Abstract
An improved understanding of the pluripotency maintenance of embryonic stem (ES) cells is important for investigations of early embryo development and for cell replacement therapy, but the mechanism behind pluripotency is still incompletely understood. Recent findings show that zinc, an essential trace element in humans, is critically involved in regulating various signaling pathways and genes expression. However, its role in ES cell fate determination remains to be further explored. Here we showed that 2μM zinc chloride (ZnCl2) transiently maintained mouse ES cell pluripotency in vitro. The cultured mouse ES cells remained undifferentiated under 2μM ZnCl2 treatment in leukemia inhibitory factor (LIF) withdrawal, retinoic acid (RA) or embryoid bodies (EBs) differentiation assays. In addition, ZnCl2 increased pluripotency genes expression and inhibited differentiation genes expression. Further mechanistic studies revealed that ZnCl2 transiently activated signal transducers and activators of transcription 3 (Stat3) signaling through promoting Stat3 phosphorylation. Inhibition of Stat3 signaling abrogated the effects of ZnCl2 on mouse ES cell pluripotency. Taken together, this study demonstrated a critical role of zinc in the pluripotency maintenance of mouse ES cells, as well as an important regulator of Stat3 signaling.
Introduction
ES cells are derived from early preimplantation embryos and they have two remarkable properties: the ability to differentiate into virtually all types of cells of the adult body, known as pluripotency; and the capacity for unlimited proliferation while maintaining the pluripotent state, known as self-renewal [1]. These characteristics render ES cells a useful and available tool for investigating the molecular and cellular control of embryogenesis and the mechanisms regarding embryonic developmental diseases [2–5].
Natural or synthetic small molecule compounds are important tools for controlling and manipulating ES cell fate, state, or function in vitro and in vivo [6–8]. 6-bromoindirubin-3’-oxime, an inhibitor of glycogen synthase kinase-3 (Gsk3), also an activator of Wnt pathway, is the first pharmacological agent shown to maintain ES cell pluripotency and self-renewal [9]. Small molecules can replace LIF and serum/BMP to maintain self-renewal and pluripotency of ES cells through regulating various signaling pathways. In a combination of three selective small-molecule inhibitors (CHIR99021, SU5402 and PD184352), which target Gsk3, fibroblast growth factor receptor tyrosine kinases, and mitogen-activated protein kinase kinase (Mek), respectively, mouse ES cells maintained an undifferentiated state and a faster self-renewal rate comparable to that in LIF plus serum/BMP medium [10]. In this new field, more and more novel small molecules functioned in ES cell fate regulation have been identified in recent years. For example, a recent breakthrough demonstrated that mouse pluripotent stem cells could be induced from somatic cells through using specific small molecule compounds without the ectopic expression of the well-known Yamanaka factors OKSM (Oct4, klf4, Sox2 and c-Myc) [11]. Importantly, compared with genetic manipulation, these small molecules provide researchers more controllable and reversible approaches for ES cell fate regulation in regenerative medicine.
Mouse ES cells can be maintained in undifferentiated state in culture medium with the presence of LIF [12, 13]. LIF stimulation leads to the phosphorylation of Stat3, which is important for the pluripotency maintenance of mouse ES cells [14, 15]. LIF/Stat3 signaling pathway plays a central role in the maintenance of the pluripotency of mouse ES cells in vitro. Although the mechanisms regarding genetic or epigenetic regulation of Stat3 pathway in ES cells have been extensively studied [16–20], the regulation of Stat3 pathway by small molecules in maintaining pluripotency remains less understood.
The complex cellular environment contains many nature small molecules, such as inorganic, organic salts and amino acids. These small molecules may participate critically in the regulation of early embryo development. Zinc, an essential trace element in humans, is an integral component of many proteins, such as metalloenzymes and zinc finger transcription factors. The regulatory roles of zinc in many biological processes including proliferation, growth, development and metabolism have been broadly explored. For example, zinc is very important for cell proliferation, especially for DNA synthesis and mitosis [21]. Zinc also regulates the metabolism of cGMP and the activities of protein kinase C [21]. In addition, some recent studies showed that zinc participated in regulating gene expression through metalresponsive transcription factor 1 [22, 23]. Recently, there is increasing evidences demonstrating that zinc has been identified as an intracellular second messenger involved in regulating various signaling pathways [24–26].
Many studies reveal that zinc is important for ES cell biology. For example, Klf4, a zinc finger transcription factor, is an essential member of the core pluripotency transcriptional network in ES cells [27–29]. A recent study demonstrated that zinc was required for embryonic development, and supplementation with adequate zinc improved the viability of porcine embryos in vitro [30]. Commercially available culture media for mouse ES cells do not contain any zinc ion. Therefore, little information exists regarding the effect of zinc on mouse ES cells in vitro, indicating that these media are ideal tools for investigating the function of zinc in mouse ES cells. A study reported that zinc chloride (ZnCl2) stimulated mouse ES cell proliferation through PI3K/Akt, MAPKs, and mTOR signaling pathways [31]. However, the effect of zinc on ES cell fate determination remains poorly understood. ZnCl2 is a natural small molecule, which is an important component of extracellular and intracellular environment. Understanding of the mechanisms by which ZnCl2 regulates mouse ES cell fate determination will be helpful to advance our knowledge about extrinsic control of pluripotent stem cells. In this study, we designed to investigate the effects of ZnCl2 on mouse ES cell pluripotency and differentiation as well as the related regulatory mechanisms.
Materials and Methods
Mouse ES cell culture and treatment
The mouse ES cell line ES-E14TG2a, purchased from the American Type Culture Collection (ATCC CRL-1821), was cultured on cell culture plates coated with 0.1% gelatin (Millipore, Billerica, MA) and in high-glucose Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY) supplemented with 15% (v/v) ES cell qualified fetal bovine serum (Gibco), 1% (v/v) penicillin and streptomycin (Gibco), 0.1mM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), 0.1mM nonessential amino acids (Gibco), 2mM L-glutamine (Gibco), and 1,000units/ml LIF (Millipore).
ZnCl2 (AMRESCO, Solon, OH) at different final concentrations of 0.02μM, 0.2μM, 2μM or 20μM were added into medium, and mouse ES cells were incubated with ZnCl2 for different time in different experiments.
For LIF withdrawal differentiation assay, mouse ES cells were cultured in medium containing all other supplements at the concentrations indicated above without LIF.
For RA differentiation assay, mouse ES cells were cultured in 6-well plates. 10μM Retinoic acid (Sigma-Aldrich) and ZnCl2 at different final concentrations were added into LIF withdrawal medium. After 48 hours, the cells were fixed for alkaline phosphatase staining or RNAs and proteins were collected for further analyses.
For EB differentiation assay, mouse ES cells were treated with 0.05%Trypsin/EDTA and then cultured in LIF withdrawal medium in Ultra Low Culture Dish (Corning, Corning, NY). ZnCl2 at different final concentrations were added into medium. Then, on day 6, RNAs and proteins were collected for further analyses.
The mouse ES cells were cultured in LIF withdrawal medium supplemented with Mek inhibitor PD0325901 (1μM) (Sigma-Aldrich) and Gsk3 inhibitor CHIR99021 (3μM) (Sigma-Aldrich) to sustain efficient self-renewal and pluripotency, known as 2i [10]. This was used as positive control in our study.
For AG490 (a Stat3 pathway inhibitor) inhibition experiment, AG490 (Sigma-Aldrich) at a final concentration of 30μM was added into LIF withdrawal medium. We treated mouse ES cells with AG490 and ZnCl2 simultaneously.
Alkaline phosphatase staining
Cells were washed twice with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich) for approximately 1 min at room temperature. Then, cells were incubated with an alkaline phosphatase substrate solution (Sigma-Aldrich) for 10–15 minutes at room temperature. After a wash with PBS, the cells were photographed.
Reverse Transcription, qRT-PCR
Total RNA was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. For each sample, 500ng of RNA was reverse transcribed to cDNA using the Prime-Script RT reagent kit (TaKaRa, Dalian, China). The obtained cDNA was amplified with the Takara Ex Taq PCR kit (TaKaRa), and qRT-PCR amplification was conducted using the Stratagene Mx3000 QPCR system (Stratagene, Foster City, CA) and analyzed via the ΔΔCT method. The primer sequences used in these assays are available in S1 Table.
Western Blotting
Cultured cells were lysed in strong RIPA buffer containing Halt Protease Inhibitor Cocktails (Thermo, Waltham, MA). The obtained protein concentrations were measured using a BCA protein assay kit (Pierce, Rockford, IL). Primary antibodies targeting OCT4 (Santa Cruz Biotechnology, Santa Cruz, CA), NANOG (Abcam, Cambridge, U.K), SOX2 (Abcam), STAT3 (Cell Signaling Technology, Boston, MA), STAT3 (pTyr705) (Cell Signaling Technology) and GAPDH (Santa Cruz Biotechnology) were incubated with the proteins overnight at 4℃, followed by incubation with the appropriate HRP (horseradish peroxidase) conjugated secondary antibodies. Detection of HRP was performed using the Super Signal West Pico Chemiluminescent Substrate (Pierce).
Immunofluorescence staining
Cells cultured in 24-well dishes were fixed in 4% paraformaldehyde and permeabilized with 0.25% Triton X-100, followed by blocking with 10% FBS in PBS. Then cells were probed with primary antibody in 10% FBS overnight at 4°C and secondary antibody in 10% FBS for 2 hours at room temperature. Then nuclei were counterstained with Hoechst 33342 for 2 minutes. Cells were washed with and imaged in PBS. Primary antibodies used were OCT4 (Santa Cruz Biotechnology), NANOG (Abcam) and Ki67 (Abcam).
Cell Counting Kit-8 (CCK8) cell proliferation assay
The cells were seeded in 96-well plates at a density of 1×103 cells/well. After different treatment, the reagent (Dojindo, Kumamoto, Japan) was thawed for approximately 10 minutes in a water bath at 37°C, and 10 μl of the reagent was added to each well of the 96-well plate. Then the plate was incubated at 37°C for 1–4 hours. Finally, the absorbance at 450 nm was recorded with SpectraMax M5.
Statistical Analysis
The error bars represent the SEM of three independent experiments. Data are represented as mean±SEM; n = 3. *, ** and *** indicate p<0.05, p<0.01, and p<0.001, respectively (Student’s t test).
Results
Zinc promotes mouse ES cell pluripotency in LIF withdrawal differentiation assay
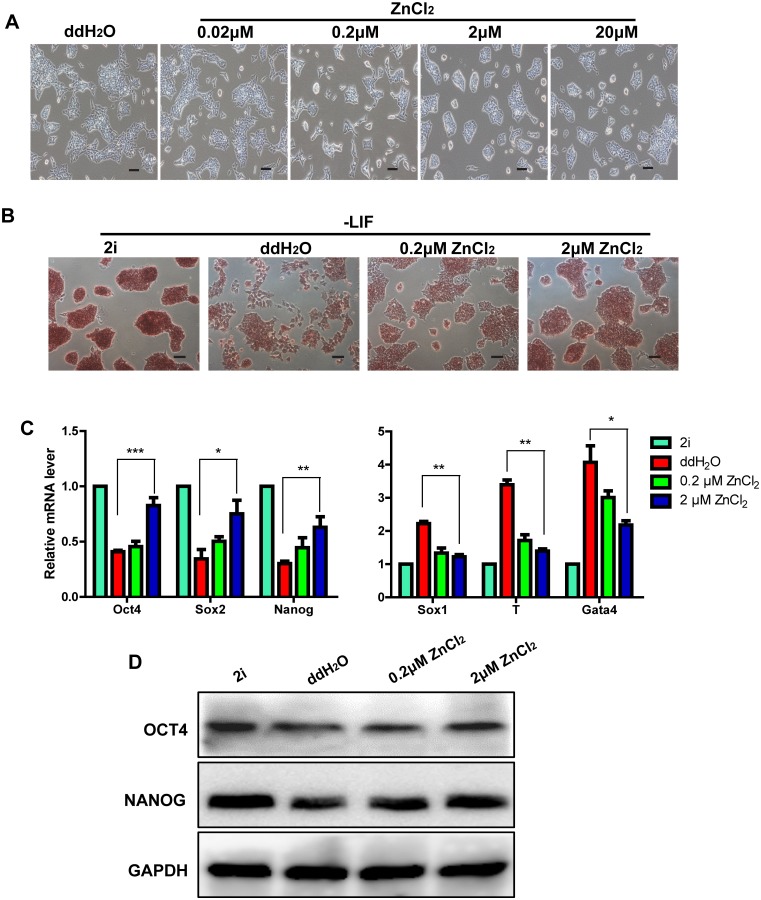
In the current in vitro culture system, mouse ES cells require LIF to maintain their pluripotent state [12, 13]. To explore whether zinc supports the pluripotency maintenance of mouse ES cells, we incubated the cells with ZnCl2 at different concentrations (0.02μM, 0.2μM, 2μM, and 20μM) for 48 hours in LIF withdrawal medium. We then examined the morphology of treated cells. Compared with the negative control (ddH2O) and low concentration (0.02μM and 0.2μM) group, high concentration ZnCl2 (2μM and 20μM) maintained the clone morphology of ES cells and markedly reduced their spontaneous differentiation (Fig 1A). When the concentration increased, the clone morphology was more obvious. However, when the concentration reached 20μM, the clone morphology was not considerably improved compared to 2μM ZnCl2 treatment, indicating that the concentration of ZnCl2 reached saturation. Therefore, we chose 2μM as experimental concentration for the following experiments. Ying et al. reported that two potent selective small molecule inhibitors, PD0325901 and CHIR99021, which target Mek and Gsk3, respectively, are sufficient to sustain efficient mouse ES cell self-renewal and pluripotency [10]. Therefore, in our following experiments, we used these two molecules, known as 2i, as positive control. 2μM ZnCl2 treated cells had similar AP enzyme activity compared to 2i treated cells (Fig 1B). However, compared with ddH2O and 0.2μM ZnCl2 treatment, 2μM ZnCl2 treatment led to a stronger alkaline phosphatase (AP) enzyme activity, an indicative of pluripotency for mouse ES cells (Fig 1B). qRT-PCR and western-blot analyses revealed that 2μM ZnCl2 treatment significantly increased the expression levels of pluripotency markers, including Oct4, Sox2 and Nanog, which are essential for the pluripotency maintenance of mouse ES cells (Fig 1C and 1D). Moreover, 2μM ZnCl2 treatment also inhibited the mRNA levels of genes related to differentiation into the three embryonic germ layers, including Sox1, T and Gata4 (Fig 1C). Therefore, our results suggested that ZnCl2 at certain concentration promoted mouse ES cell pluripotency in LIF withdrawal medium.
Fig 1. Zinc promotes mouse ES cell pluripotency in LIF withdrawal differentiation assay.
All cells were cultured in LIF withdrawal medium. (A): Morphological differences between cells treated with ddH2O and ZnCl2 at the indicated concentrations for 48 hours. Bars = 40μm. (B): Alkaline phosphatase staining of cells treated with 2i, ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. Bars = 40μm. (C): qRT-PCR analyses of the mRNA levels of pluripotency transcription factors (Oct4, Sox2 and Nanog) and lineage-specific markers (Sox1, T and Gata4) in cells treated with 2i, ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. The data are displayed relative to the results of the 2i treatment group and represented as mean±SEM; n = 3. *, ** and *** indicate p<0.05, p<0.01, and p<0.001, respectively. (D): Western blot analyses of the protein levels of OCT4 and NANOG in cells treated 2i, ddH2O and with ZnCl2 (0.2μM and 2μM) for 48 hours.
Zinc promotes mouse ES cell pluripotency in RA and EB differentiation assays
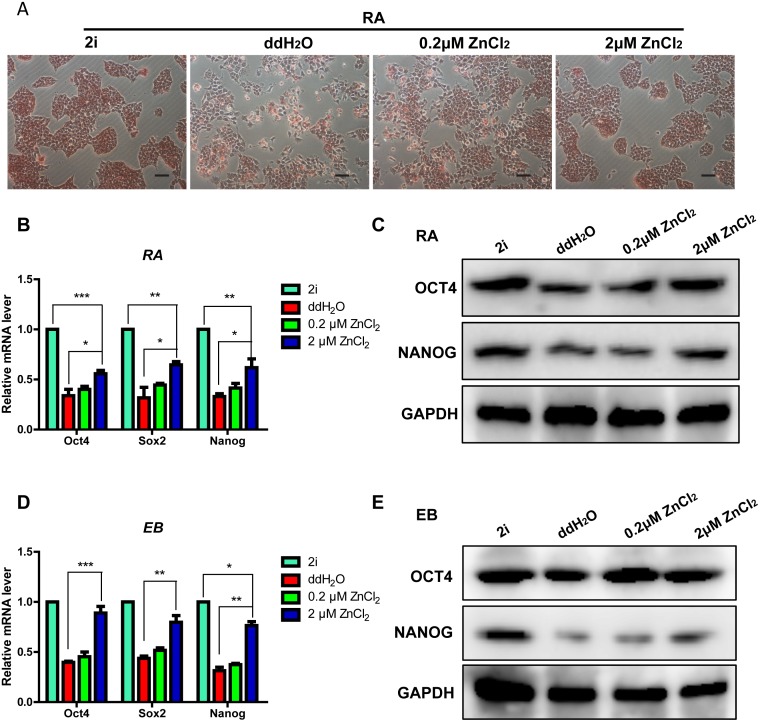
To further investigate the relationship between zinc and pluripotency, the process of differentiation was perturbed by the presence of 10μM RA, which is closely involved in mouse ES cell differentiation [32]. After 48 hours of RA exposure in LIF withdrawal medium, 2μM ZnCl2 treated cells showed a stronger AP enzyme activity and maintained a better clone morphology compared to ddH2O treated cells (Fig 2A). Moreover, compared with ddH2O treatment, 2μM ZnCl2 treatment obviously increased the mRNA and protein levels of pluripotent transcription factors Oct4, Sox2 and Nanog in RA differentiation assay (Fig 2B and 2C). In addition, we further confirmed the effect of zinc on the pluripotency by EB differentiation assay. We induced 2i, ddH2O and ZnCl2 treated mouse ES cells to form EBs and then analyzed the expression levels of pluripotent transcription factors via qRT-PCR and Western blot on EB Day 6. We found that 2μM ZnCl2 treatment elevated the mRNA and protein levels of Oct4, Sox2 and Nanog in EB differentiation assay compared to ddH2O treatment (Fig 2D and 2E).
Fig 2. Zinc promotes mouse ES cell pluripotency in RA and EB differentiation assays.
RA differentiation assay (A)-(C): all cells were treated with RA (10μM) for 48 hours and cultured in LIF withdrawal medium. (A) Alkaline phosphatase staining of cells treated with 2i, ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. (B) qRT-PCR analyses of the mRNA levels of pluripotency transcription factors (Oct4, Sox2 and Nanog) in cells treated with 2i, ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. The data are displayed relative to the results of the 2i treatment group and represented as mean±SEM; n = 3. *, ** and *** indicate p<0.05, p<0.01, and p<0.001, respectively. (C) Western blot analyses of the protein levels of OCT4 and NANOG in cells treated with 2i, ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. EB differentiation assay (D)-(E). (D) qRT-PCR analyses of the mRNA levels of pluripotency transcription factors (Oct4, Sox2 and Nanog) in cells treated with 2i, ddH2O and ZnCl2 (0.2μM and 2μM) for 6 days. The data are displayed relative to the results of the 2i treatment group and represented as mean±SEM; n = 3. *, ** and *** indicate p<0.05, p<0.01, and p<0.001, respectively. (E) Western blot analyses of the protein levels of OCT4 and NANOG in cells treated with 2i, ddH2O and ZnCl2 (0.2μM and 2μM) for 6 days.
ZnCl2 can promote pluripotent transcription factor expression. However, we also observed that the mRNA levels of Oct4, Sox2 and Nanog were slightly lower in 2μM ZnCl2 treated cells than that in 2i treated cells in RA and EB differentiation assay (Fig 2B and 2D). This may indicate that the effect of ZnCl2 on pluripotency maintenance is slightly weaker compared to 2i.
Taken together, our data presented above revealed that ZnCl2 at appropriate concentration promoted mouse ES cell pluripotency in different differentiation conditions.
Zinc transiently maintains mouse ES cell pluripotency in vitro
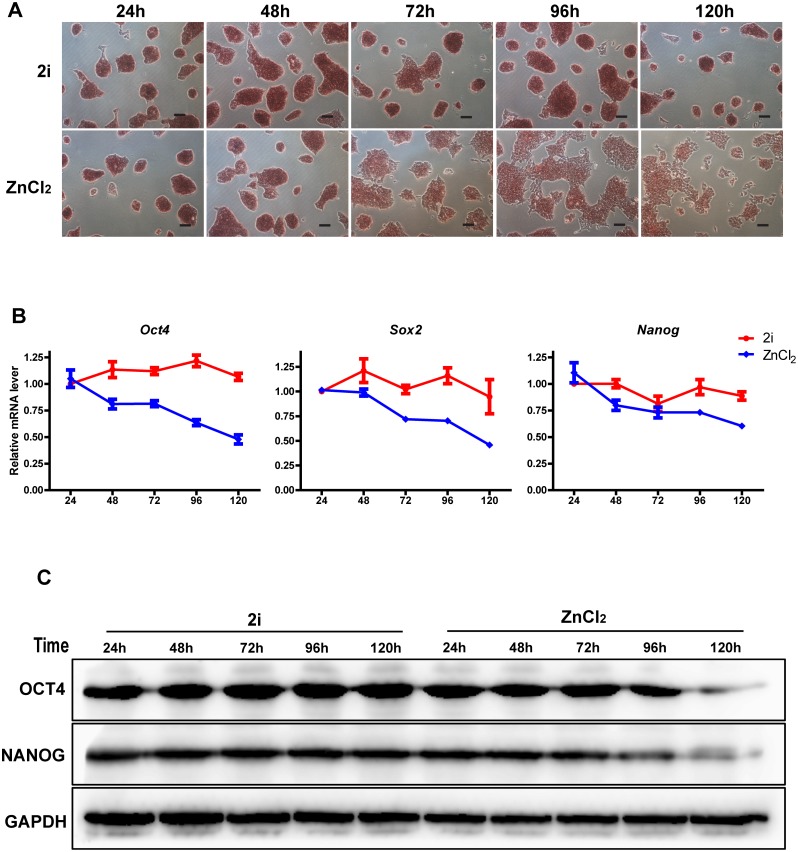
Next, we tested whether 2μM ZnCl2 could maintain the pluripotency of mouse ES cells for more than 48 hours in vitro. Compared with 2i treated cells, 2μM ZnCl2 treated cells sustained similar clone morphology and AP enzyme activity after 24 hours or 48 hours incubation in LIF withdrawal medium (Fig 3A). However, as the cells were passaged and the incubation time was prolonged to 72 hours, 96 hours or 120 hours, 2μM ZnCl2 treated ES cells started the trend of differentiation, losing their clone morphology and AP enzyme activity gradually (Fig 3A). qRT-PCR and western-blot analyses also revealed that the expression levels of pluripotency markers decreased gradually after 2μM ZnCl2 treatment for 72 hours, 96 hours or 120 hours (Fig 3B and 3C). Thus, these data indicated that zinc transiently maintained mouse ES cell pluripotency in vitro.
Fig 3. Zinc transiently maintains mouse ES cell pluripotency in vitro.
All cells were cultured in LIF withdrawal medium. (A): Alkaline phosphatase staining of cells treated with 2i and 2μM ZnCl2 at the indicated time. Bars = 40μm. (B): qRT-PCR analyses of the mRNA levels of pluripotency transcription factors (Oct4, Sox2 and Nanog) in cells treated with 2i and 2μM ZnCl2 at the indicated time. The data are displayed relative to the results of the 2i treatment for 24 hours group and represented as mean±SEM; n = 3. (C): Western blot analyses of the protein levels of OCT4 and NANOG in cells treated with 2i and 2μM ZnCl2 at the indicated time.
Zinc does not affect mouse ES cell pluripotency and proliferation in the presence of LIF
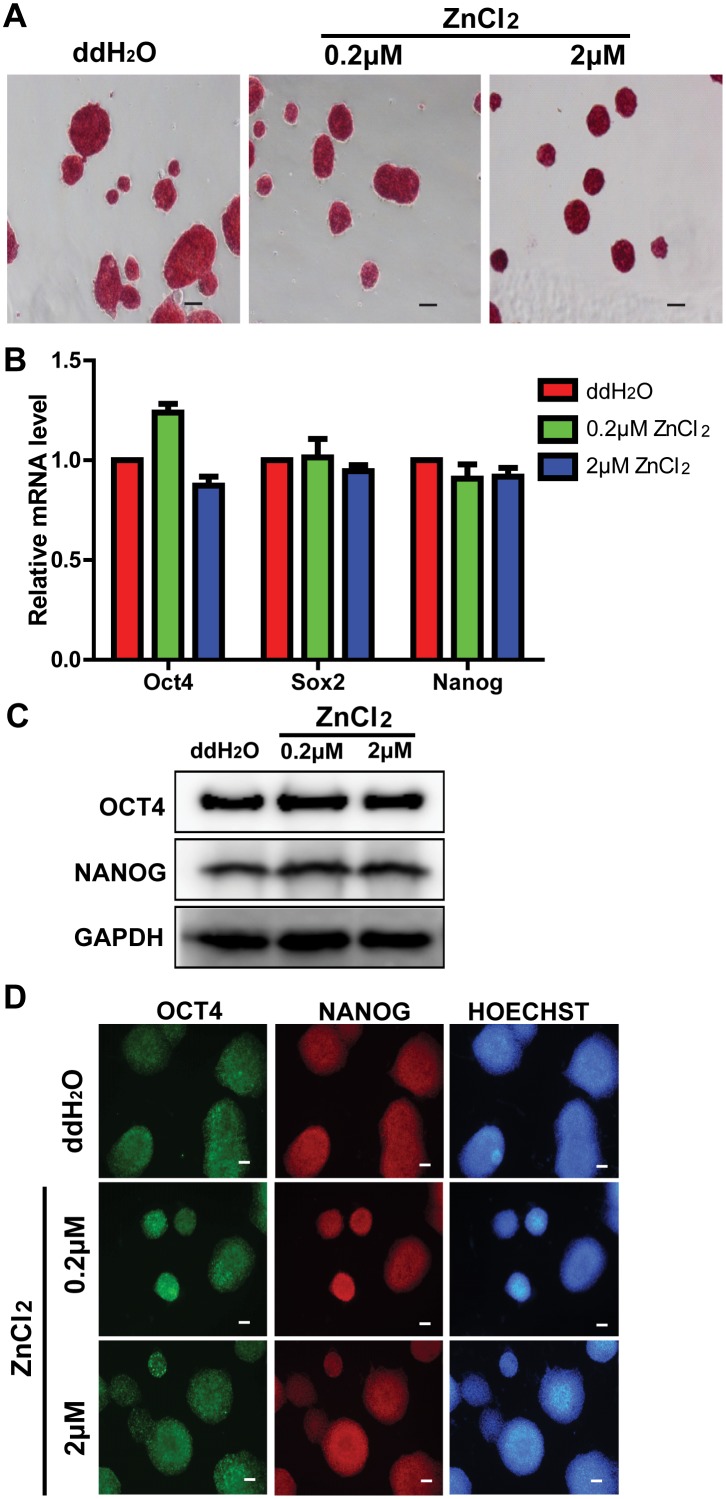
To investigate whether zinc has an impact on ES cell pluripotency when LIF is supplemented in the medium, we cultured the cells with ZnCl2 at different concentrations (0.2μM and 2μM) for 48 hours in medium supplemented with LIF and further detected the expression levels of pluripotency markers. After 48 hours of ZnCl2 exposure, ES cells maintained similar alkaline phosphatase enzyme activity (Fig 4A). The mRNA levels of Oct4, Sox2, and Nanog were without significant differences as well (Fig 4B). The protein levels of OCT4 and NANOG also maintained at similar levels as reflected by western blotting and immunocytochemistry analyses (Fig 4C and 4D). Interestingly, a previous study also showed that mouse ES cells remained undifferentiated under 72 hours of 40μM ZnCl2 exposure in LIF containing medium [31]. Therefore, our data indicated that 2μM ZnCl2 did not affect the pluripotency of mouse ES cells in the presence of LIF.
Fig 4. Zinc does not affect mouse ES cell pluripotency in the presence of LIF.
All cells were cultured in medium supplemented with LIF. (A): Alkaline phosphatase staining of cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. Bars = 40μm. (B): qRT-PCR analyses of the mRNA levels of pluripotency transcription factors Oct4, Sox2 and Nanog in cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. The data are displayed relative to the results of the ddH2O treatment group and represented as mean±SEM; n = 3. (C): Western blot analyses of the protein levels of OCT4 and NANOG in cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. (D): Immunostaining images of cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. Cells were stained with antibodies against OCT4 and NANOG. Nuclei were counterstained with Hoechst33342. Bars = 40μm.
Meanwhile, we also tested whether zinc had an impact on ES cell proliferation when LIF was supplemented in the medium. Ki67 protein is a cellular marker for proliferation, which is present during all active phases of the cell cycle, but is absent from resting cells. After 48 hours of 2μM ZnCl2 treatment, ES cells exhibited similar Ki67 expression level as reflected by immunocytochemistry analysis (S1A Fig). ES cells also sustained similar growth rate through growth curves that were constructed using the CCK-8 assay (S1B Fig). These results indicated that 2μM ZnCl2 did not affect the proliferation of mouse ES cells. A previous study demonstrated that at appropriate concentrations (≥4μM), ZnCl2 increased cell cycle regulatory protein levels, [3H]-thymidine incorporation, and total cell numbers, but higher doses of ZnCl2 (≥200μM) blocked the proliferation-promoting effect [31]. This study, together with our work, suggests that ZnCl2 at different concentrations have different effects on ES cell proliferation.
Zinc transiently activates Stat3 signaling
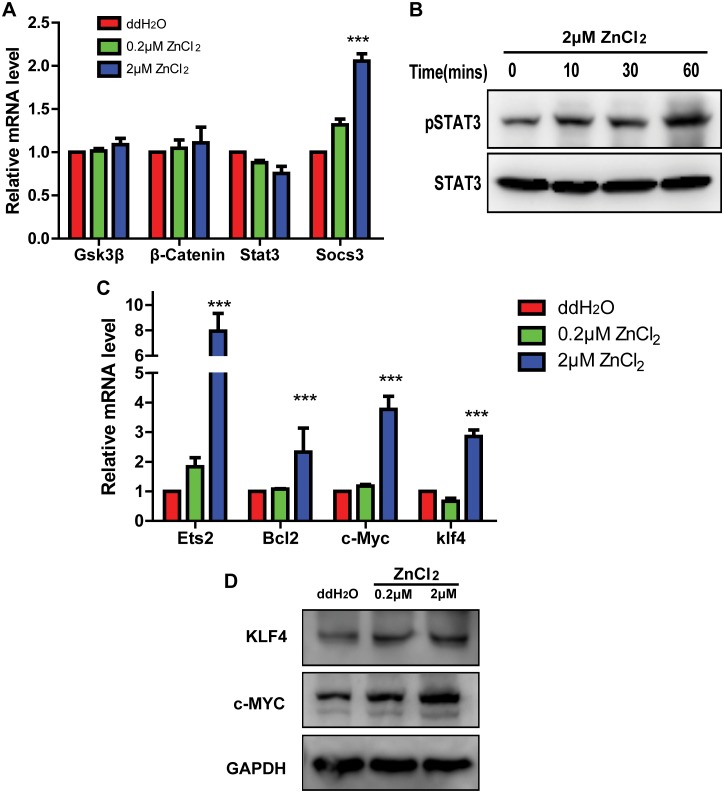
The pluripotency and self-renewal maintenance of ES cells requires a broad cell signaling network, and numerous studies showed that LIF/Stat3 signaling pathway is indispensable for the pluripotential phenotype of mouse ES cells [12–15]. A previous study demonstrated that zinc regulated inflammatory response through Jak/Stat3 pathway [33]. According to our results presented above that ZnCl2 can promote mouse ES cell pluripotency phenotype in the absence of LIF, we next examined whether ZnCl2 exerted its pro-pluripotency function on mouse ES cells through regulating Jak/Stat3 signaling pathway. Wnt and Stat3 signaling pathways are very important for mouse ES cell fate determination [34]. Therefore, we detected the expression levels of genes belong to Wnt pathway (Gsk3β, β-Catenin) and Stat3 pathway (Stat3, Socs3) via qRT-PCR. After 48 hours of 2μM ZnCl2 treatment, the expression levels of Gsk3β, β-Catenin and Stat3 remained unchanged (Fig 5A), but the expression of Stat3 target gene (Socs3) increased significantly (Fig 5A), suggesting that Stat3 signaling was upregulated, whereas Wnt signaling was not changed following ZnCl2 treatment. We then detected the posttranslational modifications of key proteins in Stat3 pathway. Stat3 is active when Tyr705 is phosphorylated. We incubated the cells with 2μM ZnCl2 for 0, 10, 30 and 60 minutes in LIF withdrawal medium and examined the protein levels of Stat3 and phosphorylated Stat3 (pTyr705) via western blotting. It showed that 2μM ZnCl2 treatment for 60 minutes elevated pSTAT3 level significantly (Fig 5B). We further analyzed the expression levels of Stat3 target genes via qRT-PCR and western blotting. After 48 hours 2μM ZnCl2 treatment, the expression levels of Ets2, Bcl2, c-Myc and Klf4 were all increased significantly (Fig 5C and 5D). Next, we prolonged the incubation time and then analyzed the effect of ZnCl2 on Stat3 signaling. Compared with 2i treated cells, the protein levels of pSTAT3 decreased gradually after 2μM ZnCl2 treatment for 72 hours,96 hours and 120 hours (S2A Fig). The mRNA levels of Stat3 target genes, Klf4 and Socs3, also decreased gradually as the incubation time was prolonged (S2B Fig). Collectively, these results suggested that zinc transiently activated Stat3 through phosphorylation and played a particularly important role in the transcriptional network controlling pluripotency of mouse ES cells.
Fig 5. Zinc activates Stat3 signaling.
All cells were cultured in LIF withdrawal medium. (A): qRT-PCR analyses of the expression levels of genes belong to Wnt signaling pathway (Gsk3β, β-Catenin) and Stat3 signaling pathway (Stat3, Socs3) in cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. The data are displayed relative to the results of the ddH2O treatment group and represented as mean±SEM; n = 3. *** indicates p<0.001. (B): Western blot analyses of the expression of STAT3 and pSTAT3 in cells treated with 2μM ZnCl2 for 0, 10, 30 and 60 minutes. (C): qRT-PCR analyses of the expression of Stat3 targeting genes in cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. The data are displayed relative to the results of the ddH2O treatment group and represented as mean±SEM; n = 3. *** indicates p<0.001. (D): Western blot analyses of the expression of Stat3 targeting genes (c-Myc and Klf4) in cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours.
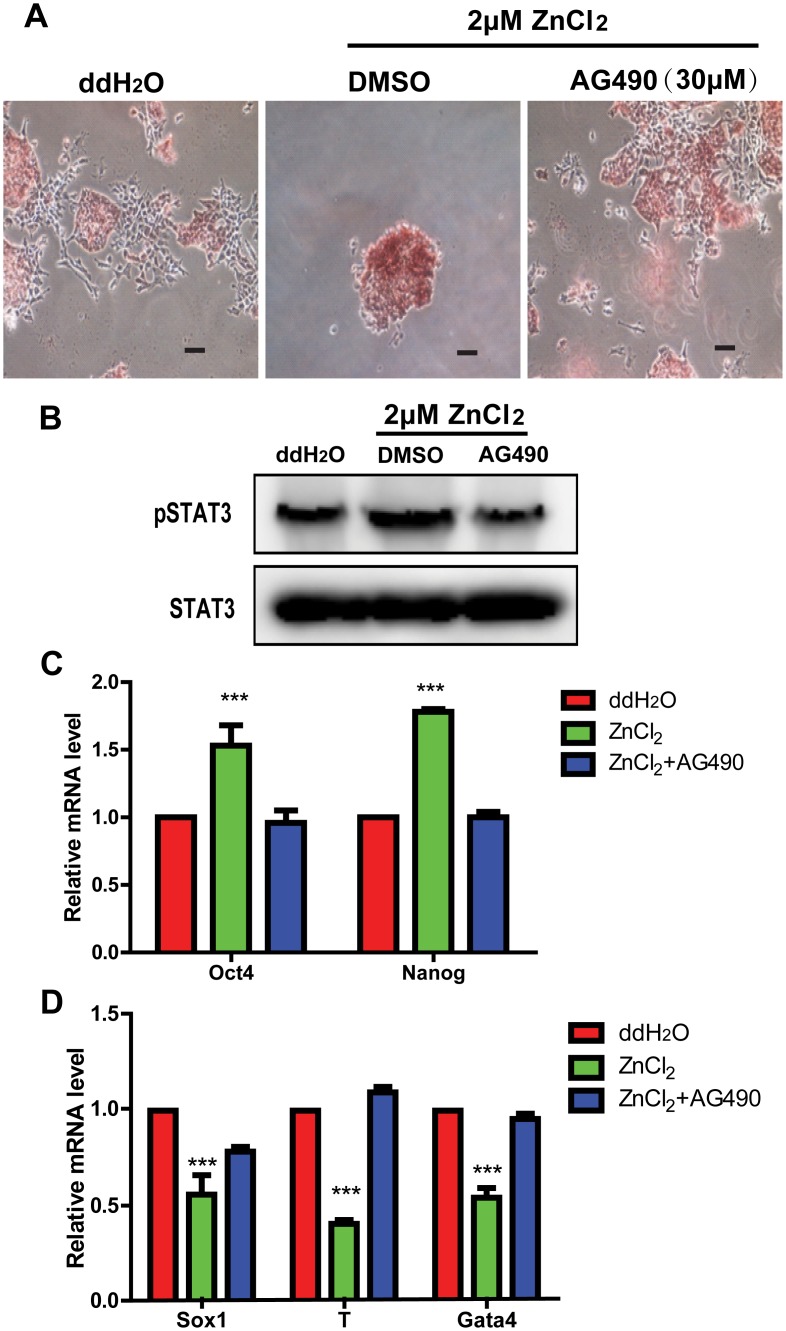
Inhibition of Stat3 signaling abrogates the effect of ZnCl2 on mouse ES cell pluripotency
Based on the findings that Stat3 activity and its target genes were altered after ZnCl2 treatment, we hypothesized that Stat3 signaling pathway might be associated with the effect of ZnCl2 in promoting the pluripotent potential of mouse ES cells. Jak is an upstream factor in Jak/Stat3 pathway, which can activate Stat3. AG490 is a selective Jak inhibitor that inhibits Jak/Stat3 signaling [35]. We thus treated mouse ES cells with AG490 (30μM) for 48 hours in LIF withdrawal medium. Compared with the ddH2O treatment, 2μM ZnCl2 treatment promoted ES cells clone morphology and increased AP enzyme activity. However, when AG490 was added into medium, the cells regained their differentiated morphology (Fig 6A). AG490 treatment also decreased the protein level of phosphorylated STAT3 (pTyr705) (Fig 6B) as well as the mRNA levels of Oct4 and Nanog (Fig 6C) in 2μM ZnCl2 treated cells. Furthermore, compared with only ZnCl2 treated cells, the expression of differentiation genes increased in ZnCl2 plus AG490 treated cells (Fig 6D). Collectively, these findings indicated that zinc regulated the mouse ES cell pluripotency potential mainly through Stat3 signaling.
Fig 6. Inhibition of Stat3 signaling abrogates the effect of ZnCl2 on mouse ES cell pluripotency.
All cells were cultured in LIF withdrawal medium and divided into three groups: cells treated with ddH2O, cells treated with 2μM ZnCl2 + DMSO for 48 hours and cells treated with 2μM ZnCl2 + AG490 (30μM) for 48 hours. (A): Alkaline phosphatase staining of three group cells. Bars = 40μm. (B): Western blot analyses of the expression of STAT3 and pSTAT3 in three group cells. (C): qRT-PCR analyses of the expression of Oct4 and Nanog in three group cells. The data are displayed relative to the results of the ddH2O treatment group and represented as mean±SEM; n = 3. *** indicates p<0.001. (D): qRT-PCR analyses of the expression of lineage-specific markers in three group cells. The data are displayed relative to the results of the ddH2O treatment group and represented as mean±SEM; n = 3. *** indicates p<0.001.
Discussion
Small molecules are useful chemical tools for manipulating cell fate, and clearly offer some distinct advantages over genetic manipulation. For example, small molecules are nonimmunogenic, more cost-effective, more easily synthesized, preserved, and standardized. Their effects are often reversible and can be finely tuned by varying the concentrations. Previous studies have shown that many small molecule compounds play essential roles in maintaining ES cell self-renewal, pluripotency and reprogramming [10, 11, 36, 37]. However, to date, the chemical regulation of ES cell fate remains to be further investigated to meet the needs of regenerative medicine. We found that, compared with ddH2O treated cells, mouse ES cells maintained the clone morphology and a stronger AP enzyme activity under ZnCl2 exposure for 48 hours at a final concentration of 2μM in LIF withdrawal medium (Fig 1A and 1B). ZnCl2 treatment also increased the expression of pluripotency markers and inhibited the expression of differentiation genes (Fig 1C and 1D). Importantly, we observed similar phenomenons in RA and EB differentiation assays (Fig 2). Our study indicates that ZnCl2 might significantly promote pluripotency of mouse ES cells. However, Corradi et al. reported that the EB formation and cardiac differentiation of mouse ES cells were inhibited by zinc oxide (ZnO) nanomaterials at noncytotoxic doses [38]. It is possible that some zinc ions are reacted to form ZnO in mouse ES cell culture medium in our study. Therefore, ZnO may also participate in the maintenance of mouse ES cell pluripotency. We also observed that the mouse ES cells started the trend of differentiation after ZnCl2 treatment for 72 hours, 96 hours and 120 hours (Fig 3). Mouse ES cells can be maintained indefinitely in the pluripotent state in LIF plus serum medium in vitro. By contrast, pluripotency is a transitory state that exists only during a short window of early embryo development in vivo. Pluripotency is also a highly regulated state in vivo. However, the regulatory mechanism is very complex which has not been well elucidated now. Zinc, which is an essential trace element for mammals, could transiently maintained mouse ES cell pluripotency in vitro in our study. Therefore, we speculate that zinc may be an important regulator for the pluripotency maintenance of early embryo development. These all need to be elucidated in future studies.
Similarly, there are studies demonstrating that other ions are also critically involved in ES cell biology. Embryos depend on a ubiquitous, persistent and highly versatile signaling system that is based around a single messenger, Ca2+ [39]. Another study showed that iron (Fe3+) is essential for porcine embryonic development [40]. Therefore, our data lend support to the notion that these important metal elements are essential regulators for embryonic pluripotency and development.
Stat3 is tightly integrated into the gene regulatory network of pluripotency. Chen and Kidder et al. found that Stat3 bound to downstream gene promoters for gene activation, including Klf4 and c-Myc. They also observed that Stat3 co-occupied pluripotency gene promoters with Oct4, Sox2 and Nanog [34, 41]. Although Stat3 target genes have been extensively identified, little is known about its upstream chemical regulators, especially its natural chemical regulator. ZnCl2 is an indispensible natural small molecule for mammal. Here, we found that ZnCl2 at appropriate concentration promoted Stat3 activities. Although ZnCl2 did not enhance Stat3 expression, it activated Stat3 through phosphorylating Tyr705. Then, activated Stat3 promoted target genes expression to maintain pluripotency. Stat3 and its target genes such as Klf4 and c-Myc are essential for maintaining mouse ES cell self-renewal and pluripotency [28, 41]. In our study, we have provided significant data to support that ZnCl2 promote mouse ES cells pluripotency, in part, through activating Stat3. However, it remains possible that ZnCl2 exerts its effects on mouse ES cell pluripotency through activating or inhibiting other factors besides Stat3. This needs to be elucidated in future studies.
Although human and mouse ES cells are differed in the signaling networks and epigenetic landscapes, it has been revealed that they share the same core regulators Oct4/Sox2/Nanog and similar transcriptional regulatory network [42]. Given that zinc is an important trace element for both human and mouse and zinc promoted the expression of Oct4, Sox2 and Nanog in our study (Figs 1C, 1D and 2B–2E), we propose that zinc is extremely possible to have similar functions regarding the maintenance and acquaintance of pluripotency in human cells, which is very worthy to be elucidated in future studies.
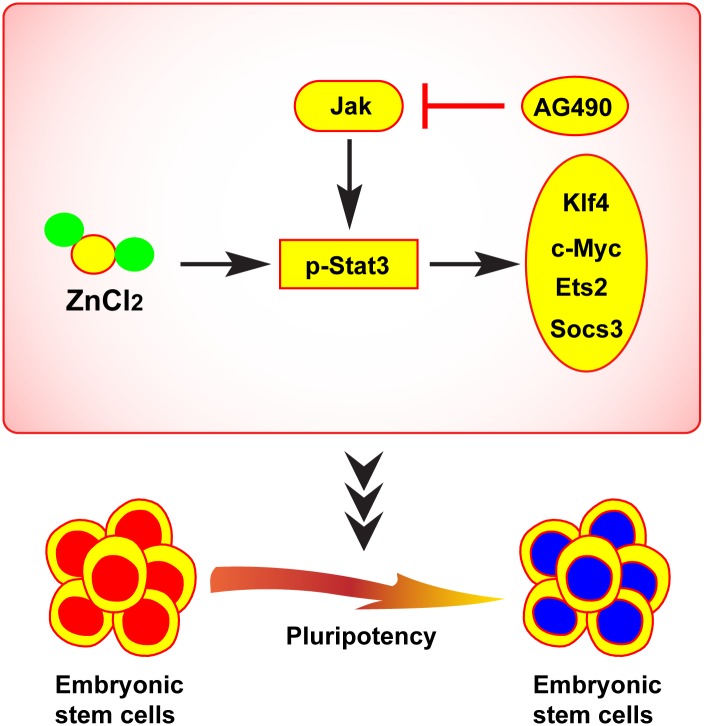
In summary, we have clearly demonstrated for the first time that ZnCl2 promoted mouse ES cell pluripotency in differentiation conditions through activating Stat3 pathway. Briefly, ZnCl2 stimulation activates Stat3 through phosphorylating. Then, activated Stat3 increases its target genes expression to promote embryonic stem cell pluripotency (Fig 7). Our finding not only has implications for zinc’s role in ES cell biology, but also elucidates another potential control switch for ES cell fate in regenerative medicine. Given the current challenges of pluripotency maintenance, this research and the questions raised by this work demonstrate the need for continued studies into the molecular basis for pluripotency and the role of zinc in mouse ES cells, as well as its relevance to human embryonic stem cells.
Fig 7. Schematic diagram of the regulatory role of ZnCl2 in the pluripotency maintenance of ES cells.
Supporting Information
All cells were cultured in medium supplemented with LIF. (A): Immunostaining images of cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. Cells were stained with antibody against Ki67. Nuclei were counterstained with Hoechst33342. Bars = 40μm. (B): Growth curves of cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) were constructed using the CCK-8 assay. The data are represented as mean±SEM; n = 3.
(TIF)
(A): Western blot analyses of the expression of STAT3 and pSTAT3 in cells treated with 2μM ZnCl2 or 2i for 24, 48, 72, 96 and 120 hours. (B): qRT-PCR analyses of the expression levels of Stat3 target genes, Klf4 and Socs3, in cells treated with 2μM ZnCl2 or 2i for 24, 48, 72, 96 and 120 hours. The data are displayed relative to the results of the 2i treatment for 24 hours group and represented as mean±SEM; n = 3.
(TIF)
All primers used for detecting mRNA expression levels by qRT-PCR.
(DOC)
Acknowledgments
This work was supported by two grants from the Shanghai Committee of Science and Technology, China (Grant No. 15ZR1432600 and Grant No. 15695841100), partly by a grant from the National Natural Science Foundation of China (Grant No.81202045). We are grateful to Bo Zhang for his foundation financial support (the National Natural Science Foundation of China, Grant No.81202045). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Shanghai Committee of Science and Technology, China; Grant No. 15ZR1432600; funding recipient: Liwen Song; URL: http://www.stcsm.gov.cn/. Shanghai Committee of Science and Technology, China; Grant No.15695841100; funding recipient: Jiajing Cheng; URL: http://www.stcsm.gov.cn/. National Natural Science Foundation of China; Grant No.81202045; URL: http://www.nsfc.gov.cn/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Smith AG. Embryo-derived stem cells: of mice and men. Annual review of cell and developmental biology. 2001;17:435–62. [DOI] [PubMed] [Google Scholar]
- 2.Bonn S, Zinzen RP, Girardot C, Gustafson EH, Perez-Gonzalez A, Delhomme N, et al. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nature genetics. 2012;44(2):148–56. 10.1038/ng.1064 [DOI] [PubMed] [Google Scholar]
- 3.Mulvey CM, Schroter C, Gatto L, Dikicioglu D, Fidaner IB, Christoforou A, et al. Dynamic Proteomic Profiling of Extra-Embryonic Endoderm Differentiation in Mouse Embryonic Stem Cells. Stem cells. Forthcoming 2015. [DOI] [PubMed] [Google Scholar]
- 4.Yazdani M, Deogracias R, Guy J, Poot RA, Bird A, Barde YA. Disease modeling using embryonic stem cells: MeCP2 regulates nuclear size and RNA synthesis in neurons. Stem cells. 2012;30(10):2128–39. 10.1002/stem.1180 [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Pasumarthi KB. Embryonic stem cell transplantation: promise and progress in the treatment of heart disease. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2008;22(6):361–74. [DOI] [PubMed] [Google Scholar]
- 6.Schugar RC, Robbins PD, Deasy BM. Small molecules in stem cell self-renewal and differentiation. Gene therapy. 2008;15(2):126–35. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453(7193):338–44. 10.1038/nature07042 [DOI] [PubMed] [Google Scholar]
- 8.Li W, Li K, Wei W, Ding S. Chemical approaches to stem cell biology and therapeutics. Cell stem cell. 2013;13(3):270–83. 10.1016/j.stem.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature medicine. 2004;10(1):55–63. [DOI] [PubMed] [Google Scholar]
- 10.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453(7194):519–23. 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–4. 10.1126/science.1239278 [DOI] [PubMed] [Google Scholar]
- 12.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. [DOI] [PubMed] [Google Scholar]
- 13.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336(6200):684–7. [DOI] [PubMed] [Google Scholar]
- 14.Boeuf H, Hauss C, Graeve FD, Baran N, Kedinger C. Leukemia inhibitory factor-dependent transcriptional activation in embryonic stem cells. The Journal of cell biology. 1997;138(6):1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. The EMBO journal. 1999;18(15):4261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho L, Miller EL, Ronan JL, Ho WQ, Jothi R, Crabtree GR. esBAF facilitates pluripotency by conditioning the genome for LIF/STAT3 signalling and by regulating polycomb function. Nature cell biology. 2011;13(8):903–13. 10.1038/ncb2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freudenberg JM, Ghosh S, Lackford BL, Yellaboina S, Zheng X, Li R, et al. Acute depletion of Tet1-dependent 5-hydroxymethylcytosine levels impairs LIF/Stat3 signaling and results in loss of embryonic stem cell identity. Nucleic acids research. 2012;40(8):3364–77. 10.1093/nar/gkr1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins K, Mohamet L, Ritson S, Merry CL, Ward CM. E-cadherin and, in its absence, N-cadherin promotes Nanog expression in mouse embryonic stem cells via STAT3 phosphorylation. Stem cells. 2012;30(9):1842–51. 10.1002/stem.1148 [DOI] [PubMed] [Google Scholar]
- 19.Huang G, Yan H, Ye S, Tong C, Ying QL. STAT3 phosphorylation at tyrosine 705 and serine 727 differentially regulates mouse ESC fates. Stem cells. 2014;32(5):1149–60. 10.1002/stem.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Aksoy I, Gonnot F, Osteil P, Aubry M, Hamela C, et al. Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nature communications. 2015;6:7095 10.1038/ncomms8095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine. 2001;14(3–4):331–41. [DOI] [PubMed] [Google Scholar]
- 22.Laity JH, Andrews GK. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1). Archives of biochemistry and biophysics. 2007;463(2):201–10. [DOI] [PubMed] [Google Scholar]
- 23.Hogstrand C, Zheng D, Feeney G, Cunningham P, Kille P. Zinc-controlled gene expression by metal-regulatory transcription factor 1 (MTF1) in a model vertebrate, the zebrafish. Biochemical Society transactions. 2008;36(Pt 6):1252–7. 10.1042/BST0361252 [DOI] [PubMed] [Google Scholar]
- 24.Azriel-Tamir H, Sharir H, Schwartz B, Hershfinkel M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. The Journal of biological chemistry. 2004;279(50):51804–16. [DOI] [PubMed] [Google Scholar]
- 25.Gruber K, Maywald M, Rosenkranz E, Haase H, Plumakers B, Rink L. Zinc deficiency adversely influences interleukin-4 and interleukin-6 signaling. Journal of biological regulators and homeostatic agents. 2013;27(3):661–71. [PubMed] [Google Scholar]
- 26.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, et al. Zinc is a novel intracellular second messenger. The Journal of cell biology. 2007;177(4):637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Z, Gao F, Kim S, Yang H, Lyu J, An W, et al. Klf4 organizes long-range chromosomal interactions with the oct4 locus in reprogramming and pluripotency. Cell stem cell. 2013;13(1):36–47. 10.1016/j.stem.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 28.Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, et al. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell stem cell. 2009;5(6):597–609. 10.1016/j.stem.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 29.Li Y, McClintick J, Zhong L, Edenberg HJ, Yoder MC, Chan RJ. Murine embryonic stem cell differentiation is promoted by SOCS-3 and inhibited by the zinc finger transcription factor Klf4. Blood. 2005;105(2):635–7. [DOI] [PubMed] [Google Scholar]
- 30.Jeon Y, Yoon JD, Cai L, Hwang SU, Kim E, Lee E, et al. Effect of zinc on in vitro development of porcine embryos. Theriogenology. 2015;84(4):531–7. 10.1016/j.theriogenology.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 31.Ryu JM, Lee MY, Yun SP, Han HJ. Zinc chloride stimulates DNA synthesis of mouse embryonic stem cells: involvement of PI3K/Akt, MAPKs, and mTOR. Journal of cellular physiology. 2009;218(3):558–67. 10.1002/jcp.21628 [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Gao Y, Yu M, Wu H, Ai Z, Wu Y, et al. Retinoic Acid Induces Embryonic Stem Cell Differentiation by Altering Both Encoding RNA and microRNA Expression. PloS one. 2015;10(7):e0132566 10.1371/journal.pone.0132566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu MJ, Bao S, Napolitano JR, Burris DL, Yu L, Tridandapani S, et al. Zinc regulates the acute phase response and serum amyloid A production in response to sepsis through JAK-STAT3 signaling. PloS one. 2014;9(4):e94934 10.1371/journal.pone.0094934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133(6):1106–17. 10.1016/j.cell.2008.04.043 [DOI] [PubMed] [Google Scholar]
- 35.Gorina R, Petegnief V, Chamorro A, Planas AM. AG490 prevents cell death after exposure of rat astrocytes to hydrogen peroxide or proinflammatory cytokines: involvement of the Jak2/STAT pathway. Journal of neurochemistry. 2005;92(3):505–18. [DOI] [PubMed] [Google Scholar]
- 36.Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500(7461):222–6. 10.1038/nature12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang T, Chen K, Zeng X, Yang J, Wu Y, Shi X, et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell stem cell. 2011;9(6):575–87. 10.1016/j.stem.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 38.Corradi S, Dakou E, Yadav A, Thomassen LC, Kirsch-Volders M, Leyns L. Morphological observation of embryoid bodies completes the in vitro evaluation of nanomaterial embryotoxicity in the embryonic stem cell test (EST). Toxicol In Vitro. 2015; 29(7): 1587–96. 10.1016/j.tiv.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 39.Webb SE, Miller AL. Calcium signalling during embryonic development. Nature reviews Molecular cell biology. 2003;4(7):539–51. [DOI] [PubMed] [Google Scholar]
- 40.Zhao MH, Liang S, Kim SH, Cui XS, Kim NH. Fe(III) Is Essential for Porcine Embryonic Development via Mitochondrial Function Maintenance. PloS one. 2015;10(7):e0130791 10.1371/journal.pone.0130791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidder BL, Yang J, Palmer S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PloS one. 2008;3(12):e3932 10.1371/journal.pone.0003932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnerch A, Cerdan C, Bhatia M. Distinguishing between mouse and human pluripotent stem cell regulation: the best laid plans of mice and men. Stem cells. 2010;28(3):419–30. 10.1002/stem.298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All cells were cultured in medium supplemented with LIF. (A): Immunostaining images of cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) for 48 hours. Cells were stained with antibody against Ki67. Nuclei were counterstained with Hoechst33342. Bars = 40μm. (B): Growth curves of cells treated with ddH2O and ZnCl2 (0.2μM and 2μM) were constructed using the CCK-8 assay. The data are represented as mean±SEM; n = 3.
(TIF)
(A): Western blot analyses of the expression of STAT3 and pSTAT3 in cells treated with 2μM ZnCl2 or 2i for 24, 48, 72, 96 and 120 hours. (B): qRT-PCR analyses of the expression levels of Stat3 target genes, Klf4 and Socs3, in cells treated with 2μM ZnCl2 or 2i for 24, 48, 72, 96 and 120 hours. The data are displayed relative to the results of the 2i treatment for 24 hours group and represented as mean±SEM; n = 3.
(TIF)
All primers used for detecting mRNA expression levels by qRT-PCR.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.