Abstract
Objectives
To evaluate whether objectively-measured sleep characteristics are associated with mortality risk independent of inflammatory burden and comorbidity.
Methods
The MrOS Sleep Study (conducted in 2003-2005) included community-dwelling older men (n=2531; average age of 76.3 (5.5 s.d.)). Sleep measures from in-home polysomnography and wrist actigraphy and assessments of serum inflammatory markers levels (C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), tumor necrosis factor-α soluble receptor II (sTNF-RII) and interferon–γ (IFN-γ)) were obtained. Vital status was ascertained over an average follow-up of 7.4 (1.9 s.d.) years.
Results
Three of the seven main sleep measures examined were independently associated with greater inflammatory burden. Mortality risk associated with prolonged (≥10% total sleep time) blood oxygen desaturation and short (<5 hours) sleep duration was attenuated to non-significance after adjusting for inflammatory burden or medical burden/lifestyle factors. Severe blood oxygen desaturation (adjusted hazard ratio (aHR)=1.57, 95% confidence interval (CI): 1.11-2.22), sleep fragmentation (aHR=1.32, 95% CI: 1.12-1.57), and a lower percentage of sleep in rapid eye movement (REM; aHR per s.d.=0.90, 95% CI: 0.93-0.97) were independently associated with mortality.
Conclusions
Short sleep duration and prolonged blood oxygen desaturation were independently associated with inflammatory burden, which attenuated associations between these sleep characteristics and mortality. Medical and lifestyle factors also substantially attenuated most sleep-mortality associations, suggesting complex relations between sleep, inflammation, and disease. Sleep fragmentation, severe blood oxygen desaturation, and the percentage of sleep time in REM were independently related to mortality risk. Future studies with repeated measures of mediators/confounds will be necessary to achieve a mechanistic understanding of sleep-related mortality risk.
Keywords: sleep, polysomnography, actigraphy, inflammation, mortality, aging, epidemiology
Both short and long sleep duration (1, 2), as well as other sleep factors such as sleep disordered breathing (SDB) (3, 4), have been linked to mortality risk. However, citing methodological concerns (regarding use of self-reported sleep measures and control of confounds/reverse causality), current literature reflects a lack of consensus regarding whether sleep directly influences mortality risk (5). Furthermore, if sleep does influence mortality risk, the biological pathways conferring such risk are unknown.
Sleep and diseases of aging may be related through inflammatory processes (6, 7). Inflammatory markers are strong predictors of disease and mortality (8-10). Evidence suggests certain sleep characteristics might affect inflammatory processes. Intermittent hypoxia can activate the sympathetic nervous system, proinflammatory transcription factors, and an inflammatory response (11). Short sleep duration may affect several factors conducive to a pro-inflammatory response, including increased blood pressure (12, 13), and altered metabolic/endocrine (14-16) and catecholamine signaling (17, 18). Sleep deprivation is associated with increased levels of circulating inflammatory markers such as C-reactive protein (CRP) (12, 19) and interleukin-6 (IL-6) (19, 20). Difficulty falling or staying asleep also plausibly affects human stress-responses, activating the above-mentioned cascades leading to heightened inflammation (i.e. (21)).
Observational evidence for the association between sleep and inflammation has produced mixed findings. Some studies have identified an independent association between sleep characteristics (namely, disordered breathing, duration, and quality ratings) and inflammation (22-33). Other studies have reported that associations between these sleep characteristics and inflammation are accounted for by confounders like disease and adiposity (34-39)). However, previous literature on sleep and inflammation has been limited by sample size and/or reliance upon subjective measures of sleep.
To our knowledge, the only prior investigation of whether inflammation attenuates sleep-related mortality risk relied on self-reported sleep duration (40). The current report therefore aimed to determine whether objectively measured sleep characteristics are associated with mortality risk independent of concurrent inflammation. Several factors other than sleep may also be associated with inflammation and mortality, and the relationship between sleep and inflammatory markers may be confounded by factors like adiposity and overt disease (34-39). Therefore, we assessed the relative roles of inflammatory burden and other health factors (that may be related to sleep, inflammation, and mortality (e.g. cardiovascular disease (41) and physical activity (42))) in attenuating associations between objectively measured sleep characteristics and mortality. We focused mainly on objective measures of sleep duration, fragmentation, and hypoxia, and secondarily include measures of sleep architecture (for comprehensiveness, and because some literature suggests potential interplay between sleep stage distributions and inflammation (e.g. (43))). Before our main analysis, we sought to replicate previously observed independent associations between sleep characteristics and inflammatory burden (44), and inflammatory burden with mortality (8-10).
Methods
Participants
From March 2000 to April 2002, the Osteoporotic Fractures in Men Study (MrOS) recruited 5,994 community-dwelling men ≥65 years of age at six clinical centers in the United States (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California) (45, 46) To be eligible to participate in the parent MrOS study, individuals had to be able to walk without assistance and be without bilateral hip replacements.
The MrOS Sleep Study was conducted between December 2003 and March 2005, and included 3,135 participants. Men were screened and excluded if they regularly used positive pressure or oral appliances during sleep for treatment of sleep apnea or used overnight nocturnal oxygen therapy (n=150). In general, men reporting nightly use of any of these devices were excluded, however seventeen men were able to forego use of their sleep devices during the night and were included in the in-home polysomnography study. Other reasons for non-participation in the Sleep Study were: death prior to sleep assessment (n=349), terminated study participation (n=39), declined sleep study (n=1997), or because MrOS Sleep Study recruitment goals had already been met prior to enrollment in the sleep study (n=324). As part of an ancillary study, inflammatory marker assays were measured from fasting serum collected at the Sleep Visit in 2562 men. A total of n=2531 men (80.7% of the Sleep Study) with complete cytokine and mortality data were included in this analysis. Ethical approval was obtained from local institutional review boards and all participants provided informed consent.
Measures
Actigraphy
The Octagonal Sleep Watch actigraphy, or SleepWatch-O, (Ambulatory Monitoring, Inc, Ardsley, NY) was used to estimate sleep/wake activity. Participants were asked to wear actigraphs on the non-dominant wrist for a minimum of 5 consecutive 24-hour periods except when bathing or during water sports; participants were also asked to keep a sleep log which was used to edit actigraph data. The actigraph measures movement using a piezoelectric biomorph-ceramic cantilevered beam, which generates a voltage each time the actigraph is moved, generating reliable estimate of sleep-wake patterns (47). These voltages are gathered continuously and stored in one minute epochs. Data collected in digital integration mode were used for this analysis. ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY) was used to score the actigraphy data (for scoring algorithms details see (48, 49)). Inter-scorer reliability for scoring of this data has been previously found to be high in our group (intra-class coefficient=0.95) (48) and this measure has been shown to have good concordance with total sleep time (TST) from polysomnography (50).
Actigraphy derived parameters used in this analysis were: TST (hours per night spent sleeping in bed after “lights off”), sleep latency (amount of time until onset of sleep, defined as when participant achieved sleep for 20 continuous minutes after getting into bed) and wake after sleep onset (WASO; minutes scored awake during the interval after sleep onset). In all analyses, actigraphy measured sleep exposures were dichotomized to represent clinically distinct sleep characteristics using the following cut-offs: short sleep duration as <5 hours (vs. 5-8 hours), long sleep duration as >8 hours (vs. 5-8 hours), sleep latency≥60 minutes, and WASO≥90 minutes.
Polysomnography
In-home sleep studies using one night of unattended polysomnography (Safiro, Compumedics, Inc., Melbourne, Australia) were performed with recording of central electroencephalography, bilateral electrooculography, chin electromyography, electrocardiogram, nasal pressure and thermistry (for airflow measurement), chest and thoracic inductance plethysmography, finger pulse oximetry, body position, and leg movements, as described before (51). Centrally trained and certified staff members performed home visits for setup of the sleep study units. After sensors were placed and calibrated, signal quality and impedance were checked, and sensors were repositioned as needed to improve signal quality, replacing electrodes if impedances were greater than 5000 ohms, using approaches similar to those in the Sleep Health Heart Study (52). Polysomnography data quality was excellent, with a failure rate of less than 4% and more than 70% of studies graded as being of excellent or outstanding quality.
Polysomnograph derived parameters included the apnea hypopnea index (AHI), computed as the average number of apneas and hypopneas per hour of recorded sleep. Apneas were defined as a complete or almost complete cessation of airflow for more than 10 seconds. Hypopneas were defined as a >30% reduction in amplitude of either respiratory effort or airflow for more than 10 seconds associated with a ≥3% oxygen desaturation (53). Parameters also included were measures of nocturnal hypoxemia: the percentage of sleep time where arterial oxygen saturation was below 90% (% TST with SaO2<90%) or below 80% (% TST with SaO2<80%). In all analyses, polysomnography measured sleep exposures were dichotomized to represent clinically significant sleep characteristics with the following cut-offs: AHI ≥30 (to represent severe SDB), ≥10% TST with SaO2<90%, ≥1% TST with SaO2<80%. The reliability determined by rescoring studies over time indicates that inter- and intra- scorer reliability for the AHI was high (ICC>0.95). Sleep stages (rapid eye movement (REM), stages 1, 2, and slow wave sleep (SWS)) were scored reliably (as previously reported (54) using standard criteria (55)) and were expressed as the percentage of sleep time spent in each stage per standard deviation unit.
Inflammatory markers
Serum was collected during morning clinic visits after an overnight fast. CRP was measured using the ELISA assay kit from ALPCO (CRP sensitive ELISA). This assay utilizes a sandwich Enzyme Immuno Assay, in which plate wells are coated with polyclonal antibodies to CRP. Inter-assay CVs have been reported previously (56). High levels of multiple inflammatory markers may reflect systemic inflammation which has been associated with greater mortality risk than single markers alone (9). Therefore we examined overall inflammatory burden, a computed variable equaling the number of inflammatory markers in the top quartile (0 to 5).
Comorbidity/other covariates
Covariates were chosen based on established clinical relationships to inflammation and/or mortality. Participants completed questionnaires including information on demographics, education, medical history, self-reported health status, physical activity (57), depressed mood (58), smoking, caffeine intake (59), and alcohol use. A history of the following medical conditions was gathered: any arthritis (including osteoarthritis or rheumatoid arthritis), cardiovascular disease (CVD), stroke, diabetes mellitus, chronic obstructive pulmonary disease (COPD), and hypertension. Although no information regarding cancer history was collected at the Sleep Visit, self-report of non-skin cancer was obtained from two other visits (Baseline and Visit 2) occurring approximately 3 years before and 1.5 years after the Sleep Visit; from this data, we computed a dichotomous variable indicating whether the participant reported any history of non-skin cancer. Participants were asked to bring all current medications used within the last 30 days with them to the Sleep Visit, whose ingredients were coded to active ingredients using a coding dictionary (60). Medications considered in analyses included: antidepressants, benzodiazepines, sedatives/hypnotics, NSAIDs, and corticosteriods. Cognitive function was measured using the Teng Modified Mini-Mental State Exam (3MS) (61). Body mass index (BMI) was calculated using standard methods. Resting SaO2 level was determined just prior to sleep using the polysomnography finger pulse oximeter.
Mortality
After the Sleep Visit, participants were contacted every four months; >97% of contact are complete. Reported deaths were confirmed with death certificates and medical records when available. Cause-specific mortality (CVD, cancer, and non-CVD/non-cancer) were based on the underlying cause of death as determined by a study physician adjudicator.
Statistical analysis
Distributions of covariates, inflammation and sleep measures were summarized by mortality status. A log-transformation was applied to cytokine measures, which were initially heavily skewed, and back-transformed for ease of interpretation. Association of the sleep and inflammatory measures were assessed with linear regression in crude and multi-variable (MV; all covariates described above) adjusted models. For descriptive purposes, adjusted survival curves were plotted for mortality by level of inflammatory burden.
A series of Cox proportional models were constructed to assess the amount of sleep-mortality risk attributable to inflammatory burden, comorbidity and/or other covariates. We first modeled the association of sleep measures with mortality after adjusting for study site, age, race, and body mass index (Model 1). Intermediate models included adjustments for only the other potential covariates listed above (Model 2a) or only inflammatory burden (Model 2b). The final model (Model 3) was adjusted for all variables. Attenuation of time-to-event associations (from Cox models) does not mathematically support a causal interpretation, and therefore we also used Aalen Additive Hazards Models to assess the pattern of attenuation using a consistent scale (mortality rates in person-years). The magnitude and pattern of attenuation were the same in Cox and Aalen models, and therefore only the former are reported. Sensitivity analyses were performed for models with SDB predictors excluding those men with a history of COPD (n=132) and then excluding men with low resting SaO2 level (≤92%, n=148).
Results
Baseline characteristics are shown by vital status (Table 1).
Table 1. Sample characteristics by all-cause and cause-specific mortality (from the Sleep Visit).
| Alive | All-Cause Mortality | CVD Mortality | Cancer Mortality | Non-CVD Non-Cancer Mortality | ||
|---|---|---|---|---|---|---|
|
|
||||||
| (n = 1903 ) | (n = 628 ) | (n = 217) | (n = 171 ) | (n = 240) | ||
|
|
||||||
| Age, mean (sd) | 75.30 (5.01) | 79.46 (5.73) | 79.82 (5.81) | 78.23 (5.60) | 80.02 (5.66) | |
| Caucasian | 1729 (90.86) | 581 (92.52) | 198 (91.24) | 159 (92.98) | 224 (93.33) | |
| African American | 58 (3.05) | 19 (3.03) | 6 (2.76) | 3 (1.75) | 10 (4.17) | |
| Other race | 116 (6.10) | 28 (4.46) | 13 (5.99) | 9 (5.26) | 6 (2.50) | |
| Smoking status | ||||||
| Current | 37 (1.94) | 12 (1.91) | 2 (0.92) | 8 (4.68) | 2 (0.83) | |
| Former | 1084 (56.96) | 381 (60.67) | 137 (63.13) | 102 (59.65) | 142 (59.17) | |
| Never | 782 (41.09) | 235 (37.42) | 78 (35.94) | 61 (35.67) | 96 (40.00) | |
| Alcohol use per week | ||||||
| <1 | 861 (45.39) | 325 (52.00) | 119 (54.84) | 79 (46.20) | 127 (53.59) | |
| 1-13 | 937(49.39) | 265 (42.40) | 88 (40.55) | 84 (49.12) | 93 (39.24) | |
| 14+ | 99 (5.22) | 35 (5.60) | 10 (4.61) | 8 (4.68) | 17 (7.17) | |
| Caffeine intake (mg/Day), median (IQR) | 184 (36 – 368) | 144 (36 – 324) | 144 (36 – 320) | 172 (36 – 404)) | 144 (0 – 324) | |
| Self-reported health status | ||||||
| Excellent or good | 1692 (88.91) | 511 (81.37) | 175 (80.65) | 151 (88.30) | 185 (77.08) | |
| Fair, poor, or very poor | 211 (11.09) | 117 (18.63) | 42 (19.35) | 20 (11.70) | 55 (22.92) | |
| Education | ||||||
| Less than high school | 96 (5.04) | 43 (6.85) | 16 (7.37) | 18 (10.53) | 9 (3.75) | |
| High school diploma | 282 (14.82) | 134 (21.34) | 52 (23.96) | 26 (15.20) | 56 (23.33) | |
| College/Graduate school | 1525 (80.14) | 451 (71.82) | 149 (68.66) | 127 (74.27) | 175 (72.92) | |
| Physical Activity (PASE) score, mean (sd) | 154.23 (70.34) | 122.86 (66.42) | 119.89 (58.64) | 133.67 (69.88) | 117.86 (69.83) | |
| Cognitive function (3MS score, 0-100), median (IQR) | 95 (91 – 97) | 92 (88 – 96) | 92 (88 – 96) | 93 (89 – 96) | 91 (86 – 95) | |
| Depression (GDS≥6) | 95 (5.00) | 61 (9.71) | 18 (8.29) | 11 (6.43) | 32 (13.33) | |
| Current antidepressant use | 144 (7.57) | 57 (9.08) | 18 (8.29) | 8 (4.68) | 31 (12.92) | |
| Current benzodiazepine use | 76 (3.99) | 42 (6.69) | 10 (4.61) | 12 (7.02) | 20 (8.33) | |
| Current non-benzodiazepine anxiolytic/hypnotic use | 43 (2.26) | 9 (1.43) | 5 (2.30) | 3 (1.75) | 1 (0.42) | |
| Current NSAID use | 423 (22.23) | 121 (19.27) | 38 (17.51) | 35 (20.47) | 48 (20.00) | |
| Current corticosteroid use(oral/nasal/inhaled) | 166 (8.76) | 65 (10.40) | 21 (9.72) | 8 (4.68) | 36 (15.13) | |
| Body mass index ( kilograms/meters2), mean (sd) | 27.19 (3.61) | 27.02 (4.16) | 27.18 (4.14) | 27.42 (4.08) | 26.58 (4.22) | |
| Chronic Disease | ||||||
| Cardiovascualar disease1 | 605 (31.79) | 305 (48.57) | 115 (53.00) | 69 (40.35) | 121 (50.42) | |
| Hypertension | 914 (48.03) | 342 (54.46) | 134 (61.75) | 86 (50.29) | 122 (50.83) | |
| Chronic obstructive pulmonary disease | 85 (4.47) | 47 (7.48) | 14 (6.45) | 9 (5.26) | 24 (10.00) | |
| Diabetes | 201 (10.56) | 94 (14.97) | 36 (16.59) | 21 (12.28) | 37 (15.42) | |
| Stroke | 60 (3.15) | 35 (5.57) | 14 (6.45) | 4 (2.34) | 17 (7.08) | |
| Arthritis | 559 (29.39) | 199 (31.69) | 63 (29.03) | 51 (29.82) | 85 (35.42) | |
| Any non-skin cancer history | 372 (19.55) | 163 (25.96) | 44 (20.28) | 65 (38.01) | 54 (22.50) | |
| Sleep Measures | ||||||
| Severe sleep apnea (AHI>30) | 310 (16.29) | 125 (19.90) | 55 (25.35) | 30 (17.54) | 40 (16.67) | |
| ≥ 10% sleep time SaO2<90% | 215 (11.30) | 102 (16.24) | 37 (17.05) | 28 (16.37) | 37 (15.42) | |
| ≥1% with sleep time SaO2<80% | 65 (3.42) | 41 (6.53) | 15 (6.91) | 17 (9.94) | 9 (3.75) | |
| Short sleep duration (<5 hours) | 206 (10.83) | 92 (14.67) | 33 (15.21) | 21 (12.35) | 38 (15.83) | |
| Long sleep duration (>8 hours) | 133 (6.99) | 50 (7.97) | 16 (7.37) | 8 (4.71) | 26 (10.83) | |
| Prolonged sleep latency (≥60 minutes) | 182 (9.57) | 84 (13.38) | 30 (13.82) | 22 (12.94) | 32 (13.33) | |
| Greater WASO (≥90 minutes) | 540 (28.39) | 256 (40.83) | 93 (42.86) | 64 (37.65) | 99 (41.25) | |
| Inflammation Markers (median (IQR)) | ||||||
| IL-6 (pg/ml) | 1 (0.71 – 1.52) | 1.40 (0.92 – 2.20) | 1.55 (1.04 – 2.69) | 1.19 ).77 – 1.71) | 1.47 (0.90 – 2.24) | |
| CRP (ug/ml) | 1.45 (0.71 – 2.82) | 1.76 (0.87 – 3.91) | 2.00 (1.00 – 4.50) | 1.56 (0.80 – 2.93) | 1.79 (0.85 – 3.95) | |
| TNF-α (pg/ml) | 4.99 (4.12 – 6.03)) | 5.63 (4.53 – 6.87) | 5.63 (4.70 – 6.78) | 5.15 (4.10 – 6.73) | 5.92 (4.78 – 7.15) | |
| TNF-αsRII (pg/ml) | 3409.60 (2792.20 – 4297.10) | 4149.60 (3189.98 – 5687.65) | 4229.90 (3196.90 – 5877.25) | 3725.20 (2888.00 – 4976.80) | 4485.15 (3389.45 – 5901.00) | |
| IFN-γ (pg/ml) | 1.83 (1.04 – 3.18) | 1.96 (1.16 – 3.33) | 1.97 (1.30 – 3.37) | 1.97 (1.08 – 3.34) | 1.91 (1.12 – 3.30) | |
| # inf markers in top quartile | 1 (0 - 2) | 1 (1 – 3) | 2 (1 – 3) | 1 (0 – 2) | 2 (1 – 3) | |
Cardiovascular disease includes myocardial infarction (MI), angina, temporary ischemic attack (TIA), claudication, or congestive heart failure (CHF)
Abbreviations: IQR=interquartile range; PASE=Physical Activity Scale for the Elderly; 3MS=Modified Mini-Mental State examination; GDS=Geriatric Depression Scale; NSAID = Nonsteroidal anti-inflammatory drug; AHI=apnea hypopnea index; SaO2=Oxygen Saturation; WASO=Wake after sleep onset; IL-6=Interleukin-6; CRP=C-Reactive Protein; TNF-α=Tumor Necrosis Factor-Alpha; TNF-αSRII=Tumor Necrosis Factor-Alpha Soluble Receptor 2; IFN-γ=Interferon Gamma
Sleep and inflammatory burden
Most participants (65.03%) had less than 2 markers in the top quartile of that markers distribution, 17.8% had 2 markers in the top quartile, 10.7% had 3 markers in the top quartile, 5.31% had 4 markers in the top quartile, and 1.8% were in the highest quartile for all markers. After adjustments for other covariates (including adiposity and chronic diseases), having ≥10% of TST with SaO2<90%, short sleep duration (<5 hours), and WASO ≥90 minutes were independently associated with inflammatory burden (Table 2). Sleep measures were also independently associated with levels of the individual markers (Supplemental Table 1; unadjusted associations available upon request). Sleep architecture measures were not independently associated with the individual inflammatory markers (stage 2 and IL-6 p=0.14, all other p's>0.30) or inflammatory burden (all p>0.29).
Table 2. Multivariable adjusted associations of sleep measures with inflammatory burden as the dependent variable.
| βϯ | SE | p | |
|---|---|---|---|
|
|
|||
| AHI ≥30 vs. <30 | 0.11 | 0.07 | 0.088 |
| ≥10% vs. <10% TST SaO2<90% | 0.24 | 0.08 | 0.002 |
| ≥1% vs. <1% TST SaO2<80% | -0.14 | 0.13 | 0.27 |
| TST <5 vs. 5-8 | 0.20 | 0.08 | 0.013 |
| TST >8 vs. 5-8 | 0.00 | 0.10 | 0.99 |
| Sleep latency ≥60 vs. <60 min | 0.12 | 0.08 | 0.14 |
| WASO ≥90 vs. <90 min | 0.13 | 0.05 | 0.024 |
β coefficient (after MV adjustments) from linear regression; similar results observed using negative binomial regression
MV=Multivariable models adjusted for: age, study site, race, BMI, probable depression, cognition, smoking status, alcohol use, education, caffeine use, physical activity, chronic disease (any arthritis (including osteoarthritis or rheumatoid arthritis), cardiovascular disease (CVD), stroke, diabetes mellitus, chronic obstructive pulmonary disease (COPD), and hypertension), self-reported health and medication use (antidepressants, benzodiazepines, sedatives/hypnotics, NSAIDs, and corticosteroids)
Inflammatory burden and mortality
In fully adjusted Cox models including sleep measures, inflammatory burden remained a significant and unique predictor of all-cause, CVD, non-CVD/non-cancer (all p trend <0.0001) but not cancer mortality.
Sleep and mortality
In Model 1, prolonged blood oxygen desaturation (≥10% TST SaO2<90%), having ≥1% TST with severe blood oxygen desaturation (SaO2<80%), short (<5 hours) sleep duration, and greater WASO (≥90 minutes) were associated with mortality risk (Table 3). The AHI, long (>8 hour) sleep duration, and prolonged sleep latency were not associated with mortality risk. Expressing the AHI continuously, or re-parameterizing the AHI (0-5 vs. ≥5-15 vs. ≥15-30 vs. ≥30) and sleep duration (≤5, ≥5-6, ≥6-7, ≥7-8, ≥8 hours) did not alter these findings.
Table 3. Hazard ratio (95% CI) of mortality by sleep measures.
| All cause (628 deaths) | CVD (217 deaths) | Cancer (171 deaths) | Non-cancer Non-CVD (240 deaths) | |
|---|---|---|---|---|
|
|
||||
| AHI ≥30 vs. <30 | ||||
| Model 1: Crude model | 1.06 (0.86-1.29) | 1.38 (1.00-1.90) | 0.89 (0.59-1.34) | 0.90 (0.63-1.27) |
| Model 2a: Model 1 + MV | 1.03 (0.84-1.27) | 1.35 (0.98-1.88) | 0.88 (0.58-1.33) | 0.86 (0.60-1.24) |
| Model 2b: Model 1 + # high inflammatory markers | 1.03 (0.84-1.26) | 1.33 (0.97-1.84) | 0.88 (0.59-1.33) | 0.87 (0.61-1.24) |
| Model 3: Model 1 + MV* + # high inflammatory markers | 1.03 (0.84-1.27) | 1.34 (0.97-1.85) | 0.87 (0.58-1.32) | 0.87 (0.61-1.26) |
| ≥10% vs. <10% TST SaO2<90% | ||||
| Model 1: Crude model | 1.28 (1.02-1.61) | 1.24 (0.85-1.80) | 1.30 (0.84-1.99) | 1.32 (0.91-1.92) |
| Model 2a: Model 1 + MV | 1.13 (0.90-1.43) | 1.12 (0.77-1.64) | 1.29 (0.83-2.00) | 1.09 (0.74-1.60) |
| Model 2b: Model 1 + # high inflammatory markers | 1.17 (0.93-1.46) | 1.08 (0.74-1.58) | 1.27 (0.83-1.96) | 1.17 (0.80-1.70) |
| Model 3: Model 1 + MV* + # high inflammatory markers | 1.07 (0.85-1.35) | 1.02 (0.69-1.49) | 1.27 (0.82-1.98) | 1.00 (0.68-1.47) |
| ≥1% vs. <1% TST SaO2<80% | ||||
| Model 1: Crude model | 1.58 (1.15-2.20) | 1.54 (0.90-2.65) | 2.46 (1.45-4.17) | 0.99 (0.50-1.94) |
| Model 2a: Model 1 + MV | 1.54 (1.09-2.16) | 1.53 (0.88-2.68) | 2.30 (1.35-3.92) | 0.81 (0.37-1.78) |
| Model 2b: Model 1 + # high inflammatory markers | 1.54 (1.11-2.14) | 1.48 (0.86-2.55) | 2.45 (1.44-4.14) | 0.94 (0.48-1.86) |
| Model 3: Model 1 + MV* + # high inflammatory markers | 1.57 (1.11-2.22) | 1.62 (0.93-2.84) | 2.31 (1.36-3.94) | 0.82 (0.38-1.81) |
| TST <5 vs. 5-8 | ||||
| Model 1: Crude model | 1.28 (1.02-1.62) | 1.25 (0.85-1.84) | 0.98 (0.61-1.58) | 1.56 (1.09-2.24) |
| Model 2a: Model 1 + MV | 1.17 (0.92-1.48) | 1.19 (0.80-1.77) | 0.94 (0.58-1.53) | 1.35 (0.92-1.97) |
| Model 2b: Model 1 + # high inflammatory markers | 1.20 (0.95-1.51) | 1.15 (0.78-1.69) | 0.97 (0.60-1.56) | 1.45 (1.01-2.08) |
| Model 3: Model 1 + MV* + # high inflammatory markers | 1.12 (0.89-1.42) | 1.12 (0.76-1.67) | 0.93 (0.58-1.52) | 1.28 (0.87-1.88) |
| TST >8 vs. 5-8 | ||||
| Model 1: Crude model | 1.02 (0.76-1.37) | 0.93 (0.55-1.55) | 0.59 (0.29-1.20) | 1.46 (0.96-2.21) |
| Model 2a: Model 1 + MV | 0.94 (0.69-1.27) | 0.92 (0.55-1.55) | 0.57 (0.27-1.17) | 1.29 (0.83-2.00) |
| Model 2b: Model 1 + # high inflammatory markers | 1.03 (0.77-1.38) | 0.94 (0.56-1.57) | 0.59 (0.29-1.20) | 1.47 (0.97-2.22) |
| Model 3: Model 1 + MV* + # high inflammatory markers | 0.83 (0.71-1.31) | 0.95 (0.56-1.61) | 0.57 (0.28-1.18) | 1.32 (0.85-2.05) |
| Sleep latency ≥60 vs. <60 min | ||||
| Model 1: Crude model | 1.25 (0.99-1.58) | 1.24 (0.84-1.84) | 1.23 (0.78-1.93) | 1.28 (0.88-1.87) |
| Model 2a: Model 1 + MV | 1.13 (0.89-1.44) | 1.14 (0.76-1.71) | 1.22 (0.77-1.94) | 1.06 (0.71-1.58) |
| Model 2b: Model 1 + # high inflammatory markers | 1.19 (0.94-1.50) | 1.15 (0.78-1.70) | 1.22 (0.77-1.92) | 1.20 (0.82-1.75) |
| Model 3: Model 1 + MV* + # high inflammatory markers | 1.12 (0.88-1.42) | 1.13 (0.75-1.69) | 1.25 (0.79-1.98) | 1.04 (0.70-1.56) |
| WASO ≥90 vs. <90 min | ||||
| Model 1: Crude model | 1.50 (1.28-1.77) | 1.57 (1.19-2.07) | 1.30 (0.95-1.79) | 1.59 (1.23-2.07) |
| Model 2a: Model 1 + MV | 1.34 (1.14-1.59) | 1.45 (1.09-1.92) | 1.24 (0.90-1.72) | 1.36 (1.03-1.79) |
| Model 2b: Model 1 + # high inflammatory markers | 1.47 (1.25-1.73) | 1.52 (1.15-2.01) | 1.29 (0.94-1.78) | 1.56 (1.20-2.03) |
| Model 3: Model 1 + MV* + # high inflammatory markers | 1.32 (1.12-1.57) | 1.44 (1.08-1.91) | 1.24 (0.89-1.71) | 1.33 (1.01-1.76) |
Crude models is adjusted for age, study site, race, and body mass index
MV=Multivariable models adjusted for: age, study site, race, BMI, probable depression, cognition, alcohol use, education, smoking status, caffeine use, physical activity, chronic disease (any arthritis (including osteoarthritis or rheumatoid arthritis), cardiovascular disease, stroke, diabetes mellitus, chronic obstructive pulmonary disease (COPD), hypertension, and any non-skin cancer history), self-reported health and medication use (antidepressants, benzodiazepines, sedatives/hypnotics, NSAIDs, and corticosteroids)
Abbreviations: CVD=Cardiovascular Disease; AHI=apnea hypopnea index; TST=Total Sleep Time; SaO2=Oxygen Saturation; WASO=Wake After Sleep Onset; Bold= Statistically significant.
Adjustments for other covariates or inflammatory burden alone substantively attenuated associations of several specific sleep factors with mortality (Table 3). Mortality risk associated with prolonged blood oxygen desaturation (≥10% TST SaO2<90%) was attenuated by 54% and 39% to non-significance after adding comorbidity/other covariates or inflammatory burden alone, respectively; full adjustments resulted in a total 75% attenuation (Model 3). Having ≥1% TST with SaO2<80% remained independently predictive of mortality after all adjustments (total attenuation was 2%); excluding lung cancer deaths did not alter this substantively association.
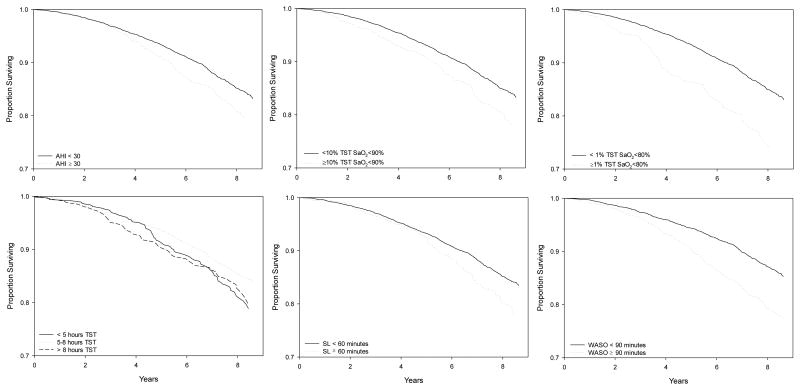
Increased mortality risk associated with short (<5 hour) sleep duration was attenuated by 40% and 29% to non-significance after adjustments in Model 2a and 2b, respectively; after all adjustments attenuation totaled 50%. Greater WASO (≥90 minutes) remained significantly associated with mortality risk after all adjustments (Model 3), although comorbidity/other covariates, inflammatory burden alone, and all adjustments attenuated this risk (by 32%, 6%, and 36%, respectively). Excluding deaths within 1 and 2 years of baseline did not alter the association of greater WASO with mortality (also see survival by WASO in Figure 1).
Figure 1.
MV* Adjusted Overall and Cause-Specific Survival by Sleep Characteristics. “*MV = Multivariable adjusted for age, study site, race, BMI, probable depression, cognition, smoking status, alcohol use, education, caffeine use, physical activity, chronic diseases, self-reported health and medication use; Abbreviations: CVD=Cardiovascular;”
A similar pattern of results was obtained after repeating analyses excluding participants with COPD, low levels of waking SaO2 (≤92%), or when adding levels of CRP and IL-6 in place of inflammatory burden.
Sleep architecture measures were not associated with all-cause mortality in any model (all crude model p's>0.20), except for the percentage of time spent in REM sleep. Greater percentage of time spent in REM sleep was associated with lower risk of all-cause mortality and non-cancer/non-CVD mortality; this association was not substantively attenuated by adjustments and retained significance in the final model (all-cause mortality HR per SD=0.90, 95% CI 0.93-0.97).
In the fully adjusted models (e.g. Model 3 including WASO), greater physical activity and higher global cognitive performance was associated with lower mortality risk (per SD on the Physical Activity Scale for the Elderly, HR 0.82, 95% CI 0.74-0.90; per SD on the 3MS, HR 0.80, 95% CI 0.75-0.86), while greater age (per year; HR 1.09, 95% CI 1.10-1.11), CVD (HR 1.32, 95% CI 1.11-1.56), diabetes (HR 1.37, 95% CI 1.09-1.73), and oral corticosteroid use (HR 1.89, 95% CI 1.22-2.94) were independently associated with increased mortality risk.
Discussion
In this large sample of community-dwelling older men, we found specific polysomnography and actigraphy assessed sleep disturbances were associated with inflammatory burden independent of BMI, lifestyle, medication, and chronic disease factors. Over 7 years of follow-up, baseline inflammatory burden independently predicted non-cancer mortality. Our main novel findings is that the mortality risk associated with prolonged nighttime blood oxygen desaturation (≥10% TST SaO2<90%) and short sleep duration was substantively attenuated by adjustment for inflammatory burden alone and other medical burden/lifestyle factors; note that the attenuation after adjusting for inflammatory burden and other health status covariates separately (Models 2a and 2b) then together (Model 3) suggests these adjustments accounted for some shared and some distinct variance in mortality risk that was attributed to these sleep characteristics (in Model 1). These findings are consistent with prior research on self-reported sleep duration, inflammation, disease, and mortality (40), and suggests these aspects of sleep may be linked to mortality through concurrent inflammation and disease.
On the other hand, the association between greater sleep fragmentation (WASO) with both CVD and non-cancer/non-CVD deaths was independent of inflammatory burden and morbidity in our study. Morbidity/other covariates somewhat attenuated the association of WASO with mortality, but inflammatory burden did not appear to have a substantial role attenuating WASO-related mortality risk. In addition, we found that the percentage of night-time sleep spent in REM stage was associated with mortality risk independent of all covariates, and this appeared to be driven by non-cancer/non-CVD deaths; future research should investigate the pathways mediating this risk.
Similarly, severe hemoglobin desaturation (SaO2<80%) during sleep predicted mortality, and this relationship was not substantively attenuated inflammatory burden, morbidity, or other covariates; this risk was associated with severe SDB rather than lung disease, as indicated by the fact that these relationships held after excluding men who had low levels of blood oxygen saturation during wakefulness, a history of chronic lung disease, or death from lung cancer. The overall association of severe night-time blood oxygen desaturation with all-cause mortality appeared to be driven by cancer mortality, a finding consistent with literature that suggests links between sleep apnea (which is associated with blood oxygen desaturation) and cancer (i.e. see (62)). In contrast, we found that the AHI, a commonly used measure of the rate of apneas and hypopneas (and intermittent hypoxia), was not independently associated with all-cause or cancer mortality. Instead, the AHI was associated with CVD mortality only prior to adjustment for concurrent health factors. Future studies are needed to assess whether and how distinct biological pathways link intermittent versus severe hypoxia to these different causes of death (CVD and cancer), including the potential carcinogenic role of severe hypoxia.
Strengths of our study include the objective assessments of sleep within a large sample of older adults that were not selected on the basis of sleep problems. Additional strengths include the careful examination of whether sleep-related mortality risk might be attributable to inflammatory burden or other health factors. We found that inflammatory burden alone attenuated crude relationships between prolonged blood oxygen desaturation and short sleep duration with mortality. Although this finding is consistent with a biologically plausible hypothesis (that inflammation mediates associations between these aspects of sleep and mortality), our study is limited by measuring sleep, inflammatory markers, and covariates cross-sectionally. Inflammatory processes may both precede and result from overt disease, and can influence and be influenced by sleep. Indeed, health covariates also substantially attenuated the mortality risk associated with prolonged blood oxygen desaturation and short sleep duration. Our study design does not enable an assessment of the temporal relations sleep, inflammation, disease, and mortality. Instead, our findings suggest sleep, inflammation, and the measured health status covariates reflect somewhat overlapping pathophysiological processes relevant to longevity. Future research including repeated measures of these factors (sleep, inflammation, and health status) is needed to test whether, in fact, sleep impacts inflammatory burden to influence mortality-risk, above and beyond pre-existing disease processes.
Additional limitations should be noted. We observed a substantial role for the health status covariates studied, but we were unable to assess other physiological markers which may have driven these effects (potentially including pro-thrombotic, cortisol, or metabolic factors like blood glucose levels). Our sample consisted of only older men who were 90% Caucasian and agreed to participate in an in-depth sleep evaluation; these findings should not be generalized to other populations including younger adults, women, and some ethnic groups. Patients with severe sleep disorders may have been less likely to participate, and therefore effects of severe sleep disorders on mortality may have been missed in our study, resulting in an under-estimate of the true mortality risk associated with sleep in the population. We present all-cause, CVD, cancer, and non-CVD/non-cancer mortality associations, however our statistical power for cause-specific groups may be limited. Given the observational nature of our study, residual confounding may be present, in particular via pre-existing sub-clinical disease. Several other sleep-wake characteristics (i.e. timing, rhythmicity, perceived quality) were not included in the current study and must be investigated in future research.
Despite these limitation, to our knowledge, our study is the first to demonstrate independent associations between objectively measured sleep characteristics and a biologically proximal factor (inflammatory burden) that attenuates observed sleep-related mortality risk. Prolonged nighttime blood oxygen desaturation and short sleep duration may be markers of mortality that reflect underlying disease processes including the inflammatory response. We also found that long sleep duration was not a marker of future mortality risk, even in crudely adjusted models; this suggests, at least in older men, greater than eight hour sleep duration may not be detrimental to longevity.
However, men in our study with severe nighttime oxygen desaturation, sleep fragmentation, and less of their sleep in REM were at increased risk for mortality. Mortality risk associated with severe nighttime oxygen desaturation, sleep fragmentation, and REM sleep was independent of a range of factors (including disease and inflammation), therefore, understanding and treating these sleep disturbances successfully among older men may be especially important to preserve longevity.
Supplementary Material
Acknowledgments
SFS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank Megan M. Marron, M.S., for her expert statistical and technical assistance. The MrOS Sleep Study was supported through the grants (see funding) with principal investigator KLS and co-investigators SR, SAI, and JAC.
Source of Funding: The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 TR000128, K24-AR04884-06. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. Inflammatory marker data was supported by National Institutes of Health funding from the National Heart, Lung, and Blood Institute (NHLBI) under the grant number HL084183-01. SFS is supported by T32 AG000181. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Abbreviations
- SDB
sleep disordered breathing
- CRP
C-reactive protein
- IL-6
interleukin-6
- MrOS
Osteoporotic Fractures in Men Study
- TST
total sleep time
- WASO
wake after sleep onset
- AHI
apnea hypopnea index
- IFN-γ
interferon gamma
- TNF-α
tumor necrosis factor alpha
- TNF-αsRII
tumor necrosis factor alpha soluble receptor two
- CVD
cardiovascular disease
- COPD
chronic obstructive pulmonary disease
- NSAID
non-steroidal anti-inflammatory drug
- 3MS
Teng Modified Mini-Mental State Exam
- SaO2
blood oxygen saturation
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. Journal of sleep research. 2009;18(2):148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 2.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurina LM, McClintock MK, Chen J-H, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Annals of Epidemiology. 23(6):361–70. doi: 10.1016/j.annepidem.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Current vascular pharmacology. 2007;5(2):93–102. doi: 10.2174/157016107780368280. Epub 2007/04/14. [DOI] [PubMed] [Google Scholar]
- 7.Miller MA, Cappuccio FP. Biomarkers of cardiovascular risk in sleep-deprived people. Journal of human hypertension. 2013;27(10):583–8. doi: 10.1038/jhh.2013.27. Epub 2013/04/19. [DOI] [PubMed] [Google Scholar]
- 8.Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clinical and experimental immunology. 2003;132(1):24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. The American journal of medicine. 1999;106(5):506–12. doi: 10.1016/s0002-9343(99)00066-2. Epub 1999/05/21. [DOI] [PubMed] [Google Scholar]
- 10.Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB. Cardiovascular Disease, Interleukin-6, and Risk of Mortality in Older Women : The Women's Health and Aging Study. Circulation. 2001;103(7):947–53. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- 11.Ryan S, McNicholas WT. Intermittent hypoxia and activation of inflammatory molecular pathways in OSAS. Arch Physiol Biochem. 2008;114(4):261–6. doi: 10.1080/13813450802307337. [DOI] [PubMed] [Google Scholar]
- 12.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology. 2004;43(4):678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32(6):760–6. doi: 10.1093/sleep/32.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiology & behavior. 2010;99(5):651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–9. doi: 10.1016/s0140-6736(99)01376-8. Epub 1999/10/30. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. The Journal of clinical endocrinology and metabolism. 2004;89(11):5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 17.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. The Journal of clinical endocrinology and metabolism. 1999;84(6):1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 18.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain, behavior, and immunity. 2003;17(5):365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 19.van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Harma M, Porkka-Heiskanen T, Alenius H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PloS one. 2009;4(2):e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trammell RA, Verhulst S, Toth LA. Effects of sleep fragmentation on sleep and markers of inflammation in mice. Comparative medicine. 2014;64(1):13–24. Epub 2014/02/12. [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–4. doi: 10.1093/sleep/32.2.200. Epub 2009/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman EM. Sleep quality, social well-being, gender, and inflammation: an integrative analysis in a national sample. Annals of the New York Academy of Sciences. 2011;1231:23–34. doi: 10.1111/j.1749-6632.2011.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lui MM, Lam JC, Mak HK, Xu A, Ooi C, Lam DC, Mak JC, Khong PL, Ip MS. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135(4):950–6. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 25.Zouaoui Boudjeltia K, Van Meerhaeghe A, Doumit S, Guillaume M, Cauchie P, Brohee D, Vanhaeverbeek M, Kerkhofs M. Sleep apnoea-hypopnoea index is an independent predictor of high-sensitivity C-reactive protein elevation. Respiration. 2006;73(2):243–6. doi: 10.1159/000090201. [DOI] [PubMed] [Google Scholar]
- 26.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30(1):29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steiropoulos P, Papanas N, Nena E, Antoniadou M, Serasli E, Papoti S, Hatzizisi O, Kyriazis G, Tzouvelekis A, Maltezos E, Tsara V, Bouros D. Inflammatory markers in middle-aged obese subjects: does obstructive sleep apnea syndrome play a role? Mediators Inflamm. 2010;2010:675320. doi: 10.1155/2010/675320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medeiros CA, de Bruin VM, Andrade GM, Coutinho WM, de Castro-Silva C, de Bruin PF. Obstructive sleep apnea and biomarkers of inflammation in ischemic stroke. Acta Neurol Scand. 2012;126(1):17–22. doi: 10.1111/j.1600-0404.2011.01589.x. [DOI] [PubMed] [Google Scholar]
- 29.Liukkonen T, Rasanen P, Ruokonen A, Laitinen J, Jokelainen J, Leinonen M, Meyer-Rochow VB, Timonen M. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosomatic medicine. 2007;69(8):756–61. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 30.Matthews KA, Zheng H, Kravitz HM, Sowers M, Bromberger JT, Buysse DJ, Owens JF, Sanders M, Hall M. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women's Health across the Nation sleep study. Sleep. 2010;33(12):1649–55. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Annals of epidemiology. 2011;21(11):799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahlman J, Miettinen K, Peuhkurinen K, Seppa J, Peltonen M, Herder C, Punnonen K, Vanninen E, Gylling H, Partinen M, Uusitupa M, Tuomilehto H, Kuopio Sleep Apnoea G. The activation of the inflammatory cytokines in overweight patients with mild obstructive sleep apnoea. Journal of sleep research. 2010;19(2):341–8. doi: 10.1111/j.1365-2869.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 33.Guven SF, Turkkani MH, Ciftci B, Ciftci TU, Erdogan Y. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012;16(1):217–21. doi: 10.1007/s11325-011-0492-2. [DOI] [PubMed] [Google Scholar]
- 34.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP) - No association with sleep duration or sleep disordered breathing. Sleep. 2007;30(8):991–6. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosomatic medicine. 2006;68(3):376–81. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 36.Sharma SK, Mishra HK, Sharma H, Goel A, Sreenivas V, Gulati V, Tahir M. Obesity, and not obstructive sleep apnea, is responsible for increased serum hs-CRP levels in patients with sleep-disordered breathing in Delhi. Sleep medicine. 2008;9(2):149–56. doi: 10.1016/j.sleep.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Fornadi K, Lindner A, Czira ME, Szentkiralyi A, Lazar AS, Zoller R, Turanyi CZ, Veber O, Novak M, Mucsi I, Molnar MZ. Lack of association between objectively assessed sleep disorders and inflammatory markers among kidney transplant recipients. Int Urol Nephrol. 2012;44(2):607–17. doi: 10.1007/s11255-011-0095-7. [DOI] [PubMed] [Google Scholar]
- 38.Brady EM, Davies MJ, Hall AP, Talbot DC, Dick JL, Khunti K. An investigation into the relationship between sleep-disordered breathing, the metabolic syndrome, cardiovascular risk profiles, and inflammation between South Asians and Caucasians residing in the United Kingdom. Metab Syndr Relat Disord. 2012;10(2):152–8. doi: 10.1089/met.2011.0073. [DOI] [PubMed] [Google Scholar]
- 39.Laugsand LE, Vatten LJ, Bjorngaard JH, Hveem K, Janszky I. Insomnia and high-sensitivity C-reactive protein: the HUNT study, Norway. Psychosomatic medicine. 2012;74(5):543–53. doi: 10.1097/PSY.0b013e31825904eb. [DOI] [PubMed] [Google Scholar]
- 40.Hall MH, Smagula SF, Boudreau RM, Ayonayon HN, Goldman SE, Harris TB, Naydeck BL, Rubin SM, Samuelsson L, Satterfield S, Stone KL, Visser M, Newman AB. Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the Health, Aging and Body Composition Study. Sleep. 2015;38(2):189–95. doi: 10.5665/sleep.4394. Epub 2014/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby P. Inflammation and cardiovascular disease mechanisms. The American journal of clinical nutrition. 2006;83(2):456s–60s. doi: 10.1093/ajcn/83.2.456S. Epub 2006/02/14. [DOI] [PubMed] [Google Scholar]
- 42.Hamer M, Sabia S, Batty GD, Shipley MJ, Tabak AG, Singh-Manoux A, Kivimaki M. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation. 2012;126(8):928–33. doi: 10.1161/circulationaha.112.103879. Epub 2012/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas KS, Motivala S, Olmstead R, Irwin MR. Sleep Depth and Fatigue: Role of Cellular Inflammatory Activation. Brain, behavior, and immunity. 2011;25(1):53–8. doi: 10.1016/j.bbi.2010.07.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.014. Epub 2015/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemporary clinical trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92. doi: 10.1093/sleep/26.3.342. Epub 2003/05/17. [DOI] [PubMed] [Google Scholar]
- 48.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28(12):1599–605. doi: 10.1093/sleep/28.12.1599. Epub 2006/01/18. [DOI] [PubMed] [Google Scholar]
- 49.Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. Journal of neuroscience methods. 2001;105(2):185–91. doi: 10.1016/s0165-0270(00)00364-2. Epub 2001/03/29. [DOI] [PubMed] [Google Scholar]
- 50.Blackwell T, Ancoli-Israel S, Redline S, Stone KL Osteoporotic Fractures in Men Study G. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2011;7(4):357–67. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehra R, Stone KL, Blackwell T, Ancoli Israel S, Dam TT, Stefanick ML, Redline S. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55(9):1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. Epub 2007/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–67. Epub 2001/04/13. [PubMed] [Google Scholar]
- 53.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–85. Epub 1998/03/11. [PubMed] [Google Scholar]
- 54.Smagula SF, Reynolds CF, Ancoli-Israel S, Barrett-Connor E, Dam T-T, Hughes-Austin JM, Paudel M, Redline S, Stone KL, Cauley JA for the Osteoporotic Fractures in Men Research G. Sleep Architecture and Mental Health Among Community-Dwelling Older Men. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015;70(5):673–81. doi: 10.1093/geronb/gbt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rechtschaffen A KA, editor. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Vol. 204. Washington DC: National Institutes of Health: NIH Publication; p. 1968. [Google Scholar]
- 56.Smagula SF, Ancoli-Israel S, Barrett-Connor E, Lane NE, Redline S, Stone KL, Cauley JA. Inflammation, sleep disturbances, and depressed mood among community-dwelling older men. Journal of psychosomatic research. 2014;76(5):368–73. doi: 10.1016/j.jpsychores.2014.02.005. Epub 2014/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. Epub 1993/02/01. [DOI] [PubMed] [Google Scholar]
- 58.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology. 1986;5:165–73. [Google Scholar]
- 59.Barone JJ, Roberts HR. Caffeine consumption. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1996;34(1):119–29. doi: 10.1016/0278-6915(95)00093-3. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 60.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. European journal of epidemiology. 1994;10(4):405–11. doi: 10.1007/BF01719664. Epub 1994/08/01. [DOI] [PubMed] [Google Scholar]
- 61.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987;48(8):314–8. Epub 1987/08/01. [PubMed] [Google Scholar]
- 62.Cao J, Feng J, Li L, Chen B. Obstructive sleep apnea promotes cancer development and progression: a concise review. Sleep & breathing = Schlaf & Atmung. 2015 doi: 10.1007/s11325-015-1126-x. Epub 2015/02/04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.