time.” For the purposes of this study, special attention was paid to the orgasm domain (two questions) and satisfaction domain (five questions; a combination of the intercourse satisfaction and overall satisfaction domains) of the IIEF. Participants completed the study questionnaire at entry into the study and at 3 and 6 months after beginning vibrator use.
Statistical Analysis
Student’s t test was used to compare the IIEF satisfaction and orgasm domain scores at baseline and 3 months after treatment and at 3 and 6 months after treatment.
RESULTS
A total of 36 men agreed to participate in the study. Their mean age was 56 ± 14 years. The mean duration of orgasmic dysfunction was 14 ± 7 months. The mean number of vascular risk factors (eg, diabetes, hypertension, dyslipidemia, and cigarette smoking exposure) was 1.4 ± 1.2. All men were in a sustained relationship. The mean partner age was 52 ± 11 years. No statistically significant differences in demographic variables or comorbidity profile existed between the responders and nonresponders.
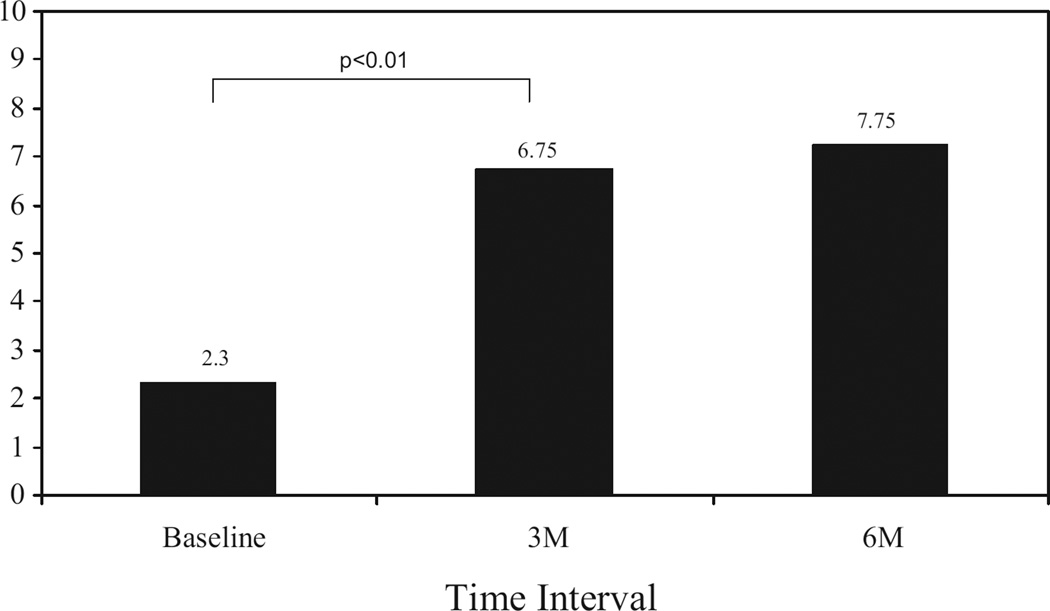
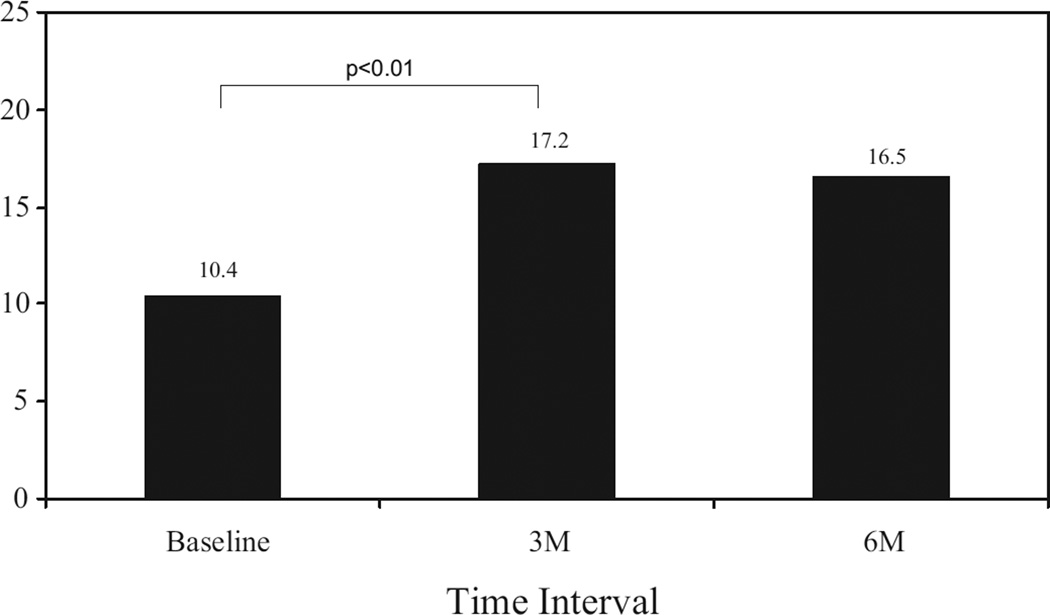
Almost three quarters of the men (72%; n = 26) had restoration of orgasm using PVS on at least some occasions. These responders self-reported that 62% ± 11% of the attempts at sexual relations resulted in an orgasmic response. The responders had a significant increase in the orgasm domain scores on the IIEF (P <0.01) from baseline to the 3-month follow-up visit (mean change from 2.30 to 6.75; Fig. 1), as well as a significant increase in the satisfaction domain score of the IIEF (P <0.01) for the same period (mean change from 10.4 to 17.2; Fig. 2). No difference was found between the 3 and 6-month assessment points.
Figure 1.
Orgasm domain.
Figure 2.
Satisfaction domain.
COMMENT
The results from our study are some of the first to present validated questionnaire-based data on the effectiveness of a treatment for idiopathic retarded orgasm. Of the 36 men in this study, 26 (72%) had restoration of orgasm using PVS. These results are consistent with the research conduced by Sonksen et al.19 that showed that PVS is an effective treatment for anorgasmia in men with spinal cord injuries above T10. The available evidence has indicated that PVS helps initiate a normal ejaculatory reflex in these men by stimulating the afferent nerves.19 Our research has helped to generalize these results to men without neurologic damage and suggests that PVS may be an effective component of treatment for men with varying etiologies of retarded ejaculation.
In addition to the high success rate of PVS, subjects also reported a significant increase in the orgasm and the satisfaction domain of the IIEF. The orgasm domain on the IIEF contains two questions and asks the subject to rate how often they achieved ejaculation and orgasm during sexual intercourse or when sexual stimulation was present. The mean total scores of responders to PVS increased from 2.30 (ie, “almost never”) to 6.75 (ie, “most of the time”). Because the IIEF asks respondents to consider the previous 4 weeks, these results indicate that PVS helped restore orgasm and ejaculation over time and was consistently effective. Also encouraging was the increase in the satisfaction domain of the IIEF (combination of the intercourse satisfaction and overall satisfaction domains). This domain contains five questions and assesses satisfaction during intercourse and with the sexual relationship. The average scores in this domain increased from 10.4 (ie, “not very enjoyable”) to 17.2 (ie, “fairly enjoyable”). It is generally believed that a 1-point improvement per question in each domain is clinically significant; thus, a 4.35-point improvement in the orgasm domain (two questions, maximal score of 10) and a 6.8-point improvement in the satisfaction domain (five questions, maximal score of 25) are consistent with a clinically meaningful change.
Important in these findings is that these results were sustained during the 6-month study period. This has significant implications for men struggling with this disorder and those attempting to treat them. As stated in the introduction, the predominant treatment for this disorder has been cognitive-behavioral therapy.9,12 Our results have indicated that PVS should be integrated into these therapies. The use of mechanical stimulation during sex therapy is not necessarily novel.20 However, the data presented in this study suggest that PVS should take a prominent role in these therapies and should be creatively integrated into existing cognitive-behavioral techniques. PVS is the only treatment for retarded ejaculation that has a body of empirical evidence supporting its use.
The integration of therapies is particularly important when examining the results of this study. Because we did not use a controlled experimental design, we cannot conclusively state that the positive response was solely a result of PVS. It is likely that it was a result of a number of factors, including couple motivation (all men had a partner), proper education about the disorder, thorough training regarding PVS, and specific subject factors (ie, these men had secondary anorgasmia, not primary anorgasmia). Additionally, PVS will obviously not directly treat psychosocial factors that might contribute to retarded orgasm such as relationship difficulties or the loss of attraction to the partner. However, the combination of approaches has the potential to increase their success rate and decrease the time needed for treatment. To illustrate, we have been encouraged by the increase in the IIEF satisfaction domains with this simple and relatively easy intervention, yet these satisfaction domains were increased to the “moderate” or “fairly” good range. The addition of sex therapy techniques might help to continue to elevate sexual satisfaction and assist the couple in eventually moving to sexual relations without PVS.
Implicit in these findings is that retarded ejaculation represents a significant sexual problem for the men with this disorder. The baseline IIEF scores of the responders and nonresponders indicated that these men were receiving almost no enjoyment from, and were very dissatisfied with, their sexual relationship. The finding that those who did not respond to PVS showed no increase in the satisfaction or orgasm domains of the IIEF indicates that this displeasure will continue for men who do not receive treatment. This underscores the importance of using effective treatments and continuing to provide empirical evidence that treatments are useful and achieve success in a timely manner.
When evaluating the results of this study, it is important to stress that this study did not use an experimental design. As stated, this limitation did not allow for the definitive conclusion that PVS caused the change in the ejaculatory response or increase in the IIEF scores. Additional limitations included the lack of specific information pertaining to these men’s sexual history and sexual functioning. For example, we did not ask whether these men could reach orgasm through masturbation, nor did we inquire about the typical length of a sexual episode with their partner or the typical intravaginal ejaculatory latency time during coitus. These men, however, did undergo a thorough examination by a Board-certified urologist and had normal neurologic history and examination findings and normal penile biothesiometry findings. Despite the above limitations, we believe this study has added novel findings and empirical evidence to published reports lacking evidence-based research.1
CONCLUSIONS
In this study, PVS was an effective treatment for retarded orgasm that increased orgasm functioning and sexual satisfaction within 3 months of the start of treatment. These gains were sustained at the 6-month assessment point. These empirical data suggest that PVS is an effective treatment of retarded orgasm that can easily be integrated into current cognitive-behavioral sex therapy techniques.
References
- 1.Waldinger MD, Schweitzer DH. Retarded ejaculation in men: an overview of psychological and neurobiological insights. World J Urol. 2005;23:76–81. doi: 10.1007/s00345-004-0487-8. [DOI] [PubMed] [Google Scholar]
- 2.Jannini EA, Simonelli C, Lenzi A. Disorders of ejaculation. J Endocrinol Invest. 2002;25:1006–1019. doi: 10.1007/BF03344077. [DOI] [PubMed] [Google Scholar]
- 3.Master VA, Turek PJ. Ejaculatory physiology and dysfunction. Urol Clin North Am. 2001;28:363–375. doi: 10.1016/s0094-0143(05)70145-2. [DOI] [PubMed] [Google Scholar]
- 4.Apfelbaum B. Retarded ejaculation: a much-misunderstood syndrome. In: Leiblum SR, Rosen RC, editors. Principles and Practice of Sex Therapy. Update for the 1990s. 2nd. New York: Guilford Press; 1990. [Google Scholar]
- 5.Waldinger MD, Berendsen HH, Blok BF, et al. Premature ejaculation and serotonergic antidepressants-induced delayed ejaculation: the involvement of the serotonergic system. Behav Brain Res. 1998;92:111–118. doi: 10.1016/s0166-4328(97)00183-6. [DOI] [PubMed] [Google Scholar]
- 6.Waldinger MD, Zwinderman AH, Olivier B. Antidepressants and ejaculation: a double-blind, randomized, placebo-controlled, fixed-dose study with paroxetine, sertraline, and nefazodone. J Clin Psychopharmacol. 2001;21:293–297. doi: 10.1097/00004714-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kiev A, Feiger A. A double-blind comparison of fluvoxamine and paroxetine in the treatment of depressed outpatients. J Clin Psychiatry. 1997;58:146–152. doi: 10.4088/jcp.v58n0402. [DOI] [PubMed] [Google Scholar]
- 8.Rosen RC, Riley A, Wagner G, et al. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 9.Perelman M. Regarding ejaculation, delayed and otherwise. J Androl. 2003;24:496. doi: 10.1002/j.1939-4640.2003.tb02699.x. [DOI] [PubMed] [Google Scholar]
- 10.Kavoussi RJ, Segraves RT, Hughes AR, et al. Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients. J Clin Psychiatry. 1997;58:532–537. doi: 10.4088/jcp.v58n1204. [DOI] [PubMed] [Google Scholar]
- 11.Shull GR, Sprenkle DH. Retarded ejaculation reconceptualization and implications for treatment. J Sex Marital Ther. 1980;6:234–246. doi: 10.1080/00926238008406089. [DOI] [PubMed] [Google Scholar]
- 12.Masters WH, Johnson VE. Human Sexual Inadequacy. Boston: Little, Brown; 1970. [Google Scholar]
- 13.Heiman JR, Meston CM. Empirically validated treatment for sexual dysfunction. Ann Rev Sex Res. 1997;8:148–194. [PubMed] [Google Scholar]
- 14.Perelman MA. Integrating sildenafil and sex therapy: unconsummated marriage secondary to erectile dysfunction and retarded ejaculation. J Sex Educ Ther. 2001;26:13–21. [Google Scholar]
- 15.Carro-Juareza M, Rodriguez-Manzo G. Yohimbine reverses the exhaustion of the coital reflex in spinal male rats. Behav Brain Res. 2003;141:43–50. doi: 10.1016/s0166-4328(02)00324-8. [DOI] [PubMed] [Google Scholar]
- 16.Smith ER, Davidson JM. Yohimbine attenuates aging-induced sexual deficiencies in male rats. Physiol Behav. 1990;47:631–634. doi: 10.1016/0031-9384(90)90069-g. [DOI] [PubMed] [Google Scholar]
- 17.Menendez Abraham E, Moran Viesca P, Velasco Plaza A, et al. Modifications of the sexual activity in male rats following administration of antiserotoninergic drugs. Behav Brain Res. 1988;30:251–258. doi: 10.1016/0166-4328(88)90167-2. [DOI] [PubMed] [Google Scholar]
- 18.McCormick S, Olin J, Brotman AW. Reversal of fluoxetine-induced anorgasmia by cyproheptadine in two patients. J Clin Psychiatry. 1990;51:383–384. [PubMed] [Google Scholar]
- 19.Sonksen J, Ohl DA, Wedemeyer G. Sphincteric events during penile vibratory ejaculation and electroejaculation in men with spinal cord injuries. J Urol. 2001;165:426–469. doi: 10.1097/00005392-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Schellen TM. Induction of ejaculation by electrovibration. Fertil Steril. 1968;19:566–569. doi: 10.1016/s0015-0282(16)36729-2. [DOI] [PubMed] [Google Scholar]