Abstract
Mechanical loading is recognized to play an important role in regulating the behaviors of cells in bone and surrounding tissues in vivo. Many in vitro studies have been conducted to determine the effects of mechanical loading on individual cell types of the tissues. In this review, we focus specifically on the use of the Flexercell system as a tool for studying cellular responses to mechanical stretch. We assess the literature describing the impact of mechanical stretch on different cell types from bone, muscle, tendon, ligament, and cartilage, describing individual cell phenotype responses. In addition, we review evidence regarding the mechanotransduction pathways that are activated to potentiate these phenotype responses in different cell populations.
Keywords: Flexercell, mechanical strain, bone, cartilage, muscle, tendon, ligament
Introduction
The bone and surrounding tissues experience many different types of mechanical loading. For example, compressive forces are experienced and tolerated well by articular cartilage. Shear stress resulting from interstitial fluid flow is tolerated in bone tissue and in tendons and ligaments, tensile stretch is tolerated in order to maintain skeletal anatomy. The effect of different forms of mechanical loading of cells has been extensively studied in vitro and in animal models and human subjects, where it is commonly reported to have stimulatory effects on those cells. In fact, fluid flow–induced shear stress actively enhances cellular events that potentiate bone formation and remodeling,1,2 and compressive loading of articular chondrocytes stimulates their proliferation capacity. In this review, we will focus specifically on the impact of mechanical stretch on bone and surrounding cell responses and in particular, the use of the Flexercell system as a means of applying that stretch.
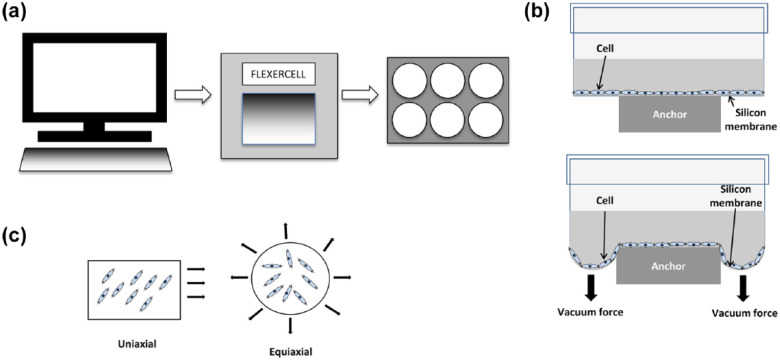
In 1985, the first reported use of flexible-bottomed plates for studying the effect of mechanical stimulation on cell cultures was made. 3 The plates used a computer-controlled vacuum device to apply negative pressure to the underside of the membrane. When the vacuum was applied, the membrane on which firmly adhered cells were attached was stretched downward and consequently, a strain was imparted on the cells adhered to that membrane. A number of parameters under the control of the operator included the magnitude of pressure, waveform, frequency, duration, and incorporation of rest period over the time course of the experiment. As the system is easy to set up and these various parameters are easy to control, the Flexercell system has been a very good platform with which to study the effect of mechanostimulation in many different contexts (Figure 1).
Figure 1.
Experimental setup of the Flexercell system for applying strain to cell populations. (a) Schematic representation of the computer-controlled Flexercell system that applies strain to cell monolayers in custom-made six-well plates. (b) Cross-sectional diagram of one well of a six-well Flexercell plate at rest (top) and upon application of the vacuum (bottom). (c) Strain can be applied in a variety of ways including uniaxial and equiaxial.
One huge benefit of the widespread use of this system is that even though the many studies of bone and surrounding cell responses use different strain regimes (magnitude, frequency, duration, etc.), the fact that the base technology applies strain using a common method of application means that some comparability between different studies can be made. Strain is applied either uniaxially or biaxially, generally in two-dimensional (2D) culture (although there are some exceptions to this, where a three-dimensional (3D) culture module is used to characterize compressive force) on silicone membranes coated with type I collagen. Dishes can also be coated with other extracellular matrix (ECM) molecules too making it possible to characterize the effect of mechanical stretch on cells anchored to different ECM components, providing a 2D model of in vivo mechanical stimulation of tissues.
In the bone and surrounding tissues, mechanical signals are continually generated and experienced by the numerous cell types that make up the component tissues. Understanding the role of mechanotransduction and its effects at the cellular level can greatly enhance our understanding of the role of mechanical signals in maintaining normal healthy musculoskeletal physiology and also provide some insight to mechanisms of pathogenesis, for instance, during chronic overuse injury. In the following sections, we review the ever-growing body of the literature that describes bone and surrounding cell responses to mechanical strain applied using the Flexercell system.
Effect of mechanical strain on bone cell phenotypes
During bone loading in vivo, macroscale forces are transmitted to osteocytes through the canalicular network in the form of microscale shear stresses caused by rapid displacement of interstitial fluid. Prolonged cyclic loading of bone leads to increased mineralized tissue density and bone mass. It was hypothesized some time ago that mechanical force was a positive regulator of bone formation creating a shift in bone homeostasis toward the anabolic state, characterized by enhanced bone-forming activity by osteoblasts and a reduction in bone resorption by osteoclasts. 4 In addition to shear stress caused by fluid flow (see above; reviewed by Brindley et al. 1 and Yeatts and Fisher 2 ), tensile stretch applied to cells via physical deformation of culture surfaces5–7 can enhance key cellular and molecular events that lead to bone formation. A summary of notable studies is presented in Table 1.
Table 1.
Summary of Flexercell studies and key findings using osteoblastic cells.
| Cell type | Stage of differentiation | Stretch device and regime | Key findings | Reference |
|---|---|---|---|---|
| Murine osteocytic (MLO-Y4) | Osteocytes | Flexercell 4000, 5% elongation, 10 min, 0.05–1 Hz | Estrogen receptor involved in strain-mediated pro-survival signaling via ERK | Aguirre et al. 8 |
| Human osteoblasts | 24-h culture in differentiation medium (early differentiation) | Flexercell, a 3%–9% strain, 8 h, 1 Hz | High strain increased proliferation, migration, VEGF and bFGF production | Bhatt et al. 9 |
| Low strain increased OCN and OPN | ||||
| Rat osteoblast (ROS 17/2.8) and mouse osteoblast (MC3T3-E1) | Early osteoblasts | Flexercell 3000, 1% elongation, 10 min, 0.25 Hz | Strain-induced rapid phosphorylation of ERK2 and FAK and activation of Ras/Raf/MEK pathway | Boutahar et al. 10 |
| Human osteosarcoma (TE-85) | Osteosarcoma | Flexercell, a intermittent strain of 3 cycles/min consisting of 10 s strain, 10 s rest, 15–60 min or 1–3 h | Strain-induced reorganizated distribution of integrins and increased β1 but not αν mRNA levels | Carvalho et al. 11 |
| Human osteosarcoma (SaOS-2) | Osteosarcoma | Flexercell, a 5%–12% elongation, 8–24 h, 0.05 Hz | Increased expression of TGFβ1, IGF1, bFGF, IL6, but no change in IL1 | Cillo et al. 12 |
| Mouse osteoblast (CIMC-4) | Pre-osteoblast | Flexercell, a biaxial strain at 0.5%–2%, 24 h, 0.16 Hz | Strain caused a reduction in RANKL, but upregulated OSX and RUNX2 via ERK1/2 | Fan et al. 13 |
| Rat osteoblast (ROS17/2.8) and human osteoblasts | Single cells; 24-h post-plating | Flexercell 3000, 1% elongation, 1.5–150 min, 0.05–5 Hz | Expression of soluble VEGF isoforms (121, 165) under low frequency | Faure et al. 14 |
| Expression of matrix-bound VEGF isoforms (206, 189, 165, 145) under high frequency | ||||
| Rat osteoblast (ROS17/2.8) | Mature osteoblasts (3 weeks in differentiation medium) | Flexercell 3000, 1% elongation, 10 min, 0.05 Hz | Stretch-induced activation of Egr-1 and nuclear translocation of NF-κB | Granet et al. 15 |
| Rat osteoblast (ROS 17/2.8) and mouse osteoblast (MC3T3-E1) | Early osteoblast | Flexercell 3000, 1% elongation, 15 cycles/min (2-s deformation period followed by a 2-s neutral position) | Induced phosphorylation of FAK, PYK2, paxillin and HIC5 | Guignandon et al. 16 |
| Murine calvarial osteoblasts | Early osteoblast (7–10 days post-plating) | Flexercell 4000, 2.5%–3% elongation, 3–24 h, 0.3 Hz | Stretch resulted in the activation of canonical Wnt signaling | Hens et al. 17 |
| Human fetal osteoblast (SV-HFO) | Differentiation medium | Bioflex loading stations, 0.4%, 5–60 min or 7–21 days, 0.05 Hz | Stretch-induced phosphorylation of ERK1/2 pathways dependent on differentiation stage | Jansen et al. 18 |
| Mouse osteoblast (MC3T3-E1) | 24 h in differentiation medium | Flexercell 4000, 0.25%–2.5%, 1 Hz, 15 min/day for 7 days | Overall increased RANKL due to increased membrane-bound RANKL but decreased soluble RANKL | Kim et al. 19 |
| Rat osteoblast and mouse osteoblast (MC3T3-E1) | Osteoblasts | Flexercell, a 10%–12%, 0.1 Hz either one single maximal strain load every 6 h or continuous strain for 24 h | Stretch increased DNA synthesis | Knoll et al. 20 |
| ALP decreased in response to strain and combined strain/TGFβ treatment | ||||
| Human osteoblast (HOB) | Osteoblasts | Flexercell 4000, 2.5% elongation, 0.1 Hz, intermittent strain 1 h, strain 3 h, and rest for 48 h | Increased DNA synthesis and IL6 production | Liegibel et al. 5 |
| No effect on ALP, COL I or OPG production | ||||
| Human osteosarcoma (SaOS-2) | 7 days post-plating | Flexercell, a 5%–12.5% strain, 24 h, 0.5 Hz | Low strain led to increased COL I and COL III expression | Liu et al. 21 |
| High strain led to decreased COL III expression | ||||
| Mouse osteoblast (MC3T3-E1) | Osteoblasts | Flexercell 2000, 7%–24% elongation, 3 cycles/min, 1–24 h | Increased expression of VEGF and M-CSF | Motokawa et al. 22 |
| Mouse osteoblast (MC3T3-E1) | Osteoblasts | Flexercell 4000, 0%–9% elongation, 3–24 h, 0.3 Hz | Stretch promoted COX2 but not COX1 expression | Narutomi et al. 23 |
| Murine osteocytic (MLO-Y4) | Osteocytes | Flexercell 4000, 2%–5%, 1–20 min | Activation of ERK via integrin/cytoskeleton/Src-mediated signaling | Plotkin et al. 24 |
| Mouse osteoblast (MC3T3-E1) | Osteoblasts | Flexercell 3000, 3400 microstrain, 2 Hz, 5 h | Increased Wnt/B-catenin target genes (Wnt10B, SFRP1, cyclin D1, FZD2, WISP2, and connexin 43) | Robinson et al. 25 |
| Mouse osteoblast (MC3T3-E1) | Osteoblasts | Flexercell 3000, 6%–18% elongation, 24 h, 0.1 Hz | Increased OPG synthesis and decreased RANKL expression | Tang et al. 6 |
| Human osteosarcoma (MG63) | Osteoblasts | Flexercell 3000, 10% elongation, 14 h, 0.5 Hz | Strain inhibited the promoter activation by vitamin D | Toyoshita et al. 26 |
| Rat osteoblast | Differentiation medium | Flexercell, a −2 kPa, 1–15 days, 0.05 Hz | Nodule formation was enhanced, depending on the timing of initiation and magnitude of the deformation regimen | Visconti et al. 27 |
| SV40 human osteoblasts | Differentiation medium | Flexercell 4000, 0.8%–3.2% elongation, 48 h, 1 Hz | High-magnitude strain led to increased expression of OC, COL I and Cbfa1/Runx2 | Zhu et al. 7 |
| Low-magnitude strain led to increased ALP activity |
ERK: extracellular-regulated protein kinase; VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor; OCN: osteocalcin; OPN: osteopontin; FAK: focal adhesion kinase; NF-κB: nuclear factor-κB; TGFβ1: transforming growth factor β1; COL: collagen; IGF1: insulin-like growth factor 1; IL: interleukin; PYK2: proline-rich tyrosine kinase 2; COX: cyclooxygenase; M-CSF: macrophage-colony stimulating factor; ALP: alkaline phosphatase; OPG: osteoprotegerin; MEK: mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK) kinase; RANKL: receptor activator of NF-kB ligand; OSX: osterix; RUNX2: Runt-related transcription factor 2; Egr-1: Early growth response protein 1; HIC5: hydrogen peroxide-inducible clone-5.
Flexercell model not specified.
Impact on cell survival, proliferation, and growth
Tensile strain applied using the Flexercell increases osteoblast proliferation5,9,20,28 although the magnitude of strain appears to have a bearing on the degree of proliferation that is permissible. 9 Application of a high strain level of 10%–12% elongation at 0.1 Hz for 24 h stimulated proliferation in primary rat osteoblasts but the even greater mitogenic effect that transforming growth factor β (TGFβ) has in the absence of strain was suppressed by strain itself, 20 suggesting that mechanotransduction competes with TGFβ signaling to dampen its effects. Application of mechanical strain to osteocytes and very mature osteoblasts failed to stimulate proliferation, 29 and it was hence concluded that these cells respond differently to proliferative osteoblasts, instead regulating metabolic responses to strain reflective of their mechanosensing role in vivo.
Lower levels of strain (2.5% elongation at 0.1 Hz) were stimulatory to proliferation of human primary osteoblasts over 48 h, without promoting alkaline phosphatase (ALP) activity or secretion of type I collagen or osteoprotegerin (OPG), suggesting that the cells were maintained in a premature state. However, pre-treating cells with testosterone prior to application of strain promoted osteogenic gene expression at the expense of proliferation, 5 highlighting that the effects of mechanotransduction are subject to a mechanistic switch regulated by androgens.
A study assaying the effects of strain over a range of 0%–9% elongation for 8 h at 1 Hz revealed that proliferation of normal human osteoblasts was magnitude dependent, with the highest strain producing the greatest level of proliferation. 9 Conditioned medium obtained from cultures stretched to 9% elongation stimulated cell proliferation in static culture, indicating the release of a soluble factor.
In summary, the collated evidence indicates that stretch signals have a positive effect on osteoblast proliferation and they do so in a magnitude-dependent fashion. However, there will inevitably be a critical upper threshold at which stretch signals are no longer stimulatory to proliferation and are indeed detrimental to cell viability. Furthermore, biological molecules such as growth factors and hormones may alter the basal cell response to stretch, and this is particularly significant in vivo, where mechanical signals are just one of many facets of the cell microenvironment.
Matrix production
When a range of stretch magnitudes from 0% to 9% was applied to human primary osteoblasts, expression of the type I collagen mRNA, COL1αII, was highest at 9% elongation, whereas in the case of mature osteoblast markers osteopontin (OPN) and osteocalcin (OCN), highest expression was seen at 3% strain, declining thereafter, 9 indicating a biphasic response to tensile strain. This parallels the authors’ observations for proliferation, which was superior at the highest magnitude of 9%. Furthermore, COL1 expression by human primary osteoblasts was only upregulated at a low magnitude of 2.5% strain in the presence of testosterone, 5 further evidence that the effect of mechanical stimulation is subject to hormonal influence. For type III collagen expression, where a “high impact” strain regime of between 5% and 12.5% strain at 0.5 Hz for 24 h on human osteosarcoma cells was used, Liu et al. 21 found that COL3 mRNA was increased at 5% but then underwent a decline in the expression after 5%, whereas COL1 transcripts were stably increased at all strain levels compared to static control cultures. As type III collagen is normally deposited as an early wound matrix component, it is surprising that it was not increased at the higher strain level.
In summary, early matrix production events, that is, deposition of type I collagenous material, appear to preferentially take place at higher strain levels in parallel with the enhanced proliferation effect. This may reflect the wound healing environment in vivo whereby early events of osteoblast proliferation and matrix deposition take place in a mechanically unstable environment prior to the formation of rigid bone, placing large amounts of strain on cells.
Impact on cytokine/growth factor production
Flexercell studies have revealed that numerous growth factors are upregulated in osteoblasts in response to stretch signals. Over a range of stretch from 0% to 9%, vascular endothelial growth factor (VEGF) expression by primary human osteoblasts was highest at 9% stretch. 9 It also seems that the frequency with which stretch is applied influences the form of VEGF synthesized, as 1% stretch applied to both human and rat osteoblasts at various frequencies from 0.05 to 5 Hz revealed that high-frequency stretch promoted the expression of VEGF isoforms that are matrix bound and low frequencies stimulated production of soluble VEGF isoforms. 14 Upregulation of VEGF, along with macrophage-colony stimulating factor (M-CSF) have been reported to be mechanistically important for the recruitment of osteoclasts in the remodeling of bone, 22 and so stretch-induced VEGF expression is pivotal for the regulation of bone remodeling events in vivo as well as in revascularization of regenerating tissue.
TGFβ expression in human osteosarcoma cells was upregulated in response to mechanical stretch applied at 12% elongation. 12 TGFβ is a potent mitogen and can also promote collagen synthesis, both of which are important during the early stages of bone healing. However, binding of TGFβ to the TGFβ receptor-III is also enhanced by mechanical stretch, 20 and this interaction has been shown to inhibit the ability of TGFβ to activate osteoblasts.30–32 Therefore, mechanical strain seems to finely regulate TGFβ signaling and promote similar mitogenic and differentiation responses to TGFβ but via a mechanotransduction pathway that competitively inhibits TGFβ signaling.
Mechanical stretch also enhanced osteoblastic expression of insulin-like growth factor (IGF) and basic fibroblast growth factor (bFGF) at 12% maximal stretch at 3 Hz, over a 24-h period. 12 Both factors stimulate early osteoblast healing responses such as osteoblast proliferation and matrix synthesis33,34 and the early wound healing events induced in osteoblasts by high-magnitude stretch, such as proliferation and type I collagen expression (see above), may be a consequence of increased expression of these factors. Indeed, osteocytes, which act as mechanosensing cell in vivo, produce increasing levels of IGF in response to mechanical strain35–37 which may be one of the primary initiation events for the recruitment of osteoblast precursors during bone remodeling and regeneration.
Increased expression of several bone morphogenetic proteins (BMPs), in particular BMP2, in response to low strain of 1% stretch was also reported in mouse osteoblastic MC3T3-E1 cells, 38 yet in human primary osteoblasts subjected to stretch over a range of 0%–9% elongation, increased gene expression was only seen at 6% and above. 9 However, the overall positive effect of mechanical stretch on osteoblastic expression of BMP2 is further evidence of the stimulatory effect of mechanical stretch signals on osteogenesis.
In terms of the bone remodeling balance, mechanical stretch also seems to have a role in regulation of bone resorption by osteoclasts. Interleukin 6 (IL6) has a well-known role in osteoclast production during normal bone homeostasis. 39 Increased expression of IL6 was observed in human osteosarcoma cells stretched at 12% elongation at 0.05 Hz 12 and in human primary osteoblasts stretched at 2.5% elongation at 0.1 Hz. 5 In the latter study, OPG, an inhibitor of osteoclastogenesis, was produced when stretch was applied in combination with testosterone, but not in its absence, demonstrating the coordinated action of mechanotransduction and hormones. The effects of stretch on IL6 are magnitude dependent, as studies of mouse MC3T3-E1 osteoblastic cells exposed to a regime of 0%–9% elongation revealed that the greatest level of IL6 production was seen at 9% stretch and was dependent on prostaglandin E2 production by cyclooxygenase 2. 23
A key downstream consequence of IL6 activity in osteoblasts that leads to osteoclast activation is production of RANKL. 39 There is some debate in the literature about the effect of mechanical strain on RANKL expression. Some researchers have reported a magnitude-dependent decline in RANKL expression,6,13 and this was accompanied by an increase in the expression of the transcription factors Osterix and Runx2 13 and OPG. 6 However, Kim et al. 19 reported an increase in RANKL expression after exposure to mechanical strain. Nevertheless, in this study, a potential mechanism for preventing osteoclast activity in order to maintain an osteoanabolic process was discovered using MC3T3-E1 cells transfected with RANKL cDNA. The expression of RANKL increased in transfected cells, but when low-level mechanical strain was applied daily for 1 week, most of the protein was in membrane-bound form with a large reduction in soluble RANKL, thus reducing its bioavailability. 19 In any case, RAW264.7 cells that are capable of osteoclastic differentiation in the presence of RANKL failed to do so effectively in the presence of mechanical strain of 10% elongation at 0.5 Hz over 1 week. 40 Together with the strong hormonal influence as demonstrated for testosterone, 5 osteoclast activity is subject to further regulation upon exposure to strain.
Overall, mechanical stretch signals induce the expression of factors that invoke early wound healing events such as mesenchymal cell proliferation and angiogenesis. Furthermore, stretch-induced upregulation of factors such as VEGF and IL6 results in osteoclast recruitment and differentiation, which is in turn carefully regulated by androgens. Studies that have assayed growth factor production over a range of different elongations generally concur that growth factor production is magnitude dependent. This likely reflects the early wound healing response where the fracture site is highly unstable and growth factors are in abundance.
Cellular maturation and differentiation
Osteoblastic differentiation, maturation, and ultimately mineralized matrix deposition can be directly enhanced by mechanical strain applied using Flexercell. For example, stretch applied to immortalized mouse calvarial osteoblasts overnight at 2% elongation enhanced the expression of osteogenic transcription factors, osterix and runx2. 13 Over a range of elongations from 0% to 3.2%, a subsequent study found that runx2 was significantly upregulated only at 3.2% stretch but not at 2.4% or lower, and type I collagen expression was upregulated in proportion to magnitude of stretch. 7 In this study, OCN was only upregulated at 2.4%–3.2% elongation, whereas ALP (expressed earlier in osteogenic differentiation) was upregulated at 0.8%–1.6% elongation but not above.
The strain magnitude-dependent expression of OCN by human primary osteoblasts was further confirmed using an elongation range of 0%–9%. OCN expression was upregulated at 3%, 6%, and 9% elongation compared to 0% control cultures, 9 whereas in the case of osteonectin and OPN, greatest gene expression was seen at 3% and then underwent a gradual decline in basal levels by 9%. 9 At stretch magnitude as low as 1% elongation, bone nodule formation was induced in monolayers of mouse MC3T3-E1 and rat primary osteoblasts.27,41 Particularly intriguing is the fact that some researchers reported that early osteogenic events such as proliferation and collagen production were best at a high strain of 9%, whereas the expression of differentiation markers OPN and OCN was most favorable at low strain of 3% elongation, 9 and bone nodule formation was reported as low as 1% elongation.27,41 Other researchers reported that the osteoanabolic phenotype was enhanced in osteoblasts with increasing magnitude of strain up to 18% elongation, characterized by enhanced expression of OPG, 6 which is a potent inhibitor of osteoclast formation and activity. 42 However, it is not just the magnitude of strain that impacts the specific response of osteoblasts but the timing of application and duration of strain that determine the exact nature of cell responses. According to the work of Visconti et al., 27 applying strain after a prolonged static culture period (either 3 or 10 days) produced a greater number of bone nodules than applying strain after just 1 day of static culture. This suggests that a regime that promotes mineralized matrix formation should be applied after early events such as collagen matrix deposition have been given time to occur.
To summarize the impact of mechanical stretch on osteogenic differentiation and maturation, it is clear that lower magnitude strain in the region of <5% elongation is superior for directing these events, a contrast to early events such as proliferation and type I collagen production, which are optimally enhanced at greater magnitudes of stretch (around 9% elongation).
Effect of mechanical strain on skeletal muscle cells
Mechanical strain is transmitted through tendons and ligaments during locomotion, when muscle contraction occurs. Skeletal muscle itself generates contractile forces that are propagated through the muscle fiber network. The nature of force generation in skeletal muscle means that as well as contraction, cells experience stretch stimuli.
In order to fully critically appraise the information in this area, a brief description of how skeletal muscle differentiates and regenerates is appropriate. The tissue itself is characterized by multinucleate muscle fibers encapsulated by connective tissue, mainly collagen, fixed at both ends (junctions).
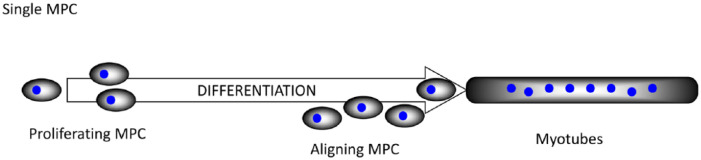
In terms of mechano-responsiveness, it could be suggested that skeletal muscle is the most relevant tissue in the human body. It is composed of elongated multinucleate muscle fibers that develop from the “end-to-end” fusing of mononuclear muscle precursor cells (MPCs) in a process known as differentiation. In development, these mononuclear cells are derived from the somites and, depending upon the anatomical location, the MPCs either migrate to differentiate at their final destination, for example, limb muscle or differentiate at or near their final destination, for example, head and neck muscles. However and astoundingly, skeletal muscle is a not a solely post-mitotic tissue. MPCs located in the periphery of post-natal muscle fibers can become activated by a variety of stimuli including mechanical. This activation triggers a series of events that lead to the asymmetric division of these cells. This process can be recapitulated with in vitro cell culture and is illustrated in Figure 2.
Figure 2.
A series of processes from MPCs to differentiated myotubes.
It is therefore very important to understand at which part of the process the cells are being stimulated using the Flexercell apparatus where a large number of studies have demonstrated that mechanical strain activates muscle cells at different stages of maturity from skeletal myoblasts to satellite cells,43–47 and a summary of muscle cell studies is presented in Table 2.
Table 2.
Summary of Flexercell studies and key findings using muscle cells.
| Cell type | Stage of differentiation | Stretch device and regime | Key findings | Reference |
|---|---|---|---|---|
| Proliferating MPCs | ||||
| Murine myoblast (C2C12) | Confluent/early fusion | Flexercell 4000, cyclical stretch at 3% at 0.05 Hz for 24–72 h | Increased MyoD, MyoG, Mef2, MHC | Bruce et al. 48 |
| Stretch abrogated the reductions in the expression of the above by TNFα treatment | ||||
| Murine myoblast (C2C12) | Single cells; 24-h post-plating | Flexercell, a 15% strain, 0.5 Hz, cyclical for up to 48 h | Increased proliferation | Iwanuma et al. 47 |
| Increased IGF1 and caspase mRNA expression in the initial 24 h of stretch | ||||
| Murine myoblast (C2C12) | MDC | Flexercell 4000, 15% strain for 10 min | Overexpression of REDD2 inhibits response of mTOR to mechanical stimulation | Miyazaki and Esser 49 |
| Rat MDCs | >95% desmin-positive single MDCs | Flexercell 2000, 12–36 h post-plating at 25% strain for 12–36 h with 12-s intervals | Increase in BrdU + cells after 12-h stretch | Tatsumi et al. 43 |
| Increase in HGF in CM at 12 h. Anti-HGF antibody prevents increase in BrdU + cells. Stretch releases pre-existing HGF from ECM | ||||
| Rat MDCs | >95% Desmin positive single MDCs | Flexercell 2000, 12–36 h post-plating at 25% strain for 24 h with 12 s intervals | Increase in LH limb BrdU + cells compared to BK and UH cultures following stretch. Increase in HGF in CM of LH cultures stretch compared to BK and UH | Tatsumi et al. 50 |
| Rat MDCs | >95% desmin-positive single MDCs | Flexercell 2000, 12–36 h post-plating at 25% strain for 24 h with 12-s intervals | Increase in BrdU + cells at an optimal pH (7.2) | Tatsumi et al. 44 |
| Increase in BrdU + cells in a HGF- and NO-dependent mechanism | ||||
| Rat MDCs | Single MDCs | Flexercell 2000, 12–36 h post-plating at 25% strain for 24 h with 12-s intervals | Stretch activation of BrdU + cells is NO dependent | Tatsumi et al. 45 |
| Rat MDCs | Single MDCs | Flexercell 2000, 25% strain, 12-s intervals for 2 h | Stretch induced an increase in the active form of MMP2 in a NO-dependent mechanism | Yamada et al. 51 |
| Aligning MPCs | ||||
| Murine myoblast (C2C12) and murine MDCs | Single cells induced to differentiate; 24-h post-seeding (C2C12) or immediately post-seeding (MDCs) | Flexercell 4000, 48 h uniaxial ramp stretch, followed by intermittent strain at 2%–6% strain for 4 days. Cyclic stretch at 1 Hz | 2D: reductions in MRF-4 and MYH-4 mRNA in both cell types. Increase in MYH-1 mRNA (C2C12 only) | Boonen et al. 52 |
| 3D: reductions in MRFs, MyoD, myogenin (both cell types) MRF-4 (C2C12) and MLP (MDCs). Increases in actin and α-actinin mRNA at day 4 (C2C12), reduction in MYH mRNAs (both cell types) | ||||
| Murine myoblast (C2C12) | Single cells induced to differentiate | Flexercell 4000, 17% cyclical stretch, 1 Hz for 1 h every 24 h for 5 days | Stretch increased proliferation and inhibited differentiation | Kumar et al. 53 |
| Murine myoblast (C2C12) | Single cells induced to differentiate | Flexercell, a 15% cyclical strain for up to 5 days at 0.1, 0.5, and 0.9 Hz | Stretch improved proliferation | Kurokawa et al. 54 |
| Stretch led to increased MHC-perinatal expression | ||||
| Fast stretch led to early expression, slow led to later expression | ||||
| Rat myoblasts (L6) | Single cells induced to differentiate | Flexercell 4000, 20% cyclical strain at 0.5 Hz for up to 24 h | Stretch caused caspase-induced apoptosis during differentiation | Liu et al. 55 |
| Murine MDCs isolated from WT and mdx tissue | 24 h in differentiation medium (early differentiation) | Flexercell, a 10% strain for 2 s, followed by 20–60 s in the non-stretched position | Increase in real time measurement of NO production during stretch (WT). No observed increase in NO in mdx MDCs | Wozniak and Anderson 56 |
| P1–P2 rat MDCs | Confluent single cells | Flexercell, a 25% strain at 6 cycles/min, each cycle consisting of 3-s strain, 3-s rest. Continuous for 24 h | Stretch induced an increase in Na+ pump activity, increased expression of α-2 subunit of Na+-K+-ATPase in the membrane fractions. Mediated through PI3-K | Yuan et al. 57 |
| Murine myoblast (C2C12) | Single cells induced to differentiate | Flexercell, a 10% strain at 0.5 Hz for 1 h on, 5 h off-0-4 days | Increase in actin fiber formation, myotube maturation (increase in α-actinin and striations) and myotube diameter with stretch. β1D and FAK proposed mediated mechanism | Zhang et al. 58 |
| Myotubes | ||||
| Rat myoblasts (L6) | Myotubes (7–9 days in culture) | Flexercell 4000, 1 Hz at 15% strain at 2, 15, 30, 60, 90, 120, and 150 min | Sarcoplasmic protein synthesis was unaltered; however, myofibrillar protein synthesis was decreased. Paradoxically, anabolic signaling (phosphorylation of key proteins) was enhanced | Atherton et al. 59 |
| Human MDCs | Stretched during differentiation or 10-min stretch after 7-day differentiation | Flexercell 2000, cyclical strain during differentiation | Stretching during differentiation increase CK activity | Clarke and Feeback 60 |
| 10%–20% cyclical strain | Acute stretching caused membrane wounding and FGF release | |||
| Mouse diaphragm MDCs | Myotubes (5 days in DM) | Flexercell, a 4 h, 10% stretch, 1.5 Hz | No effect of stretch on proinflammatory marker gene expression | Demoule et al. 61 |
| Rat myoblasts (L6) | Myotubes (7 days in DM) | Flexercell 3000, 10% stretch, 4 h, 1.5 Hz | Mechanical stretch plus oxidative stress (induced by SIN-1) causes sarcolemmal injury to a greater extent than either component alone | Ebihara et al. 62 |
| Rat myoblasts (L6) | Early myotubes | Flexercell 3000, 96 h of cyclical strain (18% stretch, 0.16 Hz) +/− heat stress | Total protein, HSP72 and 90 protein concentrations were increased by stretch and heat in a cumulative manner | Goto et al. 63 |
| Murine myoblast (C2C12) | Myotubes (5–6 days post-induction of differentiation) | Flexercell 3000, 15% stretch, 1 Hz, 10 min, cyclical | Increased phosphorylation of p70s6k following multiaxial versus uniaxial | Hornberger et al. 64 |
| Murine myoblast (C2C12) | Myotubes (5–6 days post-induction of differentiation) | Flexercell 3000, 15% stretch, 1 Hz, 10 min, cyclical | Stretch caused phosphorylation of p70s6k | Hornberger 65 |
| Addition of locally acting growth factors to myotubes did not have the same effect | ||||
| Rat MDCs | Early myotubes (8–10 days post initial plating) | Flexercell 2000, up to 24 h cyclical stretch, 0.25 Hz, 24% strain | 24 h stretching increased Ach esterase activity | Hubatsch and Jasmin 66 |
| Rat myoblasts (L6) | Myotubes; 7–8 days post-plating and in DM | Flexercell 100C, 25% cyclical stretch at 0.5 Hz for up to 48 h | Stretch caused increased glucose uptake in myotubes, not myoblasts independent of GLUT1 and 4 receptor content | Mitsumoto and Klip 67 |
| Murine myoblast (C2C12) | Early myotubes (24-h DM) | Flexercell, a 1 Hz, 20% strain consitisting of 20 s on, 20 s off. Continuous for 24 h | Increase LDH into CM. Neutrophil cytotoxicity of muscle cell membrane mediated by MPO | Nguyen et al. 68 |
| Human MDCs | Myotubes (5 days in DM) | Flexercell 4000, 0.25 Hz at 1.5, 4.9, 9.5, and 15% strains, for 30 min, 2 s on, 2 s off | Increased injury index and neutrophil chemotaxis with increased strain. Increases in IL8 and MCP-1 in conditioned media | Peterson and Pizza 69 |
| Rat myoblasts (L6) and rat MDC-sarcoglycan (SG) deficient | Myotubes | Flexercell, a 20% strain at 6 cycles/min for 1 h. Bioflex plates also used for microscopy | Increase in creatine phosphokinase in CM following stretch. Stretch-induced damage in SG-deficient cells caused by alteration in Ca2+ dynamics | Sampaolesi et al. 70 |
| Human MDCs | Myotubes (5 days in DM) | Flexercell 4000, 0.25 Hz for 2 h at 5%, 10%, 20%, or 30% strain | Lower myotube injury index at lower strains compared to higher strains and scrape injury (based on LDH in CM). Evidence for membrane rupture and an increase in lanthanum-rimmed membrane blebs (sign of injury) at 30% strain. Increased chemotaxis index of neutrophils using CM at higher strains | Tsivitse et al. 71 |
Mef2: mouse embryonic fibroblasts 2; MHC: myosin heavy chain; TNFα: tumor necrosis factor α; IGF1: insulin-like growth factor 1; MDC: myeloid-derived suppressor cell; mTOR: mammalian target of rapamycin; BrdU: bromodeoxyuridine; HGF: hepatocyte growth factor; CM: conditioned media; ECM: extracellular matrix; NO: nitric oxide; MMP2: matrix metallopeptidase 2; 2D: two-dimensional; MRF: myogenic regulatory factor; 3D: three-dimensional; PI3-K: phosphoinositide 3-kinase; FAK: focal adhesion kinase; CK: creatine kinase; FGF: fibroblast growth factor; GLUT1 and 4: glucose transporters 1 and 4; LDH: lactate dehydrogenase; IL8: interleukin 8; MCP-1: monocyte chemoattractant protein-1; MyoG: myogenin; REDD2: regulated in development and DNA damage responses 2; MYH: myosin, heavy chain; MLP: muscle LIM protein; WT: normal C57BL/6 wild-type; SIN-1: 3-morpholinosydnonimine-N-ethylcarbamide; HSP72: heat shock proteins; LH: lower hind; BK: back; UH: upper hind; Ach: acetylcholinesterase; MPO: myeloperoxidase.
Flexercell model not specified.
Impact on cell survival, proliferation, and growth
In the vast majority of studies, it is striking that cyclic stretching of single MPCs upregulates both proliferation and expression of genes and proteins that are required for further differentiation: MYOD1, myogenin (MYOG), MEF2A, p21 (CDKN1A), and IGF1. 47 Furthermore, several genes encoding muscle structural proteins are upregulated, including myosin heavy chain (MyHC) isoforms and α-tropomyosin (TPM1), and this ultimately leads to increased myotube production. 46 Intriguingly, differences in stretch-induced satellite cell responses have been reported from muscles isolated from distinct anatomical regions that contain primarily fast-twitch or slow-twitch fibers. 50 Further evidence of stretch favorably enhancing cell growth, tissue building, and regeneration includes an increase in total protein levels in response to mechanical stretch, 63 and the whole process of stretch-induced cell growth is dependent on mammalian target of rapamycin (mTOR) activation. 72 However, an antagonist, REDD2, is also expressed in skeletal muscle and is capable of inhibiting stretch-activated mTOR activity. 49 Nitric oxide synthase (NOS) becomes upregulated 46 and bFGF expression is increased, also in a strain-dependent fashion. 60 NOS drives the synthesis and release of nitric oxide (NO) from mature muscle myotubes. 56 Together with FGF, NO has well-known mitogenic potential and initiates a “wound healing” response. It does this, at least in part, by activation of satellite cells via matrix metallopeptidase 2 (MMP2)-mediated release of hepatocyte growth factor (HGF) from ECM 51 and subsequently leads to HGF signaling.43–45,73,74 It is particularly noteworthy that single MPCs are very tolerant of a wide range of strain with values ranging from 2% to 25% eliciting similar (sometimes with a dose response) effect.
Impact on the differentiation process and differentiated cells (myotubes)
As MPCs are “preparing” to fuse into multinucleate myotubes, their sensitivity to strain appears to increase 75 although the global responses remain fairly similar—including increased proliferation rates and increased indicators of fusion (Table 2).
In stark contrast to the data seen with single cells, MPCs fused into myotubes respond in quite different ways. One of the most striking observations is that “tolerance” to varying strain rates is lost. Rates nearing in excess of 20% elicited a variety of deleterious effects such as increased creatine kinase (CK) and lactate dehydrogenase (LDH) activity,60,68,70,71 membrane wounding and cell “injury,”60,71,76 inflammatory cytokine and wound repair growth factor release,60,76 sarcolemmal disruption, 62 increases in molecular chaperonins, 63 and increased acetylcholinesterase production. 66 Overall, stretching myotubes appeared to have no real effect on parameters related to cell proliferation and differentiation; however, there is some evidence of changes to cellular metabolism such as increased glucose uptake 67 and phosphorylation of a number of anabolic targets such as ribosomal S6 kinase P70S6K59,72, focal adhesion kinase (FAK), protein kinase B (Akt), 4E binding protein 1 (4EBP1), eukaryotic elongation factor 2 (eEF2), extracellular-regulated protein kinase 1/2 (ERK1/2), eukaryotic initiation factor 2α (eIF2α), and eukaryotic initiation factor 4E (eIF4). 59 Notably, however, myofibrillar protein synthesis was actually suppressed. 59
In conclusion, there is a good deal of data that have been collected, and the system has, for skeletal muscle, some utility for single MPCs work concentrating on some aspects of cellular signaling. There are clearly wider considerations when it comes to interpretation of the myotube data and also around the biomimetic nature of the systems and the interplay with other cell types. 77 The most powerful use is for comparing the same conditions across varying cell types, as described in this review.
Effect of mechanical strain on tendon and ligament cells
Tendon and ligament are important connective tissues, essential for healthy musculoskeletal physiology. Ligaments bind skeletal components together, maintaining their correct anatomical arrangement. Tendons connect muscles to bone and facilitate the transmission of contractile force from those muscles to control movement. They are therefore able to withstand high mechanical strain during normal physiological conditions. However, chronic overuse or pathologically high magnitudes of strain result in damage, causing considerable pain and impaired mobility. Using the Flexercell system, the effect of mechanical strain on cellular constituents of tendons and ligaments can be studied, facilitating our understanding of how these tissues respond to, and propagate, mechanical signals (Table 3). This in turn may help us to understand the kind of mechanical stimulation appropriate for engineering replacement tissues.
Table 3.
Summary of Flexercell studies and key findings using ligament cells.
| Cell type | Stage of differentiation | Stretch device and regime | Key findings | Reference |
|---|---|---|---|---|
| Human PDL | Confluent single cells | Flexercell, a TENS-L (3%, 6%, 8%), TENS-H (15%), 30–120 min | Suppressed IL1β-induced COX2 expression TENS-H upregulated COX2 expression and PGE2 synthesis | Agarwal et al. 78 |
| Human PDL | Single cells | Flexercell, a 20 kPa, 12 h | Increased expression of MMP1, MMP2, TIMP1, TIMP2, α6 and β1 | Bolcato-Bellemin et al. 79 |
| Human PDL | Single cells | Flexercell, a 15% 30 cycles/min consisting of 1 s strain, 1 s rest | Decreased ALP activity | Chiba and Mitani 80 |
| Canine ACL and MCL | Single cells | Flexercell 2000, 5% (0.05 strain) strain at 6 cycles/min for 2 or 22 h daily for 3 days | Increased α5 and β1 expression | Hannafin et al. 81 |
| Increased β3 expression when cells grown on laminin | ||||
| Canine ACL | Confluent single cells | Flexercell 2000, 5%–15% strain at 6 cycles/min for 2 h followed by 22-h rest for 5 consecutive days | Increased α5 and β1 expression | Henshaw et al. 82 |
| Human PDL | Confluent single cells | Flexercell 2000, 9% strain at 6 cycles/min consisting of 5 s strain, 5 s for 6 days | Increased UNCL expression | Kim et al. 83 |
| Human PDL | Single cells | Flexercell, a 5% elongation, 3 cycles/min for 24 h on 7 days | Increased TGFβ1 levels | Kimoto et al. 84 |
| Decreased M-CSF expression | ||||
| Canine ACL and MCL | Confluent single cells | Flexercell, a 60 cycles/min with 0.05–0.075 strain for 0.5–24 h | ACL: Increased COL I | Hsieh et al. 85 |
| MCL: Increased COL III | ||||
| Rat MCL | Confluent single cells | Flexercell, a 3.5% elongation, 2 h, 1 Hz, microgrooved substrate | Increased sensitivity to load and ability to propagate a calcium wave | Jones et al. 86 |
| Human PDL | Osteoblast-like characteristics | Flexercell, a 6%–15%, 0.005 Hz, 24 h | Low-magnitude strain inhibited IL1β-induced synthesis of IL11, IL6, and IL8 and induced IL10 synthesis | Long et al. 87 |
| Human PDL | Confluent single cells | Flexercell 2000, 9%–18% strain for 6 cycles/min consisting of 5 s strain, 5 s rest, 1–5 days | Increased ALP and OCN expression | Matsuda et al. 88 |
| EGF/EGF-R system acts as a negative regulator of differentiation | ||||
| Human LFC | Confluent | Flexercell 2000, 10%–20% strain, 0.16 Hz, 24–48 h | Increased COL I, III, and V via TGFβ1 | Nakatani et al. 89 |
| Bovine PDL | Confluent | Bioflex loading stations, 0.2%–18% stretch for 6 cycles/min consisting of 5 s strain, 5 s rest, 48 h | High-magnitude strain increased COL I and decorin expression but decreased ALP | Ozaki et al. 90 |
| Human PDL | Confluent | Flexercell, a 18% stretch for 6 cycles/min consisting of 5 s strain, 5 s rest, 1–5 days | Increased activity of plasminogen activator | Ozawa et al. 91 |
| Rat PDL (6-week young rats and 60-week old rats) | Confluent | Flexercell, a 9%–18% stretch for 6 cycles/min consisting of 5 s strain, 5 s rest, 1–5 days | Increased PGE2 and IL1β production by old versus young in response to strain | Shimizu et al. 92 |
| Human PDL | Confluent | Flexercell, a 15% stretch for 30 cycles/min consisting of 1 s strain, 1 s rest, 30–90 min and 6 h | Rapid, transient increase in C-FOS expression | Yamaguchi et al. 93 |
| No change in expression of osteogenic genes | ||||
| Human PDL | Confluent | Flexercell 4000, 12% for 6 s every 90 s, 6, 12, and 24 h | Increased expression of BMP2, BMP6, ALP, SOX9, MSX1, and VEGFA | Wescott et al. 94 |
| Decreased expression of BMP4 and EGF | ||||
| Human PDL | Confluent | Flexercell, a 7%–21% stretch for 12 cycles/min consisting 2.5 s strain, 2.5 s rest, 24 h | Increased VEGF expression and secretion | Yoshino et al. 95 |
PDL: periodontal ligament; IL: interleukin; COX2: cyclooxygenase 2; PGE2: prostaglandin E2; MMP: matrix metallopeptidase; TIMP: tissue inhibitors of metalloproteinases; ALP: alkaline phosphatase; ACL: anterior cruciate ligament; MCL: medial collateral ligament; UNCL: ulnar collateral ligament; TGFβ1: transforming growth factor β1; M-CSF: macrophage-colony stimulating factor; OCN: osteocalcin; EGF: epidermal growth factor; EGF-R: epidermal growth factor receptor; BMP: bone morphogenetic protein; VEGFA: vascular endothelial growth factor A; TENS-L: tensile strain of low magnitude; TENS-H: tensile strain of high magnitude; LFC: Ligamenturn flavum cells; C-FOS: FBJ murine osteosarcoma viral oncogene; SOX9: SRY (sex determining region Y)-box 9; MSX1: Msh homeobox 1.
Flexercell model not specified.
Impact on cell survival, proliferation, and growth
One of the earliest studies using Flexercell demonstrated that applied cyclic stretch alone was not capable of increasing flexor tendon proliferation, but when applied in combination with platelet-derived growth factor (PDGF), or a cocktail containing both PDGF and IGF, synthesis of DNA was significantly enhanced. 96 This could be prevented by blocking gap junction activity with the gap junction inhibitor octanol, indicating the importance of cell–cell communication for cell proliferation. 97 Therefore, in terms of proliferation, fibroblastic cells appear not to respond as favorably to mechanical stretch signals as osteoblasts or MPCs. For tissue engineering purposes, it will be necessary to screen the appropriate combination of mechanical stretch and growth factors to create functional tendon tissue with appropriate cell number or seed the scaffold with the desired number of cells required to form mature tissue at the outset.
Matrix production
Mechanical stretch has been widely reported to upregulate type I collagen synthesis by tendon cells97,98 and ligament cells.85,89,90,99 Curiously in the case of the knee joint, type I collagen is upregulated by fibroblasts of the anterior cruciate ligament (ACL)85,99 but not those from the medial collateral ligament (MCL). 85 Conversely, type III collagen is upregulated upon application of strain in MCL cells, but not ACL.85,99 These differences in collagen expression profiles might explain differences in healing of ACL versus MCL evident in vivo, with lack of healing in ACL due to lack of type III collagen production. 85 They also deliver a resounding reminder that seemingly comparable cell populations from different topographic sites can behave rather differently to each other, as demonstrated with topographically distinct skin fibroblast populations that have distinct phenotype and gene expression profiles.100–102
In fibroblasts from ligamentum flavum, mechanical stretch induces the expression of collagen types I, III, and V via a TGFβ-dependent mechanism. 89 In static culture, estrogen increases the expression of collagen types I and III,99,103 but combined with mechanical stretch signals, actually reduces their expression, along with biglycan.99,103 The compound effect of stretch and estrogen might account for the up to eightfold increase in risk of ACL injury in female athletes.104–106
Aside from synthesis of matrix components, it is important to have a controlled balance between proteases that degrade ECM proteins and their inhibitors during matrix turnover and remodeling, and a number of different proteases are upregulated in periodontal ligament (PDL) fibroblasts upon mechanical stimulation. For example, plasminogen activator (PA) is upregulated in mechanically stretched PDL cells.91,107 PA is a wide-spectrum serine protease that activates plasminogen to produce plasmin, which in turn activates MMPs to degrade ECM components and cell adhesion molecules, 108 important events during matrix remodeling, cell mobilization, and differentiation. Furthermore, transcript levels of MMPs themselves (MMP1 and MMP2), along with their inhibitors (tissue inhibitors of metalloproteinase 1 and 2 (TIMP1 and TIMP2)) are upregulated relative to unstretched controls, 79 again indicating that mechanical stimulation enhances matrix remodeling, which is essential for maintaining structural integrity and strength in ligament tissue in vivo. It has also been reported that cell aging leads to increased PA activity, 109 which may reflect the impaired healing capacity that is typically seen in aged individuals.
Exposing tendon cells to IL1 decreased their elastic modulus, thereby increasing their survival after exposure to high-magnitude stretch in 3D collagen gels (see Table 4). 115 Coupled with this, IL1 enhanced elastin expression but suppressed type I collagen expression by tendon cells cultured within the constructs, 114 which will have a bearing on the mechanical properties of bioengineered constructs that are produced this way. Therefore, a potential means of tuning the mechanical properties of engineered tendon tissues for regenerative therapy may be achievable via regulation not just of the biomaterial substrate but also of the tendon cells themselves.
Table 4.
Summary of Flexercell studies and key findings using tendon cells.
| Cell type | Stage of differentiation | Stretch device and regime | Key findings | Reference |
|---|---|---|---|---|
| Rabbit Achilles tendon | Tenocytes | Flexercell 3000, 5% elongation, 0.33 Hz for 6 h with 18 h of rest | Synergistic effect of strain and IL1β to upregulate the production of stromelysin pro-enzyme | Archambault et al. 110 |
| Strain alone induced no effect | ||||
| Canine patellar tendon | Tenocytes | Flexercell 3000, 1%–9% strain, 0.5–3.0 Hz, 15–120 min | Transient JNK activation peaking at 30 min and returning to basal levels by 2 h | Arnoczky et al. 111 |
| Avian flexor tendon | Epitenon and tendon internal fibroblasts | Flexercell 2000, 5% elongation, 1 Hz for 8 h | Strain stimulates proliferation in the presence of PDGF-BB and IGF1 and promotes phosphorylation in multiple proteins | Banes et al. 96 |
| Avian flexor tendon | Tenocytes | Flexercell, a 0.65%, 1 Hz, 8 h/day, 16-h rest | Induced expression of numerous novel genes | Banes et al. 112 |
| Porcine posterior tibial tendon | Tenocytes | Flexercell, a 5% strain, 0.5 Hz for 24 h | Increased COL I and decorin gene expression | Chen et al. 98 |
| Human tenosynovial fibroblasts | Tenosynovial fibroblasts | Flexercell, a 20%, 1 Hz, 36 h | Increased expression of PGE2 and VEGF | Hirata et al. 113 |
| Human tendon | Tendon internal fibroblasts forming bioartificial tendons | Flexercell 4000, 30% strain for 10 s | IL1β low dose upregulated elastin expression and high dose suppressed COL I expression to increase elasticity and prevent rupture due to strain | Qi et al. 114 |
| Human tendon | Tendon internal fibroblasts forming bioartificial tendons | Flexercell 4000, 3.5% elongation, 1 Hz, for 1 h/day | IL1β reduced Young’s modulus in bioartificial tendons, increasing their tolerance to mechanical strain | Qi et al. 115 |
| Avian flexor tendons | Tenocytes | Flexercell, a 75 millistrain at 1 Hz in a regime of 8 h on, 16 h resting, for 96 h | Increased junctional components n-cadherin and vinculin, but no change in actin levels | Ralphs et al. 116 |
| Human flexor tendons | Tenocytes | Flexercell, a 3.5% strain at 1 Hz for 5–30 min and 1–2 h | Stimulation of ecto-ATP activity | Tsuzaki et al. 117 |
| Human flexor tendons | Tenocytes | Flexercell, a 3.5% strain at 1 Hz for 2-h and 18-h rest | Induced IL1β, COX2 and MMP3, but not MMP1 | Tsuzaki et al. 117 |
| ATP modulated stretch-induced gene expression | ||||
| Human flexor tendons | Tendon internal fibroblasts | Flexercell 3000, 3%–5% strain at 1 Hz for 1–6 h | Connexin 43 colocalization with actin increased with strain | Wall et al. 118 |
IL1β: interleukin 1β; PDGF-BB: platelet-derived growth factor-BB; IGF1: insulin-like growth factor 1; PGE2: prostaglandin E2; VEGF: vascular endothelial growth factor; COX2: cyclooxygenase 2; MMP: matrix metallopeptidase; JNK: c-Jun N-terminal kinase.
Flexercell model not specified.
Impact on cytokine/growth factor production
Growth factors and cytokines can have both autocrine and paracrine effects in the local microenvironment where they are produced. Therefore, the effect of stretch signals applied using Flexercell can give some indication regarding the potential influence of mechanical stretch signals in vivo and also potentially enable “priming” of cells to upregulate particular factors for therapeutic applications.
Stretching PDL cells for 24 h (5% elongation at 0.05 Hz) enhanced TGFβ production after 7 days 84 but only in PDL cells from adult teeth and not deciduous teeth. This perhaps reflects the intrinsic difference in longevity between the two as TGFβ has a prominent role in matrix formation, maturation, and scar tissue formation that is not needed by deciduous teeth that are shed. On the other hand, stretch signals led to a decrease in M-CSF levels, even after pre-stimulation with vitD3, 84 which normally stimulates M-CSF production. VEGF production by PDL cells was also upregulated after stretching,94,95 as were osteogenic factors BMP2 and BMP6. 94 VEGF production by tendon fibroblasts was similarly increased and together with the simultaneous upregulation of PGE2 expression was suggested as a mediator of inflammation and fibrosis, as typically responsible for carpal tunnel syndrome. 113 This suggests that stretch-mediated upregulation of growth factors and cytokines is not necessarily a positive event for fibroblastic cells, and risk of scare tissue formation due to excessive stretch in vivo can perhaps be modeled in vitro using Flexercell systems.
The magnitude of strain seems to be an important factor in determining cell responses. Low-magnitude tensile strain (from 2% to 8%) was found to have anti-inflammatory effects on PDL cells.78,119,120 For example, IL1-mediated transcription of IL1, IL6, and IL8 was blocked, whereas production of IL10, which in turn can block synthesis of IL1, IL6, and IL8, 121 was enhanced. 119 In this low range, strain was also found to significantly inhibit IL1-mediated activation of COX2 gene expression and PGE2 synthesis in a dose-dependent fashion, 78 reinforcing the argument that low-magnitude strain is anti-inflammatory. In contrast, high strain levels (10%–18.5% strain) induce pro-inflammatory responses such as nuclear factor-κB (NF-κB)-mediated COX2 gene expression. 78 Moreover, high strain levels (9% and 18%) significantly enhanced production of IL1 and PGE2 and the effects were more prominent in PDL cells from aged tissue versus young. 92 Cellular aging was also found to impact significantly COX2 gene expression, with aged (late passage) PDL cells producing higher COX2 mRNA levels than young cells after strain. 122
High strain levels (18% elongation applied for 12 h at 0.1 Hz) were also found to significantly enhance NO production, 123 which has many physiologic functions that include inflammation, which is likely in this case given the other reported inflammatory mediators upregulated by high-magnitude strain. In rabbit Achilles tendon fibroblasts, the application of strain in the presence of IL1 also led to a 17-fold increase in pro-MMP3 production, but no difference in COX2 expression was observed compared with IL1 treatment alone. 124 Strain-induced expression of inflammatory genes could be suppressed by the addition of exogenous ATP to the culture. 117
Overall, it seems that a growth factor/cytokine expression profile associated with inflammation is produced in response to mechanical stretch signals that is magnitude dependent. However, low-magnitude stretch is actually anti-inflammatory, and this biphasic response runs parallel with physiologic scale observations where chronic overuse results in injury and inflammation.
Cellular maturation and differentiation
Functional tendons are dependent on adequate cell–cell communication and gap junctions are crucial for this. 97 Specific gap junction proteins n-cadherin and vinculin are both significantly upregulated in tendon cells after exposure to cyclic stretch 116 along with connexin-43, which is localized to gap junctions at the ends of actin filaments. 118 Enhanced cell–cell communication is very important for stretch-mediated responses, particularly so in 3D constructs populated with tendon fibroblasts, where application of mechanical strain using a non-Flexercell method resulted in a threefold increase in tensile strength of constructs compared with non-loaded constructs after 7 days of loading. 125 In addition, supplementing cultures with the anabolic steroid, nandrolone decanoate, synergistically enhances matrix remodeling and ultimately tensile strength of bioengineered tendons. 126 Therefore, applying mechanical strain to tissue engineered constructs creates a more physiologically relevant tissue. Moreover, when considering information gained from many published Flexercell studies, translating the relevance of this information from 2D to 3D microenvironment needs to be considered.
PDL cells can acquire a bone-like phenotype, and mechanical strain is generally considered to promote and enhance this. Wescott et al. 94 subjected PDL cells to 12% cyclic strain for up to 24 h and then screened an array of 78 different genes associated with osteogenic differentiation and bone metabolism. Several genes associated with osteogenesis were upregulated, including BMP2, BMP6, ALP, SOX9, MSX1, and vascular endothelial growth factor A (VEGFA), suggesting that a program of osteogenic gene expression was invoked. However, there seems to be a minimum duration of exposure to strain, or culture period following exposure, that is required for osteogenic induction to take place as short-term exposure (6 h) was insufficient to enhance the expression of any key osteogenic genes.93,94 Over a longer culture period of 48 h, the expression levels of type I collagen and decorin were elevated after stretch of up to 18% elongation. 90 Continuous cyclic strain of 9% or 18% elongation at 0.1 Hz applied to PDL cells for up to 6 days resulted in enhanced osteoblastic differentiation characterized by increased ALP activity and expression of OCN, with paralleled decrease in mitogenic potential and epidermal growth factor (EGF) receptor expression.88,127 Differentiation was inhibited by the addition of EGF to the culture medium, which reversed the profile.
In a separate study, ALP synthesis was downregulated in PDL cells subjected to cyclic stretch of 15% elongation at 0.5 Hz and even treatment with vitamin D (an agonist of ALP production) was unable to compensate. 80 Therefore, the PDL contains a source of multipotent cells that can differentiate toward a bone-forming phenotype, and a careful balance between both biological and mechanical signals is required to maintain the niche and direct cell fate. Furthermore, at high strain levels it seems that a fine balance between osteogenic differentiation and inflammation occurs. Similar to osteoblasts, in PDL cells, cyclic strain also suppresses osteoclastogenesis as although osteoclast genes OPG and RANKL are both upregulated, 128 the former inhibits the stimulatory effects of the latter.
In summary, mechanical stretch signals applied to tendon and ligament cells using Flexercell have been reported to be largely positive, with matrix synthesis and remodeling being upregulated when using moderate strain levels. Moderate strain levels also have documented anti-inflammatory properties and are able to abrogate the effects of cytokines on these cell types. Conversely, high strain levels induce inflammatory pathways and this likely reflects the potential tissue damage caused by high strains in vivo.
Effect of mechanical strain on cartilage cells
Hyaline cartilage has a crucial protective role in the bone–bone contacts, such as knee, where it protects the ends of opposing bone structures and dissipates loads evenly across the full surface area of the cartilage. Consequently, in addition to the load bearing that is typically associated with cartilage function, the constituent chondrocytes also experience tensile and shear stresses.129–131 A summary of studies assessing the impact of mechanical stretch on chondrocytes using Flexercell is presented in Table 5.
Table 5.
Summary of Flexercell studies and key findings using chondrocytes.
| Cell type | Stage of differentiation | Stretch device and regime | Key findings | Reference |
|---|---|---|---|---|
| Rabbit articular cartilage | Chondrocytes | Flexercell, a 2%–18%, 0.05 Hz, in the presence or absence of IL1β | Low stretch: inhibitor of IL1β-dependent NF-κB nuclear translocation | Agarwal et al. 132 |
| High stretch: involvement of NF-κB nuclear translocation and synthesis | ||||
| Rat articular cartilage | Chondrocytes | Flexercell 2000, 7% elongation, 0.5 Hz, cyclic stretch (1 s on, 1 s off) | Upregulated expression of MMP13 and cathepsin B | Doi et al. 133 |
| No effect on the expression of aggrecan and COL II | ||||
| Rat articular cartilage | Chondrocytes | Flexercell 4000, 3%, 0.25 Hz, in the presence or absence of IL1β | Inhibitor of IL1β-dependent NF-κB nuclear translocation and cytoplasmic degradation of IκBβ and IκBα | Dossumbekova et al. 134 |
| Human chondrosarcoma (HCS-2/8) | Chondrocytes | Flexercell, a high frequency: 30 cycles/min | High magnitude and frequency gene expression of IL1 and MMP9 | Fujisawa et al. 135 |
| Mid frequency: 1 cycle/2 min | Continuous stress induces the production of IL1 and MMP9 | |||
| Low frequency: 1 cycle/4 min | ||||
| Bovine articular cartilage | Chondrocytes | Flexercell, a higher stress at 10 cycles/min, 17% | Increased proteoglycan synthesis at low frequency and magnitude | Fukuda et al. 136 |
| Lower stress at 10 cycle/h, 5% | Decreased proteoglycan synthesis at high frequency and magnitude | |||
| Rabbit articular cartilage | Chondrocytes | Flexercell, a 0.05 Hz, 20% elongation, in the presence or absence of IL1β | Anti-inflammatory effect by inhibiting iNOS and therefore NO in IL1β-activated chondrocytes | Gassner et al. 137 |
| Rabbit articular cartilage | Chondrocytes (retain their differentiated phenotype) | Flexercell, a 3 cycles/min consisting of 10 s strain, 10 s rest | Reverses IL1β-induced suppression of proteoglycan synthesis | Gassner et al. 138 |
| Bovine articular cartilage and human chondrosarcoma (105KC) | Chondrocytes | Flexercell, a 24% maximal (average 10%), cyclic (2 s on, 2 s off), 1–20 h | Primary cells: increased COL II expression with no change in β1 integrin | Holmvall et al. 139 |
| 105KC: increased α5 expression with no change in β1, α2 or αν | ||||
| Porcine articular cartilage | Chondrocytes | Flexercell 4000, 10% strain, 0.5 Hz, 1–24 h | Increased NO, PGE2 and COX2 expression | Huang et al. 140 |
| Anabolic response: Increased COL II and aggrecan expression as an early response | ||||
| Catabolic response: Increased TGFβ and MMP1 expression at 24 h | ||||
| Human articular cartilage | Chondrocytes | Flexercell, a −20 kPa, 0.5 Hz, 24 h | Increased proliferation | Lahiji et al. 141 |
| Enhanced expression of COL II and aggrecan | ||||
| Enhanced integrin α2 but no change in α5 or β1 | ||||
| Rabbit articular cartilage | Chondrocytes (retaining their differentiated phenotype) | Flexercell, a 6%, 0.05 Hz | Abrogated TNFα-induced inhibition of proteoglycan synthesis | Long et al. 87 |
| Rat articular cartilage | Chondrocytes (retaining their differentiated phenotype) | Flexercell 4000, 3%, 0.25 Hz, 4–24 h | Blocking of IL1β-dependent pro-inflammatory gene expression (iNOS, COX2, MMP9 and MMP13) | Madhavan et al. 142 |
| Bovine articular cartilage | Chondrocytes | Flexercell 3000, 7%, the frequency (10 cycles/min, 3 s) | Enhanced NO synthesis that inhibited PG synthesis | Matsukawa et al. 143 |
| Rat articular cartilage | Chondrocytes | Flexercell, a elongation 7%, 30 cycles/min, 12–24 h | Protective effect of IL4 on matrix synthesis | Shimizu et al. 144 |
| Rat articular cartilage | Chondrocytes | Flexercell, a 5, 17% | Decreased proteoglycan synthesis and PKC activity | Tanaka et al. 145 |
| Bovine articular cartilage | Chondrocytes | Flexercell 3000, high (10 cycles/min), low (10 cycles/h) | Caused depolymerization of HA and induced ROS generation | Yamazaki et al. 146 |
| Rabbit fibrochondrocytes from TMJ | Chondrocytes (retain their differentiated phenotype) | Flexercell, a 6% stretch, 3 cycles/min, 0.05 Hz | Stretch suppresses IL1β-dependent induction of COX2 and PGE2 synthesis | Agarwal et al. 147 |
| Human chondrosarcoma (CS-OKB) | Confluent | Flexercell, a 25%, 0.05 Hz, 48 h | Induces the expression of a heat shock protein termed stress-induced chondrocytic (SIC) 1 | Chano et al. 148 |
| Rat fibrochondrocytes from TMJ | Chondrocytes (retain their differentiated phenotype) | Bioflex loading stations, 20%, 0.05 Hz, 1–20 h | IL1β-induced expression of several MMPs inhibited | Deschner et al. 149 |
| Rat fibrochondrocytes from TMJ | Chondrocytes (retain their differentiated phenotype) | Flexercell 4000, 20%, 0.05 Hz, 1–24 h | Inhibits IL1β-induced expression of TNFα, TNFR2 and iNOS, but not TNFR1 | Deschner et al. 150 |
IL1β: interleukin 1β; NF-κB: nuclear factor-κB; MMP: matrix metallopeptidase; iNOS: inducible nitric oxide synthase; NO: nitric oxide; TNFα: tumor necrosis factor α; COX2: cyclooxygenase 2; PG: proteoglycan; PKC: protein kinase C; ROS: reactive oxygen species; TMJ: temporomandibular joint; PGE2: prostaglandin E2; TNFR: tumor necrosis factor receptor; IκB: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor; HA: Hyaluronan.
Flexercell model not specified.
Impact on cell survival, proliferation, and growth
Chondrocyte proliferation is upregulated in response to strain. 151 It is not just magnitude of strain that can impact cell responses, but also frequency. This was demonstrated when mechanical stretch of 3% elongation was applied to chondrocytes at 2, 30, or 150 cycles/min (corresponding to 0.03, 0.5, or 2.5 Hz), with the higher frequency increasing DNA synthesis. 152 From this observation we can conclude that a delicate interplay between several factors including magnitude and frequency of applied stretch can influence cell behavior.
Matrix production
Multiple studies using hyaline cartilage have concluded that application of mechanical stretch increases production of the major collagen component of cartilage, type II collagen,139,140,152,153 and the integrin subunit α2,139,153 which binds to collagen. Similarly, the proteoglycan aggrecan is also upregulated140,143,152,153 as are several important cartilage glycosaminoglycans (GAGs) including chondroitin-6-sulfate, hyaluronic acid, and dermatan sulfate. 154
However, not all studies agree as stretch was found to downregulate transcript levels of aggrecan and type II collagen, but this could be reversed by the addition of IL4. 144 Hyaluronic acid was also found to undergo depolymerization in the presence of strain due to generation of reactive oxygen species. 146 These conflicting observations can perhaps be explained by the recurring theme of magnitude-dependent effects. In the case of matrix production, particularly proteoglycans that along with their component GAGs are important for producing a highly hydrated matrix, the effects of stretch are magnitude dependent. Low-magnitude strain enhances proteoglycan production and matrix synthesis, whereas high-magnitude strain diminishes it resulting in ECM degradation and onset of inflammation.132,133,135,136,155 The process is dependent on protein kinase C activity. 136 Moreover, application of high-magnitude cyclic strain to cultured chondrocytes was found to upregulate the expression of MMPs that degrade cartilage ECM.133,135,155 Cyclic strain is able to prevent suppression of proteoglycan synthesis caused by IL1138,142 and tumor necrosis factor α (TNFα) 87 but again may be magnitude dependent.
Impact on cytokine/growth factor production
In terms of inflammation, the same biphasic response as seen for tendon/ligament cells can be observed. Exposure of chondrocytes to low magnitudes of strain (<8% elongation) is reported to invoke anti-inflammatory effects, whereas high strain levels (up to 18% elongation) augment the inflammatory response. 132 This indicates some conservation in responses to different cell populations from throughout the musculoskeletal system with respect to mechanical signal responsiveness. In particular, low-level strain has generally been observed to block the expression of inflammation-related genes that augment cartilage destruction.87,132,134,137,142,147,149,150 For example, using high frequency and low strain (3% elongation at 25 MHz), IL1 pro-inflammatory activity was inhibited, due to block of IL1-mediated NF-κB nuclear translocation, 134 in a transforming growth factor-β-activated kinase-1 (TAK1)-dependent fashion. 156 This prevented subsequent transcription of inflammatory gene targets, which normally result from NF-κB activation. Similarly, TNFα-mediated inflammatory responses were blocked by strain in vitro. 87
There are some anomalies to this general trend however, with high-magnitude strain (20% elongation) applied at low frequency (0.05 Hz) reported to produce anti-inflammatory effects by downregulating IL1-dependent inducible NOS production. 137 Additionally, cyclic strain of 7% elongation at 0.5 Hz was found to induce IL1β expression and that of MMP13 and cathepsin B, 133 both proteolytic enzymes associated with cartilage pathogenesis.
Interestingly, one study reported that in the presence of a single strain regime of 10% elongation at 0.5 Hz, both catabolic/pro-inflammatory and anabolic responses were observed. 140 While production of NO, PGE2, and COX2 (characteristic of a pro-inflammatory response) were increased, so too were expression levels of type II collagen and the cartilage-specific proteoglycan aggrecan, as mentioned above. This early response was then replaced by elevations of expression of TGFβ1 and TGFβ3, along with MMP1, indicating that matrix remodeling ensues. Collectively, the above data echoes at the cellular level the patient response where gentle exercise and joint mobility are reported to be beneficial for inflamed joints.157,158
In summary, Flexercell studies indicate that low to moderate levels of strain generally promote cartilage anabolic responses, terms of chondrocyte proliferation, GAG, and proteoglycan production, while also conferring anti-inflammatory effects. However, high levels of strain have a tendency to drive inflammatory responses and provide a good deal of correlation to the trauma and injury associated with high strain and overuse in patients.
The effect of strain applied using Flexercell on mechanotransduction mediators
Most of the bone-stimulating effects of mechanical strain occur due to the activation of mechanotransduction pathways, and the major cell surface receptors involved in these pathways are the integrins. In human osteosarcoma cells, a strain regime of 20 kPa at 0.05 Hz resulted in the upregulation of β1 integrin mRNA expression11,28 along with redistribution of β1 integrin subunits from the cytoplasm to the cell membrane. 11 However, αv integrin expression and distribution were unaffected by mechanical strain, 11 indicating that functional activation and continued recruitment of integrins in response to strain are specific to certain subunits of integrin. Interestingly, vitronectin and OPN, both ligands for αv-containing αvβ3, induce greater calcium influx into osteoblasts than other matrix molecules, 159 and the lack of change in αv integrin subunit expression and distribution upon mechanical loading using Flexercell suggests that the cell response to this form of mechanical strain is not calcium-dependent. It has also been reported that both α2β1 and α5β1 can transmit the effect of stretching from the extracellular environment to activate ERK downstream. 24 However, it seems that the α-subunits are the most potent mediators, not β1, and their signal transduction capacity is actin dependent.24,160 Disruption of the actin cytoskeleton, for example, by treatment with cytochalasin D, an actin-depolymerizing agent, attenuates stress response pathways in osteoblast cells.161–163 This indicates the importance of the cytoskeleton in sustaining mechanotransduction from extracellular input signals.
Mechanotransduction downstream of integrins involves the coordinated regulation of several other key molecules. Src kinase interacts with integrin intracellular domains 24 and FAK 10 at the focal adhesion site when mechanical strain is applied. In osteoblasts, this universally leads to stretch-induced activation of mitogen-activated protein kinase (MAPK) pathways and in particular ERK, a key MAPK effector molecule that activates mechanoresponsive transcription factors.7,8,10,13,15,18,24,26,164 At the focal adhesion site formed upon mechanical activation of integrins, FAK also undergoes enhanced and sustained association with another tyrosine kinase, proline-rich tyrosine kinase 2 (PYK2),10,16 which seems to stablize FAK and remove the inhibitory Hic5 adaptor protein from the focal adhesion complex upon activation. 16 Collectively, these data confirm that integrins and their associated intracellular partners are necessary for signal transduction downstream of the strain stimulus to be executed.
Canonical wnt signaling in Flexercell-stretched osteoblasts is highly activated, with enhanced β-catenin transcriptional activity reported.17,25 This leads to upregulation of numerous wnt target genes such as those encoding cell cycle proteins, the gap junction protein connexin-43, and other transcription factors. 25 Phosphorylation of AKT can also promote β-catenin transcriptional activity via glycogen synthase kinase 3 (GSKβ) inactivation and subsequent translocation of β-catenin to the nucleus, where the wnt target genes Wisp1 and Cox2 are then transcribed. 165 Upregulation of the wnt target gene encoding connexin-43 seems to have a role in enhancing cell–cell communication among mechanically stimulated osteoblasts by enhancing gap junction communication due to increased levels of phosphorylated connexin-43 protein,166,167 and in tendon cells its colocalization with actin presumably facilitates cell communication in the direction of force application.
The role of integrins in mechanotransduction is explicit as they anchor cells to the ECM substrate in which they reside.168,169 Less obvious candidates for mechanotransduction are cell surface receptors that do not have an active role in physical anchorage of cells to ECM. It is well known that androgens, in combination with mechanical cues, can control bone metabolism and invoke a shift from high turnover to osteoanabolic mode. Typically, mechanical signaling via the estrogen receptors (ERs) enhances osteoblast proliferation. 170 ERs have a critical role in mechanotransduction and ERK activation, as osteoblasts from ERαβ−/− mice lacking both α and β forms of ER were unable to activate ERK when stimulated with 5% elongation at 0.05 Hz. 8 This phenotype could be partially rescued by transfecting ERαβ−/− osteoblasts with cDNA for either receptor or fully rescued by transfection with cDNA for both receptors. Additionally, the active estrogen compound estradiol acts in synergy with mechanical strain to promote ER-dependent ALP activity. 171 U2OS osteosarcoma cells that do not express ERs and have intrinsically low ALP activity were compared with transfected U2OS cells expressing either ERα or ERβ for COL1 expression and ALP activity after being subjected to 1.5% cyclic strain at 0.05 Hz. Mechanical strain increased ALP activity and decreased COL1 expression in ERα and ERβ mutants, with estrogen having a synergistic effect on ALP activity. However, estrogen itself is dispensable to the process of ER-mediated mechanotransduction as transfecting ERαβ−/− osteoblasts with mutant ERs incapable of binding to estrogen did not impair ERK activation, demonstrating that the process was not dependent on estrogen. Even so, it cannot be discounted that differences in the cell lines or the strain regimes applied to cells may have a bearing on the outcome.
Integrins that mediate cell adhesion initiate mechanotransduction signals and have shown significant impact on ligament cells upon stretch stimulation. In PDL fibroblasts, assessment of integrin gene expression showed that both α6 and β1 were upregulated by stretch. 79 Cells from the ACL or MCL plated onto type I collagen, fibronectin, or laminin express elevated levels of both α5 and β1 when stretched at 5% elongation for 2 h, 81 and in avian tendon cells, src kinase phosphorylation is also increased after mechanical stimulation. 96 In 3D collagen gels loaded with ACL fibroblasts, strain enhances both α5 and β1, but not in α1 expression. 82 However, the expression of the α5 integrin subunit in PDL cell monolayers subjected to stretch was actually found to be downregulated upon application of 20 kPa negative pressure to each well for 12 h. 79
As well as integrin-mediated mechanotransduction, calcium signaling has an important role in cell responsiveness to mechanical forces. Calcium signaling between MCL cells is enhanced by mechanical stretch, as determined by their ability to maintain a calcium wave between cells both aligned longitudinally in microgrooves and in non-aligned cells. 86 In the case of tendon cells, activation of JNK, a “stress-activated protein kinase,” occurs in response to mechanical strain, and the level of JNK activation is dependent on magnitude of strain applied. 111 It also seems to take place downstream of calcium signaling as increased intracellular calcium levels also enhance JNK activation independent of strain. 111 Chronic, sustained activation of JNK in tendon cells could result in cell death and tendon injury.
There was also prominent upregulation of ulnar collateral ligament (UNCL) protein in PDL cells after applied strain. 83 UNCL has a central role in mechanotransduction pathways based on evidence that loss-of-function mutations to the homolog in Drosophila result in failure of mechanotransduction 172 and so might have important function in ligament physiology.
Summary
Cell behavior is regulated by both biochemical and physical signals. The importance of biophysical cues in determining cell phenotype is becoming more widely understood, as are mechanotransduction pathways that are activated upon mechanical stimulation. Application of mechanical strain, most commonly applied using the Flexercell system, can provide physical stimulation to cells in the bone and surrounding tissues and aid our understanding of mechanical strain impact on health and injury. It may also potentially be used as a means of priming cell components of the tissues prior to seeding them into scaffolds for tissue engineering and in vivo transplantation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Priority Research Centers Program (Grant No. 2009-0093829), the Global Research Laboratory Program (Grant No. 2015032163) and KRIBB Research Initiative Program, Republic of Korea.
References
- 1. Brindley D, Moorthy K, Lee JH, et al. Bioprocess forces and their impact on cell behavior: implications for bone regeneration therapy. J Tissue Eng 2011; 2011: 620247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone 2011; 48(2): 171–181. [DOI] [PubMed] [Google Scholar]
- 3. Banes AJ, Gilbert J, Taylor D, et al. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci 1985; 75: 35–42. [DOI] [PubMed] [Google Scholar]
- 4. Burger EH, Klein-Nulend J. Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J 1999; 13(Suppl.): S101–S112. [PubMed] [Google Scholar]
- 5. Liegibel UM, Sommer U, Tomakidi P, et al. Concerted action of androgens and mechanical strain shifts bone metabolism from high turnover into an osteoanabolic mode. J Exp Med 2002; 196(10): 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang L, Lin Z, Li YM. Effects of different magnitudes of mechanical strain on Osteoblasts in vitro. Biochem Biophys Res Commun 2006; 344(1): 122–128. [DOI] [PubMed] [Google Scholar]
- 7. Zhu J, Zhang X, Wang C, et al. Different magnitudes of tensile strain induce human osteoblasts differentiation associated with the activation of ERK1/2 phosphorylation. Int J Mol Sci 2008; 9(12): 2322–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aguirre JI, Plotkin LI, Gortazar AR, et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem 2007; 282(35): 25501–25508. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt KA, Chang EI, Warren SM, et al. Uniaxial mechanical strain: an in vitro correlate to distraction osteogenesis. J Surg Res 2007; 143(2): 329–336. [DOI] [PubMed] [Google Scholar]
- 10. Boutahar N, Guignandon A, Vico L, et al. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase (FAK) and proline-rich tyrosine kinase 2 (PYK2) tyrosine sites involved in ERK activation. J Biol Chem 2004; 16: 30588–30599. [DOI] [PubMed] [Google Scholar]
- 11. Carvalho R, Bumann A, Schwarzer C, et al. A molecular mechanism of integrin regulation from bone cells stimulated by orthodontic forces. Eur J Orthod 1996; 18(1): 227–235. [DOI] [PubMed] [Google Scholar]
- 12. Cillo JE, Jr, Gassner R, Koepsel RR, et al. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: implications for distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 90(2): 147–154. [DOI] [PubMed] [Google Scholar]
- 13. Fan X, Rahnert JA, Murphy TC, et al. Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol 2006; 207(2): 454–460. [DOI] [PubMed] [Google Scholar]
- 14. Faure C, Linossier M-T, Malaval L, et al. Mechanical signals modulated vascular endothelial growth factor-A (VEGF-A) alternative splicing in osteoblastic cells through actin polymerisation. Bone 2008; 42(6): 1092–1101. [DOI] [PubMed] [Google Scholar]
- 15. Granet C, Boutahar N, Vico L, et al. MAPK and SRC-kinases control EGR-1 and NF-κB inductions by changes in mechanical environment in osteoblasts. Biochem Biophys Res Commun 2001; 284(3): 622–631. [DOI] [PubMed] [Google Scholar]
- 16. Guignandon A, Boutahar N, Rattner A, et al. Cyclic strain promotes shuttling of PYK2/Hic-5 complex from focal contacts in osteoblast-like cells. Biochem Biophys Res Commun 2006; 343(2): 407–414. [DOI] [PubMed] [Google Scholar]
- 17. Hens JR, Wilson KM, Dann P, et al. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res 2005; 20(7): 1103–1113. [DOI] [PubMed] [Google Scholar]
- 18. Jansen J, Weyts F, Westbroek I, et al. Stretch-induced phosphorylation of ERK1/2 depends on differentiation stage of osteoblasts. J Cell Biochem 2004; 93(3): 542–551. [DOI] [PubMed] [Google Scholar]
- 19. Kim DW, Lee HJ, Karmin JA, et al. Mechanical loading differentially regulates membrane-bound and soluble RANKL availability in MC3T3-E1 cells. Ann N Y Acad Sci 2006; 1068(1): 568–572. [DOI] [PubMed] [Google Scholar]
- 20. Knoll BI, McCarthy TL, Centrella M, et al. Strain-dependent control of transforming growth factor-β function in osteoblasts in an in vitro model: biochemical events associated with distraction osteogenesis. Plast Reconstr Surg 2005; 116(1): 224–233. [DOI] [PubMed] [Google Scholar]
- 21. Liu X, Zhang X, Luo Z-P. Strain-related collagen gene expression in human osteoblast-like cells. Cell Tissue Res 2005; 322(2): 331–334. [DOI] [PubMed] [Google Scholar]
- 22. Motokawa M, Kaku M, Tohma Y, et al. Effects of cyclic tensile forces on the expression of vascular endothelial growth factor (VEGF) and macrophage-colony-stimulating factor (M-CSF) in murine osteoblastic MC3T3-E1 cells. J Dent Res 2005; 84(5): 422–427. [DOI] [PubMed] [Google Scholar]
- 23. Narutomi M, Nishiura T, Sakai T, et al. Cyclic mechanical strain induces interleukin-6 expression via prostaglandin E2 production by cyclooxygenase-2 in MC3T3-E1 osteoblast-like cells. J Oral Biosci 2007; 49(1): 65–73. [Google Scholar]
- 24. Plotkin LI, Mathov I, Aguirre JI, et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol 2005; 289(3): C633–C643. [DOI] [PubMed] [Google Scholar]
- 25. Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 2006; 281(42): 31720–31728. [DOI] [PubMed] [Google Scholar]
- 26. Toyoshita Y, Iida S, Koshino H, et al. CYP24 promoter activity is affected by mechanical stress and mitogen-activated protein kinase in MG63 osteoblast-like cells. 日本補綴歯科学会雑誌 [Nihon Hotetsu Shika Gakkai Zasshi] 2008; 52(2): 171–174. [DOI] [PubMed] [Google Scholar]
- 27. Visconti L, Yen E, Johnson R. Effect of strain on bone nodule formation by rat osteogenic cells in vitro. Arch Oral Biol 2004; 49(6): 485–492. [DOI] [PubMed] [Google Scholar]
- 28. Carvalho RS, Scott JE, Yen EH. The effects of mechanical stimulation on the distribution of beta 1 integrin and expression of beta 1-integrin mRNA in TE-85 human osteosarcoma cells. Arch Oral Biol 1995; 40(3): 257–264. [DOI] [PubMed] [Google Scholar]
- 29. Mikuni-Takagaki Y, Suzuki Y, Kawase T, et al. Distinct responses of different populations of bone cells to mechanical stress. Endocrinology 1996; 137(5): 2028–2035. [DOI] [PubMed] [Google Scholar]
- 30. Centrella M, Ji C, McCarthy TL. Control of TGF-beta receptor expression in bone. Front Biosci 1998; 3: d113–d124. [DOI] [PubMed] [Google Scholar]
- 31. Ji C, Chen Y, McCarthy TL, et al. Cloning the promoter for transforming growth factor-beta type III receptor. Basal and conditional expression in fetal rat osteoblasts. J Biol Chem 1999; 274(43): 30487–30494. [DOI] [PubMed] [Google Scholar]
- 32. Centrella M, Casinghino S, Kim J, et al. Independent changes in type I and type II receptors for transforming growth factor beta induced by bone morphogenetic protein 2 parallel expression of the osteoblast phenotype. Mol Cell Biol 1995; 15(6): 3273–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. J Bone Miner Res 1993; 8(Suppl. 2): S565–S572. [DOI] [PubMed] [Google Scholar]
- 34. Neidlinger-Wilke C, Stalla I, Claes L, et al. Human osteoblasts from younger normal and osteoporotic donors show differences in proliferation and TGF beta-release in response to cyclic strain. J Biomech 1995; 28(12): 1411–1418. [DOI] [PubMed] [Google Scholar]
- 35. Kawata A, Mikuni-Takagaki Y. Mechanotransduction in stretched osteocytes–temporal expression of immediate early and other genes. Biochem Biophys Res Commun 1998; 246(2): 404–408. [DOI] [PubMed] [Google Scholar]
- 36. Lean JM, Mackay AG, Chow JW, et al. Osteocytic expression of mRNA for c-fos and IGF-I: an immediate early gene response to an osteogenic stimulus. Am J Physiol 1996; 270(6 Pt 1): E937–E945. [DOI] [PubMed] [Google Scholar]
- 37. Skerry TM, Bitensky L, Chayen J, et al. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res 1989; 4(5): 783–788. [DOI] [PubMed] [Google Scholar]
- 38. Siddhivarn C, Banes A, Champagne C, et al. Mechanical loading and delta12prostaglandin J2 induce bone morphogenetic protein-2, peroxisome proliferator-activated receptor gamma-1, and bone nodule formation in an osteoblastic cell line. J Periodontal Res 2007; 42(5): 383–392. [DOI] [PubMed] [Google Scholar]
- 39. Kwan Tat S, Padrines M, Theoleyre S, et al. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev 2004; 15(1): 49–60. [DOI] [PubMed] [Google Scholar]
- 40. Suzuki N, Yoshimura Y, Deyama Y, et al. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int J Mol Med 2008; 21(3): 291–296. [PubMed] [Google Scholar]
- 41. Siddhivarn C, Banes A, Champagne C, et al. Prostaglandin D2 pathway and peroxisome proliferator-activated receptor gamma-1 expression are induced by mechanical loading in an osteoblastic cell line. J Periodontal Res 2006; 41(2): 92–100. [DOI] [PubMed] [Google Scholar]
- 42. Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med 2001; 79(5–6): 243–253. [DOI] [PubMed] [Google Scholar]
- 43. Tatsumi R, Sheehan SM, Iwasaki H, et al. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res 2001; 267(1): 107–114. [DOI] [PubMed] [Google Scholar]
- 44. Tatsumi R, Hattori A, Ikeuchi Y, et al. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell 2002; 13(8): 2909–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tatsumi R, Hattori A, Allen RE, et al. Mechanical stretch-induced activation of skeletal muscle satellite cells is dependent on nitric oxide production in vitro. Anim Sci J 2002; 73(3): 235–239. [Google Scholar]
- 46. Chandran R, Knobloch TJ, Anghelina M, et al. Biomechanical signals upregulate myogenic gene induction in the presence or absence of inflammation. Am J Physiol Cell Physiol 2007; 293(1): C267–C276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iwanuma O, Abe S, Hiroki E, et al. Effects of mechanical stretching on caspase and IGF-1 expression during the proliferation process of myoblasts. Zoolog Sci 2008; 25(3): 242–247. [DOI] [PubMed] [Google Scholar]
- 48. Bruce SJ, Gardiner BB, Burke LJ, et al. Dynamic transcription programs during ES cell differentiation towards mesoderm in serum versus serum-freeBMP4 culture. BMC Genomics 2007; 8(1): 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyazaki M, Esser KA. REDD2 is enriched in skeletal muscle and inhibits mTOR signaling in response to leucine and stretch. Am J Physiol Cell Physiol 2009; 296(3): C583–C592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tatsumi R, Mitsuhashi K, Ashida K, et al. Comparative analysis of mechanical stretch-induced activation activity of back and leg muscle satellite cells in vitro. Anim Sci J 2004; 75(4): 345–351. [Google Scholar]
- 51. Yamada M, Sankoda Y, Tatsumi R, et al. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol 2008; 40(10): 2183–2191. [DOI] [PubMed] [Google Scholar]
- 52. Boonen KJ, Langelaan ML, Polak RB, et al. Effects of a combined mechanical stimulation protocol: value for skeletal muscle tissue engineering. J Biomech 2010; 43(8): 1514–1521. [DOI] [PubMed] [Google Scholar]
- 53. Kumar A, Murphy R, Robinson P, et al. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-κB transcription factor. FASEB J 2004; 18(13): 1524–1535. [DOI] [PubMed] [Google Scholar]
- 54. Kurokawa K, Abe S, Sakiyama K, et al. Effects of stretching stimulation with different rates on the expression of MyHC mRNA in mouse cultured myoblasts. Biomed Res 2007; 28(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 55. Liu J, Liu J, Mao J, et al. Caspase-3-mediated cyclic stretch-induced myoblast apoptosis via a Fas/FasL-independent signaling pathway during myogenesis. J Cell Biochem 2009; 107(4): 834–844. [DOI] [PubMed] [Google Scholar]
- 56. Wozniak A, Anderson J. The dynamics of the nitric oxide release-transient from stretched muscle cells. Int J Biochem Cell Biol 2009; 41(3): 625–631. [DOI] [PubMed] [Google Scholar]
- 57. Yuan X, Luo S, Lin Z, et al. Cyclic stretch translocates the α2-subunit of the Na pump to plasma membrane in skeletal muscle cells in vitro. Biochem Biophys Res Commun 2006; 348(2): 750–757. [DOI] [PubMed] [Google Scholar]
- 58. Zhang SJ, Truskey GA, Kraus WE. Effect of cyclic stretch on β1D-integrin expression and activation of FAK and RhoA. Am J Physiol Cell Physiol 2007; 292(6): C2057–C2069. [DOI] [PubMed] [Google Scholar]
- 59. Atherton P, Szewczyk N, Selby A, et al. Cyclic stretch reduces myofibrillar protein synthesis despite increases in FAK and anabolic signalling in L6 cells. J Physiol 2009; 587(14): 3719–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clarke M, Feeback DL. Mechanical load induces sarcoplasmic wounding and FGF release in differentiated human skeletal muscle cultures. FASEB J 1996; 10(4): 502–509. [DOI] [PubMed] [Google Scholar]
- 61. Demoule A, Decailliot F, Jonson B, et al. Relationship between pressure-volume curve and markers for collagen turn-over in early acute respiratory distress syndrome. Intensive Care Med 2006; 32(3): 413–420. [DOI] [PubMed] [Google Scholar]
- 62. Ebihara S, Hussain SN, Danialou G, et al. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med 2002; 165(2): 221–228. [DOI] [PubMed] [Google Scholar]
- 63. Goto K, Okuyama R, Sugiyama H, et al. Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflugers Arch 2003; 447(2): 247–253. [DOI] [PubMed] [Google Scholar]
- 64. Hornberger TA, Armstrong DD, Koh TJ, et al. Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: implications for mechanotransduction. Am J Physiol Cell Physiol 2005; 288(1): C185–C194. [DOI] [PubMed] [Google Scholar]
- 65. Hornberger TA. Regulation of skeletal muscle mass through stretch-induced signaling events. Chicago, IL: University of Illinois at Chicago, 2004. [Google Scholar]
- 66. Hubatsch DA, Jasmin BJ. Mechanical stimulation increases expression of acetylcholinesterase in cultured myotubes. Am J Physiol Cell Physiol 1997; 273(6): C2002–C2009. [DOI] [PubMed] [Google Scholar]
- 67. Mitsumoto Y, Klip A. Development regulation of the subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J Biol Chem 1992; 267(7): 4957–4962. [PubMed] [Google Scholar]
- 68. Nguyen HX, Lusis AJ, Tidball JG. Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo. J Physiol 2005; 565(2): 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peterson JM, Pizza FX. Cytokines derived from cultured skeletal muscle cells after mechanical strain promote neutrophil chemotaxis in vitro. J Appl Physiol 2009; 106(1): 130–137. [DOI] [PubMed] [Google Scholar]
- 70. Sampaolesi M, Yoshida T, Iwata Y, et al. Stretch-induced cell damage in sarcoglycan-deficient myotubes. Pflugers Arch 2001; 442(2): 161–170. [DOI] [PubMed] [Google Scholar]
- 71. Tsivitse SK, Mylona E, Peterson JM, et al. Mechanical loading and injury induce human myotubes to release neutrophil chemoattractants. Am J Physiol Cell Physiol 2005; 288(3): C721–C729. [DOI] [PubMed] [Google Scholar]
- 72. Hornberger TA, Stuppard R, Conley KE, et al. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 2004; 380(Pt 3): 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wozniak AC, Pilipowicz O, Yablonka-Reuveni Z, et al. C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem 2003; 51(11): 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wozniak AC, Anderson JE. Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn 2007; 236(1): 240–250. [DOI] [PubMed] [Google Scholar]
- 75. Kurokawa K, Itoh T, Kuwahara T, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res 2007; 14(4): 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fraser C, Galeotti C, Grandi G, et al. Neisseria meningitidis antigens and compositions. Patent, 2009, http://www.google.co.in/patents/EP2261345A3?cl=ko
- 77. Dugan JM, Cartmell SH, Gough JE. Uniaxial cyclic strain of human adipose-derived mesenchymal stem cells and C2C12 myoblasts in coculture. J Tissue Eng 2014; 5: 2041731414530138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Agarwal S, Long P, Seyedain A, et al. A central role for the nuclear factor-κB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J 2003; 17(8): 899–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bolcato-Bellemin A, Elkaim R, Abehsera A, et al. Expression of mRNAs encoding for α and β integrin subunits, MMPs, and TIMPs in stretched human periodontal ligament and gingival fibroblasts. J Dent Res 2000; 79(9): 1712–1716. [DOI] [PubMed] [Google Scholar]
- 80. Chiba M, Mitani H. Cytoskeletal changes and the system of regulation of alkaline phosphatase activity in human periodontal ligament cells induced by mechanical stress. Cell Biochem Funct 2004; 22(4): 249–256. [DOI] [PubMed] [Google Scholar]
- 81. Hannafin JA, Attia EA, Henshaw R, et al. Effect of cyclic strain and plating matrix on cell proliferation and integrin expression by ligament fibroblasts. J Orthop Res 2006; 24(2): 149–158. [DOI] [PubMed] [Google Scholar]
- 82. Henshaw DR, Attia E, Bhargava M, et al. Canine ACL fibroblast integrin expression and cell alignment in response to cyclic tensile strain in three-dimensional collagen gels. J Orthop Res 2006; 24(3): 481–490. [DOI] [PubMed] [Google Scholar]
- 83. Kim H-J, Choi YS, Jeong M-J, et al. Expression of UNCL during development of periodontal tissue and response of periodontal ligament fibroblasts to mechanical stress in vivo and in vitro. Cell Tissue Res 2007; 327(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 84. Kimoto S, Matsuzawa M, Matsubara S, et al. Cytokine secretion of periodontal ligament fibroblasts derived from human deciduous teeth: effect of mechanical stress on the secretion of transforming growth factor-β 1 and macrophage colony stimulating factor. J Periodontal Res 1999; 34(5): 235–243. [DOI] [PubMed] [Google Scholar]
- 85. Hsieh AH, Tsai CMH, Ma QJ, et al. Time-dependent increases in type-III collagen gene expression in medical collateral ligament fibroblasts under cyclic strains. J Orthop Res 2000; 18(2): 220–227. [DOI] [PubMed] [Google Scholar]
- 86. Jones BF, Wall ME, Carroll RL, et al. Ligament cells stretch-adapted on a microgrooved substrate increase intercellular communication in response to a mechanical stimulus. J Biomech 2005; 38(8): 1653–1664. [DOI] [PubMed] [Google Scholar]
- 87. Long P, Gassner R, Agarwal S. Tumor necrosis factor α-dependent proinflammatory gene induction is inhibited by cyclic tensile strain in articular chondrocytes in vitro. Arthritis Rheum 2001; 44(10): 2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Matsuda N, Yokoyama K, Takeshita S, et al. Role of epidermal growth factor and its receptor in mechanical stress-induced differentiation of human periodontal ligament cells in vitro. Arch Oral Biol 1998; 43(12): 987–997. [DOI] [PubMed] [Google Scholar]
- 89. Nakatani T, Marui T, Hitora T, et al. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-β1. J Orthop Res 2002; 20(6): 1380–1386. [DOI] [PubMed] [Google Scholar]
- 90. Ozaki S, Kaneko S, Podyma-Inoue K, et al. Modulation of extracellular matrix synthesis and alkaline phosphatase activity of periodontal ligament cells by mechanical stress. J Periodontal Res 2005; 40(2): 110–117. [DOI] [PubMed] [Google Scholar]
- 91. Ozawa Y, Shimizu N, Abiko Y. Low-energy diode laser irradiation reduced plasminogen activator activity in human periodontal ligament cells. Lasers Surg Med 1997; 21(5): 456–463. [DOI] [PubMed] [Google Scholar]
- 92. Shimizu N, Yamaguchi M, Uesu K, et al. Stimulation of prostaglandin E2 and Interleukin-1β production from old rat periodontal ligament cells subjected to mechanical stress. J Gerontol A Biol Sci Med Sci 2000; 55(10): B489–B495. [DOI] [PubMed] [Google Scholar]
- 93. Yamaguchi N, Chiba M, Mitani H. The induction of c-fos mRNA expression by mechanical stress in human periodontal ligament cells. Arch Oral Biol 2002; 47(6): 465–471. [DOI] [PubMed] [Google Scholar]
- 94. Wescott D, Pinkerton M, Gaffey B, et al. Osteogenic gene expression by human periodontal ligament cells under cyclic tension. J Dent Res 2007; 86(12): 1212–1216. [DOI] [PubMed] [Google Scholar]
- 95. Yoshino H, Morita I, Murota SI, et al. Mechanical stress induces production of angiogenic regulators in cultured human gingival and periodontal ligament fibroblasts. J Periodontal Res 2003; 38(4): 405–410. [DOI] [PubMed] [Google Scholar]
- 96. Banes AJ, Tsuzaki M, Hu P, et al. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J Biomech 1995; 28(12): 1505–1513. [DOI] [PubMed] [Google Scholar]
- 97. Banes AJ, Weinhold P, Yang X, et al. Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clin Orthop Relat Res 1999; 367(Suppl.): S356–S370. [DOI] [PubMed] [Google Scholar]
- 98. Chen CH, Marymont JV, Huang MH, et al. Mechanical strain promotes fibroblast gene expression in presence of corticosteroid. Connect Tissue Res 2007; 48(2): 65–69. [DOI] [PubMed] [Google Scholar]
- 99. Lee C-Y, Liu X, Smith CL, et al. The combined regulation of estrogen and cyclic tension on fibroblast biosynthesis derived from anterior cruciate ligament. Matrix Biol 2004; 23(5): 323–329. [DOI] [PubMed] [Google Scholar]
- 100. Chang HY, Chi JT, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 2002; 99(20): 12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Enoch S, Wall I, Peake M, et al. Increased oral fibroblast lifespan is telomerase-independent. J Dent Res 2009; 88(10): 916–921. [DOI] [PubMed] [Google Scholar]
- 102. Enoch S, Peake MA, Wall I, et al. “Young” oral fibroblasts are geno/phenotypically distinct. J Dent Res 2010; 89(12): 1407–1413. [DOI] [PubMed] [Google Scholar]
- 103. Lee CY, Smith CL, Zhang X, et al. Tensile forces attenuate estrogen-stimulated collagen synthesis in the ACL. Biochem Biophys Res Commun 2004; 317(4): 1221–1225. [DOI] [PubMed] [Google Scholar]
- 104. Gray J, Taunton JE, McKenzie DC, et al. A survey of injuries to the anterior cruciate ligament of the knee in female basketball players. Int J Sports Med 1985; 6(6): 314–316. [DOI] [PubMed] [Google Scholar]
- 105. Hutchinson MR, Ireland ML. Knee injuries in female athletes. Sports Med 1995; 19(4): 288–302. [DOI] [PubMed] [Google Scholar]
- 106. Zelisko JA, Noble HB, Porter M. A comparison of men’s and women’s professional basketball injuries. Am J Sports Med 1982; 10(5): 297–299. [DOI] [PubMed] [Google Scholar]
- 107. Yamaguchi M, Shimizu N, Ozawa Y, et al. Effect of tension-force on plasminogen activator activity from human periodontal ligament cells. J Periodontal Res 1997; 32(3): 308–314. [DOI] [PubMed] [Google Scholar]
- 108. Werb Z, Mainardi CL, Vater CA, et al. Endogenous activation of latent collagenase by rheumatoid synovial cells. N Engl J Med 1977; 296(18): 1017–1023. [DOI] [PubMed] [Google Scholar]
- 109. Miura S, Yamaguchi M, Shimizu N, et al. Mechanical stress enhances expression and production of plasminogen activator in aging human periodontal ligament cells. Mech Ageing Dev 2000; 112(3): 217–231. [DOI] [PubMed] [Google Scholar]
- 110. Archambault JM, Elfervig-Wall MK, Tsuzaki M, et al. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech 2002; 35(3): 303–309. [DOI] [PubMed] [Google Scholar]
- 111. Arnoczky SP, Tian T, Lavagnino M, et al. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. J Orthop Res 2002; 20(5): 947–952. [DOI] [PubMed] [Google Scholar]
- 112. Banes AJ, Horesovsky G, Larson C, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis Cartilage 1999; 7(1): 141–153. [DOI] [PubMed] [Google Scholar]
- 113. Hirata H, Nagakura T, Tsujii M, et al. The relationship of VEGF and PGE2 expression to extracellular matrix remodelling of the tenosynovium in the carpal tunnel syndrome. J Pathol 2004; 204(5): 605–612. [DOI] [PubMed] [Google Scholar]
- 114. Qi J, Chi L, Maloney M, et al. Interleukin-1beta increases elasticity of human bioartificial tendons. Tissue Eng 2006; 12(10): 2913–2925. [DOI] [PubMed] [Google Scholar]
- 115. Qi J, Fox AM, Alexopoulos LG, et al. IL-1beta decreases the elastic modulus of human tenocytes. J Appl Physiol 2006; 101(1): 189–195. [DOI] [PubMed] [Google Scholar]
- 116. Ralphs J, Waggett A, Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol 2002; 21(1): 67–74. [DOI] [PubMed] [Google Scholar]
- 117. Tsuzaki M, Bynum D, Almekinders L, et al. ATP modulates load-inducible IL-1β, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem 2003; 89(3): 556–562. [DOI] [PubMed] [Google Scholar]
- 118. Wall ME, Otey C, Qi J, et al. Connexin 43 is localized with actin in tenocytes. Cell Motil Cytoskeleton 2007; 64(2): 121–130. [DOI] [PubMed] [Google Scholar]
- 119. Long P, Hu J, Piesco N, et al. Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res 2001; 80(5): 1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Long P, Liu F, Piesco NP, et al. Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone 2002; 30(4): 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pertolani M. Interleukin-10: an anti-inflammatory cytokine with therapeutic potential. Clin Exp Allergy 1999; 29: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 122. Ohzeki K, Yamaguchi M, Shimizu N, et al. Effect of cellular aging on the induction of cyclooxygenase-2 by mechanical stress in human periodontal ligament cells. Mech Ageing Dev 1999; 108(2): 151–163. [DOI] [PubMed] [Google Scholar]
- 123. Kikuiri T, Hasegawa T, Yoshimura Y, et al. Cyclic tension force activates nitric oxide production in cultured human periodontal ligament cells. J Periodontol 2000; 71(4): 533–539. [DOI] [PubMed] [Google Scholar]
- 124. Archambault J, Tsuzaki M, Herzog W, et al. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res 2002; 20(1): 36–39. [DOI] [PubMed] [Google Scholar]
- 125. Garvin J, Qi J, Maloney M, et al. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng 2003; 9(5): 967–979. [DOI] [PubMed] [Google Scholar]
- 126. Triantafillopoulos IK, Banes AJ, Bowman KF, Jr, et al. Nandrolone decanoate and load increase remodeling and strength in human supraspinatus bioartificial tendons. Am J Sports Med 2004; 32(4): 934–943. [DOI] [PubMed] [Google Scholar]
- 127. Matsuda N, Morita N, Matsuda K, et al. Proliferation and differentiation of human osteoblastic cells associated with differential activation of MAP kinases in response to epidermal growth factor, hypoxia, and mechanical stress in vitro. Biochem Biophys Res Commun 1998; 249(2): 350–354. [DOI] [PubMed] [Google Scholar]
- 128. Kanzaki H, Chiba M, Sato A, et al. Cyclical tensile force on periodontal ligament cells inhibits osteoclastogenesis through OPG induction. J Dent Res 2006; 85(5): 457–462. [DOI] [PubMed] [Google Scholar]
- 129. Soltz MA, Ateshian GA. A Conewise Linear Elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J Biomech Eng 2000; 122(6): 576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Huang CY, Soltz MA, Kopacz M, et al. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng 2003; 125(1): 84–93. [DOI] [PubMed] [Google Scholar]
- 131. Seo S-J, Mahapatra C, Singh RK, et al. Strategies for osteochondral repair: focus on scaffolds. J Tissue Eng 2014; 5: 2041731414541850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Agarwal S, Deschner J, Long P, et al. Role of NF-κB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthritis Rheum 2004; 50(11): 3541–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Doi H, Nishida K, Yorimitsu M, et al. Interleukin-4 downregulates the cyclic tensile stress-induced matrix metalloproteinases-13 and cathepsin B expression by rat normal chondrocytes. Acta Med Okayama 2008; 62(2): 119–126. [DOI] [PubMed] [Google Scholar]
- 134. Dossumbekova A, Anghelina M, Madhavan S, et al. Biomechanical signals inhibit IKK activity to attenuate NF-κB transcription activity in inflamed chondrocytes. Arthritis Rheum 2007; 56(10): 3284–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Fujisawa T, Hattori T, Takahashi K, et al. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem 1999; 125(5): 966–975. [DOI] [PubMed] [Google Scholar]
- 136. Fukuda K, Asada S, Kumano F, et al. Cyclic tensile stretch on bovine articular chondrocytes inhibits protein kinase C activity. J Lab Clin Med 1997; 130(2): 209–215. [DOI] [PubMed] [Google Scholar]
- 137. Gassner R, Buckley MJ, Georgescu H, et al. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol 1999; 163(4): 2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 138. Gassner RJ, Buckley MJ, Studer RK, et al. Interaction of strain and interleukin-1 in articular cartilage: effects on proteoglycan synthesis in chondrocytes. Int J Oral Maxillofac Surg 2000; 29(5): 389–394. [PMC free article] [PubMed] [Google Scholar]
- 139. Holmvall K, Camper L, Johansson S, et al. Chondrocyte and chondrosarcoma cell integrins with affinity for collagen type II and their response to mechanical stress. Exp Cell Res 1995; 221(2): 496–503. [DOI] [PubMed] [Google Scholar]
- 140. Huang J, Ballou L, Hasty K. Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes. Gene 2007; 404(1): 101–109. [DOI] [PubMed] [Google Scholar]
- 141. Lahiji K, Polotsky A, Hungerford DS, et al. Cyclic strain stimulates proliferative capacity, α2 and α5 integrin, gene marker expression by human articular chondrocytes propagated on flexible silicone membranes. In Vitro Cell Dev Biol Anim 2004; 40(5–6): 138–142. [DOI] [PubMed] [Google Scholar]
- 142. Madhavan S, Anghelina M, Rath-Deschner B, et al. Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthritis Cartilage 2006; 14(10): 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Matsukawa M, Fukuda K, Yamasaki K, et al. Enhancement of nitric oxide and proteoglycan synthesis due to cyclic tensile strain loaded on chondrocytes attached to fibronectin. Inflamm Res 2004; 53(6): 239–244. [DOI] [PubMed] [Google Scholar]
- 144. Shimizu A, Watanabe S, Iimoto S, et al. Interleukin-4 protects matrix synthesis in chondrocytes under excessive mechanical stress in vitro. Mod Rheumatol 2004; 14(4): 296–300. [DOI] [PubMed] [Google Scholar]
- 145. Tanaka S, Hamanishi C, Kikuchi H, et al. Factors related to degradation of articular cartilagein osteoarthritis: a review. Seminars in Arthritis and Rheumatism 1998; 27(6): 392–399. [DOI] [PubMed] [Google Scholar]
- 146. Yamazaki K, Fukuda K, Matsukawa M, et al. Cyclic tensile stretch loaded on bovine chondrocytes causes depolymerization of hyaluronan: involvement of reactive oxygen species. Arthritis Rheum 2003; 48(11): 3151–3158. [DOI] [PubMed] [Google Scholar]
- 147. Agarwal S, Long P, Gassner R, et al. Cyclic tensile strain suppresses catabolic effects of interleukin-1β in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum 2001; 44(3): 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chano T, Tanaka M, Hukuda S, et al. Mechanical stress induces the expression of high molecular mass heat shock protein in human chondrocytic cell line CS-OKB. Osteoarthritis Cartilage 2000; 8(2): 115–119. [DOI] [PubMed] [Google Scholar]
- 149. Deschner J, Rath-Deschner B, Agarwal S. Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes. Osteoarthritis Cartilage 2006; 14(3): 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Deschner J, Rath-Deschner B, Wypasek E, et al. Biomechanical strain regulates TNFR2 but not TNFR1 in TMJ cells. J Biomech 2007; 40(7): 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Marques MR, Hajjar D, Franchini KG, et al. Mandibular appliance modulates condylar growth through integrins. J Dent Res 2008; 87(2): 153–158. [DOI] [PubMed] [Google Scholar]
- 152. Ueki M, Tanaka N, Tanimoto K, et al. The effect of mechanical loading on the metabolism of growth plate chondrocytes. Ann Biomed Eng 2008; 36(5): 793–800. [DOI] [PubMed] [Google Scholar]
- 153. Lahiji K, Polotsky A, Hungerford D, et al. Cyclic strain stimulates proliferative capacity, alpha-2 integrin by human articular chrondrocytes from osteoarthritic knee joints. Univ Pennsylvania Orthop J 2002; 15: 75–81. [Google Scholar]
- 154. Carvalho RS, Yen EH, Suga DM. Glycosaminoglycan synthesis in the rat articular disk in response to mechanical stress. Am J Orthod Dentofacial Orthop 1995; 107(4): 401–410. [DOI] [PubMed] [Google Scholar]
- 155. Honda K, Ohno S, Tanimoto K, et al. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol 2000; 79(9): 601–609. [DOI] [PubMed] [Google Scholar]
- 156. Madhavan S, Anghelina M, Sjostrom D, et al. Biomechanical signals suppress TAK1 activation to inhibit NF-kappaB transcriptional activation in fibrochondrocytes. J Immunol 2007; 179(9): 6246–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin 1994; 10(2): 211–219. [PubMed] [Google Scholar]
- 158. Kim HK, Kerr RG, Cruz TF, et al. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J Rheumatol 1995; 22(9): 1714–1721. [PubMed] [Google Scholar]
- 159. Miyauchi A, Gotoh M, Kamioka H, et al. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner Metab 2006; 24(6): 498–504. [DOI] [PubMed] [Google Scholar]
- 160. Geng WD, Boskovic G, Fultz ME, et al. Regulation of expression and activity of four PKC isozymes in confluent and mechanically stimulated UMR-108 osteoblastic cells. J Cell Physiol 2001; 189(2): 216–228. [DOI] [PubMed] [Google Scholar]
- 161. Hara F, Fukuda K, Asada S, et al. Cyclic tensile stretch inhibition of nitric oxide release from osteoblast-like cells is both G protein and actin-dependent. J Orthop Res 2001; 19(1): 126–131. [DOI] [PubMed] [Google Scholar]
- 162. Hara F, Fukuda K, Ueno M, et al. Pertussis toxin-sensitive G proteins as mediators of stretch-induced decrease in nitric-oxide release of osteoblast-like cells. J Orthop Res 1999; 17(4): 593–597. [DOI] [PubMed] [Google Scholar]
- 163. Yamamoto N, Fukuda K, Matsushita T, et al. Cyclic tensile stretch stimulates the release of reactive oxygen species from osteoblast-like cells. Calcif Tissue Int 2005; 76(6): 433–438. [DOI] [PubMed] [Google Scholar]
- 164. Granet C, Vico AG, Alexandre C, et al. MAP and src kinases control the induction of AP-1 members in response to changes in mechanical environment in osteoblastic cells. Cell Signal 2002; 14(8): 679–688. [DOI] [PubMed] [Google Scholar]
- 165. Case N, Ma M, Sen B, et al. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem 2008; 283(43): 29196–29205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Grimston SK, Screen J, Haskell JH, et al. Role of connexin43 in osteoblast response to physical load. Ann N Y Acad Sci 2006; 1068: 214–224. [DOI] [PubMed] [Google Scholar]
- 167. Ziambaras K, Lecanda F, Steinberg TH, et al. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J Bone Miner Res 1998; 13(2): 218–228. [DOI] [PubMed] [Google Scholar]
- 168. Ross TD, Coon BG, Yun S, et al. Integrins in mechanotransduction. Curr Opin Cell Biol 2013; 25(5): 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. McNamara LE, Sjöström T, Seunarine K, et al. Investigation of the limits of nanoscale filopodial interactions. J Tissue Eng 2014; 5: 2041731414536177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Damien E, Price JS, Lanyon LE. Mechanical strain stimulates osteoblast proliferation through the estrogen receptor in males as well as females. J Bone Miner Res 2000; 15(11): 2169–2177. [DOI] [PubMed] [Google Scholar]
- 171. Lima F, Vico L, Lafage-Proust MH, et al. Interactions between estrogen and mechanical strain effects on U2OS human osteosarcoma cells are not influenced by estrogen receptor type. Bone 2004; 35(5): 1127–1135. [DOI] [PubMed] [Google Scholar]
- 172. Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron 1994; 12(6): 1195–1206. [DOI] [PubMed] [Google Scholar]