Abstract
Disease mechanism underlying glaucoma remains unclear. Extensive research on this pathology has highlighted changes in vascular parameters and in circulation of the cerebrospinal fluid (CSF). Here, we review the most recent research on alterations in ocular blood flow and/or CSF flow in glaucoma. Ultrasound Doppler imaging studies have shown an increased resistive index in ophthalmic artery’s in glaucoma. Furthermore, changes in optic nerve CSF circulation, which can be assessed with magnetic resonance imaging, may lead to a greater translaminar pressure difference, mechanical stress, and poor clearance of toxic substances. This constitutes a new approach for understanding blood–CSF interactions involved in glaucoma.
Keywords: Glaucoma, regulation, blood flow, cerebrospinal fluid (CSF)
Introduction
Glaucomatous optic neuropathy is acknowledged to be a major cause of blindness worldwide (1). In a meta-analysis published in 2006, the mean (95% confidence interval [CI]) prevalence of primary open-angle glaucoma (POAG) was estimated to be 2.1% (95% CI, 1.6–2.7) for Caucasian populations and 4.2% (95% CI, 3.1–5.8) for black populations (2). Glaucoma is the world’s leading cause of non-reversible blindness (3). Although elevated intraocular pressure (IOP) is strongly associated with the onset and progression of optic nerve damage, it cannot alone account for the broad spectrum of patients with POAG. First, a large proportion of these patients display typical glaucomatous optic neuropathy in the absence of elevated IOP (a condition known as normal-tension glaucoma [NTG]). Second, some patients show signs of disease progression even after an effective, treatment-induced reduction in their IOP. Approximately one in six treated POAG patients will become blind in both eyes at some point in their life (4). Ocular hypertension is the main risk factor for glaucoma but is not the direct cause. The disease mechanism underlying glaucoma (and especially NTG) remains unclear. Extensive research has been performed on the condition’s etiology, methods for early diagnosis, and factors that might be predictive of disease progression. The search for risk factors other than elevated IOP has led to the recognition that patients with systemic hypotension (5) or sleep-apnea syndrome (6) are at greater risk of developing glaucoma; this suggests the involvement of vascular factors in the onset and progression of glaucoma. Several studies have reported on various aspects of orbital hemodynamics in glaucoma.
The orbital portion of the optic nerve is surrounded by a sheath of dura mater. Cerebrospinal fluid (CSF) circulates within this sheath, which communicates freely with the intracranial subarachnoid spaces. Hence, the optic nerve is exposed to two different sources of pressures: the anterior IOP and the posterior CSF pressure. Hence, the concept of translaminar pressure has recently appeared as a key parameter in the physiopathology of optic neuropathy. Recent research suggests that along with IOP, alterations in CSF pressure may be involved in glaucoma (7).
Here, we review recent findings on pathological changes in orbital blood and CSF flows in this context. We then focus on these changes’ impact on the development and progression of glaucomatous optic neuropathy.
Altered orbital blood flow in glaucoma
Many researchers have used color Doppler imaging to assess orbital blood flow. Velocity measurements have been reported for the ophthalmic, central retinal, and posterior ciliary arteries in various cohorts of glaucoma patients and healthy controls.
Impaired orbital blood flow in POAG
Studies comparing orbital blood flow in POAG patients and controls (8–22) report a trend toward an abnormally low peak-systolic velocity (PSV) and/or end-diastolic velocity (EDV), together with an elevated resistive index (RI, defined as (PSV -EDV)/PSV, a marker of vascular resistance. Ophthalmic artery (OA) velocities and RI values (including those found in above-mentioned studies) are summarized in Table 1. Disparities in the findings may have been due to inter-study methodological differences, such as the inclusion criteria (treated vs. untreated patients, differences in the severity and progression of glaucoma, etc.). Meng et al.’s (23) meta-analysis found evidence of abnormally low PSV and EDV values and abnormally high RIs in the ophthalmic, central retinal and posterior ciliary arteries in POAG groups (vs. controls).
Table 1.
Comparison of peak systolic velocity (PSV), end-diastolic velocity (EDV), and resistive index (RI) values for the ophthalmic artery in primary open-angle glaucoma (POAG) patients and controls.
| PSV (cm/s) ± SD |
EDV (cm/s) ± SD |
RI ± SD |
|||||
|---|---|---|---|---|---|---|---|
| Reference no. | Controls (n)/ POAG (n) | Controls | POAG | Controls | POAG | Controls | POAG |
| 8 | 48/49 | 41.7 ± 18.8 | 38.2 ± 5.4 | 8.3 ± 5.3 | 8.6 ± 5.9 | 0.81 ± 0.07 | 0.79 ± 0.07 |
| 11 | 26/23 | 30.8* ± 10.6 | 40.4* ± 2.2 | 8.3 ± 3.1 | 7.8 ± 3.9 | 0.73* ± 0.05 | 0.81* ± 0.05 |
| 14 | 198/252 | 54.6* ± 6.5 | 52.5* ± 7.8 | 8.8* ± 0.7 | 7.8* ± 1.1 | 0.84* ± 0.02 | 0.85* ± 0.02 |
| 15 | 30/30 | 32.7 ± 14.1 | 27.3 ± 9.6 | 9.0* ± 4.7 | 5.9* ± 3.2 | 0.74 ± 0.18 | 0.77 ± 0.11 |
| 17 | 44/95 | 40.2* ± 10.9 | 36.9* ± 6.2 | 11.7* ± 4 | 9.9* ± 4.4 | ||
| 18 | 20/20 | 30 ± 9 | 27 ± 7 | 6.5 ± 3 | 5.6 ± 2.3 | 0.79 ± 0.06 | 0.79 ± 0.07 |
| 19 | 35/60 | 43.9* ± 7.8 | 39.0* ± 7.8 | 11.9* ± 2.6 | 10.3* ± 2.6 | ||
| 20 | 25/25 | 52.6* ± 12.8 | 41.5* ± 1.2 | 14.2* ± 5.1 | 11.8* ± 4.6 | 0.66* ± 0.07 | 0.73* ± 0.06 |
| 32 | 59/102 | 40.1 ± 18.8 | 35.9 ± 3.9 | 7.4 ± 4.4 | 7.5 ± 4.9 | 0.82 ± 0.07 | 0.8 ± 0.07 |
| 35 | 20/20 | 31.4 ± 3 | 34.8 ± 0.2 | 7.6 ± 1.3 | 8.7 ± 0.8 | 0.78 ± 0.02 | 0.75 ± 0.02 |
| 39 | 16/12 | 33. 2 ± 7.8 | 26.9 ± 8.3 | 9.8 ± 2.8 | 8 ± 2.5 | 0.7 ± 0.1 | 0.7 ± 0 |
A statistically significant difference between POAG patients and controls.
Glaucoma patients appear to have a lower PSV in their central retinal artery than patients with an elevated IOP but no glaucoma (24). Hence, the lower velocities found in glaucoma patients do not result from an elevated IOP per se. This observation is strengthened by the fact that despite efficient IOP reduction, blood flow differences are still observed in both untreated and IOP-controlled groups (23).
Differences in ocular blood flow were also observed in eyes with a normal visual field in glaucoma patients with asymmetric impairment (25). These findings suggest that changes in ocular blood flow may precede damage to the optic nerve. Thus, ocular blood flow abnormalities may increase the risk of developing glaucoma in “glaucoma suspects,” with a threshold RI of 0.75 (26).
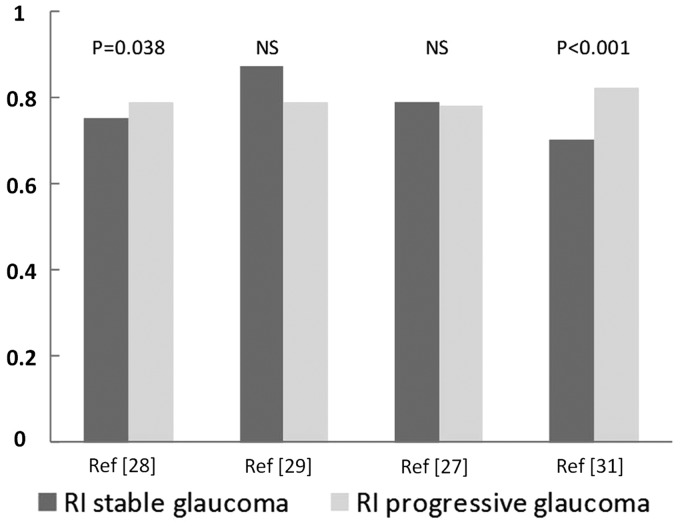
Patients with progressive disease have been compared with patients with stable disease (Fig. 1). Low blood velocity and high RI in ocular blood vessels appear to reliably predict glaucoma progression (15,27–29). In a study with a mean follow-up period of 7 years, Galassi et al. (30) proposed a threshold RI of 0.78: patients with an RI above this threshold were at a higher risk of glaucoma progression. According to Martinez and Sanchez (31), a threshold RI value of 0.72 in the OA yielded a positive predictive value of 90.5% for glaucoma progression.
Fig. 1.
The resistive index (RI) in the ophthalmic artery in stable and progressive glaucoma.
Normal tension glaucoma
Normal tension glaucoma occurs in the absence of elevated IOP. This particular form of glaucoma challenges the physiopathologic model in which optic neuropathy is caused by high IOP. Many researchers have measured alterations in ocular blood flow in NTG patients and assessed the ability of Doppler imaging to discriminate between NTG patients and healthy subjects (8,11,19,22,32–36). As was seen for POAG, these studies of NTG have generally reported low systolic, mean, and diastolic velocities, and an elevated RI (Table 2). Again, inter-study disparities are noted. For example, Chiou et al. (34) did not find significant differences in any of the studied vessels, whereas Butt et al. (11) and Abegao Pinto et al. (8) reported altered blood flow in the central retinal artery. Plange et al. (36) concluded that Doppler imaging had a sensitivity of 48% and a specificity of 90% for the identification of NTG and therefore considered that Doppler parameters should not be used as diagnostic criteria for this condition. It remains to be seen whether Doppler parameters are relevant for evaluating the risk of NTG progression (as is the case in POAG).
Table 2.
Comparison of peak systolic velocity (PSV), end diastolic velocity (EDV), and resistive index (RI) values for the ophthalmic artery in normal-tension glaucoma (NTG) patients and controls.
| PSV (cm/s) ± SD |
EDV (cm/s) ± SD |
RI ± SD |
|||||
|---|---|---|---|---|---|---|---|
| Reference no. | Controls (n)/NTG (n) | Controls | NTG | Controls | NTG | Controls | NTG |
| 8 | 48/62 | 41.7 ± 18.8 | 35.3 ± 10.7 | 8.3 ± 5.3 | 7.5 ± 3.5 | 0.81 ± 0.07 | 0.79 ± 0.07 |
| 11 | 26/25 | 30.8 ± 10.6 | 31.5 ± 8.1 | 8.3 ± 3.1 | 7.1 ± 2.9 | 0.73 ± 0.05 | 0.77 ± 0.08 |
| 19 | 35/42 | 43.9* ± 7.8 | 35.4* ± 7.8 | 11.9* ± 2.6 | 9* ± 2.7 | ||
| 32 | 59/89 | 40.1* ± 16.9 | 33.6* ± 11.2 | 7.4 ± 4.4 | 6.8 ± 3.3 | 0.82 ± 0.07 | 0.82 ± 0.07 |
| 33 | 17/34 | 30.1 ± 9.9 | 30.4 ± 7.6 | 8.4* ± 2.9 | 6.6* ± 2.9 | 0.72* ± 0.05 | 0.77* ± 0.08 |
| 34 | 25/13 | 30.9 ± 8.4 | 28.9 ± 10 | 5 ± 2.5 | 4.3 ± 1.5 | 0.84 ± 0.05 | 0.84 ± 0.07 |
| 35 | 20/20 | 31.4 ± 3 | 35.5 ± 2.2 | 7.6 ± 1.3 | 7.8 ± 0.9 | 0.78 ± 0.02 | 0.77 ± 0.02 |
A statistically significant difference between NTG and controls.
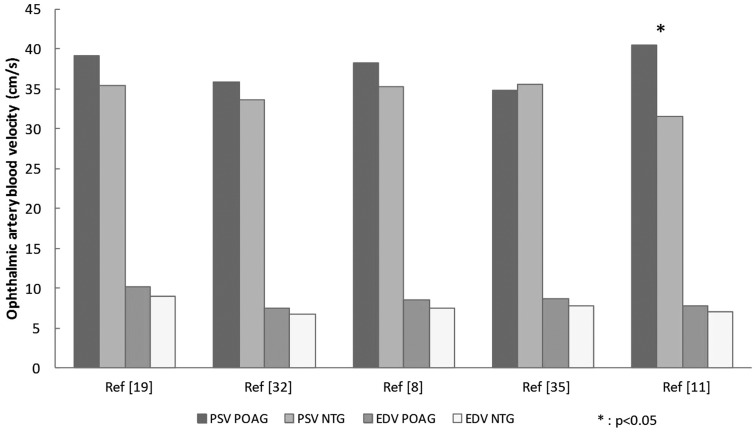
Some researchers have compared ocular blood flow in NTG patients and POAG patients (8,11,19,32,35). Again, contradictory results were obtained. For the OA, low velocities and a high RI were reported. In most studies, no NTG vs. POAG differences were observed for the central retinal or posterior cilioretinal arteries. However, Abegao Pinto et al. (32) found a significant difference only in the posterior cilioretinal artery, where the PSV was lower in NTG patients than in POAG patients (Fig. 2). Interestingly, the only above-mentioned study to report NTG vs. POAG differences in the PSV and RI (11) included only untreated patients. Moreover, the severity of glaucoma and the patients’ treatments were not fully described in the other studies – yielding potential confounding factors for the data analysis.
Fig. 2.
The peak systolic velocity (PSV) and end-diastolic velocity (EDV) in the ophthalmic artery in primary open-angle glaucoma (POAG) and normal-tension glaucoma (NTG).
Glaucoma as a consequence of impaired regulation of perfusion pressure
When arterial blood pressure rises during exercise, the body maintains normal ocular blood flow in the OA and the other ocular vessels by increasing the vascular resistance. This can be considered as a form of ocular hemodynamic regulation that protects the eye from over perfusion (37). It is possible that this regulation is impaired in patients with glaucoma. When PCO2 is artificially increased (and thus acts like a cerebral vasodilator), the ocular blood flow differences between glaucoma patients and controls are no longer significant, indicating that the differences might result from vasospasm (38,39). It has also been suggested that glaucoma patients have an altered endothelial response to nitric oxide and endothelin-1 (cell mediators involved in the regulation of IOP and ocular blood flow through vasodilation and vasoconstriction, respectively) (40,41). Some researchers have used other Doppler parameters (such as the mean systolic/diastolic ratio, which was correlated with an arterial compliance index (42)) to compare POAG and NTG patients with controls (32).
It is unclear whether patients with signs of vascular dysregulation (such as migraine, Raynaud’s phenomenon, etc.) have a higher risk of developing progressive glaucoma. In the Collaborative Normal Tension Glaucoma Study (43), treatment-naive glaucoma patients with migraine were 2.5 times more likely to display disease progression. These patients also responded favorably to treatment, suggesting that they might have a more “pressure-sensitive” form of the disease. However, migraine was not found to be a predictive factor for glaucoma progression in the Early Manifest Glaucoma trial (44) or the Ocular Hypertension Treatment Study (45). In the study by Galambos et al. (35), both POAG and NTG patients (with or without signs of vascular dysregulation) had an impaired response to postural changes. Although it is possible that subjects with systemic vascular dysregulation have altered ocular regulation, there is no reliable diagnostic test for this kind of vascular dysregulation. Impaired regulation in patients with vascular dysregulation might lead to instability of the blood supply to the optic nerve (particularly during stressful situations with elevated IOP or low arterial blood pressure) (46). Periods of relative ischemia might therefore occur, causing local inflammation and oxidative damage when perfusion is re-established. It is also possible that repeated episodes of reperfusion injury contribute to the development or progression of glaucoma (47).
Altered CSF parameters in glaucoma
Glaucoma is now defined as a degenerative optic neuropathy. As mentioned above, a specific feature of the optic nerve is its surrounding sheath of dura mater, within which CSF can circulate. This fluid motion probably has a role in the physiopathology of the optic nerve.
Translaminar pressure differences
Retinal ganglion cells join the optic nerve head and reach the orbital space by passing through the lamina cribrosa. Thus, the lamina cribrosa constitutes a border between the intraocular and orbital spaces. The translaminar pressure difference is defined as the difference between the IOP and the optic nerve CSF pressure. Jonas et al. (48) performed a histopathologic study of eyes that had been enucleated because of severe acute glaucoma. In eyes with acute glaucoma, the lamina cribrosa was thinner, the distance between the intraocular space and the CSF was shorter, and the part of the lamina cribrosa exposed to CSF was wider than in control eyes. Severely elevated IOP therefore has an anatomic impact on the lamina cribrosa. However, the mechanisms underlying glaucomatous optic neuropathy due to acute angle-closure glaucoma and POAG may be very different.
Berdal et al. postulated that retrolaminar pressure correlates well with lumbar CSF pressure (as measured by lumbar puncture) and found that the latter was 33% lower in glaucoma patients than in controls (49). Jonas et al. (50) founded that a glaucoma group displayed a lower calculated CSF pressure and greater estimated translaminar pressure difference than a non-glaucomatous group. Furthermore, NTG patients were found to have a lower CSF pressure than POAG patients and controls (51). Relative to controls, the translaminar pressure difference was significantly higher in NTG patients (because of lower CSF pressure) than in POAG patients (because of higher IOP). In terms of the pathogenesis, low CSF pressure in NTG may be similar to high IOP in POAG. The amount of glaucomatous optic nerve damage is more strongly correlated with the translaminar pressure difference than with the IOP or CSF pressure alone (7,52).
Magnetic resonance imaging (MRI) provides a non-invasive but indirect way of evaluating the optic nerve CSF pressure by measuring the optic nerve sheath diameter. An increase in orbital CSF pressure supposedly enlarges the subarachnoid spaces surrounding the optic nerve. Likewise, the diameter of the optic nerve’s sheath appears to be greater in cases of intracranial hypertension (53). Wang et al. (54) used MRI to estimate the optic nerve subarachnoid spaces by subtracting the optic nerve diameter from the total optic nerve sheath diameter; the subarachnoid space was significantly smaller in NTG patients than in POAG patients and controls (suggesting that the orbital CSF pressure is abnormally low in NTG). Conversely, Jaggi et al. (55) used computed tomography (CT) to show that the optic nerve sheath diameter is greater in NTG patients than in controls. However, the researchers failed to explain this result because a lower CSF pressure is usually expected (49,51).
Disturbances of CSF circulation: a putative optic nerve sheath compartmentalization syndrome
The above-mentioned studies may have been limited by the fact that the correlation between retrolaminar pressure and lumbar CSF pressure does not hold up under disease situations such as glaucoma. In a canine model, Morgan et al. (56) reported that retrolaminar tissue pressure was hardly influenced by intracranial CSF pressure when the latter fell below a certain level. Killer et al. demonstrated that the impairment of outflow pathways in patients with papilledema or NTG resulted in the accumulation of proteins such as lipocalin-like prostaglandin D2-synthase (a beta-trace protein that is thought to induce apoptosis) (57). By applying CT cisternography, Killer et al. also demonstrated a difference in the concentration gradients of contrast-loaded CSF between intracranial spaces and the subarachnoid spaces of the optic nerve in patients with NTG (relative to control subjects) (58). The researchers hypothesized that CSF exchanges between intracranial and orbital subarachnoid spaces were disturbed. Changes in physiological CSF circulation might lead to decreased CSF turnover and diminished clearance of toxic substances (59), causing potential damage to axons, mitochondria, and astrocytes (60). Damage to the optic nerve in glaucoma might result from “retrograde” atrophy (i.e. changes in CSF components due to compartmentalization syndrome), in contrast to the “anterograde” injuries to retinal ganglion cells caused by elevated IOP (61).
Discussion
Although glaucoma is commonly thought to be the consequence of elevated IOP, doubt has been cast on this model due to the absence of a firm correlation between IOP and glaucoma progression in some patients. At present, IOP-lowering drugs constitute the only effective treatment for glaucoma. These drugs represent a non-negligible public health cost and degrade the patients’ quality of life. Furthermore, a significant proportion of glaucoma patients do not respond to these treatments, meaning that glaucoma is still a leading cause of blindness worldwide. Hence, the disease mechanism underlying glaucoma is still the subject of intense investigation.
It appears that glaucoma is associated with low blood flow velocities and high RIs in the ophthalmic, central retinal, and short posterior cilioretinal arteries. These changes in blood flow suggest that impaired regulation of ocular blood flow results in periods of relative ischemia and repeated reperfusion damage to the optic nerve.
Furthermore, changes in the optic nerve appear to result from an elevated translaminar pressure difference (at least in NTG). Glaucoma patients appear to have a lower intracranial CSF pressure and altered CSF circulation in the subarachnoid space around the optic nerve. It has been suggested that a putative optic nerve compartmentalization syndrome can explain these observations. In fact, there is no clear evidence to show that CSF stasis can lead to neurotoxic damage to the optic nerve. One can question whether this putative compartmentalization syndrome is the cause or the consequence of low CSF motion. Considering that the retrolaminar pressure is reportedly hardly influenced by the intracranial CSF pressure (56), the optic nerve sheath might be compartmentalized under normal conditions. Given the invasive nature of direct experiments on the optic nerve, it is difficult to see how the optic nerve sheath’s anatomy can be explored in vivo in fully healthy subjects. Likewise, the evidence that CSF stasis causes neurotoxic damage (62) comes from destructive animal experiments, which can hardly be transposed directly to human pathologies. The optic nerve fibers might be injured by: (i) mechanical stress on the optic nerve fibers passing through the lamina cribrosa; and (ii) reduced clearance of toxic substances.
The CSF’s behavior is supposedly linked to blood pulsatility (63). Indeed, it has been shown that the CSF acts as a buffer between arterial and venous blood pools during the cardiac cycle. Considering that the intracranial volume is invariable, any change in CSF volume must therefore have been induced by a change in blood volume. Furthermore, recent studies have highlighted the link between hemodynamics and intracranial CSF pressure, and the latter is directly related to glaucoma (64). Consideration of the recent literature shows that these blood–CSF interactions have not yet been comprehensively addressed. Indeed, hemodynamic and/or CSF disorders are still being investigated: (i) at a very fundamental level using mathematical models (65); and (ii) in clinical trials (e.g. in the Ocular Blood Flow Assessment in Glaucoma study – NCT02178085).
Transcranial Doppler imaging has been used to evaluate intracranial pressure, with simultaneous measurements of blood flow velocities in the intracranial and orbital parts of the OA. The hypothesis is that blood flow parameters in the two segments of the OA are equal when the external pressure on the eyelid is the same as the intracranial pressure (66). Once again, further research will be required in order to explore the relationships between blood flow modifications, impaired regulation, and alterations in CSF circulation in the pathogenesis of glaucoma.
Many researchers are investigating various aspects of glaucoma; as of May 2015, 13 studies registered in the clinicaltrials.gov database were assessing ocular blood flow and its regulation, emphasizing the growing interest in this parameter. In most of these trials, blood flow is assessed with orbital ultrasound Doppler imaging or laser Doppler flowmetry. One study was designed to explore the putative correlation between optic nerve sheath diameter and blood flow in the retrobulbar vessels.
Although Doppler sonography is considered to be an operator-dependent technique, the inter-operator variability (when measured) appeared to be acceptable in the glaucoma studies; repeatability and inter-operator agreement were good (67,68). However, color Doppler examinations were performed by a single, trained operator most of the time.
Another limitation of Doppler technique relates to the direct contact between the eyelid and the probe; this could influence orbital blood flow to some extent. Identification of the target blood vessel (especially in the posterior orbit) requires good training. Furthermore, measurement of actual blood flow with ultrasound imaging is problematic because the diameter of the studied vessel cannot be reliably estimated. To solve this issue, Michelson et al. (69) and Orge et al. (70) used angiography and flow analysis software coupled with standard color Doppler measurement, respectively, to derive blood flow values.
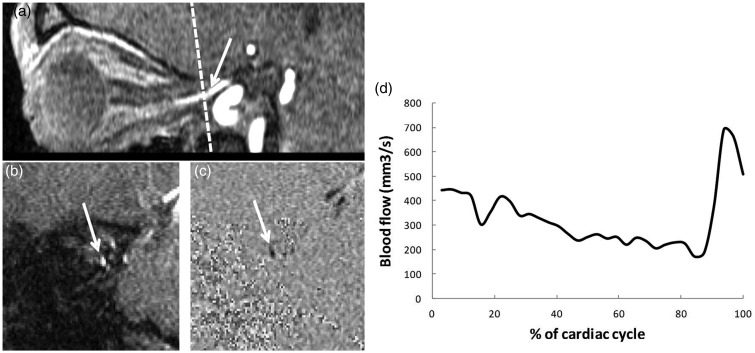
Phase contrast MRI provides new perspectives in this field of investigation. Cerebral blood and CSF flows have been quantified in both healthy subjects and pathological situations (e.g. intracranial hypertension and cerebral venous thrombosis) (71,72). It has been shown that intracranial pressure depends on volume exchanges from arterial and venous cerebral blood flow and CSF cerebral flow pulsatility during the cardiac cycle (63). Ambarki et al. (73) used phase contrast MRI to quantify blood flow in the OA in a population of healthy volunteers. It might also be possible to measure orbital venous flow and optic nerve CSF flow (74) (Fig. 3). As a reproducible, non-invasive technique, phase contrast MRI may be a way of assessing: (i) the interactions between optic nerve CSF flow and arterial and venous flows; (ii) the regulation of these flows; and (iii) the possible impairment of these mechanisms in glaucoma.
Fig. 3.
Phase-contrast (PC) MRI for the quantitative assessment of blood flow in the ophthalmic artery (OA). (a) A sagittal view of the right OA in a three-dimensional time-of-flight sequence. An oblique plane (dotted line) is placed perpendicular to the axis of the artery. (b) A coronal amplitude image (morphological image) obtained with PC MRI. The left OA appears as a white spot (arrow). (c) The corresponding phase image (functional image). The OA appears as a black spot (arrow). (d) A quantitative flow curve (mm3/s) for the left OA, obtained from the phase contrast sequence using segmentation software. The plot represents the change in blood flow over an average cardiac cycle.
In conclusion, assessments of orbital hemodynamics and hydrodynamics mainly rely on Doppler imaging and morphologic MRI. The imaging data support the hypothesis whereby glaucoma is related to alterations in ocular hemodynamic regulation and optic nerve CSF circulation. Doppler imaging can measure blood velocity but not actual blood flow. The probe has to be applied to the eyelid; deep orbital vessels cannot be measured and good repeatability requires a well-trained operator. MRI can combine morphologic and phase contrast techniques and can thus provide dynamic, absolute flow measurements within deep orbital or intracranial structures. Although further studies must now be conducted, phase contrast MRI may improve our understanding of blood–CSF flow interactions and their possible impairment in glaucoma.
Acknowledgments
The authors thank Dr David Fraser (Biotech Communication, Damery, France) for his helpful advice on the English language in this paper.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ 2004; 82: 887–888. [PMC free article] [PubMed] [Google Scholar]
- 2.Rudnicka AR, Mt-Isa S, Owen CG, et al. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci 2006; 47: 4254–4261. [DOI] [PubMed] [Google Scholar]
- 3.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82: 844–851. [PMC free article] [PubMed] [Google Scholar]
- 4.Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. Am J Ophthalmol 2013; 156: 724–730. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh S, Podhajsky P, Zimmerman MB. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica 1999; 213: 76–96. [DOI] [PubMed] [Google Scholar]
- 6.Lin C-C, Hu C-C, Ho J-D, et al. Obstructive sleep apnea and increased risk of glaucoma: a population-based matched-cohort study. Ophthalmology 2013; 120: 1559–1564. [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB, Wang N, Yang D, et al. Facts and myths of cerebrospinal fluid pressure for the physiology of the eye. Prog Retin Eye Res 2015; 46: 67–83. [DOI] [PubMed] [Google Scholar]
- 8.Abegão Pinto L, Vandewalle E, Stalmans I. Disturbed correlation between arterial resistance and pulsatility in glaucoma patients. Acta Ophthalmol 2012; 90: 214–220. [DOI] [PubMed] [Google Scholar]
- 9.Akarsu C, Bilgili MYK. Color Doppler imaging in ocular hypertension and open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 2004; 242: 125–129. [DOI] [PubMed] [Google Scholar]
- 10.Birinci H, Danacı M, Oge I, et al. Ocular blood flow in healthy and primary open-angle glaucomatous eyes. Ophthalmologica 2002; 216: 434–437. [DOI] [PubMed] [Google Scholar]
- 11.Butt Z, O’Brien C, McKillop G, et al. Color Doppler imaging in untreated high-and normal-pressure open-angle glaucoma. Invest Ophthalmol Vis Sci 1997; 38: 690–696. [PubMed] [Google Scholar]
- 12.Cellini M, Possati G, Caramazza N, et al. Colour Doppler analysis of the choroidal circulation in chronic open-angle glaucoma. Ophthalmologica 1996; 210: 200–202. [DOI] [PubMed] [Google Scholar]
- 13.Galassi F, Nuzzaci G, Sodi A, et al. Color Doppler imaging in evaluation of optic nerve blood supply in normal and glaucomatous subjects. Int Ophthalmol 1992; 16: 273–276. [DOI] [PubMed] [Google Scholar]
- 14.Garhöfer G, Fuchsjäger-Mayrl G, Vass C, et al. Retrobulbar blood flow velocities in open angle glaucoma and their association with mean arterial blood pressure. Invest Ophthalmol Vis Sci 2010; 51: 6652–6657. [DOI] [PubMed] [Google Scholar]
- 15.Janulevičienė I, Sliesoraitytė I, Siesky B, et al. Diagnostic compatibility of structural and haemodynamic parameters in open - angle glaucoma patients. Acta Ophthalmol 2008; 86: 552–557. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Ge J, Zhou W, et al. Hemodynamics of ophthalmic artery and central retinal artery and correlation with other factors in patients with primary open angle glaucoma. Yan Ke Xue Bao 1998; 14: 138–144. [PubMed] [Google Scholar]
- 17.Michelson G, Groh M, Groh M, et al. Advanced primary open-angle glaucoma is associated with decreased ophthalmic artery blood-flow velocity. Ger J Ophthalmol 1995; 4: 21–24. [PubMed] [Google Scholar]
- 18.Pemp B, Garhofer G, Lasta M, et al. The effects of moxaverine on ocular blood flow in patients with age-related macular degeneration or primary open angle glaucoma and in healthy control subjects. Acta Ophthalmol 2012; 90: 139–145. [DOI] [PubMed] [Google Scholar]
- 19.Rojanapongpun P, Drance S, Morrison B. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol 1993; 77: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekeroglu MA, Irkec M, Mocan MC, et al. The association of ocular blood flow with haemorheological parameters in primary open-angle and exfoliative glaucoma. Acta Ophthalmol 2011; 89: 429–434. [DOI] [PubMed] [Google Scholar]
- 21.Yüksel N, Karabaş V, Demirci A, et al. Comparison of blood flow velocities of the extraocular vessels in patients with pseudoexfoliation or primary open-angle glaucoma. Ophthalmologica 2001; 215: 424–429. [DOI] [PubMed] [Google Scholar]
- 22.Zhong Y, Min Y, Jiang Y, et al. Color Doppler imaging and pattern visual evoked potential in normal tension glaucoma and hypertension glaucoma. Doc Ophthalmol 2009; 119: 171–180. [DOI] [PubMed] [Google Scholar]
- 23.Meng N, Zhang P, Huang H, et al. Color Doppler imaging analysis of retrobulbar blood flow velocities in primary open-angle glaucomatous eyes: a meta-analysis. PLoS One 2013; 8: 62723–62723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolela MT, Walman BE, Buckley AR, et al. Ocular hypertension and primary open-angle glaucoma: a comparative study of their retrobulbar blood flow velocity. J Glaucoma 1996; 5: 308–310. [PubMed] [Google Scholar]
- 25.Nicolela MT, Drance SM, Rankin SJ, et al. Color Doppler imaging in patients with asymmetric glaucoma and unilateral visual field loss. Am J Ophthalmol 1996; 121: 502–510. [DOI] [PubMed] [Google Scholar]
- 26.Calvo P, Ferreras A, Polo V, et al. Predictive value of retrobulbar blood flow velocities in glaucoma suspects. Invest Ophthalmol Vis Sci 2012; 53: 3875–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gherghel D, Orgül S, Gugleta K, et al. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am J Ophthalmol 2000; 130: 597–605. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Aragon F, Garcia-Martin E, Larrosa-Lopez R, et al. Role of color Doppler imaging in early diagnosis and prediction of progression in glaucoma. Biomed Res Int 2013; 2013: 871689–871689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeitz O, Galambos P, Wagenfeld L, et al. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br J Ophthalmol 2006; 90: 1245–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galassi F, Sodi A, Ucci F, et al. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol 2003; 121: 1711–1715. [DOI] [PubMed] [Google Scholar]
- 31.Martínez A, Sánchez M. Predictive value of colour Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmol Scand 2005; 83: 716–722. [DOI] [PubMed] [Google Scholar]
- 32.Abegão PL, Vandewalle E, De Clerck E, et al. Ophthalmic artery Doppler waveform changes associated with increased damage in glaucoma patients. Invest Ophthalmol Vis Sci 2012; 53: 2448–2453. [DOI] [PubMed] [Google Scholar]
- 33.Butt Z, McKillop G, O’Brien C, et al. Measurement of ocular blood flow velocity using colour Doppler imaging in low tension glaucoma. Eye 1995; 9: 29–33. [DOI] [PubMed] [Google Scholar]
- 34.Chiou H-J, Chou Y-H, Liu C, et al. Evaluation of ocular arterial changes in glaucoma with color Doppler ultrasonography. J Ultrasound Med 1999; 18: 295–302. [DOI] [PubMed] [Google Scholar]
- 35.Galambos P, Vafiadis J, Vilchez SE, et al. Compromised autoregulatory control of ocular hemodynamics in glaucoma patients after postural change. Ophthalmology 2006; 113: 1832–1836. [DOI] [PubMed] [Google Scholar]
- 36.Plange N, Kaup M, Weber A, et al. Performance of colour Doppler imaging discriminating normal tension glaucoma from healthy eyes. Eye 2009; 23: 164–170. [DOI] [PubMed] [Google Scholar]
- 37.Michelson G, Groh M, Gründler A. Regulation of ocular blood flow during increases of arterial blood pressure. Br J Ophthalmol 1994; 78: 461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris A, Sergott R, Spaeth G, et al. Color Doppler analysis of ocular vessel blood velocity in normal-tension glaucoma. Am J Ophthalmol 1994; 118: 642–649. [DOI] [PubMed] [Google Scholar]
- 39.Hosking S, Harris A, Chung H, et al. Ocular haemodynamic responses to induced hypercapnia and hyperoxia in glaucoma. Br J Ophthalmol 2004; 88: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haefliger IO, Dettmann E, Liu R, et al. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv Ophthalmol 1999; 43: 51–58. [DOI] [PubMed] [Google Scholar]
- 41.Yorio T, Krishnamoorthy R, Prasanna G. Endothelin: is it a contributor to glaucoma pathophysiology? J Glaucoma 2002; 11: 259–270. [DOI] [PubMed] [Google Scholar]
- 42.Maruyoshi H, Kojima S, Kojima S, et al. Waveform of ophthalmic artery Doppler flow predicts the severity of systemic atherosclerosis. Circ J 2010; 74: 1251–1256. [DOI] [PubMed] [Google Scholar]
- 43.Anderson DR. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol 2003; 14: 86–90. [DOI] [PubMed] [Google Scholar]
- 44.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007; 114: 1965–1972. [DOI] [PubMed] [Google Scholar]
- 45.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 714–720. [DOI] [PubMed] [Google Scholar]
- 46.Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002; 21: 359–393. [DOI] [PubMed] [Google Scholar]
- 47.Nicolela MT. Clinical clues of vascular dysregulation and its association with glaucoma. Can J Ophthalmol 2008; 43: 337–341. [DOI] [PubMed] [Google Scholar]
- 48.Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci 2003; 44: 5189–5195. [DOI] [PubMed] [Google Scholar]
- 49.Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 2008; 115: 763–768. [DOI] [PubMed] [Google Scholar]
- 50.Jonas JB, Wang NL, Wang YX, et al. Estimated trans-lamina cribrosa pressure difference versus intraocular pressure as biomarker for open-angle glaucoma. The Beijing Eye Study 2011. Acta Ophthalmol 2015; 93: 7–13. [DOI] [PubMed] [Google Scholar]
- 51.Ren R, Jonas JB, Tian G, et al. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology 2010; 117: 259–266. [DOI] [PubMed] [Google Scholar]
- 52.Ren R, Wang N, Zhang X, et al. Trans-lamina cribrosa pressure difference correlated with neuroretinal rim area in glaucoma. Graefes Arch Clin Exp Ophthalmol 2011; 249: 1057–1063. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann J, Schmidt C, Kunte H, et al. Volumetric assessment of optic nerve sheath and hypophysis in idiopathic intracranial hypertension. Am J Neuroradiol 2014; 35: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang N, Xie X, Yang D, et al. Orbital cerebrospinal fluid space in glaucoma: the Beijing intracranial and intraocular pressure (iCOP) study. Ophthalmology 2012; 119: 2065–2073. [DOI] [PubMed] [Google Scholar]
- 55.Jaggi GP, Miller NR, Flammer J, et al. Optic nerve sheath diameter in normal-tension glaucoma patients. Br J Ophthalmol 2012; 96: 53–56. [DOI] [PubMed] [Google Scholar]
- 56.Morgan WH, Yu D-Y, Alder VA, et al. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest Ophthalmol Vis Sci 1998; 39: 1419–1428. [PubMed] [Google Scholar]
- 57.Killer H, Jaggi G, Flammer J, et al. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve.. Is it always bidirectional? Brain 2007; 130: 514–520. [DOI] [PubMed] [Google Scholar]
- 58.Killer HE, Miller NR, Flammer J, et al. Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br J Ophthalmol 2012; 96: 544–548. [DOI] [PubMed] [Google Scholar]
- 59.Wostyn P, De Groot V, Van Dam D, et al. Senescent changes in cerebrospinal fluid circulatory physiology and their role in the pathogenesis of normal-tension glaucoma. Am J Ophthalmol 2013; 156: 5–14. [DOI] [PubMed] [Google Scholar]
- 60.Killer HE. Compartment syndromes of the optic nerve and open-angle glaucoma. J Glaucoma 2013; 22: 19–20. [DOI] [PubMed] [Google Scholar]
- 61.Killer HE, Jaggi GP, Flammer J, et al. Is open-angle glaucoma caused by impaired cerebrospinal fluid circulation: around the optic nerve? Clin Experiment Ophthalmol 2008; 36: 308–311. [DOI] [PubMed] [Google Scholar]
- 62.Jaggi GP, Harlev M, Ziegler U, et al. Cerebrospinal fluid segregation optic neuropathy: an experimental model and a hypothesis. Br J Ophthalmol 2010; 94: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 63.Balédent O, Idy-Peretti I. Cerebrospinal fluid dynamics and relation with blood flow: a magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Invest Radiol 2001; 36: 368–377. [DOI] [PubMed] [Google Scholar]
- 64.Perez-Barcena J, Llompart-Pou JA, O’Phelan KH. Intracranial pressure monitoring and management of intracranial hypertension. Crit Care Clin 2014; 30: 735–750. [DOI] [PubMed] [Google Scholar]
- 65.Harris A, Guidoboni G, Arciero JC, et al. Ocular hemodynamics and glaucoma: the role of mathematical modeling. Eur J Ophthalmol 2013; 23: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siaudvytyte L, Januleviciene I, Ragauskas A, et al. Update in intracranial pressure evaluation methods and translaminar pressure gradient role in glaucoma. Acta Ophthalmol 2015; 93: 9–15. [DOI] [PubMed] [Google Scholar]
- 67.Harris A, Williamson TH, Martin B, et al. Test/retest reproducibility of color Doppler imaging assessment of blood flow velocity in orbital vessels. J Glaucoma 1995; 4: 281–286. [PubMed] [Google Scholar]
- 68.Quaranta L, Harris A, Donato F, et al. Color Doppler imaging of ophthalmic artery blood flow velocity: a study of repeatability and agreement. Ophthalmology 1997; 104: 653–658. [DOI] [PubMed] [Google Scholar]
- 69.Michelson G, Schuierer G. Absolute blood flow in the ophthalmic artery. Fortschr Ophthalmol 1991; 88: 687–689. [PubMed] [Google Scholar]
- 70.Orge F, Harris A, Kagemann L, et al. The first technique for non-invasive measurements of volumetric ophthalmic artery blood flow in humans. Br J Ophthalmol 2002; 86: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balédent O, Fin L, Khuoy L, et al. Brain hydrodynamics study by phase - contrast magnetic resonance imaging and transcranial color doppler. J Magn Reson Imaging 2006; 24: 995–1004. [DOI] [PubMed] [Google Scholar]
- 72.ElSankari S, Czosnyka M, Lehmann P, et al. Cerebral blood and CSF flow patterns in patients diagnosed for cerebral venous thrombosis-an observational study. J Clin Imaging Sci 2012; 2: 41–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ambarki K, Hallberg P, Jóhannesson G, et al. Blood flow of ophthalmic artery in healthy individuals determined by phase-contrast magnetic resonance imaging. Invest Ophthalmol Vis Sci 2013; 54: 2738–2745. [DOI] [PubMed] [Google Scholar]
- 74.Golzan SM, Avolio A, Magnussen J, et al. Visualization of orbital flow by means of phase contrast MRI. Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE. IEEE. 2012:3384–3387. [DOI] [PubMed]